Abstract
Aims
Immunomodulation in heart failure (HF) has been studied in several randomized controlled trials (RCTs) with variable effects on cardiac structure, function, and outcomes. We sought to determine the effect of immunomodulation on left ventricular ejection fraction (LVEF), LV end‐diastolic dimension (LVEDD), and all‐cause mortality in patients with HF with reduced ejection fraction (HFrEF) through meta‐analyses and trial sequential analyses (TSAs) of RCTs.
Methods and results
PubMed, Embase®, Cochrane CENTRAL, and http://ClinicalTrials.gov were systematically reviewed to identify RCTs that studied the effects of immunomodulation in patients with HFrEF. The primary endpoint in this analysis was change in LVEF. Secondary outcomes were changes in LVEDD and all‐cause mortality. TSA was used to quantify the statistical reliability of data in the cumulative meta‐analyses. Nineteen RCTs with 1341 HFrEF subjects were eligible for analyses. The aetiology of HF, specific immunomodulation strategy, and treatment duration were variable across trials. Immunomodulation led to a greater improvement in LVEF [mean difference: +5.7% 95% confidence interval (CI): 3.0–8.5%, P < 0.001] and reduction in LVEDD (mean difference: −3.7 mm, 95% CI: −7.0 to −0.4 mm, P = 0.028) than no immunomodulation in meta‐analyses and TSAs. We observed a non‐significant decrease in all‐cause mortality among those on immumomodulation (risk ratio: 0.7, 95% CI: 0.4–1.3, P = 0.234), but the Z‐curve for cumulative treatment effect of immunomodulation in the TSA did not cross the boundary of futility.
Conclusions
Immunomodulation led to improved cardiac structure and function in patients with HFrEF. While these benefits did not translate into a significant improvement in mortality, our analysis suggests that larger studies of targeted immunomodulation are needed to understand the true benefits.
Keywords: Heart failure, Inflammation, Immunomodulation, Left ventricular ejection fraction, Anti‐cytokine therapy
Introduction
Heart failure (HF) is a pro‐inflammatory state.1, 2 Several pathways including pro‐inflammatory cytokines and innate and adaptive immune cellular responses have been implicated in HF.3, 4, 5, 6, 7 It is also evident that dysregulated inflammation and immune activation contribute importantly to progressive left ventricular (LV) remodelling and dysfunction.4, 8, 9, 10 Hence, inflammatory and immune pathways are theoretically appealing as therapeutic targets in HF to improve adverse LV remodelling and prognosis. Several, generally small, randomized controlled trials (RCTs) have evaluated a variety of immunomodulation strategies in patients with HF with variable degrees of success.4, 9, 11 These approaches have ranged from broad immunomodulation targeting multiple pathways to drugs and/or biologics targeting specific cytokines or cellular components of inflammation. Anti‐cytokine approaches in HF to inhibit tumour necrosis factor (TNF)‐α1 and interleukin (IL)‐111 did not yield appreciable benefits in mortality or major adverse cardiovascular events, although a recent sub‐analysis of the Canakinumab Antiinflammatory Thrombosis Outcome Study (CANTOS) suggested that canakinumab, an IL‐1β monoclonal antibody, imparted a dose‐dependent reduction in HF‐related mortality and hospitalizations in individuals with previous myocardial infarction and increased high‐sensitivity C‐reactive protein.12
These more recent data, as well as rapid advances in our understanding of the basis for inflammation and immune activation in HF,5, 13 have underscored the need for a reappraisal of therapeutic immunomodulation in this disease.14 Nonetheless, robust data from large‐scale clinical trials regarding the combined effects of immunomodulation on cardiac remodelling and clinical outcomes in HF are few. Accordingly, we conducted a systematic review and meta‐analyses to evaluate the aggregate benefit of immunomodulation on LV structure and function and all‐cause mortality in HF with reduced ejection fraction (HFrEF) in trials that reported both outcomes. To ascertain the robustness of the treatment signals, we also employed trial sequential analysis (TSA) to improve the precision of effect sizes over time, as data have continued to accumulate in the field of immunocardiology and HF.
Methods
Protocol and registration
This study was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines ( Table S1 ). The review was registered with PROSPERO (International Prospective Register of Systematic Reviews; registration number CRD42019138909). Because this was a meta‐analysis and systematic review of trial results that have already been published, approval of institutional review board and ethics committee was not required.
Eligibility criteria
RCTs enrolling patients with HFrEF randomized to receive either immunomodulation or placebo and reporting change in LV ejection fraction (LVEF) were included in the analyses ( Data S1 ). The search strategy and data collection are detailed in the Supporting Information ( Data S2 ).
Risk of bias in individual studies
Risk of bias (RoB) was assessed using the Cochrane RoB tool modified to capture the components of random sequence generation; allocation concealment; blinding of participants; blinding of outcome assessment; and analysis of incomplete outcome data.
Outcomes
The primary endpoint in this analysis was change in LVEF in patients with HF receiving immunomodulation as compared with no immunomodulation. Secondary outcomes were the change in LV end‐diastolic dimension (LVEDD) and all‐cause mortality.
Meta‐analysis and publication bias
Random effects modelling was used to estimate pooled mean difference for continuous outcomes (change in LVEF and LVEDD; pretreatment and post‐treatment) and risk ratio (RR) for the categorical outcome (all‐cause mortality). The details for statistical analysis are presented in S4 Statistical section.
Trial sequential analysis
TSA was used to quantify the statistical reliability of data in the cumulative meta‐analyses by adjusting significance levels for sparse data and repetitive testing on accumulating data. A cumulative Z‐curve was plotted against the accrued sample size. Sequential boundaries for benefit, futility, and required information size for a conclusive meta‐analyses were constructed, assuming the cumulative treatment effect for each outcome, α = 0.05, and β = 0.20. The details for statistical analysis are presented in Data S3 . All analyses were performed using STATA V15.0 (College Station, TX, USA) statistical software and TSA software version 0.9.5.10 Beta.
Sensitivity and subgroup analyses
The effect of exclusion of studies with a high RoB on the primary outcome was estimated. The effect of immunomodulation on outcomes that showed a signal for benefit was also studied in the subgroup analyses by drug dividing into two subgroups by type of immunomodulation, that is, broad immunosuppression vs. anti‐cytokine immunotherapy, as well as by classifying studies into three groups on the basis of the aetiology of HF: ischaemic, non‐ischaemic, and mixed in each study.1 With regard to the type of immunomodulation, in addition to direct anti‐cytokine neutralization strategies (e.g. etanercept, infliximab, and anakinra), both pentoxifylline and thalidomide were considered as anti‐cytokine approaches given the known effects of these drugs in reducing TNF expression.4 All other treatments were considered as broad immunomodulation strategies. Three important trials,15, 16 which were not included based on our pre‐specified inclusion criteria (change in LVEF not available), reported all‐cause mortality in patients with HFrEF receiving immunomodulation. We also performed a sensitivity analysis for mortality after including these three trials to estimate any change in the treatment effect estimate for mortality.
Results
Baseline and treatment characteristics
Of the 685 records screened, 19 studies with 1,341 patients met our inclusion criteria ( Figure S1 ). Table 1 highlights the baseline characteristics of patients. The mean age of the trial population ranged from 43 to 70 years. The sample size in the RCTs varied from 15 to 267. Mean LVEF was 23.8% [95% confidence interval (CI): 22.0–25.6%]. There were 11 studies with both ischaemic (ICM) and non‐ischaemic cardiomyopathy (NICM): one study with only ICM and seven studies with only NICM. The percentage of patients with NICM in studies with both ICM and NICM varied from 20.8% to 83.3%. Broad immunosuppression was used in nine studies (64.9% patients), and anti‐cytokine immunosuppression was used in 10 studies (35.1% patients). Treatment duration varied from 2 weeks to 6 months. The treatment regimens of the included studies are given in Table S2 . The RoB was deemed to be acceptable ( Figures S2 and S3 ) for all except one study.35 Echocardiography was used for LVEF assessment in 10 studies (61.6% patients), while radionuclide imaging was used in nine studies (38.4% patients) ( Table S2 ).
Table 1.
