Abstract
Aims
The PREPARE‐MVR study (PRediction of Early PostoperAtive Right vEntricular failure in Mitral Valve Replacement/Repair patients) sought to investigate the alterations of right ventricular (RV) contraction pattern in patients undergoing mitral valve replacement/repair (MVR) and to explore the associations between pre‐operative RV mechanics and early post‐operative RV dysfunction (RVD).
Methods and results
We prospectively enrolled 42 patients (63 ± 11 years, 69% men) undergoing open‐heart MVR. Transthoracic three‐dimensional (3D) echocardiography was performed pre‐operatively, at intensive care unit discharge, and 6 months after surgery. The 3D model of the RV was reconstructed, and RV ejection fraction (RVEF) was calculated. We decomposed the motion of the ventricle to compute longitudinal ejection fraction (LEF) and radial ejection fraction (REF). Pulmonary artery catheterization was performed to monitor RV stroke work index (RVSWi). RVEF was slightly decreased after MVR [52 (50–55) vs. 51 (46–54)%; P = 0.001], whereas RV contraction pattern changed notably. Before MVR, the longitudinal shortening was the main contributor to global systolic RV function [LEF/RVEF vs. REF/RVEF; 0.53 (0.47–0.58) vs. 0.33 (0.22–0.42); P < 0.001]. Post‐operatively, the radial motion became dominant [0.33 (0.28–0.43) vs. 0.46 (0.37–0.51); P = 0.004]. However, this shift was temporary as 6 months later the two components contributed equally to global RV function [0.44 (0.38–0.50) vs. 0.41 (0.36–0.49); P = 0.775]. Pre‐operative LEF was an independent predictor of post‐operative RVD defined as RVSWi < 300 mmHg⋅mL/m2 [OR = 1.33 (95% CI: 1.08–1.77), P < 0.05].
Conclusions
MVR induces a significant shift in the RV mechanical pattern. Advanced indices of RV mechanics are associated with invasively measured parameters of RV contractility and may predict post‐operative RVD.
Keywords: Mitral valve regurgitation, Mitral valve surgery, Right ventricle, Right ventricular dysfunction, 3D echocardiography
Introduction
Mitral valve regurgitation (MR) is reported to be the second most frequent indication for valve surgery. 1 , 2 Severe primary MR has well‐known prognostic implications; therefore, timely diagnosis and appropriate risk stratification represent a compelling clinical demand in this patient population. 2
Chronic MR results in volume overload primarily in the left heart. However, significant adaptive and maladaptive changes are induced not only in the left ventricular (LV) but also in the right ventricular (RV) morphology and function. 3 , 4 Functional alterations of the RV are frequent but underrecognized findings in chronic primary MR patients. 5 , 6 RV function is affected early and through different pathways, including elevated pulmonary pressures, geometrical changes triggered by the altered LV morphology, and the impairment of ventricular interdependence. 3 , 7 Moreover, surgical interventions applying cardiopulmonary bypass and pericardiotomy deteriorate the native RV contraction pattern and may further predispose to develop overt dysfunction. 8 , 9 RV dysfunction (RVD) can progress into a challenging clinical scenario resulting in prolonged length of intensive care unit (ICU) stay, increased use of medical resources, and excess mortality. 5 , 10 Therefore, the identification of patients at risk for post‐operative RVD would enable a more watchful perioperative monitoring or even preventive pharmacological management. Still, functional and morphological changes of the RV owing to chronic MR and the related surgical approaches have been scarcely explored.
The PREPARE‐MVR study (PRediction of Early PostoperAtive Right vEntricular failure in Mitral Valve Replacement/Repair patients) aimed to investigate the perioperative alterations of RV mechanical pattern in patients undergoing mitral valve replacement/repair (MVR). We hypothesized that pre‐operative RV mechanical pattern is predictive of post‐operative RVD.
Methods
Study population
Between October 2016 and November 2018, we prospectively screened 154 patients with severe primary MR who were referred to open‐heart MVR at the Heart and Vascular Center of the Semmelweis University. Patients with LV or RV dysfunction [three‐dimensional (3D) LV ejection fraction (LVEF) < 50% or 3D RV ejection fraction (RVEF) < 45% at enrolment, respectively], history of cardiac surgery, pulmonary embolism, primary pulmonary hypertension, infective endocarditis, primary cardiomyopathies, moderate‐to‐severe aortic valve disease, moderate‐to‐severe mitral stenosis, severe tricuspid regurgitation, severe chronic obstructive pulmonary disease, congenital heart disease, malignancy, irregular heart rhythm inhibiting 3D reconstruction, or inadequate echocardiographic image quality [two‐dimensional (2D) or 3D] were excluded. In total, 72 patients were enrolled. In the present study, we focused on the analysis of those 42 patients who underwent perioperative pulmonary artery catheterization (PAC) as well. Thirty age‐matched and gender‐matched healthy volunteers (no history and/or symptoms of any cardiovascular or pulmonary disease, and absence of cardiovascular risk factors, such as arterial hypertension, diabetes, smoking, and dyslipidaemia) served as the control group. The protocol of the PREPARE‐MVR study (Figure 1 , http://ClinicalTrials.gov Identifier: NCT03438825) conforms with the principles outlined in the Declaration of Helsinki, 11 and it was approved by the Regional and Institutional Committee of Science and Research Ethics (approval No. 2016/175). All study participants provided written informed consent.
Figure 1.
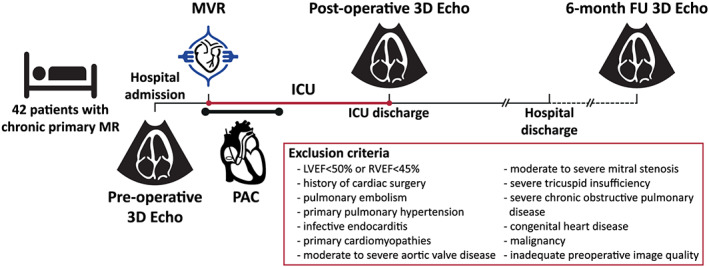
Outline of the study protocol. FU, follow‐up; ICU, intensive care unit; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; MVR, mitral valve replacement/repair; PAC, pulmonary artery catheterization; RVEF, right ventricular ejection fraction.
