Abstract
Aims
Echocardiographic measures of dyssynchrony at baseline have not demonstrated a good ability to predict response to cardiac resynchronization therapy (CRT). The purpose of this study was to determine if the acute correction of electromechanical dyssynchrony, assessed by the change in simple pulsed‐Doppler measures, was related to CRT response at 6 months.
Methods and results
Echocardiography was performed at baseline and at pre‐discharge after CRT implantation. Intraventricular, interventricular, and atrioventricular dyssynchrony were evaluated by the left pre‐ejection interval (LPEI), the interventricular mechanical delay, and the ratio of left ventricular filling time to RR interval, respectivelxy. A patient was considered responder if he/she was alive without hospitalization for heart failure and had an absolute increase of left ventricular ejection fraction (LVEF) >5 points. Forty‐eight patients (mean age 67 ± 11 years, 73% male, mean LVEF 30 ± 5%) were included. CRT led to an acute correction of intraventricular and interventricular dyssynchrony but not to an acute correction of atrioventricular dyssynchrony. There were 31 (65%) responders at 6 months. Two factors were independently associated with CRT response in multivariate analysis: ischemic cardiomyopathy (odds ratio 0.19, 95% confidence interval 0.04–0.87; P= 0.032) and delta LPEI (odds ratio 1.03 per 1 ms decrease, 95% confidence interval 1.01–1.05; P = 0.007). By receiver operating characteristic analysis, the optimal cut‐off value of delta LPEI was −16 ms. The proportion of responders in patients without ischemic cardiomyopathy and with a delta LPEI greater than −16 ms was 85%.
Conclusions
Acute correction of intraventricular electromechanical dyssynchrony evaluated by the LPEI predicted CRT response at 6 months.
Keywords: Cardiac resynchronization therapy, Electromechanical dyssynchrony, Left pre‐ejection interval, Echocardiography, Acute correction, CRT response
Introduction
Cardiac resynchronization therapy (CRT) is an established treatment for patients with symptomatic heart failure and left ventricular (LV) dysfunction.1, 2 Patient selection currently relies on electrocardiographic markers, QRS duration, and morphology. The multicentre Predictors of Response to CRT study3 demonstrated that echocardiographic parameters of mechanical dyssynchrony had, in general, unsatisfactory reproducibility and usefulness to identify clinical and echocardiographic responders to CRT. As a consequence, mechanical dyssynchrony for patient selection has not been incorporated into guidelines or standard of care.4, 5 However, the three, simple, pulsed‐Doppler parameters tested in the Predictors of Response to CRT study were interpretable in most examinations, showed good reproducibility, and had a statistically significant—although modest—performance in predicting outcomes. These parameters were left pre‐ejection interval (LPEI), interventricular mechanical delay (IVMD), and LV filling time (LVFT)/RR interval. They are termed electromechanical because they incorporate electrocardiographic and contraction timings and evaluate intraventricular, interventricular, and atrioventricular dyssynchrony, respectively.
Response to CRT is multifactorial and may depend on the effectiveness of the delivered biventricular pacing, that is, its impact on baseline dyssynchrony. This has been suggested for electrical resynchronization as manifested by the relation between the magnitude of QRS narrowing and response to therapy.6 Whether effective electromechanical resynchronization might influence outcomes has been less investigated.
The objective of this study was to determine if the acute correction of electromechanical dyssynchrony assessed by the change in the aforementioned simple echocardiographic measures was related to CRT response at 6 months.
Methods
Patient selection
Patients were included in this prospective, observational, single‐centre study if they had a standard indication for CRT.:4 LV ejection fraction (LVEF) ≤35%, New York Hear Association Class 2, 3, or ambulatory class 4, QRS width ≥ 120 ms, despite optimal medical therapy. Both de novo implantations and upgrades were included. Patients in permanent atrial fibrillation underwent concomitant atrioventricular junction ablation to ensure complete CRT delivery and could be included. Exclusion criteria were patients listed for cardiac transplantation, expected survival less than 6 months, and pacing for advanced atrioventricular block.
