Abstract
Although preconditioning strategies are growing areas of interest for therapies targeting intervertebral discs (IVDs), it is unknown whether the Wnt signals previously implicated in chondrogenesis, Wnt3A, Wnt5A, and Wnt11, play key roles in the promotion of human nucleus pulposus (NP) cell redifferentiation. In this study, NP cells isolated from herniated disc patients were transduced with lentiviral vectors to overexpress the WNT3A, WNT5A, or WNT11 genes, or CRISPR associated protein 9 (Cas9)/single guide RNA (sgRNA) vectors to knock out these genes. Following expansion, transduced NP cells were induced for redifferentiation toward the NP phenotype. The overexpression of specific WNT factors led to increases in both glycosaminoglycan (GAG) deposition and expression of redifferentiation genes. These effects were attenuated by knockout of the same WNT genes. These results indicate that specific WNT signals can regulate the expression of redifferentiation genes, unequally impact GAG deposition, and contribute to the redifferentiation of human NP cells.
Keywords: Nucleus pulposus, Wnt signaling, Intervertebral disc, Cell proliferation, Redifferentiation, Lentivirus, CRISPR-Cas9
Introduction
Located inside the intervertebral disc (IVD), nucleus pulposus (NP) tissue provides structural support and shock absorbing capacity along the spinal column.1 NP tissue functions through a hydrated collagen and proteoglycan-rich extracellular matrix. Unfortunately, IVD herniation and degeneration are among the most prevalent causes for back pain. Interestingly, low back and neck pain represented the third-highest amount of US healthcare spending from 1996 to 2013.2 These conditions can negatively impact the properties and function of the IVD due to the loss of disc height, NP cellularity, matrix remodeling, cell dedifferentiation, and other deleterious biochemical conditions.3–5
Recently, the preconditioning of NP cells and autologous NP cell-mediated therapies have aimed to rejuvenate and replenish the depleted NP tissue following injury or degeneration.3,6,7 However, many of the molecular pathways and signals that allow for successful redifferentiation and therapeutic targeting have yet to be fully elucidated.8 Wnt (Wingless protein family) signals are one of the well-conserved pathways which have been extensively studied and implicated in tissue homeostasis,9 NP apoptosis,10 and, more specifically, in cell migration and differentiation,11 with particularly notable roles in chondrogenesis.12–14 The roles of both canonical and non-canonical Wnt signals in chondrocytic tissues have been extensively reviewed15,16 and are well-demonstrated regulators in the development and differentiation of these cells into mature tissues.
Increasing evidence indicates that Wnt/β-catenin pathway signals have been implicated in IVD degeneration9,17–19. For example, Hiyama et al. found that activation of Wnt/β-catenin signaling upregulated the tumor necrosis factor alpha (TNF-α) expression and a positive-feedback loop between Wnt and TNF-α might cause the degeneration of rat NP cells17. Furthermore, Iwata et al. demonstrated that Wnt/β-catenin signaling boosted RUNX2 (Runt-related transcription factor 2) expression in degenerated IVD and led to NP calcification in a dog model19. The abovementioned findings indicate that the Wnt/β-catenin signaling pathway could be a therapeutic target for IVD degeneration. Although several studies have suggested that Wnt signals play key roles in the human disc, there are fewer studies using human derived IVDs or NP cells to fully support such hypotheses, especially for those that directly compare multiple Wnt signals at once during NP cell redifferentiation. Furthermore, recently published studies have suggested that the progression of IVD disease may be related to a crosstalk between Wnt and transforming growth factor beta (TGF-β) signaling. For instance, Hiyama et al. found that activation of Wnt/β-catenin signals significantly enhanced the expression of TGF-β320. Interestingly, Hiyama et al. also reported that TGF-β signals in IVDs inhibited Wnt/β-catenin signals21. These findings suggest that TGF-β signals could serve a therapeutic role in IVD degeneration.
In the Wnt family, three typical signals, Wnt3A, Wnt5A, and Wnt11, have been shown to be involved in mesenchymal stem cell (MSC) differentiation. Wnt3A, a typical canonical Wnt signal, has been implicated in MSC proliferation, increasing differentiation potential, and suppression of differentiation.22–24 Wnt5A and Wnt11, two typical non-canonical Wnt signals, have been more extensively implicated in differentiation and cartilage development.13,25–29 Due to the identified roles that Wnt signaling pathways play in cartilage and stem cell differentiation,13,16,25 as well as the inherent similarities and shared properties between the chondrogenic differentiation of chondrocytic tissues,30 such as those derived from the synovium (synovium derived stem cells or SDSCs) with NP cell redifferentiation,7 we hypothesize that specific Wnt signals are also significant regulators of NP cell redifferentiation in humans. We expect that non-canonical signals, Wnt5A and Wnt11, will each exhibit a more dramatic effect on NP cell redifferentiation than canonical Wnt3A.
