Abstract
Aims
Although serum uric acid (SUA) level is correlated with oxidative stress and serves as a marker of poor prognosis in heart failure patients, its possible association with subclinical left ventricular (LV) dysfunction has not been evaluated. This study aimed to investigate the association between SUA and subclinical LV dysfunction in a sample of a general population without overt cardiac disease.
Methods and results
We examined 1175 participants who underwent extensive cardiovascular health check‐up including laboratory tests and speckle‐tracking echocardiography to assess LV global longitudinal strain (GLS). The association of SUA concentration, as a continuous variable and a categorical variable using quartiles, with the presence of abnormal LVGLS was assessed. Mean age was 62 ± 12 years, and 656 (56%) were male participants. Mean SUA was 5.6 ± 1.3 mg/dL (25th–75th percentile, 4.6–6.5 mg/dL). The prevalence of abnormal LVGLS (greater than –18.6%) was greatest in the upper quartile of SUA. In multivariable analysis, SUA as a continuous variable was significantly associated with abnormal LVGLS [adjusted odds ratio (OR), 1.26 per 1 mg/dL; P = 0.008] independent of traditional cardiovascular risk factors, pertinent laboratory parameters and echocardiographic measures, and medications. In the categorical analysis, the upper quartile of SUA was independently associated with abnormal LVGLS in a fully adjusted model (adjusted OR, 2.28 vs. lowest quartile; P = 0.020).
Conclusions
In a sample of the general population, an elevated SUA was independently associated with subclinical LV dysfunction. Assessment of LVGLS may add important prognostic information in individuals with elevated SUA, even in the absence of overt cardiac disease.
Keywords: Echocardiography, Global longitudinal strain, Primary prevention, Uric acid
Introduction
Heart failure (HF) is a growing epidemic that affects nearly 5.7 million US adults, and the projected number of HF patients is 8.0 million in 2030. 1 Although survival after the onset of HF in adults has improved because of the development of various medications and device therapy, the overall 1 year HF mortality still remains high at approximately 30%. 1 These observations highlight the need for the identification of novel risk factors that may be amenable to intervention and for the early detection of individuals at higher risk for HF. Previous studies reported higher serum uric acid (SUA) levels in patients with HF 2 , 3 and suggested that SUA could serve as a marker for unfavourable prognosis. 4 , 5 , 6 , 7 , 8 The aetiology of elevated SUA in HF is likely multifactorial, including increased xanthine oxidase activity related to oxidative stress and inflammation and renal dysfunction because of hypoperfusion. 2 , 3 , 9 In recent years, several studies also reported the predictive value of SUA level for incident HF in some clinical settings, 10 , 11 , 12 , 13 suggesting that increase in SUA may in fact predict the onset of HF.
Left ventricular global longitudinal strain (LVGLS) is an echocardiographic measure of LV myocytes deformation, which can unmask subclinical abnormality in LV systolic function, even when LV ejection fraction (EF) is in the normal range. 14 , 15 LVGLS has prognostic value for cardiovascular events including HF and mortality, which is independent and additive to that of LVEF. 16 , 17 , 18 , 19 , 20 However, there was no study investigating the independent effect of SUA on subclinical LV dysfunction in the general population with normal LVEF. Understanding the association between SUA and subclinical LV dysfunction might provide insight into the pathogenesis linking elevated SUA and incident HF and may help inform the possible preventive strategies for HF. The aim of the present study was to investigate whether elevated SUA concentration is associated with impaired LVGLS in a community‐based cohort without overt cardiac disease.
Methods
Study population
The study population was derived from the Subclinical Cardiac Dysfunction in General Population (SCADGP) study. This study was designed to assess the prevalence and determinants of subclinical cardiac dysfunction in a community‐based cohort, who underwent an extensive cardiovascular health check from August 2014 to May 2018 in the University of Tokyo. Among the 1243 participants enrolled in the SCADGP study, participants who met the following criteria were excluded: history of atrial fibrillation or atrial flutter (n = 15), history of coronary artery disease (n = 29), decreased LV systolic fraction (LVEF < 50%) or significant valvular disease (n = 17), and suboptimal image quality or incomplete assessment of the echocardiographic parameters (n = 7). Thus, the final study group consisted of 1175 participants without overt cardiac disease. Written informed consent was obtained from all study participants. The study was approved by the Institutional Review Boards of the University of Tokyo.
Risk factor assessment and laboratory examination
Cardiovascular risk factors were ascertained through interviews and direct examination by research assistants. Hypertension was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg or the use of antihypertensive medication. 1 Diabetes mellitus was defined by the current use of insulin or hypoglycemic agents or a fasting glucose of ≥126 mg/dL. 1 Hypercholesterolemia was defined as total serum cholesterol >240 mg/dL, or the use of lipid‐lowering medications. 1 Body mass index (BMI) was calculated using height and weight (kg/m2).