Treatment protocol and baseline characteristics in the included trials
| S.no | Author/year | Treatment protocol | Mechanism of immunomodulation | Assessment of outcome | Total (n) | Treatment arm (n) | Control arm (n) | Mean age (years) | Women (%) | NICM (%) | LVEF (%) | LVEDD (mm) | ACEi–ARBs/beta‐blockers/Aldosterone antagonist/digoxin (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Parrillo17/1989 | Prednisone | Broad | 3 months | 101 | 49 | 52 | 43 | NR | 100 | 17.5 | 68.7 | NA/NA/NA/NA |
| 2 | Sliwa18/1998 | Pentoxifylline | Anti‐cytokine | 6 months | 28 | 14 | 14 | 52 | 32.1 | 100 | 24.2 | 64.5 | 100/NA/NA/100 |
| 3 | Deswal19/1999 | Etanercept | Anti‐cytokine | 2 weeks | 18 | 12 | 6 | 63.3 | 5.6 | 83.3 | 27.5 | NR | 100/11/NA/94.4 |
| 4 | McNamara20/2001 | IVIg | Broad | 1 year | 62 | 33 | 29 | 43 | 4 | 100 | 25 | NR | 90/18/NA/NA |
| 5 | Skudicky21/2001 | Pentoxifylline | Anti‐cytokine | 6 months | 39 | 19 | 20 | 48.5 | 33.3 | 100 | 24 | 68.5 | 100/100/NA/100 |
| 6 | Bozkurt22/2001 | Etanercept | Anti‐cytokine | 3 months | 47 | 31 | 16 | 55 | 19 | 36.2 | 18.4 | NR | 100/47/NA/87 |
| 7 | Gullestad23/2001 | IVIg | Broad | 26 weeks | 39 | 19 | 20 | 60.5 | 17.5 | 57.5 | 27 | NR | 100/75/NA/40 |
| 8 | Wojnicz24/2001 | Prednisone + azathioprine | Broad | 3 months | 84 | 41 | 43 | 40 | 17.9 | 100 | 24.4 | 67.1 | 100/100/NA/100 |
| 9 |
Sliwa25/2002 |
Pentoxifylline | Anti‐cytokine | 1 months | 15 | 8 | 7 | 46 | 16.7 | 100 | 15.4 | 69.1 | 100/NA/NA/100 |
| 10 |
Chung26/2003 |
Infliximab | Anti‐cytokine | 14 weeks | 150 | 101 | 49 | 61.3 | 19 | 35.3 | 24 | NR | 100/73/39/78 |
| 11 | Bahrmann27/2004 | Pentoxifylline | Anti‐cytokine | 6 months | 41 | 21 | 20 | 56.5 | 6.4 | 57.4 | 28 | 69 | 100/96/NA/51 |
| 12 | Sliwa28/2004 | Pentoxifylline | Anti‐cytokine | 6 months | 33 | 19 | 14 | 55.1 | 28.9 | 0 | 25 | 61.2 | 100/100/50/NA |
| 13 | Torre‐Amione29/2005 | Celecadea | Broad | 6 months | 74 | 37 | 37 | 61.7 | 31.1 | 51.4 | 22.2 | NR | 89/51/46/82 |
| 14 | Gullestad30/2005 | Thalidomide | Anti‐cytokine | 12 weeks | 48 | 22 | 26 | 66 | 25 | 32.2 | 24.6 | NR | 99/91/NA/29 |
| 15 | Gong31/2006 | Methotrexate | Broad | 12 weeks | 62 | 30 | 32 | 62.4 | 48.1 | 59.7 | 31 | 62.7 | 94/45/NA/68 |
| 16 | Frustaci32/2009 | Prednisone + azathioprine | Broad | 6 months | 85 | 43 | 42 | 42.8 | 40 | 100 | 27.1 | 68.6 | 100/100/NA/100 |
| 17 | Deftereos33/2014 | Colchicine | Broad | 6 months | 267 | 134 | 133 | 66.7 | 33 | 28 | 27.6 | 61.7 | 85/79/62/NA |
| 18 | Van Tassell34/2016 | Anakinra | Anti‐cytokine | 12 weeks | 52 | 34 | 18 | 57.7 | 27 | 65.4 | 31.2 | NR | 83/92/52/NA |
| 19 | Xiaojing35/2017 | Thymopentin | Broad | 75 days | 96 | 48 | 48 | 70.3 | 43.8 | 20.8 | 35.5 | 61.8 | 81/77/92/43 |
EF, ejection fraction; IVIg, intra‐venous immunoglobulin; LVEDD, left ventricular end‐diastolic dimension; LVEF: left ventricular ejection fraction; NA, not available; NICM, non‐ischaemic cardiomyopathy; NR, not recorded.
Autologous blood transfusion.
Effect of immunomodulation on left ventricular ejection fraction
The mean difference in change in LVEF was +5.7% (95% CI: +3.0 to 8.5%, P < 0.001) (Figure 1 A and Figure S4 ) higher with immunomodulation as compared with no immunomodulation. There was no evidence of publication bias (P > 0.05 for both Egger and Begg test for small‐study effects) ( Figure S5 ) for this estimate. The effect estimate for improvement in LVEF remained similar (5.5%) to overall estimate (5.7%) after removing the study with high RoB.35 There was directional consistency in LVEF improvement upon immunomodulation with only one study27 reporting non‐significant improvement in LVEF among those not on immunomodulation ( Figure S4 ). LVEF improvement was consistently higher when studies were stratified by type of immunomodulation; 5.0% (95% CI: 2.6–7.3, P < 0.001) with anti‐cytokine therapy and 5.6% (95% CI: 0.5–10.8, P < 0.001) with broad immunomodulation (Figure 2 and Figure S4 ). The improvement in LVEF was also seen across studies enrolling patients with ischaemic, non‐ischaemic, or mixed aetiology patients: 9.8%, 7.6%, and 2.7%, respectively (all P < 0.05) (Figure 3 and Figure S6 ). The weighted mean difference (WMD) and the standardized mean difference (SMD) for improvement in LVEF are presented in Table S3 and were consistent in magnitude and direction.
Figure 1.
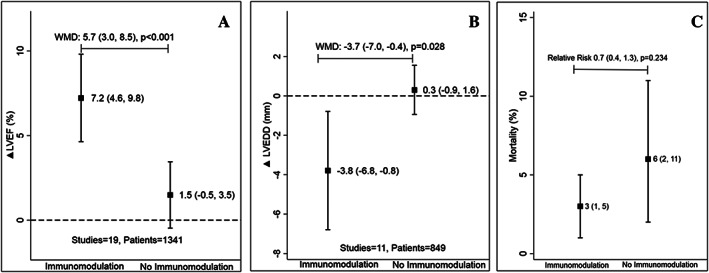
Difference in change in left ventricular ejection fraction (LVEF) (A), left ventricular end‐diastolic dimension (LVEDD) (B), and mortality (C) between immunomodulation and no immunomodulation. WMD, weighted mean difference. Data are presented as mean (95% confidence interval). The black square represents the mean change in parameters with or without immunomodulation with the error bars representing the 95% confidence interval.
Figure 2.
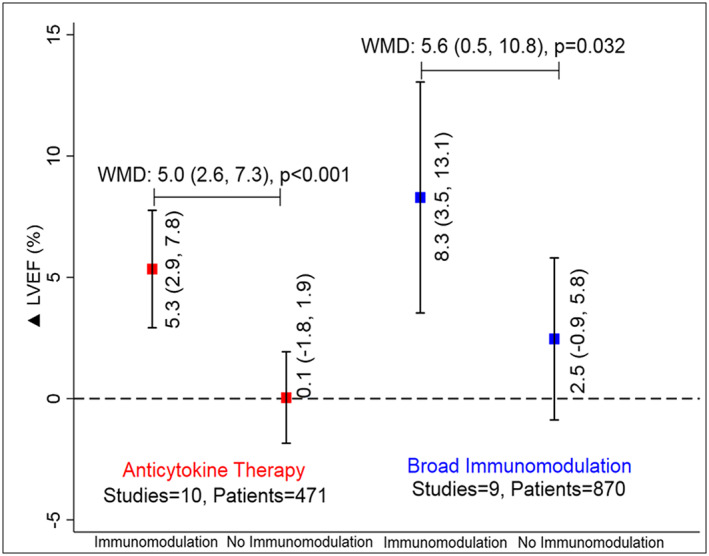
Improvement in left ventricular ejection fraction (LVEF) with or without immunomodulation with anti‐cytokine therapy (red) and broad immunomodulation (blue). WMD, weighted mean difference. Data are presented as mean (95% confidence interval). The solid square represents an improvement in LVEF with or without immunomodulation with the error bars representing the 95% confidence interval.
