Abstract
Aims
Treating patients with acute decompensated heart failure (ADHF) presenting with volume overload is a common task. However, optimal guidance of decongesting therapy and treatment targets are not well defined. The inferior vena cava (IVC) diameter and its collapsibility can be used to estimate right atrial pressure, which is a measure of right‐sided haemodynamic congestion. The CAVA‐ADHF‐DZHK10 trial is designed to test the hypothesis that ultrasound assessment of the IVC in addition to clinical assessment improves decongestion as compared with clinical assessment alone.
Methods and results
CAVA‐ADHF‐DZHK10 is a randomized, controlled, patient‐blinded, multicentre, parallel‐group trial randomly assigning 388 patients with ADHF to either decongesting therapy guided by ultrasound assessment of the IVC in addition to clinical assessment or clinical assessment alone. IVC ultrasound will be performed daily between baseline and hospital discharge in all patients. However, ultrasound results will only be reported to treating physicians in the intervention group. Treatment target is relief of congestion‐related signs and symptoms in both groups with the additional goal to reduce the IVC diameter ≤21 mm and increase IVC collapsibility >50% in the intervention group. The primary endpoint is change in N‐terminal pro‐brain natriuretic peptide from baseline to hospital discharge. Secondary endpoints evaluate feasibility, efficacy of decongestion on other scales, and the impact of the intervention on clinical endpoints.
Conclusions
CAVA‐ADHF‐DZHK10 will investigate whether IVC ultrasound supplementing clinical assessment improves decongestion in patients admitted for ADHF.
Keywords: Acute decompensated heart failure, Inferior vena cava, Congestion, NT‐proBNP, Ultrasound
Introduction
Hospitalization for acute decompensated heart failure (ADHF) is associated not only with a high risk for early re‐hospitalization but also with an increased risk of death after discharge.1 Most patients are hospitalized due to congestion‐related symptoms and signs like dyspnoea or oedema.2 Congestion is defined as elevated left ventricular filling pressure and further sub‐classified in haemodynamic and clinical congestion depending on the absence or presence of clinical signs and symptoms of congestion.3 Guidelines recommend to treat all patients with ADHF with signs and symptoms of volume overload with intravenous loop diuretics and to monitor symptoms, urine output, renal function, and electrolytes (class I, level of evidence C) without stating specific decongestion targets.4 However, this is of fundamental interest because the remaining clinical congestion at discharge is a strong predictor of mortality risk.5, 6 The reference standard for congestion assessment is invasive measurement of right atrial pressure (RAP) and pulmonary capillary wedge pressure (PCWP) by means of a pulmonary artery catheter (PAC).3 However, the routine use of PAC is limited due to its invasive nature. Therefore, other markers of decongestion are needed to guide physicians during decongesting therapy. Diagnostic studies reported that the performance of clinical signs and symptoms to detect an elevated PCWP was only mediocre.7 Estimation of RAP by measurements of inferior vena cava (IVC) diameters using hand‐carried ultrasound devices was superior to clinical examination of the jugular venous pulse.8 Findings of several other observational studies indicate that IVC size and IVC collapsibility can be used to approximate semi‐quantitatively RAP as low, intermediate, or high.9 Based on this, echocardiography guidelines recommend ultrasound assessment of the IVC to estimate RAP.10 Moreover, a post hoc analysis of the ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) trial, which investigated changes of echocardiography markers during PAC‐guided decongesting therapy, found changes in IVC diameter and collapsibility to be closely associated with the change in PCWP.11 These considerations render ultrasound measurements of IVC diameter and collapsibility promising parameters to guide decongesting therapy in patients admitted for ADHF. We therefore conduct a randomized controlled trial (CAVA‐ADHF‐DZHK10, ultrasound evaluation of the inferior vena CAVA in addition to clinical assessment to guide decongestion in Acute Decompensated Heart Failure) to study ultrasound assessment of the IVC in addition to clinical assessment to guide decongestion in ADHF. This report describes the rationale and design of this trial.
The CAVA‐ADHF trial
Design and objectives
The CAVA‐ADHF trial is a randomized, controlled, patient‐blinded, multicentre, parallel‐group trial on the impact of ultrasound assessment of the IVC in addition to clinical assessment for guiding decongestion in ADHF. The trial flowchart is shown in Figure 1 .
Figure 1.
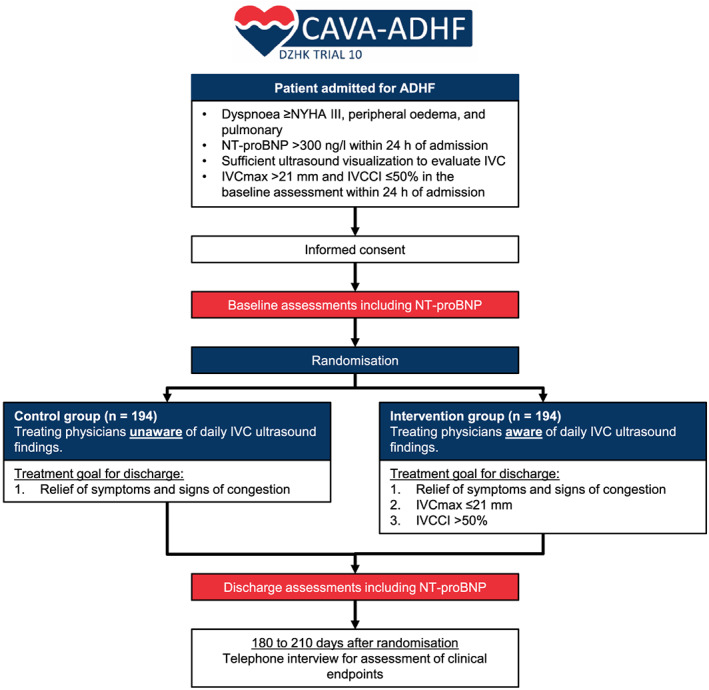
The CAVA‐ADHF‐DZHK10 trial scheme. IVC, inferior vena cava; IVCCI, inferior vena cava collapsibility index; IVC max, maximal inferior vena cava diameter; NYHA, New York Heart Association functional class; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
The primary objective is to examine patients admitted for ADHF whether decongestion guided by ultrasound assessment of IVC diameters in addition to clinical assessment leads to greater reductions in N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) levels from baseline to hospital discharge as compared with decongestion guided by clinical assessment alone. One secondary objective is to determine whether implementation of IVC ultrasound‐guided decongestion in clinical practice is feasible. The trial is registered at ClinicalTrials.gov (NCT03140566).