Venous blood samples were drawn in the fasting condition on the same day as the echocardiographic examination. The SUA level was determined using a validated enzymatic method (UA‐CL, Serotec, Chiba, Japan). Fasting blood glucose, high‐density lipoprotein (HDL) cholesterol, low‐density lipoprotein cholesterol, estimated glomerular filtration rate (eGFR), C‐reactive protein (CRP), and B‐type natriuretic peptide were also analysed in all participants.
Echocardiographic examination
Two‐dimensional echocardiography
Echocardiographic examination was performed using a commercially available system (Aplio 300, Toshiba Medical Systems, Tokyo, Japan) in accordance with a standardized protocol by trained sonographers blinded to the participant's clinical information. The dimensions of the cardiac chambers were measured in the standard manner. 21 LV mass was calculated with a validated formula 22 : LV mass = 0.8{1.04[(SWT + LVEDD + PWT)3−LVEDD3]} + 0.6, where SWT is the LV end‐diastolic septal wall thickness, LVEDD is the LV end‐diastolic diameter, and PWT is the LV end‐diastolic posterior wall thickness. Left atrial volume was measured from the apical two‐chamber and four‐chamber views using the biplane Simpson's rule. 21 LV mass and left atrial volume were then indexed for body surface area. LV diastolic parameters were assessed according to the current guideline. 23 Briefly, transmitral diastolic flow was obtained from an apical four‐chamber view. Pulsed‐wave Doppler examination of mitral inflow was performed to measure early (E) and late peak velocity. Peak early diastolic mitral annular velocity (e′) was also measured from tissue Doppler imaging in the lateral and the septal mitral annulus, and the average value was used. The ratio of E to mean e′ was then calculated (E/e′).
Speckle‐tracking echocardiography
Speckle‐tracking analysis was performed offline using vendor‐independent commercially available software (two‐dimensional cardiac performance analysis; Tomtec Imaging System, Germany). Semi‐automated border detection was performed, and LV borders were tracked throughout the entire cardiac cycle. Manual correction was performed in case of inaccurate endocardial detection. LVGLS was calculated averaging the negative peak of longitudinal strain from all three apical views including the four‐chamber, two‐chamber, and long‐axis views, according to the current guideline. 24 Impaired LVGLS was defined as a GLS greater than −18.6%, which was the 90th percentile of the strain value distribution in the SCADGP participants without any conditions associated with LV remodelling including hypertension, diabetes mellitus, coronary artery disease, significant valvular disease, arrhythmias, or BMI >25 kg/m2. Excellent correlations were observed in the inter‐observer and intra‐observer variabilities of LVGLS in 15 randomly selected participants (r = 0.94 and r = 0.95, respectively). In Bland–Altman analysis, agreement in LVGLS between the inter‐observer and intra‐observer measurements were −0.3 ± 2.1% and 0.8 ± 1.9%, respectively (mean ± 1.96 SD).
Statistical analysis
Categorical variables are presented as numbers and frequencies (%) and continuous variables as means ± standard deviations. Univariable correlation between SUA level and echocardiographic parameters was evaluated by Pearson's correlation coefficients (r). Univariable and multivariable logistic regression analyses were conducted to evaluate the association of SUA level as continuous variable as well as categorical variable using quartiles with abnormal LVGLS, adjusting for significant potential cofactors (variables with P < 0.1 in the univariable analysis) in the multivariable logistic regression models with stepwise fashion in five models (Model 1: adjustment for age and sex; Model 2: adjustment for age, sex, hypertension, diabetes mellitus, and BMI; Model 3: adjustment as in Model 2 plus laboratory parameters including fasting glucose, HDL cholesterol, eGFR, and CRP levels; Model 4: adjustment as in Model 3 plus echocardiographic parameters (LVEF, LV mass index, and E/e′); Model 5: adjustment as in Model 4 plus diuretics use.) and corresponding odds ratios (ORs) with their 95% confidence interval (CI) were reported. A value of P < 0.05 was considered significant. All statistical analyses were performed using JMP 14 software (SAS Institute, Cary, NC, USA).
Results
Clinical characteristics and echocardiographic data in the study population are shown in Table 1 . The mean age was 62 ± 12 years, and 656 (56%) were male participants. The mean value of SUA was 5.6 ± 1.3 mg/dL (25th–75th percentile, 4.6–6.5 mg/dL). The mean LVEF was 63 ± 6%, and LVGLS was −21.3 ± 2.7%.
Table 1.
