Abstract
Myocardial infection by Epstein–Barr virus (EBV) may manifest with inflammatory cardiomyopathy, coronary syndrome X, and rarely with infarct‐like myocarditis. The aim of the report is to describe a case of myocardial EBV infection causing acute myocarditis with heart failure, necrotizing coronary vasculitis, and multiple left ventricular (LV) aneurysms. A 67‐year‐old woman presented with fever, chest pain, and heart failure. She underwent non‐invasive cardiac studies including electrocardiography, 2D‐echocardiography, cardiac magnetic resonance, hematochemical exams with Troponin T determination, and invasive studies including cardiac catheterization, coronary angiography, and LV endomyocardial biopsy. Five endomyocardial samples were processed for histology and immunohistochemistry for inflammatory cells characterization and detection of viral antigens. Two additional frozen samples were evaluated by real‐time polymerase chain reaction for the presence of cardiotropic viral genomes. Routine laboratory tests revealed the presence of elevated white blood cells (17 000 103/μL) and increased Troponin T. Electrocardiogram showed sinus tachycardia with ST elevation in V2–V5. Two‐dimensional echocardiography showed normal LV dimension with reduced LV contractility (LVEF = 40%) with mild pericardial effusion. Cardiac magnetic resonance revealed the presence of a micro‐aneurism in the inferior LV wall, a diffuse oedematous imbibition of LV myocardium suggested by hyper‐intensity of T2 mapping, and increased fibrosis as suggested by areas of late gadolinium enhancement signals. Coronary arteries were normal while several micro‐aneurysms were observed at LV angiography. At histology, a lymphocytic myocarditis with necrotizing coronary vasculitis sustained by a positive real‐time polymerase chain reaction for EBV, detectable in cardiomyocytes and inflamed intramural vessels by positive immunohistochemistry for EBV latent membrane protein 1 antigen, was observed. Myocardial EBV infection is an unusual cause of acute heart failure and cardiac aneurysms, increasing the risk of electrical instability, cardiac perforation, and sudden death.
1. Introduction
Myocardial infection by Epstein–Barr virus (EBV) has been associated with inflammatory cardiomyopathy as a consequence of its cytopathic effect on cardiomyocytes1; it has been implicated as the cause of cardiac syndrome X2 because of its localization in the endothelial cells of coronary small vessels and very rarely in an infarct‐like myocarditis.3 In the present report, it is implicated in the generation of an acute myocarditis with necrotizing coronary vasculitis and formation of multiple left ventricular (LV) aneurysms.
1.1. Case report
A 67‐year‐old woman was admitted because of dyspnoea, chest pain, and fever (38°C) occurring few days earlier. Routine laboratory tests revealed the presence of elevated white blood cells count (17 000 103/μL) and increased Troponin T (max = 0.365 μg/L and normal value (NV) < 0.014). Electrocardiography showed sinus rhythm (heart rate = 105 beats per minute) with ST elevation in V2–V5. Two‐dimensional echocardiography showed normal LV dimension with akinesia of the apex and of the middle antero‐lateral LV wall and hypokinesia of the remaining LV segments (LVEF = 40%), with mild pericardial effusion. Coronary angiography was normal. Cardiac magnetic resonance confirmed a moderate LV hypokinesia with the presence of a micro‐aneurysm, predominately in the basal inferior wall of LV (Figure 1 , panels A and B, Movie S1).
Figure 1.