Patient population and eligibility
A total of 388 patients with ADHF will be enrolled at 15 trial sites in Germany. For the purpose of the trial, ADHF is diagnosed if a patient presents dyspnoea and signs of pulmonary congestion as signs for primary left heart failure as well as peripheral oedema as evidence for some kind of chronicity with secondary elevation of right‐sided filling pressure. Exclusion criteria serve primarily to exclude patients for which aggressive diuretic treatment is not the best treatment option. A detailed list of inclusion and exclusion criteria is displayed in Table 1. Figure 2 shows the geographical distribution of the trial centres. All sites have significant experience in treating patients with ADHF. Recruitment commenced in July 2017 and is estimated to be completed by the end of 2019. By 30 June 2019, a total of 348 patients (90%) have been enrolled (Figure 3 ).
Table 1.
Eligibility criteria
|
Inclusion criteria 1. Hospitalization for ADHF with dyspnoea ≥NYHA III, peripheral oedema, and pulmonary congestion (rales on auscultation or pulmonary vascular congestion on chest radiograph) 2. Age ≥ 18 years 3. NT‐proBNP >300 ng/L within 24 h after admissiona 4. Sufficient ultrasound visualization to evaluate IVC 5. IVC max >2.1 cm and IVCCI ≤50% in the baseline assessment within 24 h after admission 6. Informed consent |
|
Exclusion criteria 1. Cardiogenic shock with systolic blood pressure <90 mmHg plus end‐organ hypoperfusion 2. ADHF due to significant arrhythmiasb 3. Severe pulmonary disease as primary cause of dyspnoea 4. Simplified modification of diet in renal disease estimated glomerular filtration rate < 30 mL/min/1.73 m² 5. Need for non‐invasive or invasive ventilation support at baseline 6. Pregnancy 7. Participation in another interventional trial |
As determined by the local laboratory.
Significant arrhythmias are defined as (i) third‐degree atrioventricular block or sinus arrest with junctional or ventricular escape rhythm, (ii) sustained ventricular tachycardia, or (iii) other sustained arrhythmias leading to decompensation other than atrial fibrillation as judged by the treating physician.
ADHF, acute decompensated heart failure; IVC, inferior vena cava; IVCCCI, inferior vena cava collapsibility index; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association functional class.
Figure 2.
Geographical distribution of study sites.
Figure 3.
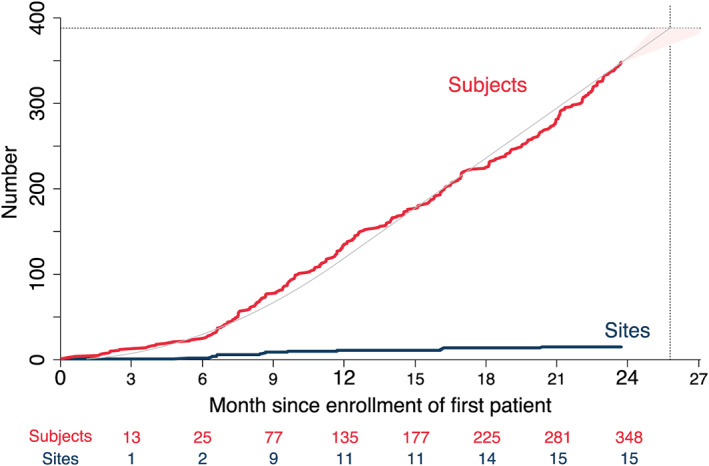
Enrolled subjects (red) and enrolling sites (blue) over time since first patient in. Prognosis (grey line, pink shading) assumes constant rates of newly enrolling sites in the first year and newly enrolled subjects per site. The CAVA‐ADHF trial aims to enrol a total of 388 patients (dotted horizontal line), which is approximated to be reached within 26 months from the start of enrolment (dotted vertical line).
Randomization
Patients are randomized either to decongestion therapy guided by ultrasound evaluated IVC diameters in addition to clinical assessment or to clinical assessment alone.
Randomization is performed within 24 h after hospital admission by a web‐based trial data base (secuTrial) with a 1:1 allocation ratio using permuted blocks of random sizes and stratified by trial site and New York Heart Association (NYHA) class (III versus IV).
Block sizes are not disclosed to ensure concealment. Allocation concealment is ensured, as the computerized system does not release the randomization code until the patient has been enrolled into the trial.
Trial intervention and blinding
From randomization to discharge, trained trial investigators not responsible for the clinical treatment examine daily IVC diameters by ultrasound in every patient irrespective of the assigned treatment group (Figure 1 ). To avoid measurement of erroneously small IVC diameters due to a transient shift of volume consecutive to an increase of venous capacitance in peripheral and splanchnic beds following application of a loop diuretic, the IVC evaluation shall be performed before the first application of a loop diuretic or at least 3 h after a preceding application. The IVC ultrasound evaluation is performed according to local infrastructure either with a handheld ultrasound device, a mobile ultrasound device at bedside, or in the ultrasound laboratory. The IVC is assessed as recommended in the current echocardiography guidelines10: The patient is kept in a supine position with the least elevation of the upper body possible. The IVC is scanned in its long axis from the subcostal view. The measurement of the IVC diameter is performed just proximal to the entrance of the hepatic veins at end‐expiration (IVC max), at inspiration (IVC min), and after a sniff manoeuvre (IVC sniff). The IVC collapsibility index (IVCCI) is derived from these parameters as percentage collapse of the maximal IVC diameter as follows: IVCCI = (IVC max − IVC min)/IVC max × 100. If IVC sniff is obtainable in acceptable quality and is smaller than IVC min, then IVC sniff is used to calculate IVCCI sniff analogously. IVC diameters (IVC max, IVC min, and IVC sniff) and IVCCI are documented by the trial investigator on a dedicated reporting form. Ultrasound images are not stored. Only for patients randomized to the intervention group, trial investigators personally communicate ultrasound findings to treating physicians and hand over the reporting form. By this process, the patient and his or her general practitioner are blinded to the allocated treatment group, and adherence is monitored. The treatment goal for every patient irrespective of the assigned treatment group is the resolution of symptoms and clinical signs of congestion (e.g. dyspnoea, oedema, rales, and elevated jugular venous pressure). Additional intervention goals in the intervention group are IVC max ≤21 mm and IVCCI >50% before discharge, which indicates that RAP is not high (Figure 4 ). The reporting form contains the recommendation to intensify and/or prolong decongesting treatment in case these interventional goals have not been met. Local trial investigators point treating physicians additionally to the treatment goals and offer advice on how decongestion treatment could be adapted in patients randomized to the intervention group. Final treatment decisions including dosing of loop diuretics, application of nitrates, and decision about unattainability of the intervention goal are ultimately left at the discretion of treating physicians.