Characteristics of the study population
| N = 1175 | |
|---|---|
| Age, years | 62 ± 12 |
| Male gender, n (%) | 656 (55.8) |
| Hypertension, n (%) | 408 (34.7) |
| Diabetes, n (%) | 120 (10.2) |
| Hypercholesterolemia, n (%) | 430 (36.6) |
| Body mass index, kg/m2 | 23.5 ± 3.5 |
| Uric acid lowering drugs, n (%) | 78 (6.6) |
| Diuretics, n (%) | 35 (3.0) |
| Laboratory examination | |
| Glucose, mg/dL | 100 ± 19 |
| Total cholesterol, mg/dL | 206 ± 34 |
| LDL cholesterol. mg/dL | 125 ± 30 |
| HDL cholesterol, mg/dL | 66 ± 19 |
| Uric acid, mg/dL | 5.6 ± 1.3 |
| eGFR, mL/min/1.73m2 | 72 ± 15 |
| C‐reactive protein, mg/dL | 0.12 ± 0.35 |
| B‐type natriuretic peptide, pg/mL | 23.6 ± 26.8 |
| Echocardiography | |
| LV end‐diastolic diameter, mm | 45 ± 4 |
| LV end‐systolic diameter, mm | 27 ± 4 |
| LV ejection fraction, % | 63 ± 6 |
| LV mass index, g/m2 | 71 ± 16 |
| LA volume index, mL/m2 | 25 ± 7 |
| E wave, cm/sec | 70 ± 15 |
| A wave, cm/sec | 68 ± 20 |
| e′, cm/sec | 8.2 ± 2.3 |
| E/e′ | 9.06 ± 2.80 |
| LVGLS, % | −21.3 ± 2.7 |
Values are mean ± standard deviation or n (percentage). A, late diastolic transmitral flow velocity; E, early diastolic transmitral flow velocity; e′, early diastolic mitral annular velocity; eGFR, estimated glomerular filtration rate; GLS, global longitudinal strain; HDL, high‐density lipoprotein; LA, left atrium; LDL, low‐density lipoprotein; LV, left ventricle.
Serum uric acid was significantly correlated with LVGLS (r = 0.23, P < 0.001; Figure 1 ). On the other hand, there was no correlation between SUA and E/e′ (r = 0.04, P = 0.20). The prevalence of abnormal LVGLS (greater than −18.6%) was greatest in the upper quartile of SUA (21.9%), followed by the third (14.9%), second (10.4%), and first (7.6%) quartiles (Figure 2 ). Table 2 shows the univariate association of clinical, laboratory, and echocardiographic variables with abnormal LVGLS. The SUA level was associated with abnormal LVGLS in the age‐adjusted and sex‐adjusted model (Table 3 , Model 1). In the multivariable model adjusted for age, sex, hypertension, diabetes mellitus, and BMI, this association persisted (Table 3 , Model 2). With further adjustment for laboratory parameters (fasting glucose, HDL cholesterol, eGFR, and CRP levels), SUA remained significantly associated with LVGLS (Table 3 , Model 3). Even after adjustment for echocardiographic parameters including LVEF, LV mass index, and E/e′, SUA was still independently associated with abnormal LVGLS (Table 3 , Model 4), and this association persisted after adjustment for diuretics (adjusted OR, 1.26 per 1 mg/dL; P = 0.008; Table 3 , Model 5). When SUA was examined as a categorical variable in the multivariable models, the upper quartile of SUA (≥6.5 mg/dL) was independently associated with abnormal LVGLS in the fully adjusted model (adjusted OR 2.28 vs. lowest quartile; 95% CI 1.14–4.58; P = 0.020; Figure 3 , Model 5).
Figure 1.
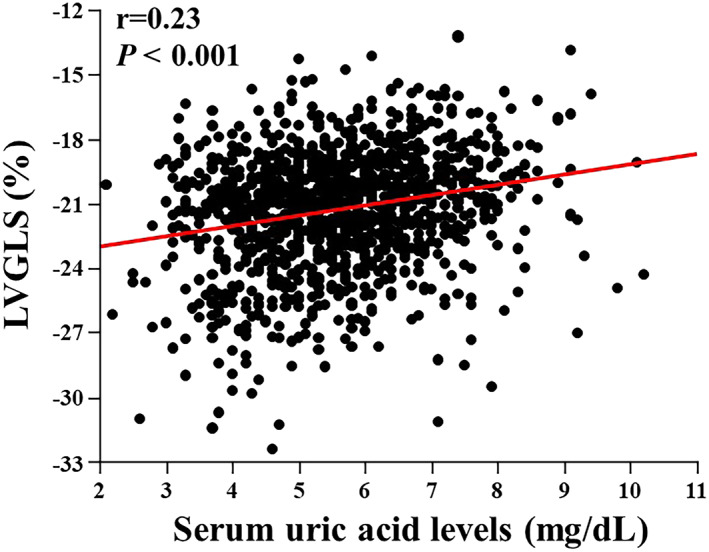
Scatter plots illustrating the association between SUA and LVGLS. LVGLS, left ventricular global longitudinal strain; SUA, serum uric acid.
Figure 2.