Echocardiography
Echocardiographic examinations were performed on a commercially available ultrasound system (GE Vivid E95, 4Vc‐D probe, Horten, Norway). All patients underwent comprehensive 2D and 3D echocardiographic evaluation pre‐operatively (1 day prior to surgery) and at ICU discharge. Follow‐up echocardiogram (2D and 3D) at the sixth month after surgery was also acquired. Owing to inadequate post‐operative image quality or new‐onset atrial fibrillation, seven patients were excluded from further follow‐up by 3D echocardiography. A standard acquisition protocol consisting of loops from parasternal, apical, and subxiphoid views was used, and 2D echocardiographic parameters were measured as per current guidelines. 12 The volume of mitral regurgitation and the effective regurgitant orifice area (EROA) were measured by the proximal isovelocity surface area (PISA) method. Severe MR was defined by an EROA > 40 mm2 and regurgitant volume > 60 mL. 13 RV basal short‐axis diameter, RV length, tricuspid annular plane systolic excursion (TAPSE), fractional area change (FAC), and tricuspid annular velocities by tissue Doppler imaging (TDI) were measured from apical four‐chamber view. Beyond the conventional echocardiographic protocol, electrocardiogram‐gated full‐volume 3D data sets reconstructed from four to six cardiac cycles optimized for the LV or RV were obtained from apical view with a minimum volume rate of 25 vol/s for further offline analysis. Data sets focused on the left heart were processed using a commercially available dedicated software (4D LV‐Analysis 3, TomTec Imaging, Unterschleissheim, Germany) and end‐diastolic volume index (EDVi), end‐systolic volume index (ESVi), and LV mass index (LVMi) were measured. To characterize global LV function, ejection fraction (EF) and parameters of myocardial deformation, such as global longitudinal strain (GLS) and global circumferential strain (GCS), were also assessed. The left atrial ESVi (LAESVi) was calculated by 4D LALV function (TomTec Imaging, Unterschleissheim, Germany). The 3D model of the RV was reconstructed from RV‐focused 3D data sets using a dedicated software (4D RV‐Function 2, TomTec Imaging, Unterschleissheim, Germany) and 3D RVEDVi, ESVi, EF, septal, and free‐wall 2D longitudinal strains were quantified as well. For the in‐depth analysis of RV mechanics, the 3D model of the RV was exported frame by frame throughout the cardiac cycle, and it was analysed using our custom software [Right Ventricular Separate Wall Motion Quantification (ReVISION) — demo version available at http://arguscognitive.com/cardio/demo]. 14 By decomposing the model's motion along the three orthogonal anatomically relevant axes, volume loss attributable to either longitudinal or radial wall motions could be separately quantified. Thus, longitudinal EF (LEF), radial EF (REF) and their ratios to global RVEF [LEF index (LEFi) = LEF/RVEF, and REF index (REFi) = REF/RVEF] could be expressed as a measure of the relative contribution of the given direction to global function. To assess longitudinal and circumferential myocardial deformation, we computed 3D RVGLS and RVGCS as described elsewhere. 15
Pulmonary artery catheterization
All patients underwent PAC pre‐operatively, and the haemodynamic monitoring was extended for the first 24 hours post‐operatively. Seven standard time points of measurements (2, 4, 6, 8, 12, 16, and 24 hours after surgery) were averaged to calculate mean post‐operative values. Central venous pressure (CVP); systolic, diastolic, and mean pulmonary artery pressure (mPAP); pulmonary artery wedge pressure (PAWP); pulmonary vascular resistance (PVR); RV stroke volume index (RVSVi); and cardiac output were monitored. Diastolic pressure gradient (DPG) was computed as the difference between diastolic PAP and PAWP. To quantify RV function invasively, RV stroke work index (RVSWi) was calculated as (mPAP − CVP) * RVSVi. Post‐operative RVD was defined as RVSWi < 300 mmHg⋅mL/m2. 10
Statistical analysis
Continuous values are expressed as median [inter‐quartile range]. Normal distribution was tested using the Shapiro–Wilk test. Group comparisons (controls vs. patients) were performed using Student's t‐test or Mann–Whitney U‐test, as appropriate. Linear regression models were created to assess the association between continuous variables. To compare echocardiographic parameters from the three predefined time points, one‐way repeated measures ANOVA with Tukey's post hoc test or Friedman test with Nemenyi post hoc test was performed depending on normality. Logistic regression models were built to predict post‐operative RVD from parameters assessed pre‐operatively. All linear and logistic regression models were adjusted for age, gender, type of surgery, and tricuspid valve repair. A P‐value of < 0.05 was considered significant in all tests. The intra‐observer and inter‐observer variabilities were evaluated using Lin's concordance correlation. To assess the intra‐observer reproducibility of the presented key parameters, the operator who performed the offline measurements repeated the 3D analysis in a randomly selected subset of 12 patients (four pre‐operative, four post‐operative, and four 6‐month follow‐up) and four control subjects blinded to previous results. The inter‐observer variability was determined by the 3D analysis of the same subjects by a second experienced operator in a blinded fashion. All statistical analyses were performed in R (version 3.4.1, R Foundation for Statistical Computing, Vienna, Austria).
Results
Population characteristics
The mean age of the MVR patient population was 63 ± 11 years, and predominantly men were included (69%). The healthy control population was age‐ and gender‐matched [60 ± 7 years, 19 (63%) men]. The underlying aetiology of primary MR was fibroelastic deficiency [n = 22 (52%)], Barlow's disease [n = 17 (41%)], and rheumatic mitral valve disease [n = 3 (7%)]. Mitral valve leaflet prolapse involved the anterior [n = 2 (5%)], posterior [n = 23 (55%)], or both leaflets [n = 14 (33%)]. Ruptured chordae tendineae were observed in 26 (62%) patients. The median of European System for Cardiac Operative Risk Evaluation (EUROSCORE) II was 1.53 [0.91–2.12]. Pre‐operative cardiovascular risk factors, laboratory results, and medical therapy are summarized in Table S1 . Severe MR was confirmed with the PISA method during the pre‐operative echocardiographic examination in each case (EROA: 73 [55–87] mm2, regurgitation volume: 102 [89–115] mL).
Surgery and intensive care unit stay
Twenty‐six patients (62%) underwent MV replacement (mechanical or bioprosthetic valve), and 16 patients (38%) had MV repair. Tricuspid valve repair (De Vega annuloplasty) was performed in nine (21%) patients. The median of cardiopulmonary bypass and aortic cross‐clamp time was 100 [89–109] and 76 [67–88] minutes, respectively. The length of stay at ICU was 47 [26–94] hours, and the length of hospital stay was 7 [6–8] days in our study cohort. During ICU stay, 16 patients (38%) required dual inotropic agent therapy (dobutamine and milrinone) and/or received inotropic support longer than 24 hours. The incidence of new‐onset atrial fibrillation was 17% (seven patients), and three patients (7%) had Stage 1 acute kidney injury (based on the criteria of Kidney Disease Improving Global Outcomes 16 ) in the post‐operative period. Among patients undergoing MV repair, none had moderate or severe residual MR, whereas trace or mild MR was observed in two (13%) patients post‐operatively. No in‐hospital or 6‐month death was observed.
