Abstract
Aims
The aims of the study are to assess the levels of coronary sinus (CS) miRNAs of systolic heart failure (HF) patients in samples obtained during cardiac resynchronization therapy (CRT) device implantation and compare them to the peripheral systemic venous miRNA expression.
Methods and Results
The cardiac specific miRNA levels were assessed in 60 patients, 39 HF patients with reduced ejection fraction and 21 control patients. The levels of four cardiac specified miRNAs (miR‐21‐5p, miR‐92b‐3p, miR‐125b‐5p, and miR‐133a‐3p) were compared between the peripheral samples of HF and controls and between peripheral venous in CS in the HF groups. Compared with controls, HF patients had higher peripheral serum venous levels of miR‐125b‐5p and miR‐133‐3p. In the HF group, the levels of expression were higher for miR‐125b‐5p and lower for miR‐92, and miR‐21‐5p in the CS, compared with the peripheral venous circulation.
Conclusions
The differences in miRNA expressions in CS compared with those in the periphery suggest that changes that may occur at the levels of the myocardial tissue in HF may be more relevant to our understanding of the biological linkage between miRNA expression and HF, than the traditional analysis of systemic serum miRNA expression.
Keywords: Coronary Sinus, miRNA, Systolic heart failure (HF), Cardiac resynchronization therapy
1. Introduction
MicroRNAs (miRNAs) are small, noncoding RNA molecules, which regulate genes in various pathophysiological processes by inhibiting the translational processing or mediating the degradation of target RNAs.1, 2, 3 Several miRNAs were associated with systolic heart failure (HF) pathophysiology.4 In fact, previous studies, including ours, reported specific changes of miRNA expression associated with HF disease in the peripheral serum of patients with HF.5, 6, 7, 8, 9, 10, 11 While the majority of the studies assessed peripheral systemic serum miRNA expression levels, fewer studies reported the potential differences between the peripheral and coronary circulation of miRNA expression in patients with several cardiac conditions, including HF.12, 13, 14, 15, 16
Identifying the origin of differentially expressed miRNAs will further improve our understanding of their involvement in HF pathophysiology. Moreover, some changes in miRNA expression of a failing heart may not be reflected in peripheral circulation. Therefore, the objective of this study is to compare cardiac venous miRNA expression levels with those of the systemic peripheral venous circulation, reflecting the net absorption and release of the failing myocardium to the systemic miRNA expression. Indeed, miRNA levels were shown to be altered in HF.5, 6, 7, 9, 10 In this study, we investigated miR‐21‐5p, miR‐92b‐3p, miR‐133a‐3p, and miR‐125b‐5p, which were shown to be involved in the pathophysiological processes of HF, including cardiotoxicity, cardiomyocyte apoptosis, hypertrophy, and inflammation.17, 18, 19, 20
Accordingly, in the current study, we assessed the levels of miRNAs of systolic HF patients in the coronary sinus (CS), the ultimate representative of the coronary blood venous circulation, using samples obtained during cardiac resynchronization therapy (CRT) implantation and compared them with the peripheral systemic venous miRNA expression in the same HF patients. The novelty of this study is that the CRT implantation designated catheter procedures enabled us to obtain CS samples from the more10 distal parts the CS,21 avoiding potential contamination with systemic venous blood returning to the right atrium.
The aim of this study is to assess differences between miRNA expression in blood directly secreted from a failing heart and peripheral blood samples, routinely used in HF research, using four HF‐related miRNAs as examples. Acknowledging such differences will further improve our understanding of miRNAs' role in HF pathophysiology.
2. Materials and Methods
2.1. Patients
The study was approved by the Helsinki Ethics Committee of Baruch Padeh Medical Center, Poriya, and signed informed consent was obtained from all study participants. In total, 60 patients were recruited from the Cardiovascular Department of Baruch Padeh Medical Center, Poriya in Tiberias, Israel, consisting of 39 heart failure patients diagnosed with class C reduced ejection fraction, fulfilling the inclusion criteria for the study, which was determined for their eligibility for CRT implantation, as determined by the European Society of Cardiology–Heart Failure Association and American College of Cardiology/American Heart Association HF guidelines.22, 23 A control group was composed of 21 patients whose inclusion criteria were fulfilled as they were referred by their treating cardiologist for an elective coronary angiogram for suspected coronary artery disease (CAD).
Exclusion criteria for both the HF and the control groups were active malignancy in the last 5 years, renal failure undergoing dialysis, ≤18 years of age, pregnancy, and acute coronary syndrome diagnosis <1 month prior to the study. For the control group, specifically, participants did not have HF symptoms and/or reduced left ventricular ejection fraction (>55%). The control group was matched for age, sex, and clinical background of known CAD risk factors.