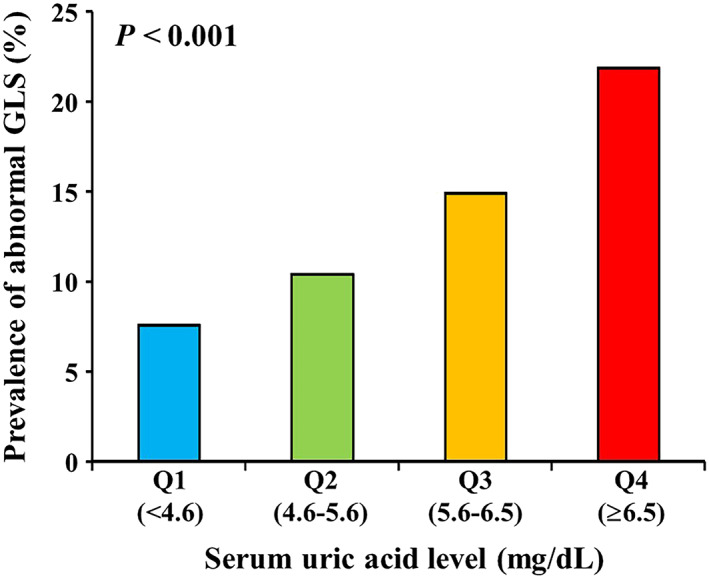
Prevalence of abnormal GLS according to the serum uric acid level. GLS, global longitudinal strain.
Table 2.
Univariable logistic regression analysis for abnormal LVGLS
| Odds ratio (95% CI) | P value | |
|---|---|---|
| Age, years | 1.02 (1.007–1.04) | 0.004 |
| Sex, male | 2.02 (1.42–2.90) | <0.001 |
| Hypertension | 1.90 (1.36–2.65) | <0.001 |
| Diabetes mellitus | 1.55 (0.93–2.48) | 0.089 |
| Hypercholesterolemia | 0.92 (0.65–1.30) | 0.641 |
| Body mass index, kg/m2 | 1.10 (1.05–1.15) | <0.001 |
| Uric acid lowering drugs | 1.53 (0.82–2.68) | 0.175 |
| Diuretics | 2.58 (1.22–5.48) | 0.022 |
| Glucose, mg/dL | 1.01 (1.001–1.02) | 0.022 |
| Total cholesterol, mg/dL | 1.00 (0.99–1.002) | 0.249 |
| LDL cholesterol, mg/dL | 1.00 (0.99–1.004) | 0.604 |
| HDL cholesterol, mg/dL | 0.98 (0.97–0.99) | <0.001 |
| Uric acid, mg/dL | 1.43 (1.26–1.63) | <0.001 |
| eGFR, mL/min/1.73m2 | 0.98 (0.97–0.99) | 0.002 |
| Log C‐reactive protein, mg/dL | 1.61 (1.12–2.28) | 0.008 |
| LV end‐diastolic diameter, mm | 1.03 (0.995–1.07) | 0.088 |
| LV end‐systolic diameter, mm | 1.09 (1.05–1.14) | <0.001 |
| LV ejection fraction, % | 0.80 (0.76–0.83) | <0.001 |
| LV mass index, g/m2 | 1.03 (1.02–1.04) | <0.001 |
| LA volume index, mL/m2 | 1.00 (0.97–1.02) | 0.771 |
| E/e′ | 1.09 (1.04–1.15) | 0.002 |
CI, confidence interval; E, early diastolic transmitral flow velocity, e′, early diastolic mitral annular velocity; eGFR, estimated glomerular filtration rate; GLS, global longitudinal strain; HDL, high‐density lipoprotein; LA, left atrium; LDL, low‐density lipoprotein; LV, left ventricle.
Table 3.
Association of SUA level with abnormal LVGLS
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | |
| SUA, mg/dL | 1.39 (1.20–1.61) | <0.001 | 1.32 (1.14–1.54) | <0.001 | 1.29 (1.10–1.51) | 0.002 | 1.26 (1.06–1.49) | 0.009 | 1.26 (1.06–1.50) | 0.008 |
CI, confidence interval; CRP, C‐reactive protein; E, early diastolic transmitral flow velocity; e′, early diastolic mitral annular velocity; eGFR, estimated glomerular filtration rate; GLS, global longitudinal strain; HDL, high‐density lipoprotein; LV, left ventricle; SUA, serum uric acid.
Model 1: age and sex adjusted.
Model 2: adjusted for age, sex, hypertension, diabetes mellitus, and body mass index.
Model 3: adjusted for variables as in Model 2 and fasting glucose, HDL cholesterol, eGFR, and CRP.
Model 4: adjusted for variables as in Model 3 and LV ejection fraction, LV mass index, and E/e′.
Model 5: adjusted for variables as in Model 4 and diuretics use.
Figure 3.

Association of SUA quartiles with abnormal LVGLS.Reference: Quartile 1. Multivariable Model 1: adjusted for age and sex. Multivariable Model 2: adjusted for age, sex, hypertension, diabetes mellitus and BMI. Multivariable Model 3: adjusted as in Model 2 with further adjustment for laboratory parameters including fasting glucose, HDL cholesterol, eGFR and CRP. Multivariable Model 4: adjusted as in Model 3 with further adjustment for LV ejection fraction, LV mass index, and E/e′. Multivariable Model 5: adjusted as in Model 4 with further adjustment for diuretics use. BMI, body mass index; CI, confidence interval; CRP, C‐reactive protein; E, early diastolic transmitral flow velocity; e′, early diastolic mitral annular velocity; eGFR, estimated glomerular filtration rate; GLS, global longitudinal strain; HDL, high‐density lipoprotein; LV, left ventricle; SUA, serum uric acid.