METHODS
Cloning of overexpression and knockout vectors
We used VSV-G pseudotyped lentiviral vectors in this study, which were approved by the Institutional Biosafety Committee (IBC) (protocol # 17-10-05). To construct overexpression (OE) vectors, the open reading frame sequences of human WNT3A, WNT5A, and WNT11 genes or control green fluorescence protein (GFP) were cloned into a lentiviral vector backbone Lenti SFFV-X-Wpre by Gibson Assembly® from New England BioLabs Inc. (NEB, Ipswich, MA). WNT3A, WNT5A, and WNT11 cDNAs were purchased from DNASU Plasmid Repository (Tempe, AZ). To knock out human WNT3A, WNT5A, and WNT11, we cloned the spacer sequence of single-guide RNA (sgRNA) into backbone Lenti-U6-sgRNA-SFFV-Cas9-E2A-Puro, in which the U6 promoter drove the expression of sgRNA and the spleen focus-forming virus (SFFV) promoter drove the expression of both CRISPR associated protein 9 (Cas9) and puromycin-resistant gene. The spacer sequences were as follows: sgWNT3A-1, GGCCATCGCCGCGCGAG; sgWNT3A-2, GCAGCTACCCGATCTGG; sgWNT3A-3, GGCACGGCCGCCATCTG; sgWNT5A-1, GACGGCCTTCACATACG; sgWNT5A-2, GGATGCGCTCCCGCTCG; sgWNT5A-3, GCTCACCGCGTATGTGA; sgWNT11–1, GGGGGCGAGCTCAATGG; sgWNT11–2, GGCGTGGCTGATGGCGG; sgWNT11–3, GTGGCTCACCTGGGACG. The sgRNA design and vector cloning have been detailed elsewhere.31,32 All of the inserts were confirmed by Sanger sequencing. The DNA-calcium phosphate co-precipitation method was used for packaging lentiviral vectors. After 100-fold concentration by ultracentrifugation, the biological titers of vectors were determined by transducing HT1080 cells followed by quantitative real-time polymerase chain reaction (qPCR).
Cell culture and lentiviral transduction
Human adult NP cells were obtained from the NP tissue of herniated disc patients (2 male and 2 female, 32–38 years old) following patient consent and Institutional Review Board approval. Pooled cells were cultured in T-25 cell culture flasks and growth medium containing Alpha Minimum Essential Medium (αMEM), 10% fetal bovine serum (FBS), 100 U/mL penicillin, 100 μg/mL streptomycin, and 0.25 μg/mL fungizone. After reaching 40–60% confluence in cell culture flasks, cell numbers were visually estimated based upon apparent density. Lentiviral vectors, produced by previously described methods,31,33 targeting each respective WNT gene, were added at a multiplicity of infection (MOI) of two in αMEM containing 10% FBS and 1× Protamine Sulfate (PS) for a 24-hour incubation at 37°C under 21% oxygen. Viral vector-containing medium was then removed and normal growth medium was added for cell expansion. For Cas9/sgRNA vectors, an additional selection for four days was performed with puromycin-enriched (1 ug/mL) selection culture media to kill non-transduced cells and increase efficiency. GFP expression, visualized by an Invitrogen™ EVOS™ FL Auto Imaging System (Thermo Fisher Scientific, Waltham, MA) at 24, 48, and 72 h, was used as a positive control for viral transduction.
Due to a lack of puromycin selection in lentiviral vectors for overexpressed WNT genes, Western blot was used to confirm transduction efficiency. Transduced NP cells were dissolved in lysis buffer (Cell Signaling, Boston, MA) with protease inhibitors. Total protein was quantified using the BCA™ Protein Assay Kit (Thermo Fisher Scientific). Thirty micrograms of protein from each sample were denatured and separated using NuPAGE_ Novex_ Bis-Tris Mini Gels in the XCell SureLock™ Mini-Cell (Life Technologies, Carlsbad, CA) at 120 V at 4°C for 3 h. Bands were transferred onto a nitrocellulose membrane using an XCell II™ Blot module (Life Technologies) at 15 V at 4°C overnight. The membrane was incubated with primary monoclonal antibodies in 5% bovine serum albumin (BSA) in TBST buffer (10 mM Tris–HCl, pH 7.5, 150 mM NaCl, 0.05% Tween-20) for 1 h (GAPDH served as an internal control), followed by the secondary antibody of horseradish peroxidase–conjugated goat anti-mouse (Thermo Fisher Scientific) for 1 h. SuperSignal West Femto Maximum Sensitivity Substrate and CL-XPosure Film (Thermo Fisher Scientific) were used for exposure. The primary antibodies used in immunoblotting included the Wnt signaling antibody sampler kit (Cell Signaling). Wnt11 polyclonal antibody was obtained from Thermo Fisher Scientific. Each set of cell samples was run in triplicate.
