Abstract
Aims
Centrifugal continuous flow pumps are currently the state of the art in left ventricular assist device therapy. This study was conducted to compare the results after implantation of the HVAD® and the HeartMate 3®.
Methods and results
We retrospectively analysed preoperative and post‐operative patient data of all 106 patients, who received a HeartMate 3 (HM3) at our centre between 2014 and 2018. A total of 392 patients receiving a sintered HVAD® served as controls. Patient matching was performed for age, sex, Interagency Registry for Mechanically Assisted Circulatory Support level at the time of implant, perioperative right heart failure, and implantation strategy, that is, bridge to transplant or destination therapy, as well as preoperative renal function, that is, as indicated by serum creatinine levels. A total of 79 matched pairs could be identified. During a median follow‐up of 15.3 months (range: 0–30 months), 23 (29.1%) and 19 (24.1%) patients died in the HVAD and HM3 groups, respectively, with a hazard ratio for mortality of 0.84 [95% confidence interval (CI): 0.46–1.54; P = 0.568]. Freedom from cerebrovascular events did not differ significantly between study groups, with a hazard ratio of 0.57 (95% CI: 0.23–1.45; P = 0.241). The risk of driveline infection was significantly lower in the HM3 (n = 33) than in the HVAD (n = 55) group (hazard ratio = 0.54; 95% CI: 0.35–0.84; P = 0.006). Eight HVAD, but no HM3, patients developed a pump thrombosis during follow‐up (P = 0.148).
Conclusions
Performance of both currently used centrifugal left ventricular assist device systems is comparable in terms of short‐term patient survival and freedom from cerebrovascular events. In our single‐centre experience, HM3 patients less frequently develop driveline infections and no pump thrombosis, which requires further evaluation.
Keywords: Mechanical circulatory support, Left ventricular assist device, Centrifugal continuous flow pump
1. Introduction
Durable mechanical circulatory support (MCS) has become an established and valuable treatment option for terminal heart failure (HF). Following the first successful implantation of a Jarvik‐7 total artificial heart in 1982, increasing numbers of HF patients were bridged to heart transplantation by implantation of durable ventricular assist devices in the 1990s.1, 2 The Randomized Evaluation of Mechanical Assistance for the treatment of Congestive Heart Failure (REMATCH) trial was published in 2001 and opened up the era of destination therapy in end‐stage HF patients who were not eligible for heart transplantation.3
The technical advances of second‐generation and third‐generation pumps addressed size, biocompatibility, durability, effectiveness, and infection issues. The third‐generation and currently most frequently used left ventricular assist devices (LVADs) are intrapericardially implantable, centrifugal continuous flow (CF) pumps. The first relevant third‐generation LVAD was the HeartWare ventricular assist device (HVAD®), which has a magnetic and hydrodynamic levitation of the internal rotor and received the CE mark in 2008, as well as Food and Drug Administration approval for bridge to transplantation in 2012.4 The most important competitive product, the HeartMate 3®, has a fully magnetically levitated internal rotor in a slightly wider constructed corpus. It was first implanted in 2014 and received CE mark in 2015.5 Both devices may be implanted by less invasive surgical techniques6, 7 and were shown to yield remarkable 1 year survival rates between 80% and 90% as well as improved quality of life and exercise capacity.8, 9, 10 However, these most recent LVADs have been compared solely with second‐generation LVADs with axial flow pumps, namely, the HeartMate II®.8, 10
Therefore, we intended to analyse our single‐centre experience implanting third‐generation LVAD devices comparing the HVAD and the HeartMate 3.