Finally, in a sensitivity analysis performed in participants without treatment with SUA lowering drugs (N = 1097), the results of the fully adjusted models were concordant with those of the general analysis, with SUA levels remaining an independent predictor of abnormal LVGLS (adjusted OR, 1.25 per 1 mg/dL; 95% CI, 1.04–1.50; P = 0.015).
Discussion
We demonstrated that an elevated SUA level was associated with impaired LVGLS in a sample of the general population without overt cardiac disease. The association was independent of traditional cardiovascular risk factors as well as pertinent laboratory and echocardiographic parameters.
Previous studies demonstrated that an elevated SUA level had prognostic value in patients with chronic as well as acute HF of varying severity. 4 , 5 , 6 , 7 , 8 Anker et al. showed that a high SUA level predicted poorer outcomes (mortality and the need for transplant) in 112 patients with moderate or severe chronic HF. 4 Pascual‐Figal et al. identified the SUA level as a strong predictor of death and HF hospital readmission in 212 acute HF patients with LVEF <40%. 5 Wu et al. also demonstrated that a higher quartile of SUA was independently associated with mortality in 1152 severe HF patients with LVEF <30% during a median follow‐up of 404 days. 6 A similar result was observed in HF patients with preserved LVEF. 8 The pathogenesis of elevated SUA in the setting of HF is likely multifactorial, involving impaired renal function as well as inflammatory cytokine activation and abnormalities in oxidative metabolism, 2 , 3 , 9 although the precise nature of the link between SUA and prognosis in HF is still uncertain. Current studies demonstrated that the SUA level is associated with incident HF in some clinical settings. 10 , 11 , 12 , 13 Krishnan et al. showed that subjects with SUA >6.3 mg/dL had a 2.1‐fold higher risk of incident HF compared with those with SUA <3.4 mg/dL in 4912 individuals from the Framingham Offspring cohort. 11 Holmes et al. demonstrated that SUA was an independent predictor of HF development in 417 734 Swedish individuals who underwent health check‐ups in outpatient clinics. 10 Kaya et al. identified hyperuricemia as an independent predictor for new‐onset HF in 2249 patients with myocardial infarction. 13 Our results are in agreement with these clinical studies and extend their findings to the subclinical LV dysfunction domain.
The quantification of myocardial systolic strain as an index of LV function is now feasible with speckle‐tracking echocardiography. 25 , 26 GLS, a measure of the myocardial systolic deformation over the longitudinal axis, is emerging as an important tool to detect early LV dysfunction and is more sensitive than LVEF. 14 , 15 Moreover, an impaired GLS is associated with unfavourable cardiovascular outcomes including HF independent of LVEF. 16 , 17 , 18 , 19 , 20 We demonstrate for the first time that an elevated SUA level was associated with impaired GLS in a general population with normal LVEF, independent of traditional cardiovascular risk factors, pertinent laboratory parameters including serum CRP levels and eGFR, and LV morphology and diastolic function. Upper quartile of SUA (≥6.5 mg/dL) carries approximately two‐fold increased risk of abnormal LVGLS. Our findings suggest that SUA may act as a surrogate marker of subclinical LV dysfunction, although the possibility that it may play a mechanistic role in its development cannot be excluded on these data. Furthermore, the relationship between elevated SUA and impaired LVGLS may be involved in explaining the increased HF risk in individuals with high SUA level. The underlying mechanisms of this association are not entirely clear, but we hypothesize several potential explanations. First, SUA is an index of oxidative stress that could be involved in ventricular remodelling, affecting inotropic and/or lusitropic function, and elevated SUA could potentially lead to myocardial fibrosis. 9 , 27 Second, SUA contributes to endothelial dysfunction via impairing nitric oxide production and subsequent vasoconstriction and altered myocyte and endothelial cell guanosine monophosphate cyclase resulting in increased cardiovascular rest tension and extracellular matrix deposition. 28 , 29 Third, elevated SUA may induce a chronic inflammatory state leading to worsened LV function. 3 Finally, elevated SUA may impair the coronary microcirculation resulting in LV dysfunction. 30 Although a high SUA level might be an epiphenomenon and just serve as a marker of reduced excretion because of renal dysfunction, which may reduce LV contractility, we demonstrated a significant association between SUA levels and LVGLS independent of renal function. Previous studies demonstrated the association between SUA and diastolic parameters in patients with established cardiac disease including HF and cardiomyopathy, 31 , 32 , 33 whereas SUA was not correlated with E/e′ in the present study. This discrepancy might be partially explained by the difference in study population, namely, we included individuals without overt cardiac disease who had relatively preserved diastolic function.