2.2. Sample collection
Blood samples were obtained early in the morning for all patients participating in the study. All HF patients continued with their medications to maintain hemodynamic indices. For the HF group, blood samples were simultaneously obtained from deep CS venous circulation and the peripheral vein. Specifically, blood samples were obtained using a CS sheath, cannulated at least 4–7 cm deep within the middistal CS, as confirmed by fluoroscopy and contrast injection, during left ventricular electrode placement as part of CRT device implantation. Peripheral blood was collected from the antecubital fossa prior to saline infusion during the catheterization procedure. It is important to note that no Heparin was given to either the HF or control groups prior to blood collection.
The blood was collected into serum‐separating tubes, stored at room temperature and processed within 10 h. The tubes were centrifuged at 2000 g for 15 min. Serum was aliquoted into Eppendorf tubes and kept at −80°C until analysis.
2.3. RNA extraction
RNA was extracted from 1 mL of whole serum, using the miRNeasy Serum/Plasma Kit (QIAGEN, catalogue number: 217184). The manufacture's protocol, originally suited for 200 μL serum, was adjusted to 1 mL of serum. For this adjusted protocol, each sample was divided into five tubes of 200 μL each, and the content of all five tubes was loaded into the same column. RNA elution from the column was done using 17 μL of RNase free water. miRNA concentration was measured using the QubitTM microRNA Assay Kit (Invitrogen, catalog number: 1810946).
2.4. cDNA synthesis
cDNA was synthesized using the TaqMan® Advanced miRNA cDNA Synthesis Kit (Applied Biosystems, catalog number A28007), using 2 μL for each RNA sample, as recommended in the protocol. The miR‐Amp reaction products from the cDNA synthesis process were diluted 1:10 with a diluted 1:10 TE buffer solution, containing 1 mM Tris‐HCl and 0.1 mM disodium EDTA. To obtain the diluted TE buffer solution, standard TE buffer solution, with a pH of 8.0 (SIGMA‐ALDRICH, catalog number: 93283), was diluted with RNase free water (Biological Industries, catalog number: 01‐866‐1A).
2.5. Real‐time polymerase chain reaction
For the real‐time polymerase chain reaction experiment, TaqMan® Advanced miRNA Assays were used. For each real‐time polymerase chain reaction, 5 μL of diluted cDNA were used. Specific probes for the miRNAs of interest were obtained from Applied Biosystems (Thermo Fisher Scientific, catalog number: A25576), including, hsa‐miR‐21‐5p, hsa‐miR‐92b‐3p, hsa‐miR‐125b‐5p, and hsa‐miR‐133a‐3p. Reactions were carried out using the CFX‐Connect Real‐Time System (BIO‐RAD), with the following protocol: 95°C for 20 s; 40 repeats on: 95°C for 3 s, 60°C for 30 s, 65°C for 5 s, and 95°C for 50 s.
As a reference, panel miR‐16‐5p and miR‐1260a‐5p were used. Although Marques et al. have found miR‐16‐5p to be unstable in the serum of HF patients,16 others have found both of these miRNA molecules to be reliable as a reference and stable in serum samples of cardiac patients.24, 25 In agreement with these studies, we found that both miRNAs are stable in HF patients and control serum samples (Appendix 1).
Real‐time polymerase chain reactions were done in triplicates and data analysis was performed using the ΔΔCt method.
2.6. Statistical analysis
All statistical tests were carried out using MedCalc® software, version 18.5 (MedCalc Software, Mariakerke, Belgium). For comparing the HF group with a control group and the peripheral serum with the CS serum in HF patients, for each miRNA tested, the Mann–Whitney test for independent samples was performed. Peripheral and CS samples of each HF patient were analysed using the Wilcoxon test for paired samples. The relationships between expression levels of different miRNAs and clinical parameters were examined using Rank correlation tests. P values for comparing clinical characteristics of HF and control groups were obtained from independent samples t‐tests. A P < 0.05 was considered statistically significant.
3. Results
In total, 39 HF patients and 21 control patients who were referred by their treating cardiologist for an elective coronary angiogram for suspected CAD were recruited for this study. Clinical characteristics of the HF and control groups are shown in Table 1. The two groups showed similar characteristics and clinical backgrounds. The percentages of patients with stable CAD were similar in the HF and control groups. Overall, 99 serum samples were collected. Peripheral and CS blood samples were obtained from each HF patient, and a peripheral blood sample was obtained from each control patient.
Table 1.