Cardiac resynchronization therapy response
The primary endpoint was CRT response at 6 months. A patient was considered responder if he/she was alive, had not been hospitalized for heart failure, and had an absolute increase of LVEF >5 points. For the purpose of this study, patients who experienced lead dislodgement or extraction during follow‐up, that is, modification or withdrawal of the initial resynchronization, were excluded from analysis.
Echocardiographic measurements
Transthoracic echocardiography was performed at baseline, at Day 1 or 2 post‐implantation (pre‐discharge), and at 6 months using Vivid 6 or 9 machines (General Electric Healthcare, Massachusetts, USA) by a physician blinded to the patient's clinical evolution. In addition to the standard examination, simple electromechanical dyssynchrony parameters were recorded on baseline and pre‐discharge examinations.3, 7 Intraventricular dyssynchrony was evaluated by the LPEI and was considered present if LPEI ≥140 ms. Interventricular dyssynchrony was evaluated by the IVMD, calculated as the difference between LPEI and right pre‐ejection interval, and was considered present if IVMD ≥40 ms. Atrioventricular dyssynchrony was evaluated in patients in sinus rhythm by the LVFT/RR ratio and was considered present if ≤40%.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation, and categorical variables were expressed as counts with percentages. Comparison between groups were performed with the Mann–Whitney test for continuous variables and a Chi‐Square test or Fischer exact test for categorical variables, as appropriate. Comparison of dyssynchrony parameters at baseline and pre‐discharge examinations was performed with a Wilcoxon signed‐rank test for continuous variables and a McNemar test for categorical variables.
Multivariate analysis using backward logistic regression was performed to identify factors independently associated with CRT response. Factors with P < 0.10 in univariate analysis were entered in the model.
A receiver operating characteristic curve was generated to evaluate the diagnostic accuracy of the parameter identified in multivariable analysis in identifying CRT response and to determine the optimal cut‐off value. This cut‐off value was used to dichotomize the population to groups ≤ cut‐off and > cut‐off. A P value <0.05 using two‐tailed analysis was considered statistically significant. Statistical analyses were performed with MedCalc v19.0.5 (MedCalc Software bvba, Ostende, Belgium).
This study complies with the Declaration of Helsinki. All patients provided written informed consent, and this study was approved by the local ethics committee.
Results
Patient population
Forty‐eight patients (mean age 67 ± 11 years, 73% male) were included (Table 1). Ischemic cardiomyopathy was present in 20 (42%) patients. Mean LVEF was 30 ± 5%, and 32 (67%) patients had left bundle branch block.
Table 1.
Patient characteristics
| Total population N = 48 | Non‐responders N = 17 | Responders N = 31 | P | |
|---|---|---|---|---|
| Age | 68 ± 11 | 68 ± 10 | 67 ± 12 | 0.81 |
| Male | 35 (73%) | 14 (82%) | 21 (68%) | 0.33 |