Adaptation to chronic primary mitral valve regurgitation
Patients' echocardiographic characteristics are presented in Table 1 . At the pre‐operative examination, patients had larger LVEDVi and LVMi (both P < 0.001 vs. controls). Higher values of EROA and regurgitant volume were associated with a more pronounced LV remodelling (LVEDVi vs. EROA: β = 0.60, R 2 = 0.33, P < 0.001; LVEDVi vs. regurgitant volume: β = 0.75, R 2 = 0.54, P < 0.001; LVMi vs. EROA: β = 0.57, R 2 = 0.28, P = 0.002; LVMi vs. regurgitant volume: β = 0.61, R 2 = 0.33, P < 0.001). The LVEF was higher in our patient population than in controls (P = 0.011), confirming the hyperdynamic state (Table 1 ). Higher RV volumes were also observed compared with those in controls (all P < 0.001). Global RV systolic function as assessed by FAC and RVEF was slightly reduced (both P < 0.001), whereas indicators of longitudinal function demonstrated preserved (tricuspid TDI S′ and LEF) or even elevated values (TAPSE, 2D RV free‐wall longitudinal strain, and 3D RVGLS, all P < 0.05) compared with those in controls (Table 1 ). Decreased radial function as quantified by REF and REFi was also noted (both P < 0.001), suggesting that the decline in radial contractions might explain the slight decrease in global RV function. At baseline, the longitudinal shortening was the main contributor to global systolic function (LEFi vs. REFi; 0.53 [0.47–0.58] vs. 0.33 [0.22–0.42]; P < 0.001), whereas in controls, the longitudinal and radial shortenings contributed equally to RVEF (0.48 [0.42–0.51] vs. 0.43 [0.37–0.48]; P = 0.251) (Figure 2 ). The magnitude of EROA and regurgitant volume were associated with RV geometrical remodelling (RVEDVi vs. EROA: β = 0.41, R 2 = 0.15, P = 0.034; RVEDVi vs. regurgitant volume: β = 0.57, R 2 = 0.31, P < 0.001). Pre‐operatively measured CVP, mPAP, PAWP, PVR, and DPG were 13 [10–16] mmHg, 28 [21–36] mmHg, 17 [14–23] mmHg, 2.5 [1.8–3.5] Wood units, and 3 [1–9] mmHg, respectively. These findings confirm the presence of isolated post‐capillary pulmonary hypertension as defined by the current guidelines. 17 Pre‐operative RVEF was inversely related to mPAP (β = −0.48, R 2 = 0.29, P = 0.002) and PAWP (β = −0.48, R 2 = 0.31, P = 0.001).
Table 1.
Echocardiographic characteristics of healthy controls and patients undergoing mitral valve replacement/repair
| Controls | Pre‐operative | Post‐operative | 6‐month FU | Overall P‐value | |
|---|---|---|---|---|---|
| n = 30 | n = 42 | n = 35 | n = 35 | ||
| Left ventricle | |||||
| 3D LVMi (g/m2) | 62 [56–67] | 95 [78–112] a | 88 [73–105] a | 67 [60–75] a , c , d | <0.001 |
| 3D LVEDVi (mL/m2) | 59 [52–64] | 91 [74–109] a | 74 [65–90] a , b | 61 [53–70] c , d | <0.001 |
| 3D LVESVi (mL/m2) | 23 [21–26] | 34 [27–40] a | 38 [29–45] a | 25 [23–29] a , c , d | <0.001 |
| 3D LVEF (%) | 61 [59–62] | 63 [61–65] a | 51 [46–57] a , b | 58 [56–61] a , c , d | <0.001 |
| 3D LVGLS (%) | −22 [−23 to −19] | −22 [−24 to −20] | −16 [−18 to −13] a , b | −18 [−20 to −16] a , c , d | <0.001 |
| 3D LVGCS (%) | −31 [−33 to −27] | −32 [−34 to −29] | −24 [−28 to −20] a , b | −29 [−31 to −27] c , d | <0.001 |
| Right ventricle | |||||
| RV basal diameter (mm) | 32 [29–33] | 35 [30–37] a | 36 [32–38] a | 34 [31–36] a | 0.139 |
| RV length (mm) | 81 [74–86] | 86 [81–93] a | 82 [76–92] | 77 [73–83] c , d | <0.001 |
| TAPSE (mm) | 24 [21–26] | 26 [23–30] a | 13 [11–15] a , b | 17 [13–18] a , c , d | <0.001 |
| RV S′ (cm/s) | 15 [14–17] | 14 [13–16] | 9 [7–11] a , b | 10 [8–12] a , c | <0.001 |
| RV 2D free wall LS (%) | −22 [−25 to −19] | −27 [−32 to −22] a | −19 [−23 to −17] a , b | −28 [−32 to −23] a , d | <0.001 |
| FAC (%) | 54 [50–58] | 39 [34–47] a | 41 [35–46] a | 47 [42–52] a , c , d | <0.001 |
| 3D RVEDVi (mL/m2) | 57 [51–60] | 66 [58–82] a | 66 [55–82] a | 60 [53–70] c , d | <0.001 |
| 3D RVESVi (mL/m2) | 21 [20–24] | 31 [28–42] a | 32 [28–44] a | 27 [24–37] a , c , d | <0.001 |
| 3D RVEF (%) | 61 [58–65] | 52 [50–55] a | 51 [46–54] a , b | 53 [49–57] a , d | 0.005 |
| LEF (%) | 29 [26–31] | 28 [23–32] | 17 [12–20] a , b | 23 [20–27] a , c , d | <0.001 |
| REF (%) | 26 [21–31] | 16 [12–23] a | 23 [17–27] a | 21 [19–27] a | 0.032 |
| LEFi | 0.48 [0.42–0.51] | 0.53 [0.47–0.58] a | 0.33 [0.28–0.43] a , b | 0.44 [0.38–0.50] c , d | <0.001 |
| REFi | 0.43 [0.37–0.48] | 0.33 [0.22–0.42] a | 0.46 [0.37–0.51] b | 0.41 [0.36–0.49] c | 0.008 |
| 3D RVGLS (%) | −24 [−26 to −21] | −28 [−33 to −22] a | −19 [−22 to −15] a , b | −24 [−28 to −21] c , d | <0.001 |
| 3D RVGCS (%) | −24 [−28 to −22] | −24 [−27 to −19] | −26 [−28 to −21] | −25 [−28 to −19] | 0.956 |
FAC, fractional area change; FU, follow‐up; LEF, longitudinal ejection fraction; LEFi, longitudinal ejection fraction index; LVEDVi, left ventricular end‐diastolic volume index; LVEF, left ventricular ejection fraction; LVESVi, left ventricular end‐systolic volume index; LVGCS, left ventricular global circumferential strain; LVGLS, left ventricular global longitudinal strain; LVMi, left ventricular mass index; REF, radial ejection fraction; REFi, radial ejection fraction index; RV, right ventricle; RVEDVi, right ventricular end‐diastolic volume; RVEF, right ventricular ejection fraction; RVESVi, right ventricular end‐systolic volume; RVGCS, right ventricular global circumferential strain; RVGLS, right ventricular global longitudinal strain; TAPSE, tricuspid annular plane systolic excursion.
P < 0.05 vs. healthy controls, unpaired Student's t‐test or Mann–Whitney U‐test.
P < 0.05 pre‐operative vs. post‐operative.
P < 0.05 pre‐operative vs. 6‐month follow‐up.
P < 0.05 post‐operative vs. 6‐month follow‐up.
Multiple groups (pre‐operative vs. post‐operative vs. 6‐month follow‐up) were compared using Friedman test with Nemenyi post hoc test, or one‐way repeated measures ANOVA with Tukey's post hoc test (the last column is the overall P‐value of ANOVA or Friedman test).
Figure 2.