2. Methods
2.1. Patients
For the present investigation, all patients who received as their first device a sintered HVAD® (HeartWare International, Inc, Framingham, MA) or a HeartMate 3® (Thoratec Corp, Pleasanton, CA) implant at the Heart and Diabetes Center North Rhine Westfalia, Germany, were eligible. In total, 498 patients fulfilled this criterion. Out of the 498 patients, 134 patients with HVAD implants were excluded because they received the implant at a time where no HeartMate 3 implants were available at our institution (before July 2014), leaving 364 patients who were finally included in the data analysis. Written informed consent for scientific use of clinical data was obtained from all patients or their relatives (in case of unconscious patients not able to sign informed consent). The investigation conforms to the principles outlined in the Declaration of Helsinki. Because of the retrospective study design, the need for an ethics committee votum was waived.
2.2. Cardiac surgery
All LVAD implants were performed in a standardized fashion via full median sternotomy using cardiopulmonary bypass with full heparinization. After suturing the fixation ring onto the left ventricular apex, the LVAD systems were inserted and fixed to the beating heart, and the outflow grafts were routinely anastomosed to the mid‐portion of the ascending aorta. Drivelines were uniformly tunnelled towards a right‐sided epigastric exit site along the rectus fascia. All patients received a complete pericardial closure with a surgical membrane.
2.3. Anticoagulation management and infection prophylaxis and treatment
The standard anticoagulation regimen for patients with LVAD implants at our clinic is phenprocoumon (international normalized ratio target range: 2.0–2.5) with 100 mg aspirin daily, which applies for both devices analysed. Outpatients performed international normalized ratio self‐monitoring and management as previously described.11
According to institutional standards, antibiotic prophylaxis was performed routinely with 2 g cephalosporin (Cefazolin‐Sandoz, Sandoz Pharmaceuticals, Basel, Switzerland) perioperatively (up to 48 h after surgery). In case of an infection, antibiotic therapy was selected according to the sensitivity of the microorganism and the clinical response of the patient.
2.4. Study design
For data analysis, patients were divided into two groups. Group 1 consisted of patients with HVAD implants (designated HVAD group; n = 258), and Group 2 consisted of patients with HeartMate 3 implants (designated HeartMate 3 group; n = 106). Baseline characteristics and data on the clinical status of the patients were available through a prospectively maintained database of our MCS program, which is based on the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) and European Registry for Patients with Mechanical Circulatory Support registry criteria.
2.5. Endpoints
Primary endpoint was overall survival during follow‐up. We followed the patients from the day of LVAD implantation, irrespectively of whether they were transplanted, weaned, or remained on the device for up to a maximum of 30 months. Secondary endpoints were pump thrombosis, driveline infection, and stroke (ischaemic/haemorrhagic). A stroke was considered present when a functional relevant motoric, sensory, or cognitive neurological deficit persisted for at least 24 h, excluding any other cause. The diagnosis was verified by multislice computed tomography and confirmed by a neurologist within 24 h. Pump thrombosis was assumed by low pump flow with an increased power consumption, haematuria, elevated lactate dehydrogenase (reference range: 100–600 unit/L), or elevated serum‐free haemoglobin (reference range: 0–40 mg/dL) concentrations and verified by computer‐gated tomography, transthoracic echocardiography, and right heart catheterization.12 A driveline infection was diagnosed when the driveline exit site showed cutaneous flush and pus formation with a positive result of microbial culture, pyrexia, tachycardia, and tachypnoea, and/or elevated C‐reactive protein concentrations (>5 mg/L) and elevated circulating white blood cell counts (>11 000 cells/μL), with or without signs of systemic infection.
2.6. Statistics
Categorical variables are summarized as percentages and number of observations. Preoperative continuous variables are presented as means and standard deviation or median and 25th–75th percentile, where appropriate.