Characteristics of HF and control groups
| Variable | HF group (n = 39) | Control group (n = 21) | P value |
|---|---|---|---|
| Age (years) | 69.5 ± 15.5 | 69.5 ± 13.5 | 0.1886 |
| Body mass index | 35 ± 15 | 37.5 ± 13.5 | 0.1954 |
| Ejection fraction (%) | 30 ± 10 | 65 ± 5 | <0.0001 |
| Sex (male) n (%) | 25 (64%) | 13 (62%) | 0.8690 |
| Hypertension n (%) | 30 (77%) | 16 (76%) | 0.9500 |
| Diabetes mellitus n (%) | 22 (56%) | 10 (48%) | 0.5232 |
| Hyperlipidemia n (%) | 31 (79.5%) | 18 (86%) | 0.5599 |
| Ischemic heart disease n (%) | 26 (67%) | 11 (52%) | 0.2855 |
| Atrial fibrillation n (%) | 9 (23%) | 1 (5%) | 0.0714 |
| Chronic renal failure n (%) | 9 (23%) | 3 (14%) | 0.4742 |
| Smoking n (%) | 5 (13%) | 2 (10%) | 0.7102 |
| ACEI/ARB n (%) | 70 (77%) | 14 (67%) | 0.4001 |
| Beta‐blockers n (%) | 34 (87%) | 13 (62%) | 0.0233 |
| Statins n (%) | 28 (72%) | 19 (90%) | 0.097 |
| Anti‐platelet therapy n (%) | 29 (74%) | 19 (90%) | 0.1413 |
| MRA n (%) | 17 (44%) | 0 | — |
ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blockers; HF, heart failure; MRA, mineralocorticoid receptor antagonists.
Clinical background and treatment of patients in the HF and the control groups. Data presented as mean ± SD or n and percentage (%). Patients referred for an elective coronary angiogram for suspected coronary artery disease.
3.1. Expression of miRNA levels
We found significant differences in the expression levels of four miRNAs in different serum samples, including miR‐125b‐5p, miR‐92‐3p, miR‐21‐5p, and miR‐133a‐3p. In the peripheral serum, miR‐125b‐5p was detected to be 1.7‐folds higher in HF patients compared with the control group (P = 0.01), as shown in Figure 1 A. In HF patients, in a paired sample analysis, miR‐125b‐5p levels were significantly higher in the serum of CS, compared with peripheral serum, with a 1.8‐fold increase (P = 0.001) (Figure 1 B). A subgroup of 11 HF patients demonstrated relatively high expression of miR‐125b‐5p in CS samples. These patients also have higher miR‐125 expression in peripheral samples than rest of the HF patients (median of 2.79 vs. 1.28, P = 0.0059). We could not identify any other special characteristics for this subgroup. No significant differences in age, gender, or ejection fraction percentage were identified for this subgroup versus the rest of the HF patients.
Figure 1.
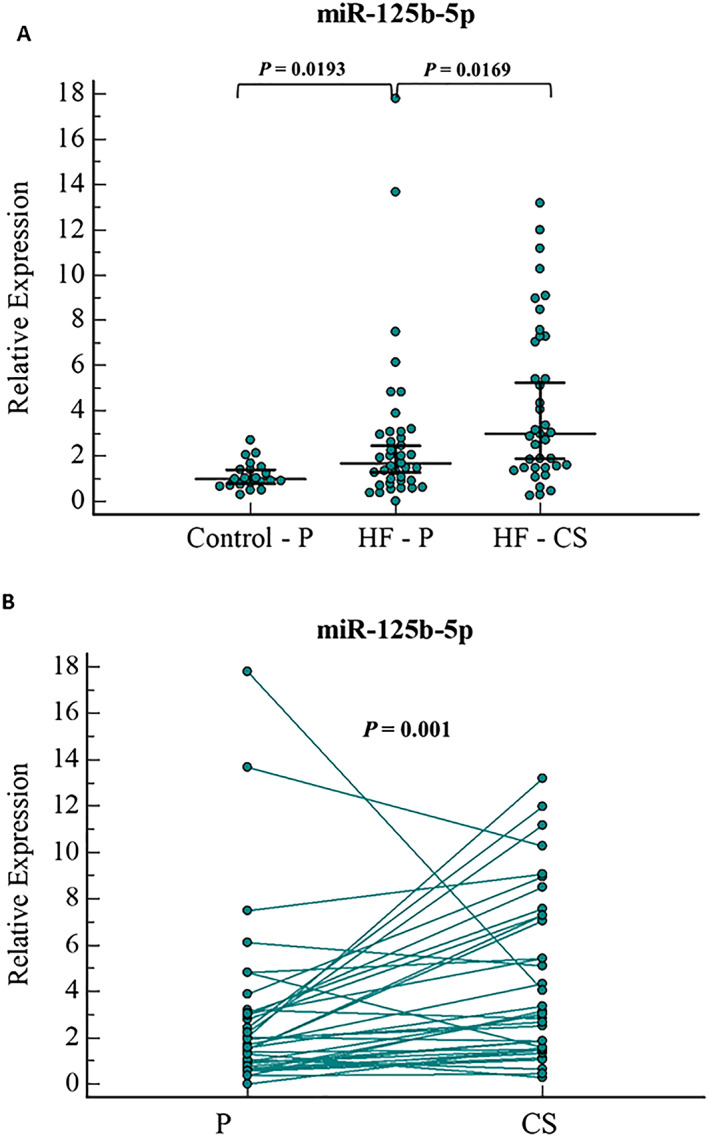
miR‐125b‐5p relative expression levels in (A) serum samples of HF(peripheral and CS samples) and control patients (peripheral samples), and (B)paired sample analysis of peripheral and CS serum samples of HF patients. Error bars indicate 95% Confidence Interval for the median. HF, heart failure; P, peripheral serum; CS, Coronary Sinus serum.