Figure 4.
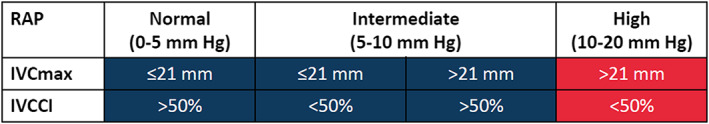
Estimation of right atrial pressure (RAP) pressure on the basis of maximal inferior vena cava diameter (IVC max) and inferior vena cava collapsibility index (IVVCI) as suggested by echocardiography guidelines.10 While a high‐estimated RAP (red) at baseline is mandatory for inclusion, the interventional goal of the CAVA‐ADHF trial is to achieve at discharge a normal to intermediate estimated RAP (blue) in addition to relief of congestion‐related symptoms and signs.
Endpoints
The primary endpoint of the CAVA‐ADHF trial is change in NT‐proBNP. Change means the ratio of NT‐proBNP at discharge to baseline. Discharge is not a fix duration in days from randomization but rather the point in time when in‐hospital treatment ends and the patient leaves the hospital. Effect measure is the ratio of geometric mean changes as a percentage difference between the two treatment groups. Blood samples for NT‐proBNP level determination are collected within 24 h of hospital admission for the baseline value and no more than 8 h before hospital discharge for the discharge value. All NT‐proBNP levels for the primary endpoint will be determined at the core laboratory (Institute of Clinical Chemistry and Laboratory Medicine, University Medicine Greifswald, Greifswald, Germany). Until then, blood samples are stored at each trial site until transportation to the core laboratory. A laboratory manual with instructions on how to sample blood, how to process it to plasma, how to store the plasma until shipment, and how to ship the samples is provided to trial centres.
Secondary endpoints are listed in Table 2. Respective information may be obtained from patients contacted via structured telephone interviews, from patient's treating physicians, or residents' registration offices. Freedom from signs of congestion at discharge will be assessed by trial investigators and is defined as the absence of orthopnoea, pulmonary rales, and jugular venous distension in conjunction with none or only a trace of oedema at discharge.
Table 2.
Secondary endpoints
|
Secondary feasibility endpoint • Proportions of patients with IVC ultrasound on two thirds of days in hospital and at discharge among all randomized patients |
|
Secondary clinical endpointsa • All‐cause death • Cardiovascular death • Unscheduled readmission for any cause • Unscheduled readmission due to worsening heart failure • Composite of all‐cause death or unscheduled readmission for any cause • Composite of cardiovascular death or unscheduled readmission due to worsening heart failure |
|
Other secondary endpoints • Haemoconcentration from baseline to discharge • Freedom from signs of congestion at discharge • Cumulative loop diuretic dose during index hospitalization • Length of index hospitalization |
|
Safety endpoints • Worsening renal function during index hospitalizationb • Worsening heart failure during index hospitalizationc |
All clinical endpoints will be analysed as time to first event with times censored at the end of 6 months of follow up.
Worsening renal function during index hospitalization is defined as an increase of serum creatinine 1.5 to 1.9 times baseline within 1 to 7 during index hospitalization or increase of serum creatinine of ≥26.5 mmol/L within 48 h after admission urine output <0.5 mL/kg/h for 6 to 12 h.
Worsening heart failure during index hospitalization is defined as worsening signs or symptoms of heart failure necessitating intensification of intravenous or mechanical heart failure treatment.
Statistical analysis
Analysis populations
The disposition of patients will be described by the CONSORT 2010 flow chart. All efficacy and feasibility analyses will be based on the intention‐to‐treat population consisting of all patients who consented, met all inclusion criteria and no exclusion criterion (Table 1), and were randomized. By this, the most likely intercurrent event, partial or total switch of treatment policy, is ignored in the efficacy analysis. Adverse events in all enrolled patients are reported on dedicated forms and are analysed by the extent to which treating physicians were aware of the IVC assessments (i.e. as‐treated population).
Primary and key secondary endpoints
The primary endpoint change in NT‐proBNP from baseline to discharge will be analysed as continuous variable and tested by means of a Mann–Whitney U test stratified by trial site and NYHA class. Trial sites contributing <20 patients to the analysis are pooled. Patients who died during index hospitalization are ranked by their survival times. Other missing values are assumed to occur completely at random and will not be imputed. In addition, 95% confidence intervals (CIs) will be estimated by means of an analysis of covariance using logarithms of NT‐proBNP at discharge with respective baseline values as covariate, stratified by NYHA class, and with trial site as random effects. For this additional analysis, multiple imputation of missing values will be performed.
In case of a significant effect on the primary endpoint, key secondary efficacy endpoints will be tested in a hierarchical testing procedure until the first test is negative. Testing will be performed in the following order:
cardiovascular death or readmission due to worsening heart failure,
all‐cause death or unscheduled readmission for any cause,
cardiovascular death, and
all‐cause death.