| Obesity | 15 (32%) | 5 (31%) | 10 (32%) | 1 |
| NYHA classification | ||||
| 2 | 16 (33%) | 5 (29%) | 11 (35%) | 0.92 |
| 3 | 32 (67%) | 12 (71%) | 20 (65%) | |
| Ischemic cardiomyopathy | 20 (42%) | 11 (65%) | 9 (29%) | 0.03 |
| Atrial fibrillation | 14 (29%) | 5 (29%) | 9 (29%) | 1 |
| QRS duration (ms) | 165 ± 29 | 165 ± 27 | 165 ± 31 | 0.89 |
| LBBB | 32 (67%) | 8 (47%) | 24 (77%) | 0.07 |
| Upgrade | 17 (35%) | 6 (35%) | 11 (35%) | 1 |
| LV lead in lateral vein | 39 (81%) | 16 (94%) | 23 (92%) | 1 |
| LVEDV (ml) | 205 ± 60 | 210 ± 64 | 202 ± 59 | 0.76 |
| LVESV (ml) | 142 ± 47 | 144 ± 46 | 140 ± 49 | 0.79 |
| LVEF | 30 ± 5 | 30 ± 5 | 29 ± 5 | 0.42 |
| TAPSE <17 | 17 (35%) | 9 (53%) | 8 (28%) | 0.16 |
| MR ≥ 2 | 17 (35%) | 7 (41%) | 10 (32%) | 0.76 |
| Creatinin | 123 ± 67 | 127 ± 71 | 121 ± 66 | 0.78 |
| Sodium | 137 ± 7 | 135 ± 10 | 137 ± 4 | 0.87 |
| Haematocrit | 42 ± 4 | 42 ± 5 | 41 ± 4 | 0.47 |
| NT‐proBNP | 3399 ± 3699 | 4106 ± 4733 | 3045 ± 3099 | 0.50 |
| ACEI/ARB | 41 (85%) | 15 (88%) | 26 (84%) | 1 |
| Betablocker | 44 (92%) | 16 (94%) | 28 (90%) | 1 |
| Aldosterone antagonists | 29 (60%) | 9 (53%) | 20 (65%) | 0.63 |
| Diuretics | 43 (90%) | 16 (94%) | 27 (87%) | 0.64 |
| Ivabradine | 6 (12%) | 1 (6%) | 5 (16%) | 0.4 |
| Amiodarone | 12 (26%) | 4 (24%) | 8 (27%) | 1 |
| Anticoagulant | 23 (49%) | 7 (41%) | 16 (53%) | 0.62 |
| Antiplatelet | 20 (42%) | 10 (59%) | 10 (33%) | 0.16 |
ACEI/ARB, ACE‐inhibitors/angiotensin receptor blockers; LBBB, left bundle branch block; LV, left ventricle; LVEDV, LV end‐diastolic volume; LVESV, LV end‐systolic volume; LVEF, LV ejection fraction; MR, mitral regurgitation; NT‐proBNP, N‐terminal pro BNP; NYHA, New York Heart Association; TAPSE, tricuspid annular plane systolic excursion.
There were 31 (65%) de novo implantations and 17 (35%) upgrades. LV lead vein location was lateral in 39 (81%) patients. Fourteen patients (29%) were in permanent atrial fibrillation at the time of implantation, and all underwent immediate atrioventricular junction ablation.
Acute correction of electromechanical dyssynchrony
At baseline, intraventricular and interventricular dyssynchrony were present in 44 (92%) and 25 (53%) patients, respectively (Table 2). Among the 32 patients in sinus rhythm in whom LVFT was measured, atrioventricular dyssynchrony was present in five (16%) patients. Acute correction of intraventricular and interventricular dyssynchrony was observed, both in absolute values and in the proportion of patients meeting the criterion (Table 2). There was no acute correction of atrioventricular dyssynchrony.
Table 2.
Acute correction of electromechanical dyssynchrony
| Baseline | Pre‐discharge | P | |
|---|---|---|---|
| LPEI (ms) | 170 ± 33 | 153 ± 35 | 0.004 |
| LPEI ≥140 ms | 44 (92%) | 29 (60%) | <0.001 |
| IVMD (ms) | 38 ± 40 | 23 ± 21 | 0.02 |
| IVMD ≥40 ms | 25 (53%) | 10 (21%) | 0.003 |
| LVFT (%) n = 32 | 47 ± 7 | 49 ± 11 | 0.65 |
| LVFT≤40% | 5 (16%) | 6 (17%) | 0.69 |
IVMD, interventricular mechanical delay; LPEI, left pre‐ejection interval; LVFT, left ventricular filling time.