In peripheral serum, miR‐92b‐3p did not show a significant difference between HF and control patients (Figure 2 A). In HF patients, paired sample analysis for miR‐92b‐3p showed reduced expression in CS compared with peripheral serum, with 1.4‐fold lower levels in the CS than in the peripheral serum (P = 0.002) (Figure 2 B).
Figure 2.
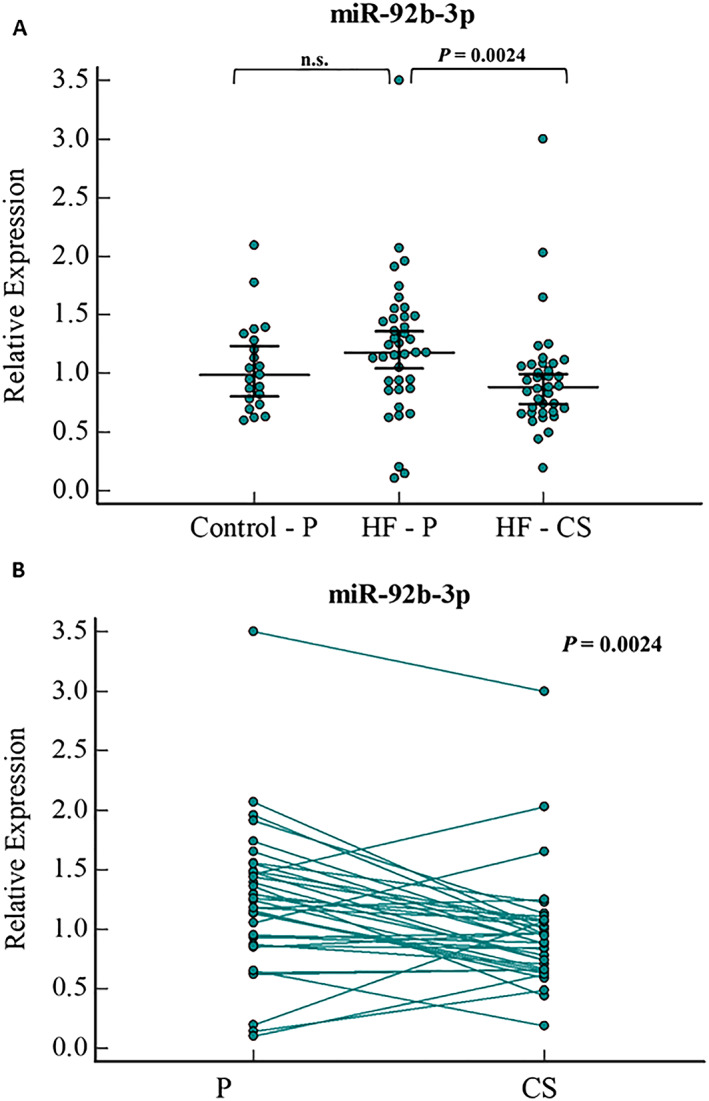
miR‐92b‐3p relative expression levels in (A) serum samples of HF (peripheral and CS samples) and control patients (peripheral samples),and (B) paired sample analysis of peripheral and CS serum samples of HF patients. Error bars indicate 95% Confidence Interval for the median. HF, heart failure; P, peripheral serum; CS, coronary sinus serum; n.s., not significant.
A comparison between HF and control patients did not show a significant difference in miR‐21‐5p in the peripheral serum (Figure 3 A). A paired sample analysis for each patient showed that miR‐21‐5p expression in the CS was significantly lower than in the peripheral serum samples, with a 1.2‐fold lower level (P = 0.03) (Figure 3 B).
Figure 3.