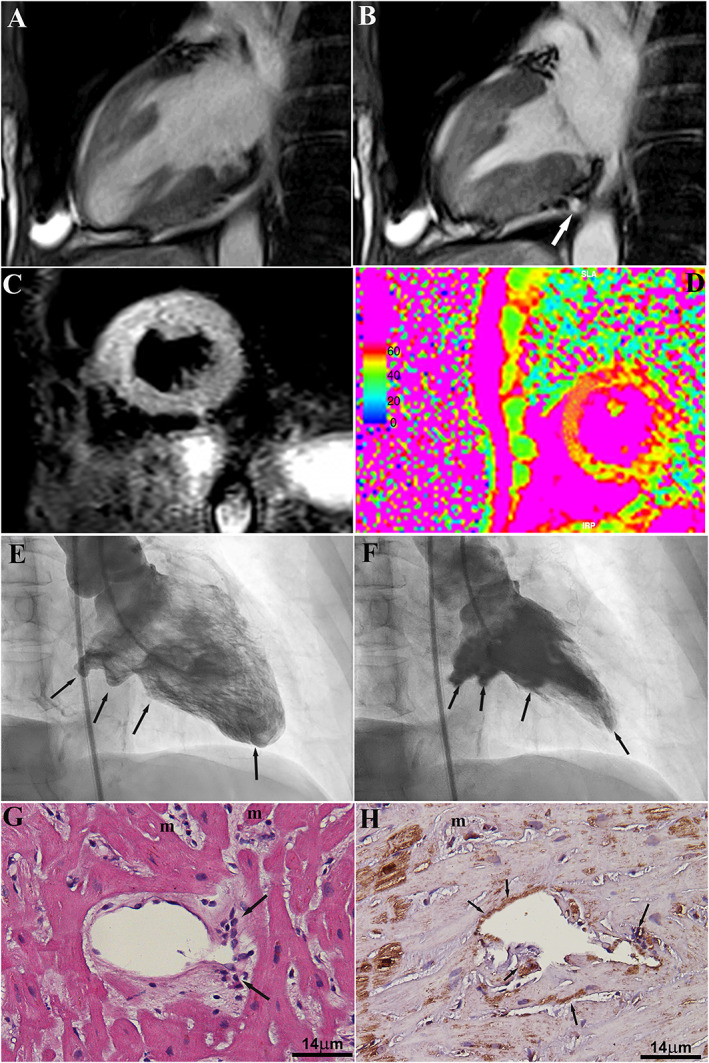
Panels A and B: Two‐chamber view cine steady‐state‐free precession cardiac magnetic resonance frames on (A) end‐diastolic and (B) end‐systolic frames show the presence of focal aneurisms located within the inferior mid‐basal left ventricular wall. Global left ventricular function is moderately reduced (ejection fraction = 40%). Panels C and D: cardiac magnetic resonance imaging showing the presence of a diffuse acute myocardial damage corresponding to a remarkable oedematous imbibition of LV myocardium, which is observed as a patchy hyper‐intense signal on T2‐weighted short‐tau inversion recovery (D) combined with globally increased T2 mapping values (e; i.e. 59 ± 11 ms; NV < 50 ms). Panels E and F: left ventricular angiography in right anterior oblique view (E = diastole and F = systole) showing multiple aneurysms in the posterior, inferior, and apical segments. Panel G: active lymphocytic inflammation of myocardium (m) and intramural coronary vessel (arrows) (haematoxylin and eosin; magnification 200×). Panel H: strong positivity of immunohistochemistry for EBV latent membrane protein 1 in cardiomyocytes (m) and inflamed vessel wall (arrows) (immunoperoxidase; magnification 200×).
Late gadolinium enhancement showed the presence of multiple patchy nodular foci of fibrosis (Figure 1, panel C). A diffuse oedematous imbibition of LV myocardium suggested by hyper‐intensity on “edema‐weighted sequences” [i.e. T2‐weighted short‐tau inversion recovery mid‐ventricular short axis combined with globally increased T2 mapping value (i.e. 59 ± 11 ms; NV < 50 ms)] (Figure 1 D) was also present. In the clinical suspicion of infarct‐like myocarditis, the patient underwent ventricular angiography with LV endomyocardial biopsy. Coronary network was normal, while LV angiography revealed the presence of multiple LV micro‐aneurysms involving posterior, inferior, and apical walls (Figures 1 E and 1F, Movie S2).
Endomyocardial biopsy showed the presence of focal myocarditis with necrotizing vasculitis of the intramural vessels (Figure 1 G). Real‐time polymerase chain reaction analysis for the most common cardiotropic viruses (including adenovirus, EBV, enterovirus, influenza A and B viruses, hepatitis C virus, parvovirus B19, cytomegalovirus, human herpes virus 6, and herpes simplex viruses) revealed a high genomic load for EBV (7.3 × 104 copies per millilitre), which was 38‐fold higher than the circulating virus load (1.9 × 103 copies per millilitre). Immunohistochemistry for EBV latent membrane protein 1 showed a strong positivity in both cardiomyocytes and inflamed vessels (Figure 1 H). The patient was treated with supportive therapy (angiotensin‐converting enzyme inhibitors and diuretics) and improved LV dimension and function at short‐term follow‐up (1 month).