Cardiac resynchronization therapy response
There were 31 (65%) responders. Two patients died (one of heart failure and one of digestive cancer), and two patients were hospitalized for heart failure. Responders had less often ischemic cardiomyopathy than non‐responders (Table 1). Other clinical parameters and baseline LV volumes and LVEF were not different between groups. Responders had greater intraventricular and interventricular dyssynchrony at baseline (Table 3). Compared to non‐responders, responders had a greater acute correction of intraventricular dyssynchrony (delta LPEI +12 ± 49 ms vs. −33 ± 38 ms, P = 0.004) and of interventricular dyssynchrony (delta IVMD +9 ± 47 ms vs. −27 ± 36 ms, P = 0.01) (Table 3). There was no difference in acute correction of atrioventricular dyssynchrony between responders and non‐responders. Electromechanical dyssynchrony at pre‐discharge, that is, residual dyssynchrony after CRT, was not different between responders and non‐responders.
Table 3.
Comparison of electromechanical dyssynchrony parameters between responders and non‐responders.
| Non‐responders N = 17 | Responders N = 31 | P | |
|---|---|---|---|
| Baseline parameters | |||
| LPEI (ms) | 153 ± 21 | 180 ± 35 | 0.009 |
| LPEI ≥140 ms | 15 (88%) | 29 (94%) | 0.61 |
| IVMD (ms) | 17 ± 39 | 49 ± 37 | 0.01 |
| IVMD ≥40 ms | 4 (25%) | 21 (68%) | 0.013 |
| LVFT/RR (%) n = 32 | 49 ± 7 | 45 ± 7 | 0.14 |
| LVFT/RR ≤ 40% | 1 (8%) | 4 (20%) | 0.63 |
| Pre‐discharge | |||
| LPEI (ms) | 165 ± 45 | 147 ± 28 | 0.31 |
| LPEI ≥140 ms | 11 (65%) | 18 (58%) | 0.89 |
| IVMD (ms) | 25 ± 21 | 21 ± 22 | 0.52 |
| IVMD≥40 ms | 5 (29%) | 5 (16%) | 0.29 |
| LVFT/RR (%) | 49 ± 12 | 49 ± 11 | 0.77 |
| LVFT/RR ≤ 40% | 2 (15%) | 4 (18%) | 1 |
| Difference baseline‐Pre‐discharge | |||
| Delta LPEI (ms) | 12 ± 49 | −33 ± 38 | 0.004 |
| Delta LPEI >10 ms | 7 (41%) | 21 (68%) | 0.14 |
| Delta IVMD (ms) | 9 ± 47 | ‐27 ± 36 | 0.01 |
| Delta LVFT/RR n = 28 | −1 ± 16 | 1 ± 9 | 1 |
IVMD, interventricular mechanical delay; LPEI, left pre‐ejection interval; LVFT, left ventricular filling time.
Multivariate analysis
Two factors remained significantly associated with CRT response in multivariable analysis: ischemic cardiomyopathy (odds ratio 0.19, 95% confidence interval 0.04–0.87; P = 0.032) and delta LPEI (odds ratio 1.03 per 1 ms decrease, 95% confidence interval 1.01–1.05; P = 0.007).
Proportion of responders according to the presence of ischemic cardiomyopathy and to the change of delta left pre‐ejection interval
Receiver operating characteristic analysis (Figure 1) showed that delta LPEI was able to identify CRT response (area under the curve: 0.750, 95% confidence interval 0.605–0.864; P = 0.001). The optimal cut‐off value was less than or equal to −16 (i.e. a decrease of LPEI of 16 ms between baseline and pre‐discharge), with a sensitivity of 68% and specificity of 71%. Patients were categorized according to this cut‐off value and to the presence of ischemic cardiomyopathy (Figure 2 ). Patients with non‐ischemic cardiomyopathy and with a delta LPEI greater than −16 ms had a higher response rate than patients with an ischemic cardiomyopathy and a delta LPEI less than −16 ms (85 vs. 36%, P = 0.004).
Figure 1.