Because of non‐randomized group assignment, we performed a matched propensity score (PS) analysis to assess treatment effects. The propensity matching score was estimated by multivariable logistic regression. In the regression model, the device type was the dependent variable. Patient matching was performed for age, sex, serum creatinine levels, INTERMACS level at the time of LVAD implantation, perioperative right HF, and the implantation strategy, that is, bridge to transplant or destination therapy. Matching was performed using a 1:1 ratio with the logit‐transformed PS. An optimal matching algorithm with a caliper width of 0.1 standard deviation from the linear predictor was used. The balance of risk factors was judged by standardized differences. The balance is considered to be satisfactory when the standardized difference is less than 10%.13
We generated Kaplan–Meier estimates by study group for the primary and secondary endpoints during follow‐up and used Cox proportional hazard models, stratified on the matched pairs for data analyses. Results are presented as hazard ratios (HRs) and 95% confidence intervals (CIs). The proportionality of hazard assumption was checked by evaluation of time‐dependent variables, which were the cross‐products of the predictor variables with event‐free outcomes. Follow‐up was assessed using the Kaplan–Meier estimate of potential follow‐up.14 In addition, we performed sensitivity analysis in the entire study cohort using multivariable‐adjusted Cox regression analysis. Those covariates, which were used for PS matching, were also used as covariates for the multivariable‐adjusted analysis. Moreover, we used PS‐adjusted Cox regression analysis to compare clinical outcomes between study groups.15 P‐values <0.05 were considered statistically significant. We applied the statistical software package IBM SPSS, version 24 (IBM Corp, Armonk, NY, USA), R (version 2.15.3), and the SPE file in SPSS to perform the analyses.
3. Results
Out of the cohort of 364 patients, PS matching was possible in 79 pairs (Table 1). PS matching reduced the standardized differences in preoperative covariates between the study groups substantially. In the PS‐matched groups, all standardized differences were <10%, with the exception of implantation strategy. The mean distance in the estimated PS was 0.02 and resulted in two generally well‐matched populations of patients with similar preoperative characteristics.
Table 1.
Baseline characteristics of the study groups
| Parameter | All patients n = 364 | PS‐matched pairs | ||||
|---|---|---|---|---|---|---|
| HeartWare | HeartMate 3 | HeartWare | HeartMate 3 | |||
| n = 258 | n = 106 | Std diff (%) | n = 79 | n = 79 | Std diff (%) | |
| Age (years) | 54.4 ± 13.0 | 58.4 ± 11.5 | −32.6 | 56.4 ± 11.9 | 56.3 ± 12.0 | 0.8 |
| Gender, male | 218 (85) | 94 (89) | −18.7 | 70 (89) | 70 (89) | 0.0 |
| INTERMACS level | 1.80 ± 1.26 | 2.48 ± 1.06 | −58.4 | 2.27 ± 1.25 | 2.30 ± 1.11 | −2.5 |
| Creatinine (mg/dL) | 1.51 ± 0.78 | 1.61 ± 0.85 | −12.3 | 1.58 ± 0.80 | 1.58 ± 0.73 | 0.0 |
| Right heart failure | 101 (39) | 35 (33) | 18.1 | 30 (38) | 29 (37) | −2.9 |
| Bridge to transplant | 194 (75) | 72 (68) | 22.0 | 59 (75) | 56 (71) | 11.7 |
| Destination therapy | 64 (25) | 34 (32) | −22.0 | 20 (25) | 23 (29) | −11.7 |
| Time of inclusion after start of recruitment (days) | 601 ± 398 | 1056 ± 309 | −127.7 | 961 ± 341 | 990 ± 311 | −8.9 |
| Propensity score | 0.80 ± 0.21 | 0.49 ± 0.23 | 140.7 | 0.58 ± 0.22 | 0.56 ± 0.21 | 9.3 |
Right heart failure was considered when central venous pressures were elevated >18 mmHg, and cardiac index was diminished <2.0 L/min/m2, in the presence of peripheral oedema or ascites. Data are presented as mean (standard deviation) or n (%). INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; PS, propensity score.