While our observations raise the possibility of HF prevention in individuals with elevated SUA levels, conflicting results were obtained on the effects of therapeutic interventions to reduce SUA. Oxypurinol reduced SUA without any clinical benefits in 405 symptomatic HF patients, although a post hoc analysis suggested possible benefits in patients with SUA >9.5 mg/dL. 34 Furthermore, Givertz et al. found that allopurinol failed to improve clinical status as well as LVEF at 24 weeks in 253 symptomatic HF patients with LVEF ≤40% and SUA ≥9.5 mg/dL. 35 On the other hand, a recent multicentre, prospective, randomized open‐label study demonstrated the beneficial effect of xanthine oxidase inhibitors on cardiovascular outcomes in 1070 hyperuricaemic subjects. 36 These conflicting results might be partially explained by the difference in study population, namely, xanthine oxidase inhibitor might be useful in individuals free of HF but not in the setting of established HF. Future studies are warranted to assess whether therapeutic strategies aimed at lowering SUA levels may improve subclinical LV dysfunction and prevent or delay the development of HF. Furthermore, although we demonstrated the association between SUA and subclinical LV dysfunction, future studies are needed to explore the pathophysiological mechanisms linking SUA and subclinical LV dysfunction, which might provide valuable information regarding the possible preventive therapeutic strategies for LV dysfunction and subsequent HF development in individuals with elevated SUA.
Study limitations
Because of the cross‐sectional nature of our study, we could not confirm a cause–effect relationship between SUA and subclinical LV dysfunction or estimate the impact of SUA on HF development. Although some previous studies demonstrated the association between SUA and the extent of coronary atherosclerosis, 37 we cannot clearly ascertain the absence of obstructive coronary artery disease. In addition, the impact of differences in diet on our observations could not be assessed, because complete information of diet was not uniformly available in our study. These factors might affect our observations about the independent association between SUA and subclinical LV dysfunction.
Conclusions
This study demonstrated a significant association between elevated SUA and impaired LVGLS in a sample of the general population without overt cardiac disease. This finding may be important in explaining the underlying pathophysiological mechanism for the higher incidence of HF in individuals with elevated SUA. In addition, the assessment of LVGLS may add important information in the prognostic assessment of individuals with elevated SUA, even in the absence of overt cardiac disease.
Conflict of interest
None declared.
Funding
None.
Author contributions
K.N. and Y.Y. contributed to the conception and design of the work. K.N., M.D., Y.Y., T.N., Y.M., and I.K. contributed to the acquisition, analysis, or interpretation of data for the work. K.N. drafted the manuscript. M.D., Y.Y., J.I., N.S., M.H., H.K., T.N., Y.M., H.M., M.R.D., S.H., and I.K. critically revised the manuscript. All gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Acknowledgements
The authors wish to thank Yutaka Yatomi, MD for the general support.
Nakanishi, K. , Daimon, M. , Yoshida, Y. , Ishiwata, J. , Sawada, N. , Hirokawa, M. , Kaneko, H. , Nakao, T. , Mizuno, Y. , Morita, H. , Di Tullio, M. R. , Homma, S. , and Komuro, I. (2020) Serum uric acid level and subclinical left ventricular dysfunction: a community‐based cohort study. ESC Heart Failure, 7: 1031–1038. 10.1002/ehf2.12691.
References
- 1. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O'Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, On behalf of the American Heart Association Council on Epidemiology , Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart Disease and Stroke Statistics‐2018 Update: A Report From the American Heart Association. Circulation 2018; 137: e67–e492. [DOI] [PubMed] [Google Scholar]
- 2. Leyva F, Anker S, Swan JW, Godsland IF, Wingrove CS, Chua TP, Stevenson JC, Coats AJ. Serum uric acid as an index of impaired oxidative metabolism in chronic heart failure. Eur Heart J 1997; 18: 858–865. [DOI] [PubMed] [Google Scholar]
- 3. Leyva F, Anker SD, Godsland IF, Teixeira M, Hellewell PG, Kox WJ, Poole‐Wilson PA, Coats AJ. Uric acid in chronic heart failure: a marker of chronic inflammation. Eur Heart J 1998; 19: 1814–1822. [DOI] [PubMed] [Google Scholar]