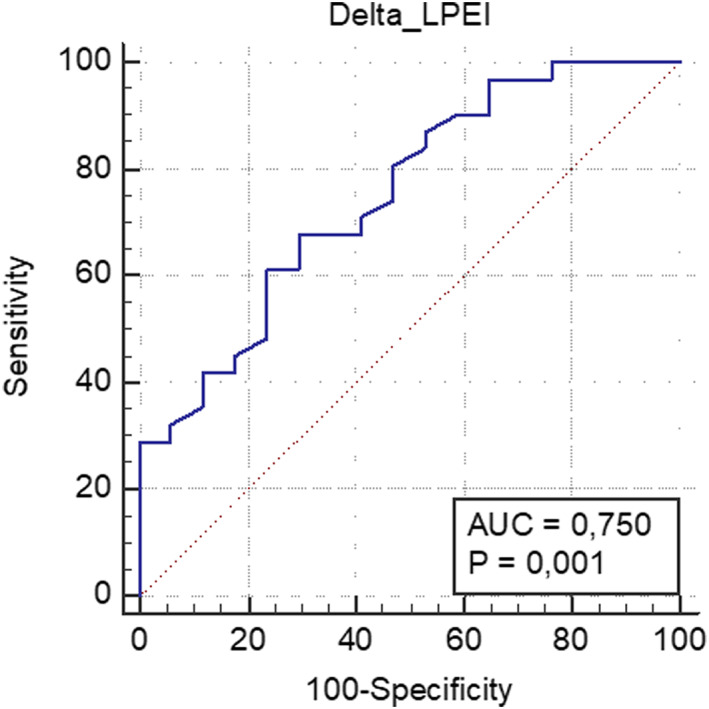
Receiver operating characteristic (ROC) analysis for the ability of delta left pre‐ejection interval (LPEI) to predict cardiac resynchronization therapy (CRT) response.
Figure 2.
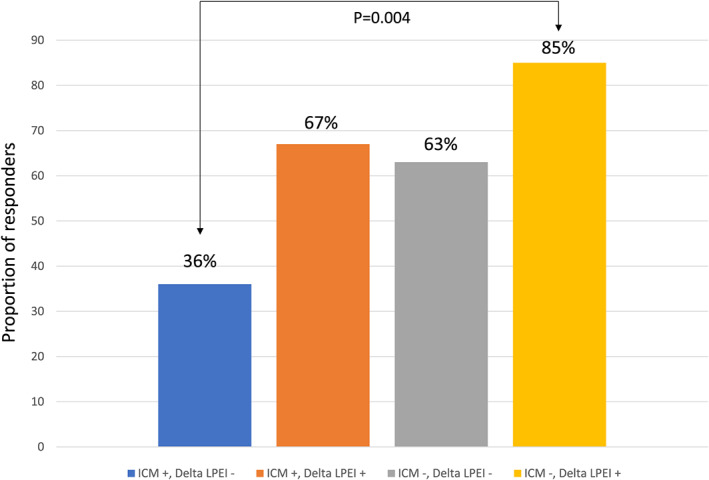
Responder rates according to the etiology of heart failure and to the magnitude of LPEI reduction. Patients without ischemic cardiomyopathy and with a delta LPEI greater than ≤‐16ms had a higher response rate than patients with an ischemic cardiomyopathy and a delta LPEI less than ‐16ms (85% vs. 36%, P=0.004).
Discussion
The main result of this study was that acute correction of electromechanical intraventricular dyssynchrony evaluated by a simple echocardiographic parameter predicted CRT response at 6 months.
In this study, baseline LPEI and IVMD were predictors of CRT response in univariate analysis but not in multivariate analysis. There has been a debate about the value of echocardiographic parameters of mechanical dyssynchrony in predicting CRT response. The randomized Cardiac Resynchronization–Heart Failure trial required in patients with a QRS duration between 120 and 149 ms the presence of two of the three following criteria: LPEI >140 ms, IVMD >40 ms, and a diastolic overlap of LV lateral wall contraction.1 In subgroup analysis, patients with QRS duration <160 ms did not derive benefit in terms of survival without hospitalization for a major cardiovascular event.1 Likewise, Bordachar et al.8 showed that these three parameters measured at baseline did not predict clinical and echocardiographic response to CRT, both when considered alone or in combination. While other studies using the same echocardiographic parameters showed conflicting results,9 our results are in line with the greatest body of evidence suggesting that no parameter can currently be recommended for patient selection.