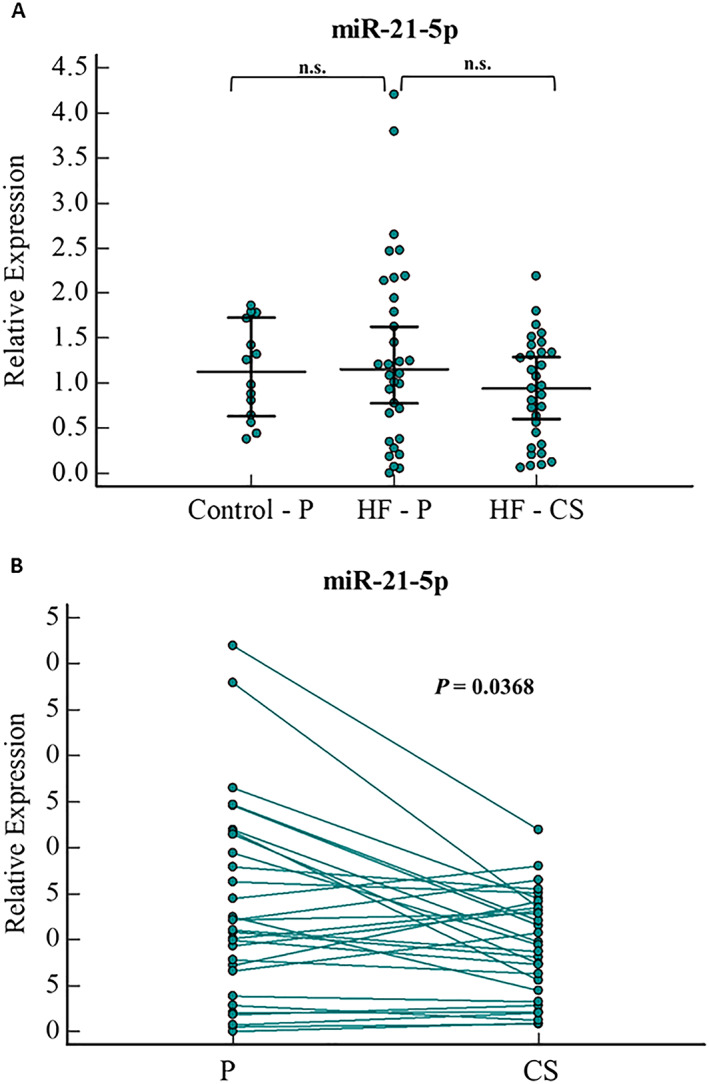
miR‐21‐5p relative expression levels in (A) serum samples of HF(peripheral and CS samples) and control patients (peripheral samples), and (B)paired samples analysis of peripheral and CS serum sample of HF patients. Error bars indicate 95% Confidence Interval for the median. HF, heart failure; P, peripheral serum; CS, Coronary Sinus serum; n.s., not significant.
In the peripheral serum, miR‐133a‐3p was found to be significantly higher in HF patients compared with the controls, with a 1.4‐fold increase (P = 0.02) (Figure 4 ). However, paired sample analysis showed no significant difference between the CS and peripheral serum samples of HF patients (Appendix 1).
Figure 4.
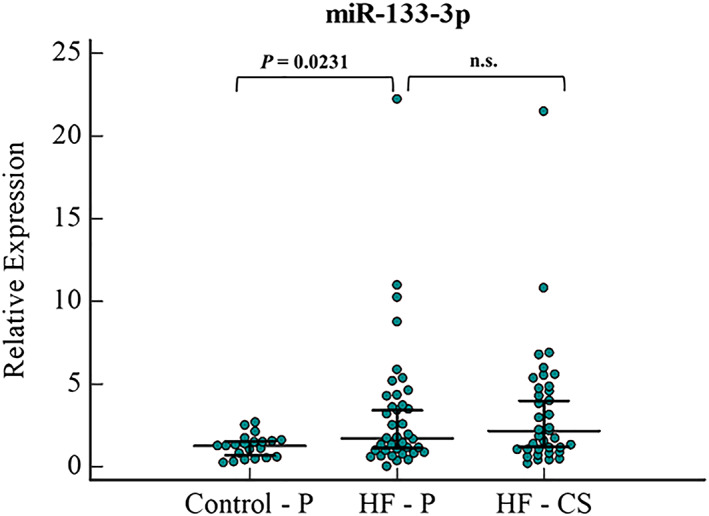
miR‐133a‐3p relative expression levels in serum samples of HF (peripheral and CS samples) and control patients (peripheral samples). Error bars indicate 95% Confidence Interval for the median. HF, heart failure; P, peripheral serum; CS, coronary sinus serum; n.s., not significant.
4. Discussion
The main finding of this study is that HF patients diagnosed with class C reduced ejection fraction have significant differences in the expression of their venous CS miRNAs, as compared with their peripheral venous serum. Specifically, higher levels of miR‐125b‐5p and lower levels of miR‐92 and miR‐21‐5p were documented in the CS, compared with the peripheral venous circulation.
The miRNAs investigated in this study are involved in pathophysiological processes that are known to occur in HF. Indeed, miR‐125b‐5p17 and miR‐133a‐3p18 have been shown to suppress proapoptotic pathways in the cardiomyocytes, and miR‐92b‐3p contributes to cardiac hypertrophic growth,19 whereas miR‐21‐5p is associated with cardiotoxicity and inflammatory cell infiltration in the cardiac injury model.20 Although miRNAs are powerful regulators of protein translation, contributing to the pathogenesis of HF, their cardiac origin and nature remain unclear.6, 7, 9 The novelty of this study is that we tested expression levels of selected miRNAs in venous blood taken directly from the deep part of the CS, for example, directly sampling the myocardial venous circulation and comparing them with systemic peripheral venous blood. This comparison provided us with a unique glimpse into the molecular environment of the failing heart.