Figure 3.
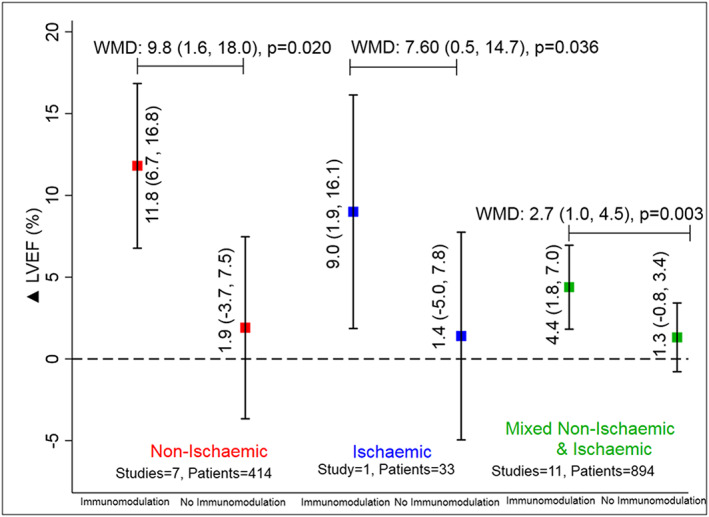
Improvement in left ventricular ejection fraction (LVEF) with or without immunomodulation in studies with non‐ischaemic (red), ischaemic (blue), and mixed non‐ischaemic and ischaemic aetiology (green) of heart failure. WMD, weighted mean difference. Data are presented as mean (95% confidence interval). The solid square represents an improvement in parameters with or without immunomodulation with the error bars representing the 95% confidence interval.
Effect of immunomodulation on left ventricular end‐diastolic dimension
Eleven studies reported a change in LVEDD (n = 849). Of them, five (18.3% patients) used anti‐cytokine immunotherapy and six (81.7% patients) used broad immunomodulation (Table 1). The mean change in LVEDD was 3.7 mm lower (95% CI: −7.0 to −0.4, P = 0.028) with immunomodulation as compared with no immunomodulation (Figure 1 B). The improvement varied from −19.2 to 0.0 mm ( Figure S7 ). The LVEDD was numerically lower with both with anti‐cytokine immunotherapy (−1.6 mm, 95% CI: −4.2 to 1.0, P = 0.231) and broad immunomodulation (−5.2 mm, 95% CI: −10.0 to −0.4, P = 0.035) (Figure 4). This improvement in LVEDD was seen across studies enrolling patients with non‐ischaemic or mixed aetiology patients, −5.2 and −3.7 mm, respectively (Figure 5 and Figure S8 ), but did not reach statistical significance individually in either group individually. The WMD and SMD for improvement in LVEDD for the overall meta‐analysis are presented in Table S3 and were consistent in magnitude and direction.
Figure 4.
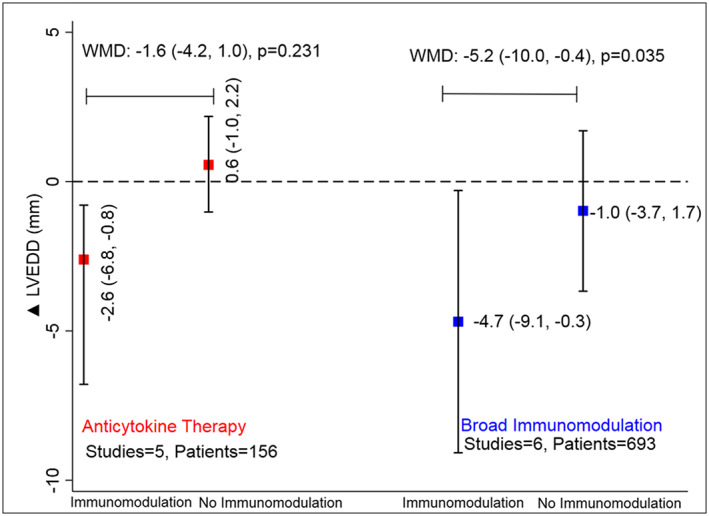
Improvement in left ventricular end‐diastolic dimension (LVEDD) with or without immunomodulation with anti‐cytokine therapy (red) and broad immunomodulation (blue). WMD, weighted mean difference. Data are presented as mean (95% confidence interval). The solid square represents the mean change in parameters with or without immunomodulation with the error bars representing the 95% confidence interval.
Figure 5.
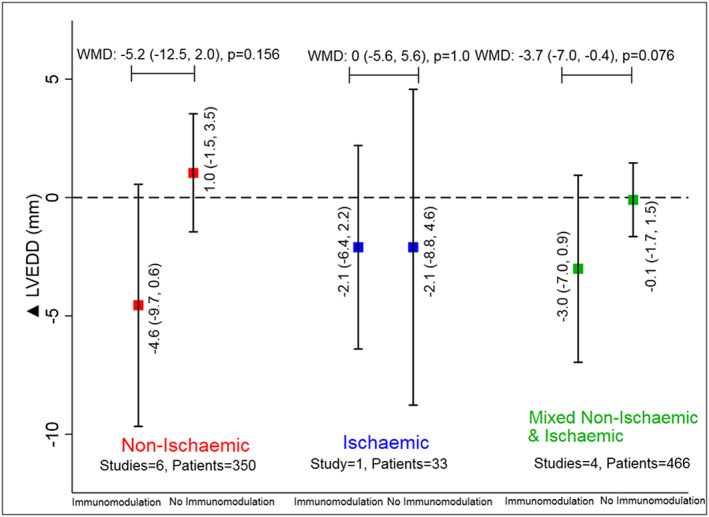
Improvement in left ventricular end‐diastolic dimension (LVEDD) with or without immunomodulation in studies with non‐ischaemic (red), ischaemic (blue), and mixed non‐ischaemic and ischaemic aetiology (green) of heart failure. WMD, weighted mean difference. Data are presented as mean (95% confidence interval). The solid square represents the mean change in parameters with or without immunomodulation with the error bars representing the 95% confidence interval.
Effect of immunomodulation on mortality
In 14 studies (n = 1099) reporting mortality, the risk of mortality was 2.7% vs. 6.0% (RR 0.7, 95% CI: 0.4–1.3, P = 0.234) with immunomodulation as compared with no immunomodulation, respectively, over a mean follow‐up of 5 months (Figure 1 C and Figure S9 ). The sensitivity analysis after the inclusion of three trials that did not report ΔLVEF suggested no significant change in effect size or direction for mortality ( Figure S10 ).
Trial sequential analysis
The cumulative Z‐score to study the improvement in LVEF with immunomodulation as compared with no immunomodulation crossed the Lan–DeMets boundary for the true benefit, increasing the robustness of pooled trial results ( Figure S11 ). Similar findings were seen for improvement in LVEDD ( Figure S12 ). The Z‐score for mortality with immunomodulation did not cross the statistical boundary of benefit or harm or futility, indicating the lack of currently available data to support conclusions regarding mortality differences ( Figure S13 ).
Discussion
In this study, we observed that in patients with HFrEF, immunomodulation therapy led to a significant improvement in LVEF and reduction in LVEDD, without conclusive differences in the risk of mortality. The improvement in LVEF was seen with both anti‐cytokine therapy and broad immunomodulation and was consistent across aetiologies of HF. Similarly, there was a trend towards a reduction in LVEDD with both anti‐cytokine therapy and broad immunomodulation. Further, the TSA suggested a lack of sufficient extant data in these trials to demonstrate a difference in mortality with immunomodulation as compared with placebo.