Our study suggests that the modification of baseline dyssynchrony by biventricular pacing could be a better predictor of CRT response than baseline dyssynchrony. Response to CRT depends on the myocardial substrate10 and on the interplay between lead positions and the underlying substrate.11, 12 A recent study showed that improvement in LV end‐systolic volume and global longitudinal strain at 6 months post‐CRT was associated with better survival at 8 years.13 It would be interesting to be able to predict CRT response at an even earlier time point after device implantation, immediately after implantation. Few studies have examined the impact of acute resynchronization on outcomes.14, 15, 16 Bleeker et al.14 assessed LV dyssynchrony with tissue‐Doppler imaging and considered it present if the maximum delay between peak systolic velocities among the four walls within the LV was of 65 ms or greater. Only responders (defined as a >10% reduction of LV end‐systolic volume at 6 months) demonstrated a significant reduction of dyssynchrony immediately after implantation. Stankovic et al.15 showed that correction of septal flash and apical rocking by CRT were associated with reverse remodelling at 1 year. Wang et al.16 showed that echocardiographic responders at 6 months had a significant reduction in acute discoordination assessed with speckle‐tracking echocardiography.
In our study, acute correction of LPEI was associated with a positive response to CRT. Compared with the parameters used in the aforementioned studies, LPEI is easy and quick to measure and has very low intraobserver and interobserver variability,3, 17 thus strengthening the validity of the difference observed between baseline and pre‐discharge. Moreover, LPEI is obtained with pulsed‐Doppler and is therefore vendor‐independent.
LPEI is equal to the electromechanical delay (corresponding to excitation–contraction coupling) plus isovolumic contraction time. LPEI is correlated with LVEF, dP/dtmax, global longitudinal strain, and stroke volume.17 A normal ventricle has a short LPEI whereas an abnormal ventricle (such as in heart failure) has a long LPEI. LPEI can be considered a good maker of global systolic function and therefore a decrease of LPEI is indicative of improved systolic function. Cazeau et al. studied 18 parameters of dyssynchrony and showed that LPEI was the one correlated with the greatest number of other parameters, 13 out of 17.18 A decrease of LPEI was associated with directionally similar changes of the other parameters indicating improved synchrony. We found that a 16 ms decrease in LPEI was the optimal cut‐off for predicting CRT response at 6 months. In a previous pilot study,11 a strategy of CRT implantation aiming at reducing LPEI by at least 10 ms (by repositioning the right ventricular lead and if necessary, adding a second right ventricular lead) was acutely associated with an improved diastolic filling duration, an interventricular delay reduction, and an increase in LVEF. The present study suggests that a greater reduction in LPEI might be required to observe CRT response at 6 months.
Finally, our study is in line with the well‐known fact that ischemic cardiomyopathy is associated with a lower likelihood of LV reverse remodelling after CRT.19, 20 In our study, the aetiology of heart failure was the second independent factor of CRT response. Patients with non‐ischemic cardiomyopathy and with a delta LPEI above the cut‐off value of 16 ms had a very high response rate (85%) while patients with ischemic cardiomyopathy and a poor LPEI reduction had a very low likelihood of response (36%).
Study limitations
The number of patients and the follow‐up duration limited to 6 months are the main limitations of this study. There were few deaths and hospitalizations for heart failure, and the CRT response was mainly driven by LV reverse remodelling. Other dyssynchrony parameters were not studied, and perhaps a combination of parameters might have a better prediction capability than any parameter considered alone. A relatively high proportion of patients with atrial fibrillation was included, precluding conclusions regarding atrioventricular dyssynchrony. Finally, atrioventricular and interventricular delays optimization was not performed, and this might also influence response to CRT.
Conclusions
In a standard CRT population, acute correction of intraventricular electromechanical dyssynchrony evaluated by a simple parameter, the LPEI, predicted CRT response at 6 months.