Feasibility is analysed by absolute and relative frequency and exact binomial confidence intervals. Feasibility is studied to determine whether (i) ultrasound evaluation of the IVC with measurement of IVC diameters is possible in the majority of ADHF patients and (ii) its daily repetition is feasible in clinical practice. Secondary time to event endpoints are analysed by means of Kaplan–Meier curves and hazard ratios with 95% confidence intervals estimated from Cox regression. Haemoconcentration (i.e. relative increase in haemoglobin from baseline to discharge) and freedom from signs of congestion at discharge are analysed by absolute and relative frequencies together with 95% confidence score intervals for the difference of proportions. Other secondary endpoints will also be descriptively analysed and are presented together with power calculations of detectable effects for key secondary endpoints, analysis of adverse events, and pre‐specified subgroup analyses in the supplement (please see the online appendix for more details).
Sample size rationale
For the ratio of NT‐proBNP at baseline to NT‐proBNP at day 2, a substudy of the RELAX‐AHF trial found geometric means of 0.61 with placebo and 0.49 with serelaxin. This corresponds to a ratio of geometric means of 0.812 (95% confidence interval, 0.753 to 0.876).12 Assuming normal distributions of logarithms, this was converted to mean difference of 0.21 logarithmic units and standard deviation of 0.7. Sample size analysis is based on an unpaired (two sample) t‐test. To detect an effect size of 0.21/0.7 = 0.3 standard deviations with a power of 80% at the two‐sided 5% level after 1:1 allocation, 176 patients per group have to be analysed. To compensate for a loss of 10% due to reasons other than death during index hospitalization, about 388 patients shall be recruited. Loss may be caused by intercurrent events like withdrawal of consent, discharge to another hospital, or missing NT‐proBNP values at admission or discharge (please see the online appendix for power calculations of other endpoints than the primary endpoint).
Study organization and funding
The CAVA‐ADHF trial is an investigator‐initiated trial fully funded by the DZHK. The sponsor of the trial is the University Medical Centre Schleswig‐Holstein, Campus Lübeck (UKSH). The coordinating centre is the Department of Cardiology, Angiology, and Intensive Care Medicine of the University Hospital of Schleswig‐Holstein in Lübeck together with the Heart Centre Leipzig at University of Leipzig. Local Ethics Committees of participating trial sites approved the trial protocol. Project management is located at the Centre for Clinical Trials Lübeck, which also conducts on‐site monitoring in a risk‐adaptive manner. The Steering Committee (A. J., S. D., and H. T.) designed the trial and ensures scientific quality in all study‐related aspects. The Steering Committee will submit the final report for peer‐reviewed publication, which will be referenced in the trial registry and disseminated on cardiologist's conferences. An independent data safety and monitoring board consists of three members not directly involved in the trial conduct, with expertise in the management of heart failure patients and/or large‐scale clinical trials (two clinicians and one biostatistician). The data safety and monitoring board is monitoring unblinded safety data. The DZHK infrastructure provides independent storage of patient identifiable data (independent trustee, Greifswald) and clinical trial data (data management, Göttingen). By this, the highest level of privacy and data quality is ensured. Clinical endpoints are adjudicated by local trial investigators. All statistical analyses will be performed by the Institute of Medical Biometry and Statistics (University of Lübeck, Lübeck, Germany).
Discussion
The CAVA‐ADHF trial is a randomized, controlled, patient‐blinded, multicentre, parallel‐group trial in patients admitted for ADHF assessing the efficacy and feasibility of a decongestion therapy guided by ultrasound evaluation of IVC diameters in addition to clinical assessment. Typically, patients admitted for ADHF present with congestion‐related signs and symptoms.2 Besides relative volume overload following redistribution due to haemodynamic changes with resulting pulmonary congestion, absolute volume overload due to increased water and sodium retention or reduced urine output is common.13 Interestingly, despite different underlying causes, congestion patterns between patients with heart failure with reduced versus preserved ejection fraction are similar.14 We therefore chose not to confine the CAVA‐ADHF population to either one of these heart failure types. Loop diuretics are used in the vast majority of ADHF patients with volume overload. Guidelines recommend (i) an inital dose of 20 to 40 mg intravenous furosemide for diuretic naive patientsand (ii) an initial intravenous dose at least equivalent to oral furosemide dose for patients on chronic diuretic therapy.4 Several observational studies and post hoc analyses of randomized controlled trials reported that residual congestion at the end of treatment is of paramount prognostic importance.15, 16 Further observations indicate that an aggressive decongestion therapy might even offer a survival benefit despite a moderate drop in the estimated glomerular filtration rate.17, 18 However, specific targets for such an aggressive decongestion strategy are not well defined. Targeted decongestion based on PAC‐obtained PCWP and RAP values compared with clinical congestion assessment alone did not affect the risk of all‐cause mortality and re‐hospitalization in the ESCAPE trial.19 Of note, length of hospital stay was also similar in both groups (approximately 8.5 days in mean), but in the intervention group, PACs were only in place for a median of 1.9 days. Hence, the tool providing the information for gearing the intensity of decongestion treatment was not available throughout the hospitalization period. Moreover, the ESCAPE trial was stopped prematurely after enrolment of 433 of 500 planned patients due to concerns of in‐hospital adverse events related to the PAC. Therefore, PAC‐guided therapy was abandoned, but other measures are urgently needed. Numerous markers for congestion were found to predict mortality and/or re‐hospitalization after an episode of ADHF including NT‐proBNP,20 B‐lines on lung ultrasound,21 IVC diameter,22 and haemoconcentration.23 With the exception of NT‐proBNP, none of these parameters have been tested in a randomized controlled trial. In the PRIMA II (Can NT‐ProBNP‐Guided Therapy During Hospital Admission for Acute Decompensated Heart Failure Reduce Mortality and Readmissions?) trial, which enrolled 405 ADHF patients, NT‐proBNP‐guided treatment did not improve the composite endpoint of all‐cause mortality and heart failure readmissions in comparison with conventional treatment (hazard ratio, 0.96; 95% confidence interval, 0.72 to 1.37; P = 0.99).24 This raised concerns whether other parameters known to be of prognostic importance might be able to improve decongestion therapy and/or alter prognosis when in‐hospital treatment is tailored to one of these parameters or whether all these parameters are only an expression of disease severity and thereby signifying an unfavourable disease progression. Among the ultrasound parameters, the IVC diameter holds several practical advantages: it is easily obtainable by any standard ultrasound equipment including handheld devices, it requires little skills of the echocardiographer, and it is—together with lung ultrasound—the quickest to obtain. These are important features of a bioassay that may be considered for use in clinical routine (e.g. assessed during ward rounds in several patients by a young physician). The IVC collects blood from the veins of the abdominal organs and lower extremities and transports it to the right atrium. The IVC is a highly compliant vessel without prominent internal and/or external mechanisms actively affecting its tone. Thereby, the IVC diameter is predominantly determined by intravascular volume status and right‐sided filling pressure. In contrast to laboratory biomarkers, where serum levels often depend on secretion and degradation/excretion, and chest X‐ray‐assessed pulmonary congestion, the IVC diameter is a fast responding gauge of these measures. In contrast to lung ultrasound, which can be used to assess the amount of extravascular lung water and its change during treatment,25 IVC diameters are stronger, influenced by total volume overload and right‐sided congestion, which is a major problem in many patients presenting with ADHF. Therefore, ultrasound assessment of the IVC is an attractive candidate as target for in‐hospital treatment with the goal to improve decongestion and normalize volume status as used in the CAVA‐ADHF trial. Ultrasound evaluation was limited to the IVC to keep the intervention as simple and easy to implement as possible.