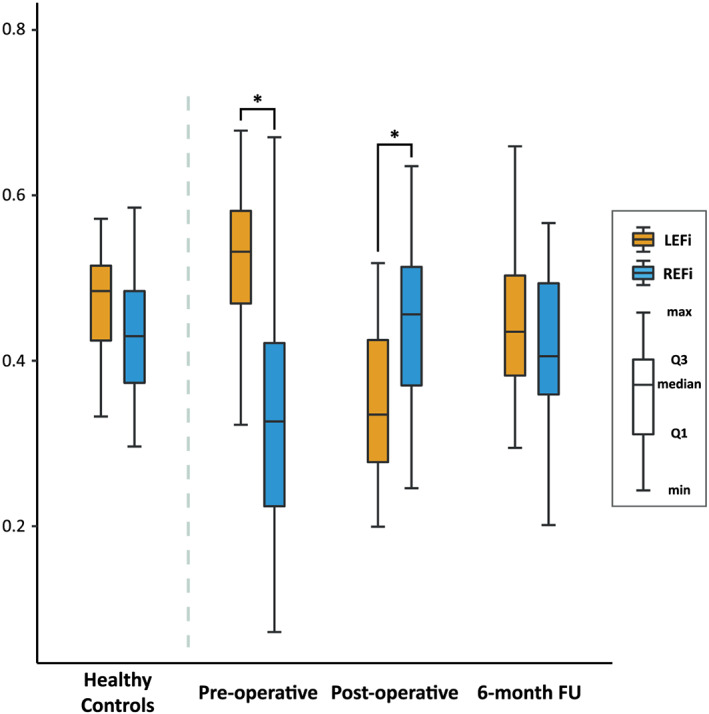
Contribution of longitudinal and radial contractions to global systolic RV function in healthy controls and patients undergoing MVR. Before surgery, the longitudinal shortening was the major contributor to global RV function, whereas after MVR, the radial contraction became the dominant component. However, this shift was temporary as 6 months later the contribution of the two components equalized, and the contraction pattern became similar to healthy controls'. * P < 0.05, paired Student's t‐test or paired Wilcoxon test. FU, follow‐up; LEFi, longitudinal ejection fraction index; MVR, mitral valve replacement/repair; REFi, radial ejection fraction index; RV, right ventricle.
Morphological and functional changes in left ventricle following mitral valve replacement/repair
LVEDVi decreased after MVR, while 6 months after the surgery, it became comparable with controls' (Table 1 ). LVMi did not change significantly immediately after surgery, but it showed a pronounced reduction at 6‐month follow‐up. LVEF and LV myocardial deformation exhibited lower values after surgery and recovered only partially at 6 months (Table 1 ). Sixteen (46%) patients exhibited LVEF < 50% at the post‐operative echocardiographic assessment, and LVEF reduction > 10% in comparison with the pre‐operative values was observed in 18 (51%) patients. Higher pre‐operative 3D LVGLS was found to be associated with a greater reduction in LVEF (β = −0.73, R 2 = 0.50, P < 0.001).
Post‐operative shift in right ventricular contraction pattern
RV geometry as assessed by RVEDVi did not change right after surgery; however, it showed some reduction at 6‐month follow‐up (Table 1 ). While RVEF decreased only slightly after MVR (52 [50–55] vs. 51 [46–54]%, P = 0.001), there were marked changes in RV mechanical pattern (Figures 2 and 3 ): the radial motion became the dominant component after surgery (LEFi vs. REFi; 0.33 [0.28–0.43] vs. 0.46 [0.37–0.51]; P = 0.004). However, this temporary shift recovered 6 months later, and the contraction pattern was found to be similar to that of controls showing the equal contribution of the two components (0.44 [0.38–0.50] vs. 0.41 [0.36–0.49]; P = 0.775). The post‐operative decline and the tendency towards normalization could be observed in other indices of longitudinal RV function (TAPSE, RV S′, and 2D free‐wall longitudinal strain) as well (Table 1 ). We did not find differences in LV and RV echocardiographic parameters (perioperative and 6 month follow‐up) between MV repair and replacement patients (Table S2 ).
Figure 3.
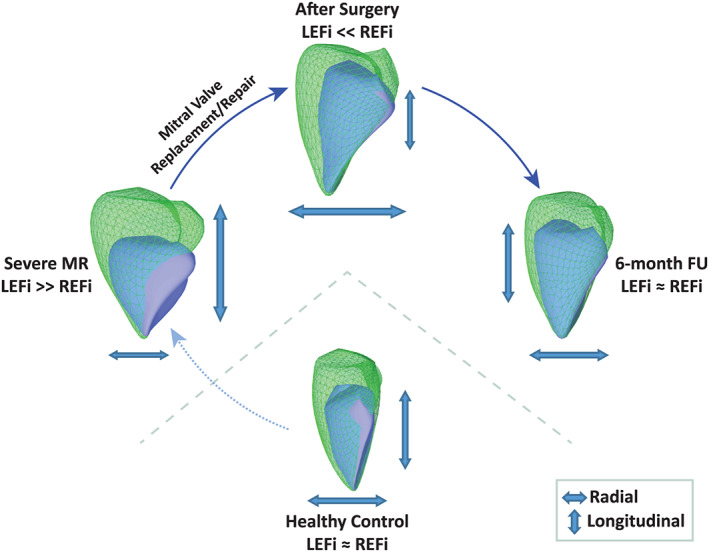
RV mechanics in healthy controls and patients undergoing surgical treatment of severe primary MR: representative cases. By decomposing the motion of the 3D RV model, the different wall motion components can be quantified separately. The green mesh represents the end‐diastolic volume, and the blue surface is the end‐systolic volume of the RV. In the healthy subject, the longitudinal and radial motion contributed equally to global RV function (LEFi vs. REFi: 0.48 vs. 0.44). In the patient with severe MR, the pre‐operatively observed longitudinal dominance (LEFi vs. REFi: 0.54 vs. 0.29) shifted to radial dominance (LEFi vs. REFi: 0.39 vs. 0.51). However, this shift was temporary as 6 months later the contribution of the two components equalized, and the contraction pattern became similar to healthy control's (LEFi vs. REFi: 0.46 vs. 0.43). FU, follow‐up; LEFi, longitudinal ejection fraction index; MR, mitral regurgitation; REFi, radial ejection fraction index; RV, right ventricle.
Predictive value of pre‐operative parameters on post‐operative right ventricular dysfunction
Post‐operative RVSWi was 336 [279–488] mmHg⋅mL/m2 in our patient cohort, and no difference was noted between MV repair and replacement patients (Table S3 ). Among pre‐operative parameters, LEF (β = −0.55, R 2 = 0.28, P < 0.001) and 3D RVGLS (β = 0.55, R 2 = 0.30, P < 0.001) were associated with post‐operative RVSWi. Nonetheless, post‐operative RVSWi demonstrated no correlation with pre‐operative LVEDVi, LVEF, LVGLS, MR regurgitant volume, LAESVi, RVEF, RVEDVi, and pulmonary artery systolic pressure (PASP, estimated by echocardiography). Post‐operative RVD (defined as RVSWi < 300 mmHg⋅mL/m2) was detected in 14 (32%) patients. In these patients, lower pre‐operative values of LVMi (P = 0.020), RV basal diameter (P = 0.012), and RVESVi (P = 0.023) were measured than in patients without RVD (Table 2 ). However, RV 2D free‐wall longitudinal strain (P = 0.031), LEF (P = 0.006), LEFi (P = 0.011), and 3D RVGLS (P = 0.007) were found to be increased compared with those in patients without post‐operative RVD (Table 2 ). Except for the higher prevalence of chronic anaemia among RVD patients, there were no other differences regarding baseline clinical and intraoperative characteristics between the two groups (Table 2 ). Importantly, the advanced 3D parameters of longitudinal RV function were predictors of post‐operative RVD (pre‐operative LEF: OR = 1.33 [95% CI: 1.08–1.77], P < 0.05; pre‐operative 3D RVGLS: OR = 0.82 [95% CI: 0.68–0.94], P < 0.05). Despite their established prognostic value, 18 , 19 neither pre‐operative LAESVi nor PASP (by echo) was predictive of RVD in our study cohort.