Emerging evidence indicates that HF is composed of a dysregulated inflammatory milieu with altered immune cell functional networks.1, 2 A persistent inflammatory response to injury (ischaemic, infectious, pressure, or volume overload), or a failure to resolve inflammation following acute injury, can lead to chronic expression of pro‐inflammatory cytokines that have been suggested to contribute to HF progression.36 Moreover, the failing heart exhibits expanded populations and activation of both innate and adaptive immune cells that are essential contributors to progressive LV remodelling.2, 3, 5, 7, 10 Pro‐inflammatory cytokines such as TNF‐α, IL‐1β, and IL‐6 are associated with increasing severity of HF1, 37, 38 and contribute to progressive LV remodelling and systolic dysfunction.39, 40
The improvement in LVEF and LVEDD seen with immunomodulation in our analyses suggests that suppression of chronic inflammation, properly targeted to the appropriate patients, may have a therapeutic benefit on LV remodelling in HFrEF. Of the 19 studies included in our meta‐analysis, eight studies23, 25, 26, 27, 30, 31, 34, 35 reported changes in the levels of inflammatory cytokines TNF‐α, IL‐1β, or IL‐6 with and without immunomodulation. Only two studies25, 30 reported significantly greater reductions in blood TNF‐α levels with immunomodulatory treatment as compared with no immunomodulation. This suggests that aside from suppression of cytokine elaboration, additional mechanisms such as decreased free radical production (as seen with pentoxifylline41) might play a synergistic role.
Existing studies suggest that during hospitalization for acute decompensated HF, circulating IL‐1β levels are associated with a higher concentration of natriuretic peptides (myocardial stretch) and troponin T (myocardial injury), along with a higher hazard of death at 1 year.42 Further, in murine models, administration of anti‐IL‐1β antibody given early or late after reperfused myocardial infarction improved LV remodelling and function.43 These findings, the benefits seen with canakinumab in reducing cardiovascular outcomes,44 and incident HF after myocardial infarction12 identify IL‐1β as an especially promising therapeutic target for immunomodulation.
A previous meta‐analysis of six studies with 221 patients (LVEF ≤ 40%) that evaluated the effect of pentoxifylline in HF suggested a 70% reduced odds of mortality as compared with placebo.45 In our study, we grouped pentoxifylline with other anti‐cytokine immunotherapy and demonstrated a comparable 51% reduced risk of mortality, although this was not statistically significant. Evidence for the use of other immunomodulatory agents is limited and has not been consistently demonstrated in humans. Anakinra (a recombinant IL‐1 receptor antagonist) has been shown to reduce C‐reactive protein levels and improve oxygen consumption in patients with HF.46
There is an increased expression of autoantibodies in human HF.47 These may subsequently induce a persistent immune response contributing to cardiac dysfunction.47, 48 Extraction of circulating antibodies by immunoadsorption has been shown to improve cardiac index in patients with dilated cardiomyopathy.49 Intra‐venous immunoglobulin is a broad immunomodulatory agent that acts similarly to immunoadsorption and suppresses the inflammatory response via neutralization of autoantibodies and cytokines.50 Other broad immunomodulators including prednisone, azathioprine, and methotrexate have diverse effects on both the cellular and cytokine arms of inflammation.4
Despite goal‐directed medical therapy, HF hospitalizations and mortality remain high with nearly 40% of patients with HF succumbing to their disease within 5 years of diagnosis.51 Current practice guidelines for HF are focused mainly on neurohormonal blockade,52 with absolute improvements in LVEF generally less than 5% with currently approved neurohormonal blockers (i.e. angiotensin‐converting enzyme inhibitors, beta‐blockers, and aldosterone antagonists).53, 54, 55 The addition of sacubitril–valsartan on the background of optimized therapy improves LVEF ~5% after 3 months.56 LVEF improvement with neurohormonal blockade has been suggested to reduce hospitalization and improve quality of life in patients with HF.57, 58 Our study indicates a similar improvement in LVEF with immunomodulation after a mean duration of 4.5 months as compared with placebo. While neurohormonal blockade in HF has been shown to reduce markers of inflammation,59 it is difficult to determine whether this is an indirect result of decreased LV remodelling or a direct mechanism of action.60 The marked LVEF improvement (~10%) seen in studies of immunomodulation in patients with non‐ischaemic cardiomyopathy suggests that there may be differential therapeutic effects of immunomodulation across HF aetiologies, possibly the result of a different profile of immune system activation in non‐ischaemic vs. ischaemic HF.61, 62, 63
Despite potential beneficial effects of immunomodulation on LV function and remodelling, such therapy has not translated into the improvement of hard cardiovascular endpoints in several trials, with some trials even suggesting harm.11 Possible explanations for these seemingly paradoxical results may relate to treatment‐related factors such as timing, duration, and type of therapy; patient‐related factors such as underlying HF aetiology and co‐morbidities; and the complex nature of the immune and inflammatory response itself. Indeed, while there are established detrimental effects of sustained inflammation on cardiac remodelling and function,1, 63 immunoinflammatory activation is also necessary for the initiation of reparative and defence pathways essential for restoring tissue integrity (which may be hampered by immunomodulation that is not precisely targeted).2 Hence, a better understanding of specific immune activation pathways and their temporality after myocardial injury would be critical for eliciting beneficial effects while reducing harm after immunomodulation.
To the best of our knowledge, this is the first and largest meta‐analysis inclusive of most studies aimed at evaluating the combined response of immunomodulation on LV structure, function, and outcomes in HFrEF. The observed improvements in LVEF and LVEDD suggest a beneficial reverse‐remodelling effect that should be further explored in large‐scale RCTs. The lack of mortality benefit could be due to competing co‐morbidities, lack of precise target inhibition that increases untoward effects, or suboptimal duration of follow‐up. It can also be argued that immunomodulation improves echocardiographic parameters without clinical outcome benefit. Indeed, large‐scale trials of TNF neutralization failed to show benefit with regard to mortality, although changes in remodelling were not assessed.15, 26 However, as our TSA of mortality did not cross the boundary of futility, future trials should be pursued to understand whether clinical outcomes are beneficially impacted by adding immunomodulatory therapy to currently approved goal‐directed medical therapy in HFrEF.
Meta‐analyses have well‐acknowledged limitations.64 Heterogeneity in treatment protocols, background treatment, and patient characteristics, and short follow‐up duration in the studies limit understanding of the ideal patient population that might benefit from immunomodulation. Further, besides all‐cause mortality, data regarding the risk of hospitalization and cardiovascular mortality were not uniformly available for quantitative analysis. Improvement in HF outcomes has to be balanced against an increased risk of infections with immunosuppression.
Conclusions
To summarize, our meta‐analysis suggests that in HFrEF patients, immunomodulation leads to a significant improvement in LV remodelling, as indexed by LVEF and LVEDD. The improvement in LVEF was seen with both anti‐cytokine therapy and broad immunomodulation and across non‐ischaemic and ischaemic aetiologies of HFrEF. The TSA suggested limited available data to definitively define differences in mortality risk. Future investigations are required to understand the ideal HFrEF patient population that would benefit from immunomodulation and the specific immunomodulatory therapies that should be considered.
Conflict of Interest
None of the authors had any conflicts of interest or financial disclosures to declare.
Funding
Dr. Bajaj is supported by Walter B. Frommeyer, Jr. Fellowship in Investigative Medicine awarded by the University of Alabama at Birmingham, American College of Cardiology Presidential Career Development Award, and National Center for Advancing Translational Research of the National Institutes of Health under award number UL1TR001417. Dr. Prabhu is supported by NIH‐NHLBI R01 grants HL125735 and HL147549 and a Department of Veterans Affairs VA Merit Award I01 BX002706.The content of the manuscript does not necessarily represent the views of NIH, VA, ACC and/or UAB.
Supporting information
Data S1. PRISMA checklist
Data S2. Inclusion exclusion criteria for all trials
Data S3. Search Strategy and data collection
Data S4. Statistics
Figure 1. Flow diagram for study selection
Figure 2. Risk‐of‐bias summary for randomized trials (RoB 2.0) assessed using the Cochrane RoB tool28
Figure S3. Review author's judgements about each risk‐of‐bias for each trial included. Green, yellow and red solid circles represent low, some concern and high risk‐of‐bias, respectively.
Figure S4. Effect of immunomodulation on LVEF as compared to no immunomodulation according to drug class. Black solid square diamonds and associated solid lines represent summary RR and 95% CI of each trial listed in the left column. The numerical estimates in the right columns are weighted mean difference(s) (WMD) with 95% CI of each trial listed in the left column. The hollow blue diamond is summary WMD and 95% CI for immunomodulation as compared to no immunomodulation. The hollow red diamond is the overall summary WMD with 95% CI. CI: Confidence interval
Figure S5. Funnel plot for publication bias with each blue dot representing a randomized trial and the dotted lines representing the pseudo 95% confidence intervals.