2. Discussion
Myocardial infection by EBV may provide a different clinical phenotype, depending partly on the variable genomic properties of the infectious agent. It includes an inflammatory cardiomyopathy,1 where chronically infected cardiomyocytes lead to progressive cardiac dilatation and dysfunction; a coronary syndrome X2 in which EBV electively localizes in the endothelial cells of intramural vessels causing an endothelial dysfunction and angina with normal coronary arteries and an acute infarct‐like myocarditis3, 4, 5, 6, 7, 8 with extensive myocytolysis and cardiogenic shock. EBV infection has also been implicated in the generation of pulmonary and systemic vasculitis.4, 5, 6, 7
At variance with previous descriptions, the present report documents a combined EBV inflammation of myocardium and intramural coronary vessels with acute myocarditis and necrotizing coronary vasculitis. Causal role of EBV has been suggested by the elevated myocardial viral load at real‐time polymerase chain reaction by a significant (>38 times) gap between myocardial and blood viral copies and by the positive immunohistochemistry for EBV latent membrane protein 1 antigen localizing the agent in cardiomyocytes and inflamed coronary intramural vessels. Myocardial inflammation and vessel involvement results in our case in the generation of multiple LV aneurysms. The occurrence of vasculitis of intramural vessels may have contributed to local ischaemia of the ventricular wall participating in wall damage and micro‐aneurysms formation, making the treatment more challenging and prognosis more severe. Indeed, occurrence of myocardial aneurysms increases the risk of cardiac perforation, electrical instability, and death.
Conflict of interest
None declared.
Funding
This work was supported by the European Project ERA‐CVD “Transnational Research Projects on Cardiovascular Diseases” (JTC 2016 IKDT‐IGCM) and Italian Ministry of Health (Ricerca corrente) given to IRCCS Spallanzani and IRCCS San Raffaele Pisana.
Supporting information
Movie S1. Cine images performed in vertical long axis view nicely depicted the presence of a micro‐aneurism, predominately in the basal‐inferior wall of left ventricle.
Movie S2. Left ventricular angiography showing multiple LV aneurysms caused by EBV myocarditis with coronary vasculitis.
Chimenti, C. , Verardo, R. , Grande, C. , Francone, M. , and Frustaci, A. (2020) Infarct‐like myocarditis with coronary vasculitis and aneurysm formation caused by Epstein–Barr virus infection. ESC Heart Failure, 7: 938–941. 10.1002/ehf2.12611.
References
- 1. Chimenti C, Russo A, Pieroni M, Calabrese F, Verardo R, Thiene G, Russo MA, Maseri A, Frustaci A. Intramyocyte detection of Epstein‐Barr virus genome by laser capture microdissection in patients with inflammatory cardiomyopathy. Circulation 2004; 7: 3534–3539. [DOI] [PubMed] [Google Scholar]
- 2. Chimenti C, Sale P, Verardo R, Cicalini S, Petrosillo N, Russo MA, Fedele F, Frustaci A. High prevalence of intramural coronary infection in patients with drug‐resistant cardiac syndrome X: comparison with chronic stable angina and normal controls. Heart 2010; 96: 1926–1931 [DOI] [PubMed] [Google Scholar]
- 3. Tyson AA Jr, Hackshaw BT, Kutcher MA. Acute Epstein‐Barr virus myocarditis simulating myocardial infarction with cardiogenic shock. South Med J 1989; 82: 1184–1187. [DOI] [PubMed] [Google Scholar]
- 4. Ba H, Xu L, Peng H. Chronic active Epstein‐Barr virus infection with systemic vasculitis and pulmonary arterial hypertension in a child. Front Pediatr 2019; 7: 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Faria R, Pereira S, Santos W. Fulminant myocarditis‐case report. Rev Port Cardiol 2012; 31: 503–507. [DOI] [PubMed] [Google Scholar]
- 6. Hashimoto T, Sakata Y, Fukushima K. Pulmonary arterial hypertension associated with chronic active Epstein‐Barr virus infection. Intern Med 2011; 50: 119–124. [DOI] [PubMed] [Google Scholar]
- 7. Sahu SK, Giri S, Malik S. Unusual infectious mononucleosis complicated by vasculitis. Medical Journal of Dr DY Patil University Case Report 2016; 9: 104–106. [Google Scholar]
- 8. Sarvari KP, Zolyomi S, Agoston G. A rare case of acute myocarditis. J Med Microb Diagn 2015; 4: 3. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie S1. Cine images performed in vertical long axis view nicely depicted the presence of a micro‐aneurism, predominately in the basal‐inferior wall of left ventricle.
Movie S2. Left ventricular angiography showing multiple LV aneurysms caused by EBV myocarditis with coronary vasculitis.