Table 2.
Comparison of patients with and without post‐operative right ventricular dysfunction
|
No post‐operative RVD n = 28 |
Post‐operative RVD n = 14 |
P‐value | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 67 [54–70] | 64 [61–69] | 0.666 |
| Male | 20 (71) | 9 (64) | 0.729 |
| BMI (kg/m2) | 27 [24–30] | 25 [24–27] | 0.270 |
| HTN | 21 (75) | 10 (71) | 1.000 |
| HLD | 15 (54) | 5 (36) | 0.338 |
| DM | 5 (18) | 0 (0) | 0.151 |
| CKD (GFR < 60 mL/min/1.73 m2) | 1 (4) | 1 (7) | 1.000 |
| Chronic anaemia a | 1 (4) | 4 (29) | 0.035 |
| Tobacco abuse | 7 (25) | 2 (14) | 0.692 |
| History of atrial fibrillation (any type) | 7 (25) | 3 (21) | 1.000 |
| NYHA | |||
| I | 8 (29) | 1 (7) | |
| II | 5 (18) | 4 (29) | |
| III | 15 (54) | 9 (64) | |
| IV | 0 (0) | 0 (0) | 0.340 |
| EUROSCORE II (%) | 1.60 [1.08–2.26] | 1.21 [0.88–1.76] | 0.379 |
| Pre‐operative echocardiogram | |||
| 3D LAESVi (mL/m2) | 66 [53–81] | 54 [44–78] | 0.470 |
| 3D LVMi (g/m2) | 108 [84–114] | 80 [76–91] | 0.020 |
| 3D LVEDVi (mL/m2) | 97 [81–111] | 74 [67–95] | 0.075 |
| 3D LVESVi (mL/m2) | 37 [29–41] | 31 [25–33] | 0.063 |
| 3D LVEF (%) | 63 [62–65] | 63 [60–65] | 0.576 |
| 3D LVGLS (%) | −23 [−24 to −21] | −21 [−22 to −19] | 0.357 |
| 3D LVGCS (%) | −32 [−33 to −29] | −32 [−35 to −30] | 0.988 |
| RV basal diameter (mm) | 35 [33–38] | 30 [28–35] | 0.012 |
| RV length (mm) | 86 [82–94] | 84 [77–91] | 0.485 |
| TAPSE (mm) | 26 [23–30] | 26 [23–28] | 0.965 |
| RV S′ (cm/s) | 14 [13–17] | 13 [12–15] | 0.143 |
| RV 2D free wall LS (%) | −26 [−29 to −20] | −31 [−35 to −25] | 0.031 |
| Fractional area change (%) | 38 [34–43] | 44 [38–49] | 0.143 |
| 3D RVEDVi (mL/m2) | 73 [62–88] | 60 [54–70] | 0.071 |
| 3D RVESVi (mL/m2) | 35 [29–47] | 29 [24–33] | 0.023 |
| 3D RVEF (%) | 52 [49–55] | 53 [52–55] | 0.189 |
| LEF (%) | 26 [21–30] | 31 [27–34] | 0.006 |
| REF (%) | 18 [12–24] | 15 [12–20] | 0.416 |
| LEFi | 0.49 [0.46–0.55] | 0.57 [0.53–0.63] | 0.011 |
| REFi | 0.35 [0.22–0.43] | 0.29 [0.24–0.37] | 0.141 |
| 3D RVGLS (%) | −27 [−32 to −21] | −35 [−39 to −29] | 0.007 |
| 3D RVGCS (%) | −25 [−29 to −21] | −21 [−25 to −19] | 0.531 |
| Intraoperative parameters | |||
| Type of surgery | |||
| Annuloplasty or valvuloplasty | 9 (32) | 7 (50) | |
| Biological valve implantation | 7 (25) | 2 (14) | |
| Mechanical valve implantation | 12 (43) | 5 (36) | 0.536 |
| De Vega annuloplasty | 8 (29) | 1 (7) | 0.230 |
| Cross‐clamp time (min) | 79 [69–87] | 71 [61–88] | 0.584 |
| Cardiopulmonary bypass time (min) | 100 [93–112] | 98 [82–104] | 0.461 |
BMI, body mass index; CKD, chronic kidney disease; DM, diabetes mellitus; FAC, fractional area change; GFR, glomerular filtration rate; HLD, hyperlipidaemia; HTN, hypertension; LAESVi, left atrial end‐systolic volume index; LEF, longitudinal ejection fraction; LEFi, longitudinal ejection fraction index; LVEDVi, left ventricular end‐diastolic volume index; LVEF, left ventricular ejection fraction; LVESVi, left ventricular end‐systolic volume index; LVGCS, left ventricular global circumferential strain; LVGLS, left ventricular global longitudinal strain; LVMi, left ventricular mass index; NYHA, New York Heart Association; REF, radial ejection fraction; REFi, radial ejection fraction index; RV, right ventricle; RVD, right ventricular dysfunction; RVEDVi, right ventricular end‐diastolic volume; RVEF, right ventricular ejection fraction; RVESVi, right ventricular end‐systolic volume; RVGCS, right ventricular global circumferential strain; RVGLS, right ventricular global longitudinal strain; TAPSE, tricuspid annular plane systolic excursion.
Statistical test: unpaired Student's t‐test or Mann–Whitney U‐test.
Chronic anaemia was defined as haemoglobin concentration < 120 g/L in women or <130 g/L in men.
Intra‐observer and inter‐observer variability of three‐dimensional echocardiographic parameters
The assessed values of intra‐observer and inter‐observer variability of key 3D echocardiographic parameters were comparable with previous data (Table 3 ). 20 , 21 Our custom ReVISION method is a fully automated technique; it adds no further variability on top of the 3D contouring performed using the dedicated software.
Table 3.