Figure S6. : Effect of immunomodulation on LVEF as compared to no immunomodulation according to heart failure aetiology. Black solid square diamonds and associated solid lines represent summary RR and 95% CI of each trial listed in the left column. The numerical estimates in the right columns are weighted mean difference(s) (WMD) with 95% CI of each trial listed in the left column. The hollow blue diamond is summary WMD and 95% CI for immunomodulation as compared to no immunomodulation. The hollow red diamond is the overall summary WMD with 95% CI. CI: Confidence interval
Table 3. Mean difference, weighted (WMD) and standardized (SMD) for primary and secondary outcomes.
Figure 7. Effect of immunomodulation on LVEDD as compared to no immunomodulation according to drug class. Black solid square diamonds and associated solid lines represent summary RR and 95% CI of each trial listed in the left column. The numerical estimates in the right columns are weighted mean difference(s) (WMD) with 95% CI of each trial listed in the left column. The hollow blue diamond is summary WMD and 95% CI for each class of immunomodulation as compared to no immunomodulation. The hollow red diamond is the overall summary WMD with 95% CI. CI: Confidence interval
Figure 8. Effect of immunomodulation on LVEDD as compared to no immunomodulation according to heart failure aetiology. Black solid square diamonds and associated solid lines represent summary RR and 95% CI of each trial listed in the left column. The numerical estimates in the right columns are weighted mean difference(s) (WMD) with 95% CI of each trial listed in the left column. The hollow blue diamond is summary WMD and 95% CI for immunomodulation as compared to no immunomodulation. The hollow red diamond is the overall summary WMD with 95% CI.CI: Confidence interval
Figure S9. Effect of immunomodulation on mortality as compared to no immunomodulation according to heart failure aetiology. Black solid square diamonds and associated solid lines represent summary RR and 95% CI of each trial listed in the left column. The numerical estimates in the right columns are the weighted mean difference(s) (WMD) with 95% CI of each trial listed in the left column. The hollow blue diamond is a summary WMD and 95% CI for immunomodulation as compared to no immunomodulation. The hollow red diamond is the overall summary RR with 95% CI.CI: Confidence interval
Figure S10. Sensitivity analysis for effect of immunomodulation on mortality as compared to no immunomodulation according to heart failure aetiology in all trials reporting mortality data. Black solid square diamonds and associated solid lines represent summary RR and 95% CI of each trial listed in the left column. The numerical estimates in the right columns are weighted mean difference(s) (WMD) with 95% CI of each trial listed in the left column. The hollow blue diamond is summary WMD and 95% CI for immunomodulation as compared to no immunomodulation. The hollow red diamond is the overall summary RR with 95% CI.CI: Confidence interval
Figure 11. Trial sequential analysis of immunomodulation vs. no immunomodulation in patients with heart failure for improvement in left ventricular ejection fraction (LVEF). The solid black line represents the line of no difference. The green lines above and below the line of no difference represent the O'Brien‐Fleming trial sequential boundary for no benefit and benefit with immunomodulation, respectively. The solid black lines are upper and lower bounds for 95% CI. The green vertical line is the required information size for conclusive meta‐analyses, given two‐sided α=0.05 and β=0.20. The solid blue line is Z‐curve derived from a random‐effects meta‐analysis of individual RCTs. The inner wedge represents the O'Brien‐Fleming β‐spending function at 80% power. The Z‐curve surpassed the trial sequential boundary and the information size, indicating a true improvement in LVEF with immunomodulation as compared to no immunomodulation.
Figure S11. Trial sequential analysis of immunomodulation vs. no immunomodulation in patients with heart failure for improvement in left ventricular ejection fraction (LVEF). The solid black line represents the line of no difference. The green lines above and below the line of no difference represent the O'Brien‐Fleming trial sequential boundary for no benefit and benefit with immunomodulation, respectively. The solid black lines are upper and lower bounds for 95% C.
Figure S12 Trial sequential analysis of immunomodulation vs. no immunomodulation in patients with heart failure for improvement in left ventricle end‐diastolic dimension (LVEDD). The solid black line represents the line of no difference. The green lines above and below the line of no difference represent the O'Brien‐Fleming trial sequential boundary for no benefit and benefit with immunomodulation, respectively. The solid black lines are upper and lower bounds for 95% CI. The green vertical line is the required information size for conclusive meta‐analyses, given two‐sided α=0.05 and β=0.20. The solid blue line is Z‐curve derived from a random‐effects meta‐analysis of individual RCTs. The inner wedge represents the O'Brien‐Fleming β‐spending function at 80% power. The Z‐curve surpassed the trial sequential boundary and the information size, indicating a true improvement in LVEDD with immunomodulation as compared to no immunomodulation.
Figure S13 Trial sequential analysis of immunomodulation vs. no immunomodulation in patients with heart failure for improvement in mortality. The solid black line represents the line of no difference. The solid black line represents the line of no difference. The green lines above and below the line of no difference represent the O'Brien‐Fleming trial sequential boundary for no benefit and benefit with immunomodulation, respectively. The solid black lines are upper and lower bounds for 95% CI. The green vertical line is the required information size for conclusive meta‐analyses, given two‐sided α=0.05 and β=0.20. The solid blue line is Z‐curve derived from a random‐effects meta‐analysis of individual RCTs. The inner wedge represents the O'Brien‐Fleming β‐spending function at 80% power. The Z‐curve did not surpass the trial sequential boundary and the information size, indicating a lack of sufficient evidence to conclude effect on mortality with or without immunomodulation.
Bajaj, N. S. , Gupta, K. , Gharpure, N. , Pate, M. , Chopra, L. , Kalra, R. , and Prabhu, S. D. (2020) Effect of immunomodulation on cardiac remodelling and outcomes in heart failure: a quantitative synthesis of the literature. ESC Heart Failure, 7: 1319–1330. 10.1002/ehf2.12681.
References
- 1. Mann DL. Inflammatory mediators and the failing heart. Circ Res 2002; 91: 988–998. [DOI] [PubMed] [Google Scholar]
- 2. Prabhu SD, Frangogiannis NG. The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ Res 2016; 119: 91–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bansal SS, Ismahil MA, Goel M, Zhou G, Rokosh G, Hamid T, Prabhu SD. Dysfunctional and proinflammatory regulatory T‐lymphocytes are essential for adverse cardiac remodeling in ischemic cardiomyopathy. Circulation 2019; 139: 206–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mann DL. Innate immunity and the failing heart: the cytokine hypothesis revisited. Circ Res 2015; 116: 1254–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nahrendorf M. Myeloid cell contributions to cardiovascular health and disease. Nat Med 2018; 24: 711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yndestad A, Holm AM, Muller F, Simonsen S, Froland SS, Gullestad L, Aukrust P. Enhanced expression of inflammatory cytokines and activation markers in T‐cells from patients with chronic heart failure. Cardiovasc Res 2003; 60: 141–146. [DOI] [PubMed] [Google Scholar]
- 7. Patel B, Bansal SS, Ismahil MA, Hamid T, Rokosh G, Mack M, Prabhu SD. CCR2(+) Monocyte‐derived infiltrating macrophages are required for adverse cardiac remodeling during pressure overload. JACC Basic Transl Sci 2018; 3: 230–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Prabhu SD. Cytokine‐induced modulation of cardiac function. Circ Res 2004; 95: 1140–1153. [DOI] [PubMed] [Google Scholar]
- 9. Zhang Y, Bauersachs J, Langer HF. Immune mechanisms in heart failure. Eur J Heart Fail 2017; 19: 1379–1389. [DOI] [PubMed] [Google Scholar]