We and others have demonstrated alteration of miRNA expression in peripheral venous serum of HF patients.5, 6, 7, 8, 9, 10, 11 Indeed, in this study, we found significant differences in the peripheral venous serum levels of miRNA expression in HF patients compared with controls, with higher expression of miR‐125b‐5p and miR‐133‐3p in the peripheral serum of HF patients.
Interestingly, several previous studies focused on the differences between the coronary and systemic peripheral miRNA expression in HF patients. Notably, most of these studies focused on the differences in miRNA expression comparing CS versus peripheral arterial serum hence termed ‘transcoronary gradient’.14, 15, 16 It is however, important to note that there are known significant differences between arterial and venous miRNA expression per se, which may be related to the local physical differences of the arterial and venous blood, even in the same patients.15, 26 Therefore, it may be more appropriate to compare the venous CS blood directly with peripheral venous blood, rather than with arterial blood samples of miRNA expression, as we did in the current study.
Similar to our study, only one previous small study by Goldraich et al. compared venous myocardial miRNA expression directly with peripheral venous blood in HF patients.15 In contrast to this study of Goldraich et al.15 we found that the expression of miR‐133a 3p in the venous periphery of HF patients was not significantly different than the CS expression levels. However, several important differences should be noted in comparison with this previous study. First, we had a significantly larger patient cohort. Secondly, in contrast to the traditional healthy control group comparison, we deliberately chose a more compatible relevant control group regarding the prevalence of CAD and its related comorbidities. By doing so, we could specifically focus on the impact of systolic HF on miRNA expression per se, as it was the key difference between the two groups. Another important difference from most previous studies, which assessed the CS miRNA expression, was that we obtained the CS venous sample via specified CS catheterization equipment rather than using the routine right heart hemodynamic catheter, which was used for example in the study of Goldraich et al.15 This procedure allowed us to obtain ‘pure’ unmixed CS samples, a true representation of the venous myocardial circulation. Altogether, to the best of our knowledge, our study is the largest and the first to specifically compare cardiac miRNAs in the coronary venous circulation with the systemic peripheral venous circulation in HF patients.
The mechanism by which myocardial venous miRNA expression may differ from the peripheral systemic venous circulation in the same HF patient is intriguing and is beyond the scope of our current study goals. While specific tissue cells were suggested in the miRNA degradation process, it is not clear whether myocardial cells are involved in miRNA degradation.27 It was suggested that alteration in miRNA expression in HF disease occurs because of the absorption/release of miRNAs.17 Our findings that lower levels of miR‐92 and miR‐21‐5p suggest that they are absorbed into the myocardial tissue, and higher levels of miR‐125b‐5p may suggest that they are released from the myocardium in HF.
Indeed, Widmer et al.28 assessed CS–aorta miRNA expression gradients in patients with early atherosclerosis and coronary endothelial dysfunction, suggesting that these miRNA are released into the coronary circulation during myocardial injury.12 Moreover, cardiac intracellular transfer of miRNA was also reported.29 Whatever the mechanism for the local myocardial changes in miRNA expression, it may pose a key role in the pathophysiological process occurring in HF.30
In our study, the expression levels of miR‐125b‐5p were almost twice as high in the CS than in the peripheral venous circulation. Interestingly, a previous study identified miR‐125b‐5p as one of the five most abundant miRNAs in pericardial fluid of HF patients,31 while it was not detected in other body fluids,32 further supporting our finding that miR‐125b‐5p is secreted from a failing heart. miR‐125b‐5p is associated with several inflammatory cytokines and most notably with the proinflammatory mediators, including tumour necrosis factor alpha, interleukin‐6 and interleukin‐1β and the NF‐κB pathway, suggesting that elevated miR‐125b expression promotes inflammation by the activation of the NF‐κB pathway.33
The current limitation is that the study was conducted in a single centre with a small sample size. Nevertheless, our sample is larger than those of most previous studies, and we investigated different miRNAs that are related to the pathophysiological processes of HF. However, it is possible that other miRNAs, which were not investigated in this study, are involved in HF pathophysiological processes. In addition, because of technical and ethical considerations, we did not obtain deep CS samples from the controls, and therefore, we cannot determine whether the observed changes in miRNA expressions in the CS versus peripheral blood are related to the occurrence of HF or to the origin of the miRNAs.
5. Conclusions
In conclusion, we identified miR‐125b‐5p and miR‐133a‐3p to be expressed differently in HF patients compared with the control group and found significant differences in the expression levels of miR‐125b‐5p, miR‐92b‐3p, and miR‐21‐5p in the CS compared with peripheral serum samples. Changes in miRNA expression in CS samples may suggest that they originate from or are absorbed by the cardiac tissue. Further studies are needed in order to determine the exact cells that secrete these molecules.