Intra‐observer and inter‐observer variability of key 3D echocardiographic parameters
| Intra‐observer variability (95% confidence interval) | Inter‐observer variability (95% confidence interval) | |
|---|---|---|
| 3D LAESV | 0.970 (0.913–0.990) | 0.964 (0.875–0.990) |
| 3D LVEDV | 0.982 (0.950–0.994) | 0.971 (0.921–0.990) |
| 3D LVESV | 0.981 (0.950–0.992) | 0.963 (0.901–0.987) |
| 3D RVEDV | 0.992 (0.978–1.000) | 0.954 (0.879–0.983) |
| 3D RVESV | 0.977 (0.936–0.992) | 0.900 (0.758–0.961) |
| 3D RVESV after decomposition, longitudinal motion only | 0.895 (0.728–0.961) | 0.856 (0.673–0.940) |
| 3D RVESV after decomposition, radial motion only | 0.933 (0.823–0.976) | 0.896 (0.756–0.958) |
LAESV, left atrial end‐systolic volume; LVEDV, left ventricular end‐diastolic volume; LVESV, left ventricular end‐systolic volume; RVEDV, right ventricular end‐diastolic volume; RVESV, right ventricular end‐systolic volume.
Statistical test: Lin's concordance correlation.
Discussion
Advanced diagnostic and therapeutic options have become increasingly available concerning the LV, while RVD remained a frequent clinical challenge in the perioperative care. It is common that RVD remains unrecognized, which can lead to adverse outcomes. 10 In the PREPARE‐MVR study, we intended to provide further insights into RV mechanics in patients undergoing MVR and to identify its association with the development of early post‐operative RVD. We have found that severe MR resulted in decreased radial and increased longitudinal contribution to global RV function, which pattern underwent an instantaneous shift due to open‐heart surgery. The observed increase in pre‐operative longitudinal contractions was associated with decreased post‐operative RV contractility as assessed by PAC. However, at 6‐month follow‐up, normal RV contraction pattern was restored.
The impact of LV remodelling and dysfunction on post‐operative outcomes after MVR has been extensively studied. 2 Recently, it has been also demonstrated that global RVD, especially when coupled with LV dysfunction, is a powerful predictor of long‐term outcomes. 3 RVEF is a conventional and useful measure of RV systolic performance. However, just as it has been described concerning the LV, the elemental components of myocardial mechanics could be more sensitive and predictive because the native RV contraction pattern could be disrupted even before the RVEF starts to decline. 22 In our current study, patients with severe primary MR but with preserved biventricular function were recruited. Despite this fact, a reduction in radial and an increase in longitudinal wall motion could be observed pointing to a functional shift induced by the severe valvular disease and the subsequent post‐capillary pulmonary hypertension.
Adaptation to chronic primary mitral valve regurgitation
Chronic primary MR triggers eccentric hypertrophy with geometric changes of the LV cavity as an adaptive mechanism to volume overload. Its shape becomes more spherical, and the consequent rise of LV and pericardial constraint likely contributes to the alterations of RV function. 3 Chronic MR results in a progressive deterioration of LV contractile function, although the LVEF is maintained over a relatively long period. Owing to the aforementioned mechanisms, the normal function of the interventricular septum may be hampered early during the course of the disease. 3 Furthermore, longstanding MR induces elevation in LA pressure, PAWP, and PAP, that result in the pressure overload of the RV. 23 Still, the overall RV performance was influenced by pulmonary pressures in our patients as confirmed by the inverse relation of RVEF to the levels of mPAP and PAWP. Moreover, the functional consequences of pressure overload could be observed in the RV contraction pattern: the radial motion (the so‐called bellows effect) was impaired despite the preserved RVEF. A similar response can be recognized in patients with pulmonary arterial hypertension, and the prognostic value of this phenomenon is well established. 24 , 25
Post‐operative shift in right ventricular contraction pattern
The pre‐operative alterations attributable to MR make the RV susceptible to develop overt RVD during and immediately after the open‐heart surgery. MVR diminishes LV volume overload, resulting in the reduction of LV and pericardial constraint. The apparent deficit in RV function after MV surgery, when evaluated exclusively by the parameters of the longitudinal contractions, has been described in previous studies. 26 , 27 Nevertheless, the observed alteration of RV contraction pattern is not specific to MVR as similar shift was reported by Raina et al. in patients undergoing heart transplantation or isolated coronary artery bypass surgery. 28 Different causes have been proposed to explain this loss in longitudinal RV performance, such as geometric changes in the RV, intraoperative ischaemia, pericardial disruption, and suboptimal cardioprotection. 29 , 30 The decline in longitudinal function occurs immediately after the opening of the pericardium, which supports the hypothesis on the fundamental role of the pericardium in maintaining physiological RV geometry and function. 9 However, the reductions in longitudinal measures after surgery might reflect a single aspect of a modified and complex contraction pattern. Thus, conventional M‐mode and 2D indices may be inadequate for the post‐operative assessment of RV function. 20 Our results clearly demonstrated that the radial motion could effectively compensate for the post‐operative decline of longitudinal motion to maintain RVEF. Interestingly, we have found that 6 months after the successful operation, the native contraction pattern is restored in MVR patients as RV longitudinal and radial contributions (LEFi, REFi) are comparable with healthy controls'. However, the M‐mode and 2D indices of longitudinal RV function indicate still incomplete recovery as also demonstrated by Tamborini et al. 31 To the best of our knowledge, this is the first observational study characterizing the dynamic perioperative changes of RV mechanics comprehensively in MR patients using 3D echocardiography.
Prognostic value of pre‐operative cardiac mechanics
Most studies and guideline recommendations prominently focus on the prognostic value of LV function, while the assessment of RV function is still underemphasized. 13 Previously, Pandis and co‐workers showed that high pre‐operative LVGLS by 2D speckle tracking echocardiography was predictive of a steeper decline in LVEF after MVR. 32 This phenomenon was confirmed in our study. Importantly, our findings are similar regarding the RV as well: hyperdynamic RV longitudinal motion is associated with post‐operative contractile dysfunction. Accordingly, we may hypothesize that the similarity in the contraction characteristics is a manifestation of the LV–RV interplay.
Nevertheless, not only LV dysfunction but also RVD contributes significantly to perioperative adverse outcomes. 10 RVD is a challenging perioperative clinical scenario without a clean‐cut method for an accurate diagnosis. Main causes include myocardial ischaemia and acute RV pressure overload, besides the effect of the pericardial sac opening. 8 , 33 While intraoperative RV failure can be evident as it hinders the weaning from cardiopulmonary bypass, early post‐operative RVD often remains subclinical and requires an integrated diagnostic approach involving physical examination, blood tests for end‐organ damage, haemodynamic, and imaging‐based monitoring. 10 We have chosen a robust parameter of RV contractility (i.e. RVSWi) to define RVD; however, performing PAC is not part of the routine care in MVR patients. Increased pre‐operative 3D parameters of RV longitudinal function were predictive of the decreased contractility after the operation. According to our results, 3D echocardiography may facilitate a more profound pre‐operative risk stratification of MVR patients. By improving the perioperative care, it might enable timely diagnosis and, potentially, the prevention of severe RVD. The more in‐depth understanding of perioperative changes in RV haemodynamics and mechanics may also contribute to establish the real‐life prevalence of RVD and to evaluate its detrimental consequences.