- 10. Ismahil MA, Hamid T, Bansal SS, Patel B, Kingery JR, Prabhu SD. Remodeling of the mononuclear phagocyte network underlies chronic inflammation and disease progression in heart failure: critical importance of the cardiosplenic axis. Circ Res 2014; 114: 266–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Panahi M, Papanikolaou A, Torabi A, Zhang JG, Khan H, Vazir A, Hasham MG, Cleland JGF, Rosenthal NA, Harding SE, Sattler S. Immunomodulatory interventions in myocardial infarction and heart failure: a systematic review of clinical trials and meta‐analysis of IL‐1 inhibition. Cardiovasc Res 2018; 114: 1445–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Everett BM, Cornel JH, Lainscak M, Anker SD, Abbate A, Thuren T, Libby P, Glynn RJ, Ridker PM. Anti‐inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation 2019; 139: 1289–1299. [DOI] [PubMed] [Google Scholar]
- 13. Lavine KJ, Pinto AR, Epelman S, Kopecky BJ, Clemente‐Casares X, Godwin J, Rosenthal N, Kovacic JC. The macrophage in cardiac homeostasis and disease: JACC Macrophage in CVD Series (Part 4). J Am Coll Cardiol 2018; 72: 2213–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Frantz S, Falcao‐Pires I, Balligand JL, Bauersachs J, Brutsaert D, Ciccarelli M, Dawson D, de Windt LJ, Giacca M, Hamdani N, Hilfiker‐Kleiner D, Hirsch E, Leite‐Moreira A, Mayr M, Thum T, Tocchetti CG, van der Velden J, Varricchi G, Heymans S. The innate immune system in chronic cardiomyopathy: a European Society of Cardiology (ESC) scientific statement from the Working Group on Myocardial Function of the ESC. Eur J Heart Fail 2018; 20: 445–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mann DL, McMurray JJ, Packer M, Swedberg K, Borer JS, Colucci WS, Djian J, Drexler H, Feldman A, Kober L, Krum H, Liu P, Nieminen M, Tavazzi L, van Veldhuisen DJ, Waldenstrom A, Warren M, Westheim A, Zannad F, Fleming T. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation 2004; 109: 1594–1602. [DOI] [PubMed] [Google Scholar]
- 16. Moreira DM, Vieira JL, Gottschall CAM. The effects of METhotrexate therapy on the physical capacity of patients with ISchemic heart failure: a randomized double‐blind, placebo‐controlled trial (METIS trial). J Cardiac Fail 2009; 15: 828–834. [DOI] [PubMed] [Google Scholar]
- 17. Parrillo JE, Cunnion RE, Epstein SE, Parker MM, Suffredini AF, Brenner M, Schaer GL, Palmeri ST, Cannon RO, Alling D, Wittes JT, Ferrans VJ, Rodriguez ER, Fauci AS. A prospective, randomized, controlled trial of prednisone for dilated cardiomyopathy. New Engl J Med 1989; 321: 1061–1068. [DOI] [PubMed] [Google Scholar]
- 18. Sliwa K, Skudicky D, Candy G, Wisenbaugh T, Sareli P. Randomised investigation of effects of pentoxifylline on left‐ventricular performance in idiopathic dilated cardiomyopathy. Lancet 1998; 351: 1091–1093. [DOI] [PubMed] [Google Scholar]
- 19. Deswal A, Bozkurt B, Seta Y, Parilti‐Eiswirth S, Hayes FA, Blosch C, Mann DL. Safety and efficacy of a soluble P75 tumor necrosis factor receptor (Enbrel, etanercept) in patients with advanced heart failure. Circulation 1999; 99: 3224–3226. [DOI] [PubMed] [Google Scholar]
- 20. McNamara DM, Holubkov R, Starling RC, Dec GW, Loh E, Torre‐Amione G, Gass A, Janosko K, Tokarczyk T, Kessler P, Mann DL, Feldman AM. Controlled trial of intravenous immune globulin in recent‐onset dilated cardiomyopathy. Circulation 2001; 103: 2254–2259. [DOI] [PubMed] [Google Scholar]
- 21. Skudicky D, Bergemann A, Sliwa K, Candy G, Sareli P. Beneficial effects of pentoxifylline in patients with idiopathic dilated cardiomyopathy treated with angiotensin‐converting enzyme inhibitors and carvedilol: results of a randomized study. Circulation 2001; 103: 1083–1088. [DOI] [PubMed] [Google Scholar]
- 22. Bozkurt B, Torre‐Amione G, Warren MS, Whitmore J, Soran OZ, Feldman AM, Mann DL. Results of targeted anti‐tumor necrosis factor therapy with etanercept (ENBREL) in patients with advanced heart failure. Circulation 2001; 103: 1044–1047. [DOI] [PubMed] [Google Scholar]
- 23. Gullestad L, Aass H, Fjeld JG, Wikeby L, Andreassen AK, Ihlen H, Simonsen S, Kjekshus J, Nitter‐Hauge S, Ueland T, Lien E, Froland SS, Aukrust P. Immunomodulating therapy with intravenous immunoglobulin in patients with chronic heart failure. Circulation 2001; 103: 220–225. [DOI] [PubMed] [Google Scholar]
- 24. Wojnicz R, Nowalany‐Kozielska E, Wojciechowska C, Glanowska G, Wilczewski P, Niklewski T, Zembala M, Poloński L, Rozek Marius M, Wodniecki J. Randomized, placebo‐controlled study for immunosuppressive treatment of inflammatory dilated cardiomyopathy. Circulation 2001; 104: 39–45. [DOI] [PubMed] [Google Scholar]
- 25. Sliwa K, Woodiwiss A, Candy G, Badenhorst D, Libhaber C, Norton G, Skudicky D, Sareli P. Effects of pentoxifylline on cytokine profiles and left ventricular performance in patients with decompensated congestive heart failure secondary to idiopathic dilated cardiomyopathy. Am J Cardiol 2002; 90: 1118–1122. [DOI] [PubMed] [Google Scholar]
- 26. Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT. Randomized, double‐blind, placebo‐controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor‐alpha, in patients with moderate‐to‐severe heart failure: results of the anti‐TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation 2003; 107: 3133–3140. [DOI] [PubMed] [Google Scholar]
- 27. Bahrmann P, Hengst UM, Richartz BM, Figulla HR. Pentoxifylline in ischemic, hypertensive and idiopathic‐dilated cardiomyopathy: effects on left‐ventricular function, inflammatory cytokines and symptoms. Eur J Heart Fail 2004; 6: 195–201. [DOI] [PubMed] [Google Scholar]
- 28. Sliwa K, Woodiwiss A, Kone VN, Candy G, Badenhorst D, Norton G, Zambakides C, Peters F, Essop R. Therapy of ischemic cardiomyopathy with the immunomodulating agent pentoxifylline: results of a randomized study. Circulation 2004; 109: 750–755. [DOI] [PubMed] [Google Scholar]
- 29. Torre‐Amione G, Sestier F, Radovancevic B, Young J. Broad modulation of tissue responses (immune activation) by celacade may favorably influence pathologic processes associated with heart failure progression. Am J Cardiol 2005; 95: 30C–37C discussion 38C‐40C. [DOI] [PubMed] [Google Scholar]
- 30. Gullestad L, Ueland T, Fjeld JG, Holt E, Gundersen T, Breivik K, Folling M, Hodt A, Skardal R, Kjekshus J, Andreassen A, Kjekshus E, Wergeland R, Yndestad A, Froland SS, Semb AG, Aukrust P. Effect of thalidomide on cardiac remodeling in chronic heart failure: results of a double‐blind, placebo‐controlled study. Circulation 2005; 112: 3408–3414. [DOI] [PubMed] [Google Scholar]
- 31. Gong K, Zhang Z, Sun X, Zhang X, Li A, Yan J, Luo Q, Gao Y, Feng Y. The nonspecific anti‐inflammatory therapy with methotrexate for patients with chronic heart failure. Am Heart J 2006; 151: 62–68. [DOI] [PubMed] [Google Scholar]
- 32. Frustaci A, Russo MA, Chimenti C. Randomized study on the efficacy of immunosuppressive therapy in patients with virus‐negative inflammatory cardiomyopathy: the TIMIC study. Eur Heart J 2009; 30: 1995–2002. [DOI] [PubMed] [Google Scholar]
- 33. Deftereos S, Giannopoulos G, Panagopoulou V, Bouras G, Raisakis K, Kossyvakis C, Karageorgiou S, Papadimitriou C, Vastaki M, Kaoukis A, Angelidis C, Pagoni S, Pyrgakis V, Alexopoulos D, Manolis AS, Stefanadis C, Cleman MW. Anti‐inflammatory treatment with colchicine in stable chronic heart failure: a prospective, randomized study. JACC Heart Fail 2014; 2: 131–137. [DOI] [PubMed] [Google Scholar]
- 34. Van Tassell BW, Abouzaki NA, Oddi Erdle C, Carbone S, Trankle CR, Melchior RD, Turlington JS, Thurber CJ, Christopher S, Dixon DL, Fronk DT, Thomas CS, Rose SW, Buckley LF, Dinarello CA, Biondi‐Zoccai G, Abbate A. Interleukin‐1 blockade in acute decompensated heart failure: a randomized, double‐blinded, placebo‐controlled pilot study. J Cardiovasc Pharmacol 2016; 67: 544–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xiaojing C, Yanfang L, Yanqing G, Fangfang C. Thymopentin improves cardiac function in older patients with chronic heart failure. Anatol J Cardiol 2017; 17: 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Seta Y, Shan K, Bozkurt B, Oral H, Mann DL. Basic mechanisms in heart failure: the cytokine hypothesis. J Cardiac Fail 1996; 2: 243–249. [DOI] [PubMed] [Google Scholar]