Median follow‐up was 15.3 months (25th–75th percentile: 8.3–25.3 months). Figure 1 illustrates the results of the primary endpoint by study group. Survival rates in the HVAD and HeartMate 3 groups were 75.5% and 76.0% at 1 year and 63.8% and 70.2% at 2 years of follow‐up, respectively. The HR of mortality for the HeartMate 3 group vs. the HVAD group was 0.84 (95% CI: 0.46–1.54; P = 0.568). Multi‐organ failure was the primary cause of death in both groups, that is, 11 (58%) and 15 (65%) patients in the HeartMate 3 and HVAD groups, respectively. Minor numbers of patients died due to neurological complications (stroke/haemorrhage, n = 4 vs. n = 1) and other reasons (n = 4 vs. n = 7) in the HeartMate 3 and HVAD groups, respectively. Seven patients were transplanted in both the HVAD and HeartMate 3 groups. Median survival rates on device were 13.9 and 13.4 months, respectively, and did not differ significantly between study groups [HR for the HeartMate 3 vs. HVAD group = 0.76 (95% CI: 0.41–1.43; P = 0.397)]. None of the patients were weaned during follow‐up, neither in the HVAD group nor in the HeartMate 3 group.
Figure 1.
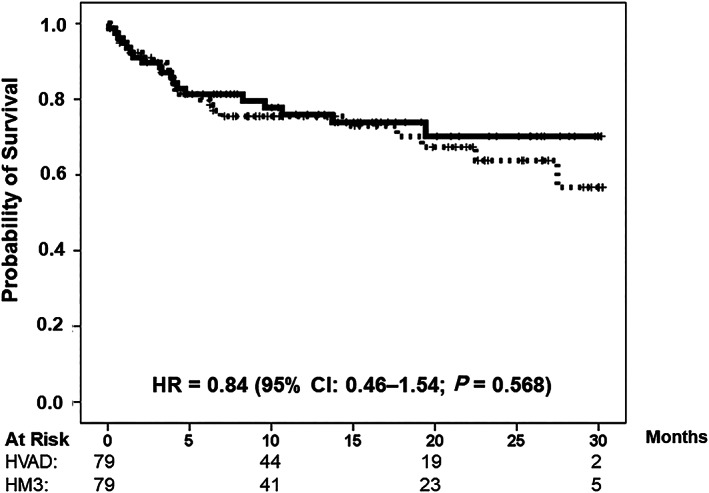
Overall survival in the propensity score‐matched study groups. HeartMate 3 group (solid line); HVAD group (dotted line). CI, confidence interval; HR, hazard ratio.
Freedom from secondary clinical endpoints is illustrated in Figure 2 A– 2 C. Briefly, freedom from any kind of cerebrovascular event, embolic stroke or haemorrhage, was 86.9% and 89.5% at 1 year and 74.0% and 86.2% at 3 years in the HVAD and HeartMate 3 groups, respectively. The HR of an event for the HeartMate 3 group vs. the HVAD group was 0.57 (95% CI: 0.23–1.45; P = 0.241). Freedom from driveline infection in the HVAD and HeartMate 3 groups was 25.3% and 50.3% at 1 year and 25.3% and 38.9% at 2 years of follow‐up, respectively, with an HR of an event for the HeartMate 3 group vs. the HVAD group of 0.54 (95% CI: 0.35–0.84; P = 0.006). Freedom from pump thrombosis in the HVAD and HeartMate 3 groups was 87.1% and 100% at 1 year and 82.5% and 100% at 2 years of follow‐up, respectively. The HR of an event for the HeartMate 3 group vs. the HVAD group was 0.02 (95% CI: 0.00–4.43; P = 0.148). The proportional hazard assumption of the model was satisfied for all clinical endpoints (all P‐values >0.05).
Figure 2.