- 4. Anker SD, Doehner W, Rauchhaus M, Sharma R, Francis D, Knosalla C, Davos CH, Cicoira M, Shamim W, Kemp M, Segal R, Osterziel KJ, Leyva F, Hetzer R, Ponikowski P, Coats AJ. Uric acid and survival in chronic heart failure: validation and application in metabolic, functional, and hemodynamic staging. Circulation 2003; 107: 1991–1997. [DOI] [PubMed] [Google Scholar]
- 5. Pascual‐Figal DA, Hurtado‐Martinez JA, Redondo B, Antolinos MJ, Ruiperez JA, Valdes M. Hyperuricaemia and long‐term outcome after hospital discharge in acute heart failure patients. Eur J Heart Fail 2007; 9: 518–524. [DOI] [PubMed] [Google Scholar]
- 6. Wu AH, Ghali JK, Neuberg GW, O'Connor CM, Carson PE, Levy WC. Uric acid level and allopurinol use as risk markers of mortality and morbidity in systolic heart failure. Am Heart J 2010; 160: 928–933. [DOI] [PubMed] [Google Scholar]
- 7. Hamaguchi S, Furumoto T, Tsuchihashi‐Makaya M, Goto K, Goto D, Yokota T, Kinugawa S, Yokoshiki H, Takeshita A, Tsutsui H, Investigators J‐C. Hyperuricemia predicts adverse outcomes in patients with heart failure. Int J Cardiol 2011; 151: 143–147. [DOI] [PubMed] [Google Scholar]
- 8. Palazzuoli A, Ruocco G, De Vivo O, Nuti R, McCullough PA. Prevalence of hyperuricemia in patients with acute heart failure with either reduced or preserved ejection fraction. Am J Cardiol 2017; 120: 1146–1150. [DOI] [PubMed] [Google Scholar]
- 9. Bergamini C, Cicoira M, Rossi A, Vassanelli C. Oxidative stress and hyperuricaemia: pathophysiology, clinical relevance, and therapeutic implications in chronic heart failure. Eur J Heart Fail 2009; 11: 444–452. [DOI] [PubMed] [Google Scholar]
- 10. Holme I, Aastveit AH, Hammar N, Jungner I, Walldius G. Uric acid and risk of myocardial infarction, stroke and congestive heart failure in 417,734 men and women in the Apolipoprotein MOrtality RISk study (AMORIS). J Intern Med 2009; 266: 558–570. [DOI] [PubMed] [Google Scholar]
- 11. Krishnan E. Hyperuricemia and incident heart failure. Circ Heart Fail 2009; 2: 556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ekundayo OJ, Dell'Italia LJ, Sanders PW, Arnett D, Aban I, Love TE, Filippatos G, Anker SD, Lloyd‐Jones DM, Bakris G, Mujib M, Ahmed A. Association between hyperuricemia and incident heart failure among older adults: a propensity‐matched study. Int J Cardiol 2010; 142: 279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kaya MG, Uyarel H, Akpek M, Kalay N, Ergelen M, Ayhan E, Isik T, Cicek G, Elcik D, Sahin O, Cosgun SM, Oguzhan A, Eren M, Gibson CM. Prognostic value of uric acid in patients with ST‐elevated myocardial infarction undergoing primary coronary intervention. Am J Cardiol 2012; 109: 486–491. [DOI] [PubMed] [Google Scholar]
- 14. Ng AC, Delgado V, Bertini M, van der Meer RW, Rijzewijk LJ, Shanks M, Nucifora G, Smit JW, Diamant M, Romijn JA, de Roos A, Leung DY, Lamb HJ, Bax JJ. Findings from left ventricular strain and strain rate imaging in asymptomatic patients with type 2 diabetes mellitus. Am J Cardiol 2009; 104: 1398–1401. [DOI] [PubMed] [Google Scholar]
- 15. Potter E, Marwick TH. Assessment of left ventricular function by echocardiography: The case for routinely adding global longitudinal strain to ejection fraction. JACC Cardiovasc Imaging 2018; 11: 260–274. [DOI] [PubMed] [Google Scholar]
- 16. Stanton T, Leano R, Marwick TH. Prediction of all‐cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging 2009; 2: 356–364. [DOI] [PubMed] [Google Scholar]
- 17. Motoki H, Borowski AG, Shrestha K, Troughton RW, Tang WH, Thomas JD, Klein AL. Incremental prognostic value of assessing left ventricular myocardial mechanics in patients with chronic systolic heart failure. J Am Coll Cardiol 2012; 60: 2074–2081. [DOI] [PubMed] [Google Scholar]
- 18. Ersboll M, Valeur N, Mogensen UM, Andersen MJ, Moller JE, Velazquez EJ, Hassager C, Sogaard P, Kober L. Prediction of all‐cause mortality and heart failure admissions from global left ventricular longitudinal strain in patients with acute myocardial infarction and preserved left ventricular ejection fraction. J Am Coll Cardiol 2013; 61: 2365–2373. [DOI] [PubMed] [Google Scholar]
- 19. Russo C, Jin Z, Elkind MS, Rundek T, Homma S, Sacco RL, Di Tullio MR. Prevalence and prognostic value of subclinical left ventricular systolic dysfunction by global longitudinal strain in a community‐based cohort. Eur J Heart Fail 2014; 16: 1301–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Park JJ, Park JB, Park JH, Cho GY. Global longitudinal strain to predict mortality in patients with acute heart failure. J Am Coll Cardiol 2018; 71: 1947–1957. [DOI] [PubMed] [Google Scholar]