- 37. Tsutamoto T, Hisanaga T, Wada A, Maeda K, Ohnishi M, Fukai D, Mabuchi N, Sawaki M, Kinoshita M. Interleukin‐6 spillover in the peripheral circulation increases with the severity of heart failure, and the high plasma level of interleukin‐6 is an important prognostic predictor in patients with congestive heart failure. J Am Coll Cardiol 1998; 31: 391–398. [DOI] [PubMed] [Google Scholar]
- 38. Torre‐Amione G, Kapadia S, Benedict C, Oral H, Young JB, Mann DL. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the Studies of Left Ventricular Dysfunction (SOLVD). J Am Coll Cardiol 1996; 27: 1201–1206. [DOI] [PubMed] [Google Scholar]
- 39. Kubota T, McTiernan CF, Frye CS, Slawson SE, Lemster BH, Koretsky AP, Demetris AJ, Feldman AM. Dilated cardiomyopathy in transgenic mice with cardiac‐specific overexpression of tumor necrosis factor‐alpha. Circ Res 1997; 81: 627–635. [DOI] [PubMed] [Google Scholar]
- 40. Sivasubramanian N, Coker ML, Kurrelmeyer KM, MacLellan WR, DeMayo FJ, Spinale FG, Mann DL. Left ventricular remodeling in transgenic mice with cardiac restricted overexpression of tumor necrosis factor. Circulation 2001; 104: 826–831. [DOI] [PubMed] [Google Scholar]
- 41. Sonkin PL, Freedman SF, Needham D, Rao KMK, Hatchell DL. Pentexifylline modulates deformability, F‐actin content, and superoxide anion production of polymorphonuclear leukocytes from diabetic cats. Exp Eye Res 1992; 55: 831–838. [DOI] [PubMed] [Google Scholar]
- 42. Pascual‐Figal DA, Bayes‐Genis A, Asensio‐Lopez MC, Hernández‐Vicente A, Garrido‐Bravo I, Pastor‐Perez F, Díez J, Ibáñez B, Lax A. The interleukin‐1 axis and risk of death in patients with acutely decompensated heart failure. J Am Coll Cardiol 2019; 73: 1016–1025. [DOI] [PubMed] [Google Scholar]
- 43. Harouki N, Nicol L, Remy‐Jouet I, Henry J‐P, Dumesnil A, Lejeune A, Renet S, Golding F, Djerada Z, Wecker D, Bolduc V, Bouly M, Roussel J, Richard V, Mulder P. The IL‐1β antibody gevokizumab limits cardiac remodeling and coronary dysfunction in rats with heart failure. JACC Basic Transl Sci 2017; 2: 418–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida‐Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ. Antiinflammatory therapy with canakinumab for atherosclerotic disease. New Engl J Med 2017; 377: 1119–1131. [DOI] [PubMed] [Google Scholar]
- 45. Champion S, Lapidus N, Cherié G, Spagnoli V, Oliary J, Solal AC. Pentoxifylline in heart failure: a meta‐analysis of clinical trials. Cardiovasc Ther 2014; 32: 159–162. [DOI] [PubMed] [Google Scholar]
- 46. Van Tassell BW, Canada J, Carbone S, Trankle C, Buckley L, Oddi Erdle C, Abouzaki NA, Dixon D, Kadariya D, Christopher S, Schatz A, Regan J, Viscusi M, Del Buono M, Melchior R, Mankad P, Lu J, Sculthorpe R, Biondi‐Zoccai G, Lesnefsky E, Arena R, Abbate A. Interleukin‐1 blockade in recently decompensated systolic heart failure: results from REDHART (Recently Decompensated Heart Failure Anakinra Response Trial). Circ Heart Fail 2017; 10:e004373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kaya Z, Leib C, Katus Hugo A, Rosenzweig A. Autoantibodies in heart failure and cardiac dysfunction. Circ Res 2012; 110: 145–158. [DOI] [PubMed] [Google Scholar]
- 48. Levin MJ, Hoebeke J. Cross‐talk between anti‐β1‐adrenoceptor antibodies in dilated cardiomyopathy and Chagas' heart disease. Autoimmunity 2008; 41: 429–433. [DOI] [PubMed] [Google Scholar]
- 49. Felix SB, Staudt A, Dörffel WV, Stangl V, Merkel K, Pohl M, Döcke WD, Morgera S, Neumayer HH, Wernecke KD, Wallukat G, Stangl K, Baumann G. Hemodynamic effects of immunoadsorption and subsequent immunoglobulin substitution in dilated cardiomyopathy: three‐month results from a randomized study. J Am Coll Cardiol 2000; 35: 1590–1598. [DOI] [PubMed] [Google Scholar]
- 50. Aukrust P, Müller F, Svenson M, Nordøy I, Bendtzen K, Frøland SS. Administration of intravenous immunoglobulin (IVIG) in vivo—down‐regulatory effects on the IL‐1 system. Clin Exp Immunol 1999; 115: 136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P. Heart disease and stroke statistics‐2017 update: a report from the American Heart Association. Circulation 2017; 135: e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yancy Clyde W, Jessup M, Bozkurt B, Butler J, Casey Donald E, Colvin Monica M, Drazner Mark H, Filippatos Gerasimos S, Fonarow Gregg C, Givertz Michael M, Hollenberg Steven M, Lindenfeld J, Masoudi Frederick A, McBride Patrick E, Peterson Pamela N, Stevenson Lynne W, Westlake C. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017; 136: e137–e161. [DOI] [PubMed] [Google Scholar]
- 53. Chatterjee S, Biondi‐Zoccai G, Abbate A, D'Ascenzo F, Castagno D, Van Tassell B, Mukherjee D, Lichstein E. Benefits of beta blockers in patients with heart failure and reduced ejection fraction: network meta‐analysis. BMJ 2013; 346: f55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cicoira M, Zanolla L, Rossi A, Golia G, Franceschini L, Brighetti G, Marino P, Zardini P. Long‐term, dose‐dependent effects of spironolactone on left ventricular function and exercise tolerance in patients with chronic heart failure. J Am Coll Cardiol 2002; 40: 304–310. [DOI] [PubMed] [Google Scholar]
- 55. Cohn JN, Johnson G, Ziesche S, Cobb F, Francis G, Tristani F, Smith R, Dunkman WB, Loeb H, Wong M, Bhat G, Goldman S, Fletcher RD, Doherty J, Hughes CV, Carson P, Cintron G, Shabetai R, Haakenson C. A comparison of enalapril with hydralazine–isosorbide dinitrate in the treatment of chronic congestive heart failure. New Engl J Med 1991; 325: 303–310. [DOI] [PubMed] [Google Scholar]
- 56. Almufleh A, Marbach J, Chih S, Stadnick E, Davies R, Liu P, Mielniczuk L. Ejection fraction improvement and reverse remodeling achieved with sacubitril/valsartan in heart failure with reduced ejection fraction patients. Am J Cardiovasc Dis 2017; 7: 108–113. [PMC free article] [PubMed] [Google Scholar]
- 57. Beta‐Blocker Evaluation of Survival Trial Investigators , Eichhorn EJ, Domanski MJ, Krause‐Steinrauf H, Bristow MR, Lavori PW. A trial of the beta‐blocker bucindolol in patients with advanced chronic heart failure. New Engl J Med 2001; 344: 1659–1667. [DOI] [PubMed] [Google Scholar]
- 58. Tate CW, Robertson AD, Zolty R, Shakar SF, Lindenfeld J, Wolfel EE, Bristow MR, Lowes BD. Quality of life and prognosis in heart failure: results of the Beta‐Blocker Evaluation of Survival Trial (BEST). J Cardiac Fail 2007; 13: 732–737. [DOI] [PubMed] [Google Scholar]
- 59. Ohtsuka T, Hamada M, Hiasa G, Sasaki O, Suzuki M, Hara Y, Shigematsu Y, Hiwada K. Effect of beta‐blockers on circulating levels of inflammatory and anti‐inflammatory cytokines in patients with dilated cardiomyopathy. J Am Coll Cardiol 2001; 37: 412–417. [DOI] [PubMed] [Google Scholar]
- 60. Tsutamoto T, Wada A, Maeda K, Mabuchi N, Hayashi M, Tsutsui T, Ohnishi M, Sawaki M, Fujii M, Matsumoto T, Kinoshita M. Angiotensin II type 1 receptor antagonist decreases plasma levels of tumor necrosis factor alpha, interleukin‐6 and soluble adhesion molecules in patients with chronic heart failure. J Am Coll Cardiol 2000; 35: 714. [DOI] [PubMed] [Google Scholar]
- 61. Baldetti L, Gallone G, Melillo F, Pagnesi M, Beneduce A. Another call to address inflammation in heart failure. J Am Coll Cardiol 2019; 74: 477–478. [DOI] [PubMed] [Google Scholar]
- 62. Lynch TL, Ismahil MA, Jegga AG, Zilliox MJ, Troidl C, Prabhu SD, Sadayappan S. Cardiac inflammation in genetic dilated cardiomyopathy caused by MYBPC3 mutation. J Mol Cell Cardiol 2017; 102: 83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Adamo L, Rocha‐Resende C, Prabhu SD, Mann DL. Reappraising the role of inflammation in heart failure. Nat Rev Cardiol 2020. [DOI] [PubMed] [Google Scholar]
- 64. Kalra R, Arora P, Morgan C, Hage FG, Iskandrian AE, Bajaj NS. Conducting and interpreting high‐quality systematic reviews and meta‐analyses. J Nucl Cardiol 2017; 24: 471–481. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. PRISMA checklist
Data S2. Inclusion exclusion criteria for all trials
Data S3. Search Strategy and data collection
Data S4. Statistics
Figure 1. Flow diagram for study selection
Figure 2. Risk‐of‐bias summary for randomized trials (RoB 2.0) assessed using the Cochrane RoB tool28
Figure S3. Review author's judgements about each risk‐of‐bias for each trial included. Green, yellow and red solid circles represent low, some concern and high risk‐of‐bias, respectively.