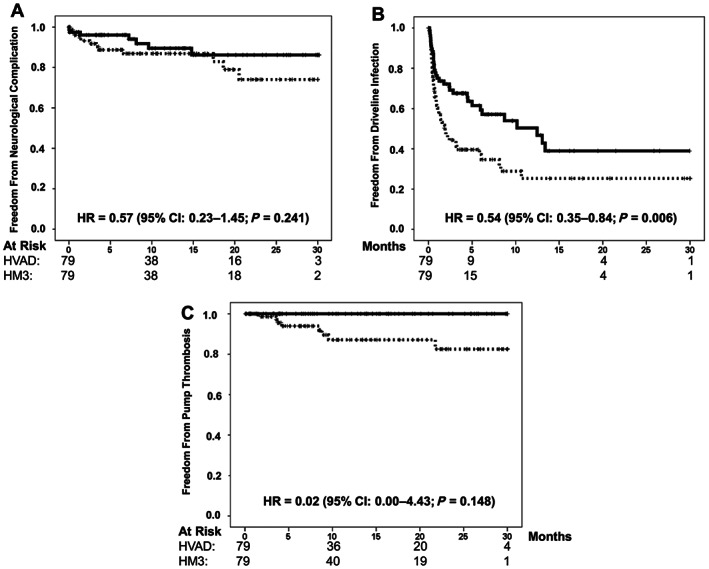
Major complications after left ventricular assist device implantation. Freedom from stroke (A), driveline infection (B), and pump thrombosis (C) in the propensity score‐matched study groups. HeartMate 3 group (solid line); HVAD group (dotted line).
Table 2 shows the results of the sensitivity analyses. Briefly, unadjusted results differed considerably from the PS‐matched results. However, data in the PS‐adjusted and the multivariable‐adjusted models did not differ substantially from the PS‐matched results. Similar to the PS‐matched results, the only significant difference between study groups was the risk of driveline infection in the PS‐adjusted and multivariable‐adjusted models.
Table 2.
Sensitivity analyses of clinical outcomes during follow‐up in the entire study cohort of HeartMate 3 vs. HeartWare patients
| Parameter | HeartMate 3 | HVAD | Unadjusted model | PS‐adjusted model | Multivariablea‐adjusted model | |||
|---|---|---|---|---|---|---|---|---|
| n = 106 | n = 258 | HR (95% CI) | P‐value | HR (95% CI) | P‐value | HR (95% CI) | P‐value | |
| Overall mortality, n (%) | 23 (23.6) | 121 (46.9) | 0.66 (0.42–1.02) | 0.059 | 1.08 (0.65–1.80) | 0.773 | 0.99 (0.59–1.67) | 0.975 |
| Stroke, n (%) | 8 (7.5) | 54 (20.9) | 0.43 (0.21–0.92) | 0.028 | 0.54 (0.24–1.26) | 0.155 | 0.58 (0.25–1.34) | 0.205 |
| Driveline infection, n (%) | 42 (39.6) | 131 (50.8) | 0.84 (0.59–1.18) | 0.312 | 0.58 (0.38–0.89) | 0.013 | 0.48 (0.32–0.74) | 0.001 |
| Pump thrombosis, n (%) | 0 (0) | 28 (10.9) | 0.03 (0.01–1.27) | 0.067 | 0.00 (0.00–∞) | 0.941 | 0.00 (0.00–∞) | 0.941 |
CI, confidence interval; HR, hazard ratio; PS, propensity score.
Adjusted for age, gender, serum creatinine, Interagency Registry for Mechanically Assisted Circulatory Support level, right heart failure, implant strategy, and time of inclusion after start of recruitment.
4. Discussion
Durable MCS therapy has become an alternative to transplantation for many patients with terminal HF, not only because of the dramatic organ shortage but also in the presence of contraindications for transplant. The widespread use of durable MCS is due to meticulous refinements in ventricular assist device technology during the last decades, specifically addressing size and effectiveness and reduction of adverse events. The introduction of the so‐called third‐generation and fourth‐generation CF centrifugal LVADs, that is, the HVAD® and HeartMate 3®, has virtually replaced the use of any other device in clinical settings. Both devices have been studied solely in industry‐sponsored trials and proven to be non‐inferior to the previous state‐of‐the‐art and so‐called second‐generation CF axial flow pump, that is, the HeartMate II®. In fact, MCS‐related and anti‐coagulation‐related complications remain relevant issues after implantation of both novel devices.5, 8, 9, 10, 16 INTERMACS has documented the increased use of CF centrifugal pumps in recent times together with a relatively low freedom from the combined major event of infection, bleeding, device malfunction, stroke, or death within the first year after durable MCS implantation.17
Such data are a mandate to continue the search for the optimal durable MCS device, and it remains an intriguing question which of the latest and increasingly used devices, that is, the HVAD or the HeartMate 3, may potentially offer best patient outcomes. A randomized trial comparing MCS therapy using these two LVADs is still not available, leaving the choice of device to physicians' preferences.