Future studies should address whether the changes in miRNA expression contribute to the initiation, progression, and potential treatments of HF. Targeting miRNAs that are involved in the pathophysiological process of HF may allow for the development of a therapeutic strategy for HF.
Conflict of interest
Inbar Ben‐Zvi, Natalia Volinsky, Liza Grosman‐Rimon, Izhak Haviv, Guy Rozen, Nizar Andria, Nofar Asulin, Nufar Margalit, Ibrahim Marai, and Offer Amir declare that they have no conflict of interest.
Appendix 1 1.
Figure A1.
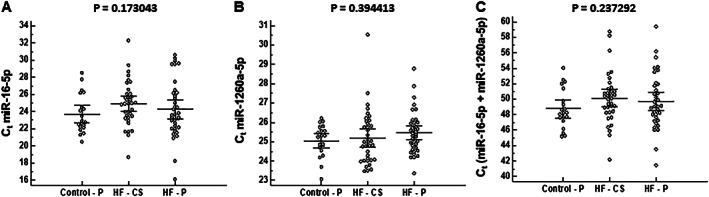
Expression levels of miR‐16‐5p (A), miR‐1260a‐5p (B) and sum of miR‐16‐5p+miR‐1260a‐5p (C) are presented as cycle threshold (Ct) values for control peripheral, HF peripheral and HF CS samples. Kruskal–Wallis test was performed to evaluate differences between three groups of samples.
Ben‐Zvi, I. , Volinsky, N. , Grosman‐Rimon, L. , Haviv, I. , Rozen, G. , Andria, N. , Asulin, N. , Margalit, N. , Marai, I. , and Amir, O. (2020) Cardiac‐peripheral transvenous gradients of microRNA expression in systolic heart failure patients. ESC Heart Failure, 7: 835–843. 10.1002/ehf2.12597.
References
- 1. Small EM, Olson EN. Pervasive roles of microRNAs in cardiovascular biology. Nature 2011; 469: 336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004; 116: 281–297. [DOI] [PubMed] [Google Scholar]
- 3. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009; 136: 215–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Romaine SP, Tomaszewski M, Condorelli G, Samani NJ. MicroRNAs in cardiovascular disease: an introduction for clinicians. Heart 2015; 101: 921–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barwari T, Joshi A, Mayr M. MicroRNAs in cardiovascular disease. J Am Coll Cardiol 2016; 68: 2577–2584. [DOI] [PubMed] [Google Scholar]
- 6. Deng J, Zhong Q. Advanced research on the microRNA mechanism in heart failure. Int J Cardiol 2016; 220: 61–64. [DOI] [PubMed] [Google Scholar]
- 7. Goren Y, Kushnir M, Zafrir B, Tabak S, Lewis BS, Amir O. Serum levels of microRNAs in patients with heart failure. Eur J Heart Fail 2012; 14: 147–154. [DOI] [PubMed] [Google Scholar]
- 8. Gupta SK, Foinquinos A, Thum S, Remke J, Zimmer K, Bauters C, de Groote P, Boon RA, de Windt LJ, Preissl S, Hein L, Batkai S, Pinet F, Thum T. Preclinical development of a microRNA‐based therapy for elderly patients with myocardial infarction. J Am Coll Cardiol 2016; 68: 1557–1571. [DOI] [PubMed] [Google Scholar]
- 9. Schulte C, Karakas M, Zeller T. microRNAs in cardiovascular disease—clinical application. Clin Chem Lab Med 2017; 55: 687–704. [DOI] [PubMed] [Google Scholar]
- 10. Vegter EL, van der Meer P, de Windt LJ, Pinto YM, Voors AA. MicroRNAs in heart failure: from biomarker to target for therapy. Eur J Heart Fail 2016; 18: 457–468. [DOI] [PubMed] [Google Scholar]
- 11. Huang ZP, Wang DZ. miR‐22 in cardiac remodeling and disease. Trends Cardiovasc Med 2014; 24: 267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. De Rosa S, Fichtlscherer S, Lehmann R, Assmus B, Dimmeler S, Zeiher AM. Transcoronary concentration gradients of circulating microRNAs. Circulation 2011; 124: 1936–1944. [DOI] [PubMed] [Google Scholar]
- 13. Xu G, Cui Y, Jia Z, Yue Y, Yang S. The values of coronary circulating miRNAs in patients with atrial fibrillation. PLoS ONE 2016; 11: e0166235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. De Rosa S, Eposito F, Carella C, Strangio A, Ammirati G, Sabatino J, Abbate FG, Iaconetti C, Liguori V, Pergola V, Polimeni A, Coletta S, Gareri C, Trimarco B, Stabile G, Curcio A, Indolfi C, Rapacciuolo A. Transcoronary concentration gradients of circulating microRNAs in heart failure. Eur J Heart Fail 2018; 20: 1000–1010. [DOI] [PubMed] [Google Scholar]
- 15. Goldraich LA, Martinelli NC, Matte U, Cohen C, Andrades M, Pimentel M, Biolo A, Clausell N, Rohde LE. Transcoronary gradient of plasma microRNA 423‐5p in heart failure: evidence of altered myocardial expression. Biomarkers: biochem indicat expo, response, susceptibility chem 2014; 19: 135–141. [DOI] [PubMed] [Google Scholar]