Conflicts of interest
Dr. Anselme reports speaker and consulting fees from Boston Scientific, Medtronic, and MicroPort. Drs. Moubarak and Viart declare no conflicts of interest.
Funding
None.
Moubarak, G. , Viart, G. , and Anselme, F. (2020) Acute correction of electromechanical dyssynchrony and response to cardiac resynchronization therapy. ESC Heart Failure, 7: 1302–1308. 10.1002/ehf2.12654.
References
- 1. Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L, Cardiac Resynchronization‐Heart Failure (CARE‐HF) Study Investigators . The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005; 352: 1539–1549. [DOI] [PubMed] [Google Scholar]
- 2. Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, Estes NA 3rd, Foster E, Greenberg H, Higgins SL, Pfeffer MA, Solomon SD, Wilber D, Zareba W. MADIT‐CRT Trial Investigators. Cardiac‐resynchronization therapy for the prevention of heart‐failure events. N Engl J Med 2009; 361: 1329–1338. [DOI] [PubMed] [Google Scholar]
- 3. Chung ES, Leon AR, Tavazzi L, Sun JP, Nihoyannopoulos P, Merlino J, Abraham WT, Ghio S, Leclercq C, Bax JJ, Yu CM, Gorcsan J 3rd, St John Sutton M, De Sutter J, Murillo J. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation 2008; 117: 2608–2616. [DOI] [PubMed] [Google Scholar]
- 4. Brignole M, Auricchio A, Baron‐Esquivias G, Bordachar P, Boriani G, Breithardt OA, Cleland J, Deharo JC, Delgado V, Elliott PM, Gorenek B, Israel CW, Leclercq C, Linde C, Mont L, Padeletti L, Sutton R, Vardas PE, ESC Committee for Practice Guidelines (CPG) , Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Document Reviewers , Kirchhof P, Blomstrom‐Lundqvist C, Badano LP, Aliyev F, Bänsch D, Baumgartner H, Bsata W, Buser P, Charron P, Daubert JC, Dobreanu D, Faerestrand S, Hasdai D, Hoes AW, le Heuzey JY, Mavrakis H, McDonagh T, Merino JL, Nawar MM, Nielsen JC, Pieske B, Poposka L, Ruschitzka F, Tendera M, van Gelder I, Wilson CM. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J 2013; 34: 2281–2329. [DOI] [PubMed] [Google Scholar]
- 5. Mele D, Luisi GA, Malagù M, Laterza A, Ferrari R, Bertini M. Echocardiographic evaluation of cardiac dyssynchrony: does it still matter? Echocardiography 2018; 35: 707–715. [DOI] [PubMed] [Google Scholar]
- 6. Korantzopoulos P, Zhang Z, Li G, Fragakis N, Liu T. Meta‐analysis of the usefulness of change in QRS width to predict response to cardiac resynchronization therapy. Am J Cardiol 2016; 118: 1368–1373. [DOI] [PubMed] [Google Scholar]
- 7. Cazeau S, Gras D, Lazarus A, Ritter P, Mugica J. Multisite stimulation for correction of cardiac asynchrony. Heart 2000; 84: 579–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bordachar P, Lafitte S, Réant P, Reuter S, Clementy J, Mletzko RU, Siegel RM, Goscinska‐Bis K, Bowes R, Morgan J, Bénard S, Leclercq C. Low value of simple echocardiographic indices of ventricular dyssynchrony in predicting the response to cardiac resynchronization therapy. Eur J Heart Fail 2010; 12: 588–592. [DOI] [PubMed] [Google Scholar]
- 9. Cazeau SJ, Daubert JC, Tavazzi L, Frohlig G, Paul V. Responders to cardiac resynchronization therapy with narrow or intermediate QRS complexes identified by simple echocardiographic indices of dyssynchrony: the DESIRE study. Eur J Heart Fail 2008; 10: 273–280. [DOI] [PubMed] [Google Scholar]