To our knowledge, this present study is the first risk‐adjusted single‐centre experience comparing the outcomes after HVAD and HeartMate 3 implantation. Obviously, risk adjustment is mandatory, as the univariate analysis suggested an overall survival benefit after implantation of HeartMate 3®, but this did not withstand the multivariable nor the matched pairs analyses.
The major findings herein were that the use of the HVAD and the HeartMate 3 yielded comparable survival and cerebrovascular stroke rates but a higher risk of driveline infections and pump thrombosis in HVAD patients. Regarding the relevant differences, we cannot explain at present why the driveline exit site in HVAD patients may be more susceptible to inflammation and infection. The surgical principle for driveline tunnelling along the rectus fascia is routinely followed by all implanting surgeons at our institution. Also, exit site dressing is uniformly performed and trained, irrespective of the implanted pump type. Device‐specific differences in driveline flexibility, cable diameters, vibration conduction, or potential heat emission may explain our results, but this issue truly needs further investigation.
Pump thrombosis is, despite optimized anti‐coagulation therapy, a major complication after LVAD implantation,17 impacting on clinical status and quality of life. It may be treated with fibrinolytic agents or pump exchange surgery. The risk of developing a second pump thrombosis is increased after the first occurrence. Pump thrombosis is considered to adversely affect survival,18 but the fact that it did not occur in the presently investigated HeartMate 3 cohort with equal midterm survival may challenge this assumption. Truly, this requires further larger‐scale investigations on a multi‐centre level. Nevertheless, the absence of pump thrombosis reported elsewhere and herein9, 10 may favour use of the HeartMate 3 device over the HVAD for durable MCS therapy. The fully magnetically levitated internal rotor and the relatively wide rotor housing together with thegeneration of an internal pulse every 30 s in the Heartmate 3 might explain the strikingly different incidences of pump thrombosis. It needs to be noted that the outflow graft was found twisted in some HeartMate 3 patients19 and that its occurrence required complex and risky redo surgery. Growing awareness of this seldom but potential complication prompted the most recent introduction of a fixation device, additionally mounted onto the pump's outflow socket. Although this seems to exclude outflow graft twists in the future, it makes the HeartMate 3 relatively big and thus may favour use of the smaller HVAD in an anatomically restricted situs.
Although inclusion of risk adjustment and uniform surgical procedures strengthen the presented data, it has to be noted that this single‐centre and retrospective design of the present study limits interpretation of the results, also because of relatively small patient numbers. Prospective multi‐centre analyses have to follow up on improving patient outcomes in modern durable MCS therapy. Because survival is comparable between the groups studied herein, such future studies need to address whether differences in driveline infections and pump thrombosis may affect outcome distinctively, namely, in terms of quality of life. A prospective randomized trial could also exclude a potential selection bias. Although we intended to use the two devices equally, we cannot exclude a confounder, for example, a learning curve with implantation of the HeartMate 3, or a tendency to prefer the HVAD in an anatomically limited situs for its smaller size.
Conflict of interest
Rene Schramm has received speaker honoraria and travel expense reimbursement from Abbott. Jan Gummert and Michiel Morshuis have received consulting honoraria and travel expense reimbursement from Medtronic and Abbott.