An analysis of 1291 patients enrolled in a prospective multicentre cohort study suggested that the concept of door‐to‐balloon time for STEMI might be transferred to ADHF as door‐to‐furosemide time. In this analysis, in‐hospital mortality was significantly lower in patients receiving the first intravenous furosemide injection in <60 min after arrival at the emergency department as compared with patients who received it later (2.3% vs. 6.0%, respectively; P = 0.002).26 A rapid reduction in congestion through an early intravenous furosemide injection might limit subendocardial ischaemia and organ injury and thereby improve short‐term and long‐term prognosis.27 However, the CAVA‐ADHF protocol aims to achieve a superior level of decongestion at hospital discharge rather than a fast decongestion. We argue against the idea of a fast decongestion for several reasons. First, the finding of a prognostic benefit due to a short door‐to‐furosemide time is inconsistent. Almost a year after the aforementioned observational study, a South Korean group published neutral findings based on a similar strategy.28 Second, outcome trials investigating novel intravenous vasodilators (i.e. nesiritide, serelaxin, and ularitide), which induce rapid reductions in filling pressures, failed to impact hard endpoints.29, 30 Third, ADHF may not always be as hyperacute as its terminus suggests. Haemodynamic congestion usually evolves over a period of days to weeks, followed by the development of clinical signs and symptoms. Ultimately, unbearable symptoms may trigger hospital admission.31 Therefore, achieving an optimal level of decongestion at hospital discharge may be key, and this concept is central for the CAVA‐ADHF trial. However, respective thresholds for good or optimal decongestion have not been defined.4 Relief of dyspnoea and weight loss are commonly used metrics to guide decongestion therapy in clinical practice. However, both have considerable shortcomings, and their use is based on convenience for assessment and established clinical routine.32 A recent scientific statement on the use of diuretics in heart failure highlights a multimodal evaluation of congestion (i.e. clinical assessment, biomarkers, and technical assessments) as the best contemporary strategy. The authors, however, also state that such strategy might be dependent on local expertise and has never been tested prospectively. The CAVA‐ADHF trial intends to close this gap by investigating whether a bimodal evaluation of congestion (i.e. clinical assessment supplemented by ultrasound‐estimated RAP) is superior to clinical assessment alone and whether this bimodal evaluation is feasible in a multicentre setting. The CAVA‐ADHF trial defines a good response to decongesting therapy in the intervention group as the combination of (i) relief of congestion‐related symptoms, (ii) reduction of the IVC diameter to ≤21 mm, and (iii) increase in the IVC collapsibility to >50%. Because the guidelines published by the European Society of Cardiology did not define relief of congestion‐related symptoms precisely,4 no specific targets were defined in the CAVA‐ADHF protocol either. However, as outlined earlier, the achievement of IVC goals would translate to the estimation of a normal or just intermediately elevated RAP and thereby improvement in haemodynamic congestion (Figure 4 ).
There are several limitations to ultrasound assessment of the IVC. Obesity, meteorism, or other anatomical obstacles might preclude visualization of the IVC by ultrasound. In such case, the patients are not eligible for enrolment and randomization. Estimating the RAP in patients under positive pressure ventilation is not possible. Therefore, the need for non‐invasive or invasive ventilation support at baseline is also an exclusion criterion. The ultrasound assessment of the IVC will be paused in patients suffering worsening heart failure requiring positive pressure ventilation during in‐hospital treatment after randomization. In addition, IVC ultrasound does assess only right‐sided congestion. Thereby, RAP estimated by ultrasound assessment of IVC diameters will fail to detect changes in left‐sided filling pressure in a minority of patients (approximately 15% of patients).33, 34
Due to the pilot trial‐nature of the CAVA‐ADHF trial, we chose change in NT‐proBNP from baseline to discharge as a surrogate endpoint. An individual patient data meta‐analysis of several observational studies and a post‐hoc analysis of the RELAX‐AHF trial indicated that the reduction in NT‐proBNP during decongesting therapy is of prognostic importance.12, 20 As NT‐proBNP is measured in a core laboratory, the endpoint of change in NT‐proBNP will be unbiased and reliably assessed.
Conclusions
In patients admitted for ADHF, assessment of congestion during decongesting therapy and determination of a ‘good level’ of decongestion is an everyday clinical problem without satisfactory scientific evidence guiding the treating physician. CAVA‐ADHF is designed to test whether IVC assessment in addition to clinical assessment is superior to clinical assessment alone with regard to the surrogate efficacy endpoint NT‐proBNP. If the CAVA‐ADHF trial shows promising results for this efficacy endpoint and/or other secondary endpoints, this approach should be tested in a sufficiently powered confirmatory trial to detect meaningful effects on hard clinical endpoints.