Limitations
The small sample size represents a limitation; however, in our current analysis, the main aim was to correlate 3D echocardiographic data to invasively measured RV contractility, and PAC is not part of the routine perioperative care in case of MVR surgery. Patients were selected for perioperative PAC on the basis of the individual decision of the cardiac anaesthesiologist in adherence to the institutional protocol. During ICU stay, only focused 2D echocardiographic studies were performed as multi‐beat 3D acquisitions are extremely challenging and are of low success rate in this setting. Therefore, post‐operative 3D measurements refer to the time point of ICU discharge. Nevertheless, RV functional shift was still prominent. We have chosen a PAC‐based contractility index to indicate RVD; however, we could not couple these low‐risk patients with decreased post‐operative RVSWi to other clinical hard endpoints, such as mortality. Further studies are warranted in higher‐risk populations and with pre‐operatively decreased RV function. The repair rate in this specific cohort of patients was rather low, which might lead to a biased interpretation of our results. Thus, we have thoroughly compared the early and late post‐operative clinical and echocardiographic characteristics of patients who underwent MV repair vs. replacement, and no significant differences were found. Moreover, linear and logistic regression models were adjusted for the type of surgery as well. Because there is no true reference method for assessing RV longitudinal and radial wall motions, we have no possibility to validate our echocardiographic results to a gold standard. Inter‐observer and intra‐observer variabilities confirm the reliability of our method and are consistent with previous studies. 20 , 21
Conclusions
In the PREPARE‐MVR study, we explored the shift in RV mechanical pattern that occurs parallel with complex haemodynamic changes in patients with severe primary MR undergoing MVR. Before the surgery, the longitudinal shortening was the main contributor to RVEF, while after MVR, the radial motion became the dominant component of RV systolic function. At 6‐month follow‐up, the two components contributed equally, and the contraction pattern became similar to controls', suggesting functional RV reverse remodelling. Moreover, the pre‐operative 3D indices of longitudinal RV function were related to invasively measured post‐operative RV contractility; thus, they might predict perioperative RVD.
Conflict of interest
None declared.
Supporting information
Table S1. Pre‐operative clinical characteristics of the study cohort.
Table S2. Echocardiographic characteristics of patients undergoing different types of mitral valve surgery.
Table S3. Haemodynamic parameters and ICU data of patients undergoing different types of mitral valve surgery
Acknowledgements
This work was supported by the National Research, Development, and Innovation Office of Hungary (NVKP_16‐1‐2016‐0017 — National Heart Programme), and the Higher Education Institutional Excellence Programme of the Ministry for Innovation and Technology, Hungary, within the framework of the Therapeutic Development thematic programme of the Semmelweis University.
Tokodi, M. , Németh, E. , Lakatos, B. K. , Kispál, E. , Tősér, Z. , Staub, L. , Rácz, K. , Soltész, Á. , Szigeti, S. , Varga, T. , Gál, J. , Merkely, B. , and Kovács, A. (2020) Right ventricular mechanical pattern in patients undergoing mitral valve surgery: a predictor of post‐operative dysfunction?. ESC Heart Failure, 7: 1246–1256. 10.1002/ehf2.12682.
Márton Tokodi, M.D. and Endre Németh, M.D., Ph.D. contributed equally to this work and are joint first authors. Béla Merkely, M.D., Ph.D., D.Sc. and Attila Kovács, M.D., Ph.D. contributed equally to this work and are joint last authors. Clinical Trial Registration: https://www.clinicaltrials.gov. Unique identifier: NCT03438825.
References
- 1. Monteagudo Ruiz JM, Galderisi M, Buonauro A, Badano L, Aruta P, Swaans MJ, Sanchis L, Saraste A, Monaghan M, Theodoropoulos KC, Papitsas M, Liel‐Cohen N, Kobal S, Bervar M, Berlot B, Filippatos G, Ikonomidis I, Katsanos S, Tanner FC, Cassani D, Faletra FF, Leo LA, Martinez A, Matabuena J, Grande‐Trillo A, Alonso‐Rodriguez D, Mesa D, Gonzalez‐Alujas T, Sitges M, Carrasco‐Chinchilla F, Li CH, Fernandez‐Golfin C, Zamorano JL. Overview of mitral regurgitation in Europe: results from the European Registry of mitral regurgitation (EuMiClip). Eur Heart J Cardiovasc Imaging 2018; 19: 503–507. [DOI] [PubMed] [Google Scholar]
- 2. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Muñoz D, Rosenhek R, Sjögren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL, Group ESD . 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017; 38: 2739–2791. [DOI] [PubMed] [Google Scholar]
- 3. Le Tourneau T, Deswarte G, Lamblin N, Foucher‐Hossein C, Fayad G, Richardson M, Polge AS, Vannesson C, Topilsky Y, Juthier F, Trochu JN, Enriquez‐Sarano M, Bauters C. Right ventricular systolic function in organic mitral regurgitation: impact of biventricular impairment. Circulation 2013; 127: 1597–1608. [DOI] [PubMed] [Google Scholar]
- 4. Apor A, Nagy AI, Kovacs A, Manouras A, Andrassy P, Merkely B. Three‐dimensional dynamic morphology of the mitral valve in different forms of mitral valve prolapse—potential implications for annuloplasty ring selection. Cardiovasc Ultrasound 2016; 14: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Denault AY, Pearl RG, Michler RE, Rao V, Tsui SS, Seitelberger R, Cromie M, Lindberg E, D'Armini AM. Tezosentan and right ventricular failure in patients with pulmonary hypertension undergoing cardiac surgery: the TACTICS trial. J Cardiothorac Vasc Anesth 2013; 27: 1212–1217. [DOI] [PubMed] [Google Scholar]
- 6. Chrustowicz A, Gackowski A, El‐Massri N, Sadowski J, Piwowarska W. Preoperative right ventricular function in patients with organic mitral regurgitation. Echocardiography 2010; 27: 282–285. [DOI] [PubMed] [Google Scholar]
- 7. Hochreiter C, Niles N, Devereux RB, Kligfield P, Borer JS. Mitral regurgitation: relationship of noninvasive descriptors of right and left ventricular performance to clinical and hemodynamic findings and to prognosis in medically and surgically treated patients. Circulation 1986; 73: 900–912. [DOI] [PubMed] [Google Scholar]
- 8. Haddad F, Couture P, Tousignant C, Denault AY. The right ventricle in cardiac surgery, a perioperative perspective: II. Pathophysiology, clinical importance, and management. Anesth Analg 2009; 108: 422–433. [DOI] [PubMed] [Google Scholar]