- 21. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28: 1–39. [DOI] [PubMed] [Google Scholar]
- 22. Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation 1977; 55: 613–618. [DOI] [PubMed] [Google Scholar]
- 23. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016; 29: 277–314. [DOI] [PubMed] [Google Scholar]
- 24. Voigt JU, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R, Pedri S, Ito Y, Abe Y, Metz S, Song JH, Hamilton J, Sengupta PP, Kolias TJ, d'Hooge J, Aurigemma GP, Thomas JD, Badano LP. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. J Am Soc Echocardiogr 2015; 28: 183–193. [DOI] [PubMed] [Google Scholar]
- 25. Smiseth OA, Torp H, Opdahl A, Haugaa KH, Urheim S. Myocardial strain imaging: how useful is it in clinical decision making? Eur Heart J 2016; 37: 1196–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Collier P, Phelan D, Klein A. A test in context: myocardial strain measured by speckle‐tracking echocardiography. J Am Coll Cardiol 2017; 69: 1043–1056. [DOI] [PubMed] [Google Scholar]
- 27. Jia G, Habibi J, Bostick BP, Ma L, DeMarco VG, Aroor AR, Hayden MR, Whaley‐Connell AT, Sowers JR. Uric acid promotes left ventricular diastolic dysfunction in mice fed a Western diet. Hypertension 2015; 65: 531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kanellis J, Kang DH. Uric acid as a mediator of endothelial dysfunction, inflammation, and vascular disease. Semin Nephrol 2005; 25: 39–42. [DOI] [PubMed] [Google Scholar]
- 29. Maruhashi T, Hisatome I, Kihara Y, Higashi Y. Hyperuricemia and endothelial function: from molecular background to clinical perspectives. Atherosclerosis 2018; 278: 226–231. [DOI] [PubMed] [Google Scholar]
- 30. Gullu H, Erdogan D, Caliskan M, Tok D, Kulaksizoglu S, Yildirir A, Muderrisoglu H. Elevated serum uric acid levels impair coronary microvascular function in patients with idiopathic dilated cardiomyopathy. Eur J Heart Fail 2007; 9: 466–468. [DOI] [PubMed] [Google Scholar]
- 31. Cicoira M, Zanolla L, Rossi A, Golia G, Franceschini L, Brighetti G, Zeni P, Zardini P. Elevated serum uric acid levels are associated with diastolic dysfunction in patients with dilated cardiomyopathy. Am Heart J 2002; 143: 1107–1111. [DOI] [PubMed] [Google Scholar]
- 32. Nogi S, Fujita S, Okamoto Y, Kizawa S, Morita H, Ito T, Sakane K, Sohmiya K, Hoshiga M, Ishizaka N. Serum uric acid is associated with cardiac diastolic dysfunction among women with preserved ejection fraction. Am J Physiol Heart Circ Physiol 2015; 309: H986–H994. [DOI] [PubMed] [Google Scholar]
- 33. Norvik JV, Schirmer H, Ytrehus K, Storhaug HM, Jenssen TG, Eriksen BO, Mathiesen EB, Lochen ML, Wilsgaard T, Solbu MD. Uric acid predicts mortality and ischaemic stroke in subjects with diastolic dysfunction: the Tromso Study 1994‐2013. ESC Heart Fail 2017; 4: 154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hare JM, Mangal B, Brown J, Fisher C Jr, Freudenberger R, Colucci WS, Mann DL, Liu P, Givertz MM, Schwarz RP, Investigators O‐C. Impact of oxypurinol in patients with symptomatic heart failure. Results of the OPT‐CHF study. J Am Coll Cardiol 2008; 51: 2301–2309. [DOI] [PubMed] [Google Scholar]
- 35. Givertz MM, Anstrom KJ, Redfield MM, Deswal A, Haddad H, Butler J, Tang WH, Dunlap ME, LeWinter MM, Mann DL, Felker GM, O'Connor CM, Goldsmith SR, Ofili EO, Saltzberg MT, Margulies KB, Cappola TP, Konstam MA, Semigran MJ, McNulty SE, Lee KL, Shah MR, Hernandez AF, NHLBI Heart Failure Clinical Research Network . Effects of xanthine oxidase inhibition in hyperuricemic heart failure patients: the xanthine oxidase inhibition for hyperuricemic heart failure patients (EXACT‐HF) study. Circulation 2015; 131: 1763–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kojima S, Matsui K, Hiramitsu S, Hisatome I, Waki M, Uchiyama K, Yokota N, Tokutake E, Wakasa Y, Jinnouchi H, Kakuda H, Hayashi T, Kawai N, Mori H, Sugawara M, Ohya Y, Kimura K, Saito Y, Ogawa H. Febuxostat for Cerebral and CaRdiorenovascular Events PrEvEntion StuDy. Eur Heart J 2019; 40: 1778–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Biscaglia S, Ceconi C, Malagu M, Pavasini R, Ferrari R. Uric acid and coronary artery disease: an elusive link deserving further attention. Int J Cardiol 2016; 213: 28–32. [DOI] [PubMed] [Google Scholar]