Conflict of interest
None declared.
Funding
This work is supported by the DZHK (German Centre for Cardiovascular Research) (grants 81X1700106, 81X1700107). The study/trial/project is carried out using the clinical‐scientific infrastructure of the DZHK.
Appendix A.
A..1.
A..1.1. Statistical analysis
A..1.1.1. Descriptive statistics
Success of randomization is demonstrated by descriptive statistics of baseline demographics and measures of disease severity by treatment group. Scores summarizing physical examination use ordinal scales, so that absolute and relative frequency distributions and 95% confidence Wald interval for the odds ratio from an ordinal logistic regression on allocated treatment are reported. Items of history and most aspects of the intervention are dichotomous and, consequently, described by absolute and relative frequencies together with 95% confidence score intervals for the difference of proportions. Lengths of stay and drug doses are metric variables and are described by median and interquartile range (IQR) for each treatment group and 95% confidence Hodges–Lehmann intervals for the difference of medians. Concomitant medications are nominal variables, for which tables of absolute and relative frequencies are presented with modal values highlighted.
A..1.1.2. Detectable effects on secondary endpoints
With regard to some of the secondary endpoints, missing values can be avoided by assuming feasibility, death, and readmission only where data exist. An exact binomial test with a nominal 5% two‐sided significance level will have 80% power to detect the difference between the null hypothesis, a feasible proportion of 80%, and the alternative proportion of 85.8%, if 2 × 176 = 352 cases are analysed.
According to a retrospective analysis of ADHF patients treated from April 2008 to December 2014 in our department [Heart Vessels. 2017 Jul;32(7):856‐864.], the composite endpoint of all‐cause mortality or unscheduled readmission for any cause follows a Weibull distribution and occurs with a rate of 54.4% 180 days after admission. Based on this, a two‐sided level 5% log‐rank test has a power of 80% to detect a hazard ratio of 0.657 when the rate is 54.4% in the inferior management and 40.3% in the superior management, if 2 × 194 = 388 randomized cases exhibit 180 day censoring rate of 0.05. This would mean observing 178 events. Calculations used nQuery Advisor 6.01.
A..1.1.3. Safety and exploratory analyses
Frequencies of AE and SAE are tabulated by system organ class. Numbers of patients, causality (dichotomized), and resolution, worsening renal function during index hospitalization and worsening heart failure during index hospitalization, are analysed as absolute and relative frequencies together with χ 2 for trend, while intensities and durations are analysed like the other ordinal and metric variables, respectively. Variables measured by echocardiography will be analysed in a multivariate t‐test and 95% confidence ellipsoid. Biomarkers are explored, to what extent they are predictive, prognostic, or surrogate, in analysis of covariance models similar to the primary analysis. Additionally, modern regularized regression methods are applied like elastic net with subsampling, boosting, or random forests using R.
Analysis of covariance with baseline as covariate is used for all metric measurements made at baseline and at discharge. Adjustments of NT‐proBNP changes by baseline values for body mass index, atrial fibrillation, and estimated glomerular filtration rate are explored.
Multiple logistic regression with baseline as second factor is used for all ordinal measurements made at baseline and at discharge.
Exploratory subgroup analyses of event times and NT‐proBNP changes will be performed regarding patients' sex, NYHA class, and left ventricular ejection fraction (<40% vs. ≥40%) by estimating Cox regressions adjusted for age and a linear model for logarithms adjusted for baseline, respectively, with treatment as a factor and the interaction with treatment. Treatment effects within subgroups are estimated, and 95% confidence intervals are computed and shown in a forest plot augmented by descriptive P values for the interaction.
Jobs, A. , Vonthein, R. , König, I. R. , Schäfer, J. , Nauck, M. , Haag, S. , Fichera, C. F. , Stiermaier, T. , Ledwoch, J. , Schneider, A. , Valentova, M. , von Haehling, S. , Störk, S. , Westermann, D. , Lenz, T. , Arnold, N. , Edelmann, F. , Seppelt, P. , Felix, S. , Lutz, M. , Hedwig, F. , Borggrefe, M. , Scherer, C. , Desch, S. , and Thiele, H. (2020) Inferior vena cava ultrasound in acute decompensated heart failure: design rationale of the CAVA‐ADHF‐DZHK10 trial. ESC Heart Failure, 7: 973–983. 10.1002/ehf2.12598.