- 9. Unsworth B, Casula RP, Kyriacou AA, Yadav H, Chukwuemeka A, Cherian A, Stanbridge Rde L, Athanasiou T, Mayet J, Francis DP. The right ventricular annular velocity reduction caused by coronary artery bypass graft surgery occurs at the moment of pericardial incision. Am Heart J 2010; 159: 314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Konstam MA, Kiernan MS, Bernstein D, Bozkurt B, Jacob M, Kapur NK, Kociol RD, Lewis EF, Mehra MR, Pagani FD, Raval AN, Ward C. Evaluation and management of right‐sided heart failure: a scientific statement from the American Heart Association. Circulation 2018; 137: e578–e622. [DOI] [PubMed] [Google Scholar]
- 11. Human Experimentation . Code of ethics of W.M.A. Br Med J 1964; 2: 177–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt J‐U. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28: 1–39.e14. [DOI] [PubMed] [Google Scholar]
- 13. Lancellotti P, Tribouilloy C, Hagendorff A, Popescu BA, Edvardsen T, Pierard LA, Badano L, Zamorano JL. Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2013; 14: 611–644. [DOI] [PubMed] [Google Scholar]
- 14. Lakatos B, Toser Z, Tokodi M, Doronina A, Kosztin A, Muraru D, Badano LP, Kovacs A, Merkely B. Quantification of the relative contribution of the different right ventricular wall motion components to right ventricular ejection fraction: the ReVISION method. Cardiovasc Ultrasound 2017; 15: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lakatos BK, Kiss O, Tokodi M, Toser Z, Sydo N, Merkely G, Babity M, Szilagyi M, Komocsin Z, Bognar C, Kovacs A, Merkely B. Exercise‐induced shift in right ventricular contraction pattern: novel marker of athlete's heart? Am J Physiol Heart Circ Physiol 2018; 315: H1640–H1648. [DOI] [PubMed] [Google Scholar]
- 16. Kellum JA, Lameire N, Aspelin P, Barsoum RS, Burdmann EA, Goldstein SL, Herzog CA, Joannidis M, Kribben A, Levey AS, MacLeod AM, Mehta RL, Murray PT, Naicker S, Opal SM, Schaefer F, Schetz M, Uchino S. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012; 2: 1–138. [Google Scholar]
- 17. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 18. Le Tourneau T, Messika‐Zeitoun D, Russo A, Detaint D, Topilsky Y, Mahoney DW, Suri R, Enriquez‐Sarano M. Impact of left atrial volume on clinical outcome in organic mitral regurgitation. J Am Coll Cardiol 2010; 56: 570–578. [DOI] [PubMed] [Google Scholar]
- 19. Hyllén S, Nozohoor S, Ingvarsson A, Meurling C, Wierup P, Sjögren J. Right ventricular performance after valve repair for chronic degenerative mitral regurgitation. Ann Thorac Surg 2014; 98: 2023–2030. [DOI] [PubMed] [Google Scholar]
- 20. Lakatos BK, Tokodi M, Assabiny A, Toser Z, Kosztin A, Doronina A, Racz K, Koritsanszky KB, Berzsenyi V, Nemeth E, Sax B, Kovacs A, Merkely B. Dominance of free wall radial motion in global right ventricular function of heart transplant recipients. Clin Transplant 2018; 32: e13192. [DOI] [PubMed] [Google Scholar]
- 21. Maffessanti F, Muraru D, Esposito R, Gripari P, Ermacora D, Santoro C, Tamborini G, Galderisi M, Pepi M, Badano LP. Age‐, body size‐, and sex‐specific reference values for right ventricular volumes and ejection fraction by three‐dimensional echocardiography: a multicenter echocardiographic study in 507 healthy volunteers. Circ Cardiovasc Imaging 2013; 6: 700–710. [DOI] [PubMed] [Google Scholar]
- 22. Kovacs A, Lakatos B, Tokodi M, Merkely B. Right ventricular mechanical pattern in health and disease: beyond longitudinal shortening. Heart Fail Rev 2019; 24: 511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barbieri A, Bursi F, Grigioni F, Tribouilloy C, Avierinos JF, Michelena HI, Rusinaru D, Szymansky C, Russo A, Suri R, Bacchi Reggiani ML, Branzi A, Modena MG, Enriquez‐Sarano M. Prognostic and therapeutic implications of pulmonary hypertension complicating degenerative mitral regurgitation due to flail leaflet: a multicenter long‐term international study. Eur Heart J 2011; 32: 751–759. [DOI] [PubMed] [Google Scholar]
- 24. Kind T, Mauritz GJ, Marcus JT, van de Veerdonk M, Westerhof N, Vonk‐Noordegraaf A. Right ventricular ejection fraction is better reflected by transverse rather than longitudinal wall motion in pulmonary hypertension. J Cardiovasc Magn Reson 2010; 12: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moceri P, Duchateau N, Baudouy D, Schouver ED, Leroy S, Squara F, Ferrari E, Sermesant M. Three‐dimensional right‐ventricular regional deformation and survival in pulmonary hypertension. Eur Heart J Cardiovasc Imaging 2018; 19: 450–458. [DOI] [PubMed] [Google Scholar]
- 26. Zanobini M, Saccocci M, Tamborini G, Veglia F, Di Minno A, Poggio P, Pepi M, Alamanni F, Loardi C. Postoperative echocardiographic reduction of right ventricular function: is pericardial opening modality the main culprit? Biomed Res Int 2017; 2017: 4808757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maffessanti F, Gripari P, Tamborini G, Muratori M, Fusini L, Alamanni F, Zanobini M, Fiorentini C, Caiani EG, Pepi M. Evaluation of right ventricular systolic function after mitral valve repair: a two‐dimensional Doppler, speckle‐tracking, and three‐dimensional echocardiographic study. J Am Soc Echocardiogr 2012; 25: 701–708. [DOI] [PubMed] [Google Scholar]
- 28. Raina A, Vaidya A, Gertz ZM, Susan C, Forfia PR. Marked changes in right ventricular contractile pattern after cardiothoracic surgery: implications for post‐surgical assessment of right ventricular function. J Heart Lung Transplant 2013; 32: 777–783. [DOI] [PubMed] [Google Scholar]
- 29. Forsberg LM, Tamas E, Vanky F, Nielsen NE, Engvall J, Nylander E. Left and right ventricular function in aortic stenosis patients 8 weeks post‐transcatheter aortic valve implantation or surgical aortic valve replacement. Eur J Echocardiogr 2011; 12: 603–611. [DOI] [PubMed] [Google Scholar]
- 30. Lindqvist P, Holmgren A, Zhao Y, Henein MY. Effect of pericardial repair after aortic valve replacement on septal and right ventricular function. Int J Cardiol 2012; 155: 388–393. [DOI] [PubMed] [Google Scholar]
- 31. Tamborini G, Muratori M, Brusoni D, Celeste F, Maffessanti F, Caiani EG, Alamanni F, Pepi M. Is right ventricular systolic function reduced after cardiac surgery? A two‐ and three‐dimensional echocardiographic study. Eur J Echocardiogr 2009; 10: 630–634. [DOI] [PubMed] [Google Scholar]
- 32. Pandis D, Sengupta PP, Castillo JG, Caracciolo G, Fischer GW, Narula J, Anyanwu A, Adams DH. Assessment of longitudinal myocardial mechanics in patients with degenerative mitral valve regurgitation predicts postoperative worsening of left ventricular systolic function. J Am Soc Echocardiogr 2014; 27: 627–638. [DOI] [PubMed] [Google Scholar]
- 33. Zochios V, Protopapas AD, Parhar K. Markers of right ventricular dysfunction in adult cardiac surgical patients. J Cardiothorac Vasc Anesth 2017; 31: 1570–1574. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Pre‐operative clinical characteristics of the study cohort.
Table S2. Echocardiographic characteristics of patients undergoing different types of mitral valve surgery.
Table S3. Haemodynamic parameters and ICU data of patients undergoing different types of mitral valve surgery