- 16. Marques FZ, Vizi D, Khammy O, Mariani JA, Kaye DM. The transcardiac gradient of cardio‐microRNAs in the failing heart. Eur J Heart Fail 2016; 18: 1000–1008. [DOI] [PubMed] [Google Scholar]
- 17. Bayoumi AS, Park KM, Wang Y, Teoh JP, Aonuma T, Tang Y, Su H, Weintraub NL, Kim IM. A carvedilol‐responsive microRNA, miR‐125b‐5p protects the heart from acute myocardial infarction by repressing pro‐apoptotic bak1 and klf13 in cardiomyocytes. J Mol Cell Cardiol 2018; 114: 72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li M, Ding W, Tariq MA, Chang W, Zhang X, Xu W, Hou L, Wang Y, Wang J. A circular transcript of ncx1 gene mediates ischemic myocardial injury by targeting miR‐133a‐3p. Theranostics 2018; 8: 5855–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hu ZQ, Luo JF, Yu XJ, Zhu JN, Huang L, Yang J, Fu YH, Li T, Xue YM, Feng YQ, Shan ZX. Targeting myocyte‐specific enhancer factor 2D contributes to the suppression of cardiac hypertrophic growth by miR‐92b‐3p in mice. Oncotarget 2017; 8: 92079–92089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gryshkova V, Fleming A, McGhan P, De Ron P, Fleurance R, Valentin JP, Nogueira da Costa A. miR‐21‐5p as a potential biomarker of inflammatory infiltration in the heart upon acute drug‐induced cardiac injury in rats. Toxicol Lett 2018; 286: 31–38. [DOI] [PubMed] [Google Scholar]
- 21. Morgan JM, Delgado V. Lead positioning for cardiac resynchronization therapy: techniques and priorities. Europace: Eur pacing, arrhythmias, card electrophysiol: j work groups card pacing, arrhythmias, card cell electrophysiol Eur Soc Cardio 2009; 11: v22–v28. [DOI] [PubMed] [Google Scholar]
- 22. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 23. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA Guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017; 70: 776–803. [DOI] [PubMed] [Google Scholar]
- 24. Kok MG, Halliani A, Moerland PD, Meijers JC, Creemers EE, Pinto‐Sietsma SJ. Normalization panels for the reliable quantification of circulating microRNAs by RT‐qPCR. FASEB J 2015; 29: 3853–3862. [DOI] [PubMed] [Google Scholar]
- 25. Kroh EM, Parkin RK, Mitchell PS, Tewari M. Analysis of circulating microRNA biomarkers in plasma and serum using quantitative reverse transcription‐PCR (qRT‐PCR). Methods (San Diego, Calif) 2010; 50: 298–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xu W, Zhou Y, Xu G, Geng B, Cui Q. Transcriptome analysis reveals non‐identical microRNA profiles between arterial and venous plasma. Oncotarget 2017; 8: 28471–28480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang Z, Qin YW, Brewer G, Jing Q. MicroRNA degradation and turnover: regulating the regulators. Wiley interdisciplinary reviews RNA 2012; 3: 593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Widmer RJ, Chung WY, Herrmann J, Jordan KL, Lerman LO, Lerman A. The association between circulating microRNA levels and coronary endothelial function. PLoS ONE 2014; 9: e109650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ottaviani L, Sansonetti M, da Costa Martins PA. Myocardial cell‐to‐cell communication via microRNAs. Non‐coding RNA res 2018; 3: 144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thum T. Noncoding RNAs and myocardial fibrosis. Nat Rev Cardiol 2014; 11: 655–663. [DOI] [PubMed] [Google Scholar]
- 31. Kuosmanen SM, Hartikainen J, Hippelainen M, Kokki H, Levonen AL, Tavi P. MicroRNA profiling of pericardial fluid samples from patients with heart failure. PLoS ONE 2015; 10: e0119646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Weber JA, Baxter DH, Zhang S, Huang DY, Huang KH, Lee MJ, Galas DJ, Wang K. The microRNA spectrum in 12 body fluids. Clin Chem 2010; 56: 1733–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang B, Wang LS, Zhou YH. Elevated microRNA‐125b promotes inflammation in rheumatoid arthritis by activation of NF‐kappaB pathway. Biomed pharmacother = Biomed pharmacother 2017; 93: 1151–1157. [DOI] [PubMed] [Google Scholar]