References
- 1. Solomon SD, Dobson J, Pocock S, Skali H, McMurray JJ, Granger CB, Yusuf S, Swedberg K, Young JB, Michelson EL, Pfeffer MA. Candesartan in Heart failure: Assessment of Reduction in M, morbidity I. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation 2007; 116: 1482–1487. [DOI] [PubMed] [Google Scholar]
- 2. Chioncel O, Mebazaa A, Harjola VP, Coats AJ, Piepoli MF, Crespo‐Leiro MG, Laroche C, Seferovic PM, Anker SD, Ferrari R, Ruschitzka F, Lopez‐Fernandez S, Miani D, Filippatos G, Maggioni AP, Investigators ESCHFL‐TR . Clinical phenotypes and outcome of patients hospitalized for acute heart failure: the ESC heart failure long‐term registry. Eur J Heart Fail 2017; 19: 1242–1254. [DOI] [PubMed] [Google Scholar]
- 3. Gheorghiade M, Follath F, Ponikowski P, Barsuk JH, Blair JE, Cleland JG, Dickstein K, Drazner MH, Fonarow GC, Jaarsma T, Jondeau G, Sendon JL, Mebazaa A, Metra M, Nieminen M, Pang PS, Seferovic P, Stevenson LW, van Veldhuisen DJ, Zannad F, Anker SD, Rhodes A, McMurray JJ, Filippatos G, European Society of C, European Society of Intensive Care M . Assessing and grading congestion in acute heart failure: a scientific statement from the acute heart failure committee of the heart failure association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. Eur J Heart Fail 2010; 12: 423–433. [DOI] [PubMed] [Google Scholar]
- 4. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force M, Document R. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 5. Chioncel O, Mebazaa A, Maggioni AP, Harjola VP, Rosano G, Laroche C, Piepoli MF, Crespo‐Leiro MG, Lainscak M, Ponikowski P, Filippatos G, Ruschitzka F, Seferovic P, Coats AJS, Lund LH, Investigators E‐EHHFL‐TR . Acute heart failure congestion and perfusion status—impact of the clinical classification on in‐hospital and long‐term outcomes; insights from the ESC‐EORP‐HFA heart failure long‐term registry. Eur J Heart Fail 2019; 21: 1338–1352. [DOI] [PubMed] [Google Scholar]
- 6. Rubio‐Gracia J, Demissei BG, Ter Maaten JM, Cleland JG, O'Connor CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, Davison BA, Givertz MM, Bloomfield DM, Dittrich H, Damman K, Perez‐Calvo JI, Voors AA. Prevalence, predictors and clinical outcome of residual congestion in acute decompensated heart failure. Int J Cardiol 2018; 258: 185–191. [DOI] [PubMed] [Google Scholar]
- 7. Mullens W, Damman K, Harjola VP, Mebazaa A, Brunner‐La Rocca HP, Martens P, Testani JM, Tang WHW, Orso F, Rossignol P, Metra M, Filippatos G, Seferovic PM, Ruschitzka F, Coats AJ. The use of diuretics in heart failure with congestion—a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2019; 21: 137–155. [DOI] [PubMed] [Google Scholar]
- 8. Brennan JM, Blair JE, Goonewardena S, Ronan A, Shah D, Vasaiwala S, Brooks E, Levy A, Kirkpatrick JN, Spencer KT. A comparison by medicine residents of physical examination versus hand‐carried ultrasound for estimation of right atrial pressure. Am J Cardiol 2007; 99: 1614–1616. [DOI] [PubMed] [Google Scholar]
- 9. Beigel R, Cercek B, Luo H, Siegel RJ. Noninvasive evaluation of right atrial pressure. J Am Soc Echocardiogr 2013; 26: 1033–1042. [DOI] [PubMed] [Google Scholar]
- 10. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010; 23: 685–713 quiz 86‐8. [DOI] [PubMed] [Google Scholar]
- 11. Ramasubbu K, Deswal A, Chan W, Aguilar D, Bozkurt B. Echocardiographic changes during treatment of acute decompensated heart failure: insights from the ESCAPE trial. J Card Fail 2012; 18: 792–798. [DOI] [PubMed] [Google Scholar]
- 12. Metra M, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Ponikowski P, Unemori E, Voors AA, Adams KF Jr, Dorobantu MI, Grinfeld L, Jondeau G, Marmor A, Masip J, Pang PS, Werdan K, Prescott MF, Edwards C, Teichman SL, Trapani A, Bush CA, Saini R, Schumacher C, Severin T, Teerlink JR, Investigators R‐A . Effect of serelaxin on cardiac, renal, and hepatic biomarkers in the Relaxin in Acute Heart Failure (RELAX‐AHF) development program: correlation with outcomes. J Am Coll Cardiol 2013; 61: 196–206. [DOI] [PubMed] [Google Scholar]
- 13. Anand IS, Ferrari R, Kalra GS, Wahi PL, Poole‐Wilson PA, Harris PC. Edema of cardiac origin. Studies of body water and sodium, renal function, hemodynamic indexes, and plasma hormones in untreated congestive cardiac failure. Circulation 1989; 80: 299–305. [DOI] [PubMed] [Google Scholar]
- 14. Van Aelst LNL, Arrigo M, Placido R, Akiyama E, Girerd N, Zannad F, Manivet P, Rossignol P, Badoz M, Sadoune M, Launay JM, Gayat E, Lam CSP, Cohen‐Solal A, Mebazaa A, Seronde MF. Acutely decompensated heart failure with preserved and reduced ejection fraction present with comparable haemodynamic congestion. Eur J Heart Fail 2018; 20: 738–747. [DOI] [PubMed] [Google Scholar]
- 15. Wattad M, Darawsha W, Solomonica A, Hijazi M, Kaplan M, Makhoul BF, Abassi ZA, Azzam ZS, Aronson D. Interaction between worsening renal function and persistent congestion in acute decompensated heart failure. Am J Cardiol 2015; 115: 932–937. [DOI] [PubMed] [Google Scholar]
- 16. Ambrosy AP, Pang PS, Khan S, Konstam MA, Fonarow GC, Traver B, Maggioni AP, Cook T, Swedberg K, Burnett JC Jr, Grinfeld L, Udelson JE, Zannad F, Gheorghiade M, Investigators ET. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur Heart J 2013; 34: 835–843. [DOI] [PubMed] [Google Scholar]
- 17. Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, LeWinter MM, Deswal A, Rouleau JL, Ofili EO, Anstrom KJ, Hernandez AF, McNulty SE, Velazquez EJ, Kfoury AG, Chen HH, Givertz MM, Semigran MJ, Bart BA, Mascette AM, Braunwald E, O'Connor CM, Network NHFCR. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 2011; 364: 797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Testani JM, Chen J, McCauley BD, Kimmel SE, Shannon RP. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation 2010; 122: 265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Binanay C, Califf RM, Hasselblad V, O'Connor CM, Shah MR, Sopko G, Stevenson LW, Francis GS, Leier CV, Miller LW, Investigators E, Coordinators ES. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA 2005; 294: 1625–1633. [DOI] [PubMed] [Google Scholar]