Schramm, R. , Zittermann, A. , Morshuis, M. , Schoenbrodt, M. , von Roessing, E. , von Dossow, V. , Koster, A. , Fox, H. , Hakim‐Meibodi, K. , and Gummert, J. F. (2020) Comparing short‐term outcome after implantation of the HeartWare® HVAD® and the Abbott® HeartMate 3®. ESC Heart Failure, 7: 908–914. 10.1002/ehf2.12649.
References
- 1. Cai AW, Islam S, Hankins SR, Fischer W, Eisen HJ. Mechanical circulatory support in the treatment of advanced heart failure. Am J Transplant 2017; 17: 3020–3032. [DOI] [PubMed] [Google Scholar]
- 2. Miller LW, Pagani FD, Russell SD, John R, Boyle AJ, Aaronson KD, Conte JV, Naka Y, Mancini D, Delgado RM, MacGillivray TE, Farrar DJ, Frazier OH. HeartMate II Clinical Investigators. Use of a continuous‐flow device in patients awaiting heart transplantation. N Engl J Med 2007; 357: 885–896. [DOI] [PubMed] [Google Scholar]
- 3. Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, Long JW, Ascheim DD, Tierney AR, Levitan RG, Watson JT, Meier P, Ronan NS, Shapiro PA, Lazar RM, Miller LW, Gupta L, Frazier OH, Desvigne‐Nickens P, Oz MC, Poirier VL. Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group. Long‐term use of a left ventricular assist device for end‐stage heart failure. N Engl J Med 2001; 345: 1435–1443. [DOI] [PubMed] [Google Scholar]
- 4. Wieselthaler GM, O'Driscoll G, Jansz P, Khaghani A, Strueber M. HVAD Clinical Investigators. Initial clinical experience with a novel left ventricular assist device with a magnetically levitated rotor in a multi‐institutional trial. J Heart Lung Transplant 2010; 29: 1218–1225. [DOI] [PubMed] [Google Scholar]
- 5. Heatley G, Sood P, Goldstein D, Uriel N, Cleveland J, Middlebrook D, Mehra MR. MOMENTUM 3 Investigators. Clinical trial design and rationale of the Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy With HeartMate 3 (MOMENTUM 3) investigational device exemption clinical study protocol. J Heart Lung Transplant 2016; 35: 528–536. [DOI] [PubMed] [Google Scholar]
- 6. Chatterjee A, Feldmann C, Dogan G, Hanke JS, Ricklefs M, Deniz E, Haverich A, Schmitto JD. Clinical overview of the HVAD: a centrifugal continuous‐flow ventricular assist device with magnetic and hydrodynamic bearings including lateral implantation strategies. J Thorac Dis 2018; 10: S1785–S1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chatterjee A, Feldmann C, Hanke JS, Ricklefs M, Shrestha M, Dogan G, Haverich A, Schmitto JD. The momentum of HeartMate 3: a novel active magnetically levitated centrifugal left ventricular assist device (LVAD). J Thorac Dis 2018; 10: S1790–S1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rogers JG, Pagani FD, Tatooles AJ, Bhat G, Slaughter MS, Birks EJ, Boyce SW, Najjar SS, Jeevanandam V, Anderson AS, Gregoric ID, Mallidi H, Leadley K, Aaronson KD, Frazier OH, Milano CA. Intrapericardial left ventricular assist device for advanced heart failure. N Engl J Med 2017; 376: 451–460. [DOI] [PubMed] [Google Scholar]
- 9. Mehra MR, Naka Y, Uriel N, Goldstein DJ, Cleveland JC Jr, Colombo PC, Walsh MN, Milano CA, Patel CB, Jorde UP, Pagani FD, Aaronson KD, Dean DA, McCants K, Itoh A, Ewald GA, Horstmanshof D, Long JW, Salerno C. MOMENTUM 3 Investigators. A fully magnetically levitated circulatory pump for advanced heart failure. N Engl J Med 2017; 376: 440–450. [DOI] [PubMed] [Google Scholar]