- 10. Poole JE, Singh JP, Birgersdotter‐Green U. QRS duration or QRS morphology: what really matters in cardiac resynchronization Therapy? J Am Coll Cardiol; 67: 1104–1117. [DOI] [PubMed] [Google Scholar]
- 11. Moubarak G, Ritter P, Daubert JC, Cazeau S. First experience of intraoperative echocardiography‐guided optimization of cardiac resynchronization therapy delivery. Arch Cardiovasc Dis 2014; 107: 169–177. [DOI] [PubMed] [Google Scholar]
- 12. Singh JP, Klein HU, Huang DT, Reek S, Kuniss M, Quesada A, Barsheshet A, Cannom D, Goldenberg I, McNitt S, Daubert JP, Zareba W, Moss AJ. Left ventricular lead position and clinical outcome in the multicenter automatic defibrillator implantation trial‐cardiac resynchronization therapy (MADIT‐CRT) trial. Circulation 2011; 123: 1159–1166. [DOI] [PubMed] [Google Scholar]
- 13. van der Bijl P, Kostyukevich MV, Khidir M, Ajmone Marsan N, Delgado V, Bax JJ. Left ventricular remodelling and change in left ventricular global longitudinal strain after cardiac resynchronization therapy: prognostic implications. Eur Heart J Cardiovasc Imaging 2019; 20: 1112–1119. [DOI] [PubMed] [Google Scholar]
- 14. Bleeker GB, Mollema SA, Holman ER, Van de Veire N, Ypenburg C, Boersma E, van der Wall EE, Schalij MJ, Bax JJ. Left ventricular resynchronization is mandatory for response to cardiac resynchronization therapy: analysis in patients with echocardiographic evidence of left ventricular dyssynchrony at baseline. Circulation 2007; 116: 1440–1448. [DOI] [PubMed] [Google Scholar]
- 15. Stankovic I, Prinz C, Ciarka A, Daraban AM, Kotrc M, Aarones M, Szulik M, Winter S, Belmans A, Neskovic AN, Kukulski T, Aakhus S, Willems R, Fehske W, Penicka M, Faber L, Voigt JU. Relationship of visually assessed apical rocking and septal flash to response and long‐term survival following cardiac resynchronization therapy (PREDICT‐CRT). Eur Heart J Cardiovasc Imaging 2016; 17: 262–269. [DOI] [PubMed] [Google Scholar]
- 16. Wang CL, Wu CT, Yeh YH, Wu LS, Chang CJ, Ho WJ, Hsu LA, Luqman N, Kuo CT. Recoordination rather than resynchronization predicts reverse remodeling after cardiac resynchronization therapy. J Am Soc Echocardiogr 2010; 23: 611–620. [DOI] [PubMed] [Google Scholar]
- 17. Reant P, Dijos M, Donal E, Mignot A, Ritter P, Bordachar P, Dos Santos P, Leclercq C, Roudaut R, Habib G, Lafitte S. Systolic time intervals as simple echocardiographic parameters of left ventricular systolic performance: correlation with ejection fraction and longitudinal two‐dimensional strain. Eur J Echocardiogr 2010; 11: 834–844. [DOI] [PubMed] [Google Scholar]
- 18. Cazeau S, Toulemont M, Ritter P, Reygner J. Statistical ranking of electromechanical dyssynchrony parameters for CRT. Open Heart 2019; 6: e000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wikstrom G, Blomström‐Lundqvist C, Andren B, Lönnerholm S, Blomström P, Freemantle N, Remp T, Cleland JG, CARE‐HF study investigators . The effects of aetiology on outcome in patients treated with cardiac resynchronization therapy in the CARE‐HF trial. Eur Heart J 2009; 30: 782–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barsheshet A, Goldenberg I, Moss AJ, Eldar M, Huang DT, McNitt S, Klein HU, Hall WJ, Brown MW, Goldberger JJ, Goldstein RE, Schuger C, Zareba W, Daubert JP. Response to preventive cardiac resynchronization therapy in patients with ischaemic and nonischaemic cardiomyopathy in MADIT‐CRT. Eur Heart J 2011; 32: 1622–1630. [DOI] [PubMed] [Google Scholar]