- 20. Salah K, Kok WE, Eurlings LW, Bettencourt P, Pimenta JM, Metra M, Bayes‐Genis A, Verdiani V, Bettari L, Lazzarini V, Damman P, Tijssen JG, Pinto YM. A novel discharge risk model for patients hospitalised for acute decompensated heart failure incorporating N‐terminal pro‐B‐type natriuretic peptide levels: a European coLlaboration on Acute decompeNsated Heart Failure: ELAN‐HF Score. Heart 2014; 100: 115–125. [DOI] [PubMed] [Google Scholar]
- 21. Platz E, Lewis EF, Uno H, Peck J, Pivetta E, Merz AA, Hempel D, Wilson C, Frasure SE, Jhund PS, Cheng S, Solomon SD. Detection and prognostic value of pulmonary congestion by lung ultrasound in ambulatory heart failure patients. Eur Heart J 2016; 37: 1244–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goonewardena SN, Gemignani A, Ronan A, Vasaiwala S, Blair J, Brennan JM, Shah DP, Spencer KT. Comparison of hand‐carried ultrasound assessment of the inferior vena cava and N‐terminal pro‐brain natriuretic peptide for predicting readmission after hospitalization for acute decompensated heart failure. JACC Cardiovasc Imaging 2008; 1: 595–601. [DOI] [PubMed] [Google Scholar]
- 23. Vaduganathan M, Greene SJ, Fonarow GC, Voors AA, Butler J, Gheorghiade M. Hemoconcentration‐guided diuresis in heart failure. Am J Med 2014; 127: 1154–1159. [DOI] [PubMed] [Google Scholar]
- 24. Stienen S, Salah K, Moons AH, Bakx AL, van Pol P, Kortz RAM, Ferreira JP, Marques I, Schroeder‐Tanka JM, Keijer JT, Bayes‐Genis A, Tijssen JGP, Pinto YM, Kok WE. NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide)‐guided therapy in acute decompensated heart failure: PRIMA II randomized controlled trial (Can NT‐ProBNP‐Guided Therapy During Hospital Admission for Acute Decompensated Heart Failure Reduce Mortality and Readmissions?). Circulation 2018; 137: 1671–1683. [DOI] [PubMed] [Google Scholar]
- 25. Picano E, Pellikka PA. Ultrasound of extravascular lung water: a new standard for pulmonary congestion. Eur Heart J 2016; 37: 2097–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Matsue Y, Damman K, Voors AA, Kagiyama N, Yamaguchi T, Kuroda S, Okumura T, Kida K, Mizuno A, Oishi S, Inuzuka Y, Akiyama E, Matsukawa R, Kato K, Suzuki S, Naruke T, Yoshioka K, Miyoshi T, Baba Y, Yamamoto M, Murai K, Mizutani K, Yoshida K, Kitai T. Time‐to‐furosemide treatment and mortality in patients hospitalized with acute heart failure. J Am Coll Cardiol 2017; 69: 3042–3051. [DOI] [PubMed] [Google Scholar]
- 27. Unverferth DV, Magorien RD, Lewis RP, Leier CV. The role of subendocardial ischemia in perpetuating myocardial failure in patients with nonischemic congestive cardiomyopathy. Am Heart J 1983; 105: 176–179. [DOI] [PubMed] [Google Scholar]
- 28. Park JJ, Kim SH, Oh IY, Choi DJ, Park HA, Cho HJ, Lee HY, Cho JY, Kim KH, Son JW, Yoo BS, Oh J, Kang SM, Baek SH, Lee GY, Choi JO, Jeon ES, Lee SE, Kim JJ, Lee JH, Cho MC, Jang SY, Chae SC, Oh BH. The effect of door‐to‐diuretic time on clinical outcomes in patients with acute heart failure. JACC Heart Fail 2018; 6: 286–294. [DOI] [PubMed] [Google Scholar]
- 29. Packer M, O'Connor C, McMurray JJV, Wittes J, Abraham WT, Anker SD, Dickstein K, Filippatos G, Holcomb R, Krum H, Maggioni AP, Mebazaa A, Peacock WF, Petrie MC, Ponikowski P, Ruschitzka F, van Veldhuisen DJ, Kowarski LS, Schactman M, Holzmeister J, Investigators T‐A. Effect of ularitide on cardiovascular mortality in acute heart failure. N Engl J Med 2017; 376: 1956–1964. [DOI] [PubMed] [Google Scholar]
- 30. O'Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, Heizer GM, Komajda M, Massie BM, McMurray JJ, Nieminen MS, Reist CJ, Rouleau JL, Swedberg K, Adams KF Jr, Anker SD, Atar D, Battler A, Botero R, Bohidar NR, Butler J, Clausell N, Corbalan R, Costanzo MR, Dahlstrom U, Deckelbaum LI, Diaz R, Dunlap ME, Ezekowitz JA, Feldman D, Felker GM, Fonarow GC, Gennevois D, Gottlieb SS, Hill JA, Hollander JE, Howlett JG, Hudson MP, Kociol RD, Krum H, Laucevicius A, Levy WC, Mendez GF, Metra M, Mittal S, Oh BH, Pereira NL, Ponikowski P, Tang WH, Tanomsup S, Teerlink JR, Triposkiadis F, Troughton RW, Voors AA, Whellan DJ, Zannad F, Califf RM. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med 2011; 365: 32–43. [DOI] [PubMed] [Google Scholar]
- 31. Zile MR, Bennett TD, St John Sutton M, Cho YK, Adamson PB, Aaron MF, Aranda JM Jr, Abraham WT, Smart FW, Stevenson LW, Kueffer FJ, Bourge RC. Transition from chronic compensated to acute decompensated heart failure: pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation 2008; 118: 1433–1441. [DOI] [PubMed] [Google Scholar]
- 32. Testani JM, Brisco MA, Kociol RD, Jacoby D, Bellumkonda L, Parikh CR, Coca SG, Tang WH. Substantial discrepancy between fluid and weight loss during acute decompensated heart failure treatment. Am J Med 2015; 128: 776–783 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Campbell P, Drazner MH, Kato M, Lakdawala N, Palardy M, Nohria A, Stevenson LW. Mismatch of right‐ and left‐sided filling pressures in chronic heart failure. J Card Fail 2011; 17: 561–568. [DOI] [PubMed] [Google Scholar]
- 34. Drazner MH, Brown RN, Kaiser PA, Cabuay B, Lewis NP, Semigran MJ, Torre‐Amione G, Naftel DC, Kirklin JK. Relationship of right‐ and left‐sided filling pressures in patients with advanced heart failure: a 14‐year multi‐institutional analysis. J Heart Lung Transplant 2012; 31: 67–72. [DOI] [PubMed] [Google Scholar]