- 10. Mehra MR, Goldstein DJ, Uriel N, Cleveland JC Jr, Yuzefpolskaya M, Salerno C, Walsh MN, Milano CA, Patel CB, Ewald GA, Itoh A, Dean D, Krishnamoorthy A, Cotts WG, Tatooles AJ, Jorde UP, Bruckner BA, Estep JD, Jeevanandam V, Sayer G, Horstmanshof D, Long JW, Gulati S, Skipper ER, O'Connell JB, Heatley G, Sood P, Naka Y. MOMENTUM 3 Investigators. Two‐year outcomes with a magnetically levitated cardiac pump in heart failure. N Engl J Med 2018; 378: 1386–1395. [DOI] [PubMed] [Google Scholar]
- 11. Koertke H, Zittermann A, Minami K, Tenderich G, Wagner O, El‐Arousy M, Krian A, Ennker J, Taborski U, Klövekorn WP, Moosdorf R, Saggau W, Morshuis M, Koerfer J, Seifert D, Koerfer R. Low‐dose international normalized ratio self‐management: a promising tool to achieve low complication rates after mechanical heart valve replacement. Ann Thorac Surg 2005. Jun; 79: 1909–1914. [DOI] [PubMed] [Google Scholar]
- 12. Oezpeker C, Zittermann A, Ensminger S, Kizner L, Koster A, Sayin A, Schoenbrodt M, Milting H, Gummert JF, Morshuis M. Systemic thrombolysis versus device exchange for pump thrombosis management: a single‐center experience. ASAIO J 2016. May‐Jun; 62: 246–251. [DOI] [PubMed] [Google Scholar]
- 13. Börgermann J, Hakim K, Renner A, Parsa A, Aboud A, Becker T, Masshoff M, Zittermann A, Gummert JF, Kuss O. Clampless off‐pump versus conventional coronary artery revascularization: a propensity score analysis of 788 patients. Circulation 2012. Sep 11; 126: S176–S182. [DOI] [PubMed] [Google Scholar]
- 14. Schemper M, Smith TL. A note on quantifying follow‐up in studies of failure time. Control Clin Trials 1996; 17: 343–346. [DOI] [PubMed] [Google Scholar]
- 15. D'Agostino RB Jr. Propensity scores in cardiovascular research. Circulation 2007; 115: 2340–2343. [DOI] [PubMed] [Google Scholar]
- 16. Aaronson KD, Slaughter MS, Miller LW, McGee EC, Cotts WG, Acker MA, Jessup ML, Gregoric ID, Loyalka P, Frazier OH, Jeevanandam V, Anderson AS, Kormos RL, Teuteberg JJ, Levy WC, Naftel DC, Bittman RM, Pagani FD, Hathaway DR, Boyce SW. HeartWare Ventricular Assist Device (HVAD) Bridge to Transplant ADVANCE Trial Investigators. Use of an intrapericardial, continuous‐flow, centrifugal pump in patients awaiting heart transplantation. Circulation 2012. Jun 26; 125: 3191–3200. [DOI] [PubMed] [Google Scholar]
- 17. Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, Miller MA, Baldwin JT, Young JB. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant 2015. Dec; 34: 1495–1504. [DOI] [PubMed] [Google Scholar]
- 18. Kirklin JK, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, Miller MA, Baldwin JT, Young JB, Naftel DC. Eighth annual INTERMACS report: special focus on framing the impact of adverse events. J Heart Lung Transplant 2017. Oct; 36: 1080–1086. [DOI] [PubMed] [Google Scholar]
- 19. Grüger T, Kaufmann F, Dreysse S, Falk V, Krabatsch T, Potapov E. Late post‐pump blood flow obstruction in a novel left ventricular assist device: the unusual case of a twisted outflow graft. J Thorac Cardiovasc Surg 2018. Jan; 155: e33–e35. [DOI] [PubMed] [Google Scholar]