Figure S4. Effect of immunomodulation on LVEF as compared to no immunomodulation according to drug class. Black solid square diamonds and associated solid lines represent summary RR and 95% CI of each trial listed in the left column. The numerical estimates in the right columns are weighted mean difference(s) (WMD) with 95% CI of each trial listed in the left column. The hollow blue diamond is summary WMD and 95% CI for immunomodulation as compared to no immunomodulation. The hollow red diamond is the overall summary WMD with 95% CI. CI: Confidence interval
Figure S5. Funnel plot for publication bias with each blue dot representing a randomized trial and the dotted lines representing the pseudo 95% confidence intervals.
Figure S6. : Effect of immunomodulation on LVEF as compared to no immunomodulation according to heart failure aetiology. Black solid square diamonds and associated solid lines represent summary RR and 95% CI of each trial listed in the left column. The numerical estimates in the right columns are weighted mean difference(s) (WMD) with 95% CI of each trial listed in the left column. The hollow blue diamond is summary WMD and 95% CI for immunomodulation as compared to no immunomodulation. The hollow red diamond is the overall summary WMD with 95% CI. CI: Confidence interval
Table 3. Mean difference, weighted (WMD) and standardized (SMD) for primary and secondary outcomes.
Figure 7. Effect of immunomodulation on LVEDD as compared to no immunomodulation according to drug class. Black solid square diamonds and associated solid lines represent summary RR and 95% CI of each trial listed in the left column. The numerical estimates in the right columns are weighted mean difference(s) (WMD) with 95% CI of each trial listed in the left column. The hollow blue diamond is summary WMD and 95% CI for each class of immunomodulation as compared to no immunomodulation. The hollow red diamond is the overall summary WMD with 95% CI. CI: Confidence interval
Figure 8. Effect of immunomodulation on LVEDD as compared to no immunomodulation according to heart failure aetiology. Black solid square diamonds and associated solid lines represent summary RR and 95% CI of each trial listed in the left column. The numerical estimates in the right columns are weighted mean difference(s) (WMD) with 95% CI of each trial listed in the left column. The hollow blue diamond is summary WMD and 95% CI for immunomodulation as compared to no immunomodulation. The hollow red diamond is the overall summary WMD with 95% CI.CI: Confidence interval
Figure S9. Effect of immunomodulation on mortality as compared to no immunomodulation according to heart failure aetiology. Black solid square diamonds and associated solid lines represent summary RR and 95% CI of each trial listed in the left column. The numerical estimates in the right columns are the weighted mean difference(s) (WMD) with 95% CI of each trial listed in the left column. The hollow blue diamond is a summary WMD and 95% CI for immunomodulation as compared to no immunomodulation. The hollow red diamond is the overall summary RR with 95% CI.CI: Confidence interval
Figure S10. Sensitivity analysis for effect of immunomodulation on mortality as compared to no immunomodulation according to heart failure aetiology in all trials reporting mortality data. Black solid square diamonds and associated solid lines represent summary RR and 95% CI of each trial listed in the left column. The numerical estimates in the right columns are weighted mean difference(s) (WMD) with 95% CI of each trial listed in the left column. The hollow blue diamond is summary WMD and 95% CI for immunomodulation as compared to no immunomodulation. The hollow red diamond is the overall summary RR with 95% CI.CI: Confidence interval
Figure 11. Trial sequential analysis of immunomodulation vs. no immunomodulation in patients with heart failure for improvement in left ventricular ejection fraction (LVEF). The solid black line represents the line of no difference. The green lines above and below the line of no difference represent the O'Brien‐Fleming trial sequential boundary for no benefit and benefit with immunomodulation, respectively. The solid black lines are upper and lower bounds for 95% CI. The green vertical line is the required information size for conclusive meta‐analyses, given two‐sided α=0.05 and β=0.20. The solid blue line is Z‐curve derived from a random‐effects meta‐analysis of individual RCTs. The inner wedge represents the O'Brien‐Fleming β‐spending function at 80% power. The Z‐curve surpassed the trial sequential boundary and the information size, indicating a true improvement in LVEF with immunomodulation as compared to no immunomodulation.
Figure S11. Trial sequential analysis of immunomodulation vs. no immunomodulation in patients with heart failure for improvement in left ventricular ejection fraction (LVEF). The solid black line represents the line of no difference. The green lines above and below the line of no difference represent the O'Brien‐Fleming trial sequential boundary for no benefit and benefit with immunomodulation, respectively. The solid black lines are upper and lower bounds for 95% C.
Figure S12 Trial sequential analysis of immunomodulation vs. no immunomodulation in patients with heart failure for improvement in left ventricle end‐diastolic dimension (LVEDD). The solid black line represents the line of no difference. The green lines above and below the line of no difference represent the O'Brien‐Fleming trial sequential boundary for no benefit and benefit with immunomodulation, respectively. The solid black lines are upper and lower bounds for 95% CI. The green vertical line is the required information size for conclusive meta‐analyses, given two‐sided α=0.05 and β=0.20. The solid blue line is Z‐curve derived from a random‐effects meta‐analysis of individual RCTs. The inner wedge represents the O'Brien‐Fleming β‐spending function at 80% power. The Z‐curve surpassed the trial sequential boundary and the information size, indicating a true improvement in LVEDD with immunomodulation as compared to no immunomodulation.
Figure S13 Trial sequential analysis of immunomodulation vs. no immunomodulation in patients with heart failure for improvement in mortality. The solid black line represents the line of no difference. The solid black line represents the line of no difference. The green lines above and below the line of no difference represent the O'Brien‐Fleming trial sequential boundary for no benefit and benefit with immunomodulation, respectively. The solid black lines are upper and lower bounds for 95% CI. The green vertical line is the required information size for conclusive meta‐analyses, given two‐sided α=0.05 and β=0.20. The solid blue line is Z‐curve derived from a random‐effects meta‐analysis of individual RCTs. The inner wedge represents the O'Brien‐Fleming β‐spending function at 80% power. The Z‐curve did not surpass the trial sequential boundary and the information size, indicating a lack of sufficient evidence to conclude effect on mortality with or without immunomodulation.