Cell proliferation
To determine the proliferation index of each group, before cell expansion, NP cells were labeled with CellVue® Claret at 2×10−6 M for 5 min according to the manufacturer’s protocol (Sigma-Aldrich, St. Louis, MO). After six days of proliferation, expanded cells were collected and measured using a BD FACS LSRFORTESSA™ flow cytometer (BD Biosciences, San Jose, CA). Twenty thousand events of each sample were collected using CellQuest Pro software (BD Biosciences); cell proliferation index was analyzed using ModFit LT™ version 3.1 (Verity Software House, Topsham, ME).
Redifferentiation induction and evaluation
Expanded cells (3×105) were centrifuged at 500 g for 5 min in a 15-mL polypropylene tube to form a pellet. After overnight incubation (day 0), the pellets were cultured in a serum-free chondrogenic medium consisting of high-glucose Dulbecco’s Modified Eagle’s Medium (DMEM), 40 μg/mL proline, 100 nM dexamethasone, 100 U/mL penicillin, 100 μg/mL streptomycin, 0.1 mM L-ascorbic acid-2-phosphate, and 1×ITS™ Premix (BD Biosciences) with the supplementation of 10 ng/mL TGF-β3 (PeproTech, Inc., Rocky Hill, NJ) in a 5% O2 incubator for as long as 28 days. The pellets were evaluated for NP cell redifferentiation using biochemical analysis and qPCR.
Representative pellets (n=4) were digested at 60°C for 4 h with 125 μg/mL papain in PBE buffer (100 mM phosphate, 10 mM ethylenediaminetetraacetic acid, pH 6.5) containing 10 mM cysteine. To quantify cell density, the amount of DNA in the papain digestion was measured using the Quant-iT™ Pico Green® dsDNA assay kit (Life Technologies) with a CytoFluor® Series 4000 (Applied Biosystems, Foster City, CA). Glycosaminoglycan (GAG) was measured using dimethylmethylene blue (DMMB) dye and a Spectronic™ BioMate™ 3 Spectrophotometer (Thermo Fisher Scientific) with bovine chondroitin sulfate (Sigma-Aldrich) as a standard. All experiments were done in triplicate.
Total RNA was extracted from representative samples (n=4) using an RNase-free pestle in TRIzol® (Life Technologies). Two micrograms of mRNA were used for reverse transcriptase with a High-Capacity cDNA Archive Kit (Applied Biosystems) at 37°C for 120 min. NP cell redifferentiation related genes, type I collagen (COL1A1; Assay ID Hs00164004_m1), aggrecan (ACAN; Assay ID Hs00153936_m1), type II collagen (COL2A; Assay ID Hs00156568_m1), paired box 1 (PAX1; Assay ID Hs01071293_g1), and forkhead box F1 (FOXF1; Assay ID Hs00230962_m1) were customized by Applied Biosystems as part of their Custom TaqMan® Gene Expression Assays. Wnt signals were also evaluated using qPCR, including WNT3A (Assay ID Hs00263977_m1), WNT5A (Assay ID Hs00998537_m1), and WNT11 (Assay ID Hs00182986_m1), following cell expansion of virally transduced cells and after NP cell redifferentiation. All reactions were completed in triplicate. Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH; Assay ID Hs02758991_g1) was carried out as the endogenous control gene.34 TaqMan® real-time PCR was performed with iCycler iQ™ Multi Color Real-Time PCR Detection and calculated by computer software (Perkin-Elmer, Wellesley, MA). Relative transcript levels were calculated as χ=2−ΔΔCt, in which ΔΔCt=ΔE-ΔC, ΔE=Ctexp-CtGAPDH, and ΔC=Ctct1-CtGAPDH.
Statistical analysis
Because of the small sample size (n=4) and uncertainty about the normality of the measurement, the Mann-Whitney Test was used to determine if the 0.05 level of significance was achieved. Inferences from the Mann-Whitney Test relate to the medians of the respective distributions instead of means. All calculations were carried out using JMP/Pro13.2 software (SAS Institute, Cary, NC).
RESULTS
Lentiviral vectors effectively overexpressed WNT genes
Spinal magnetic resonance imaging (MRI) of herniated disc patients prior to surgery and tissue collection revealed Grade II (moderately severe) disc degeneration35,36. Representative MRI showed that herniated NP tissue appeared lighter in color with comparable density to the adjacent discs and maintained a reasonably distinct border between the annulus fibrosus (AF) and NP tissues (Fig. 1A). The lentiviral vectors transduced with PS supplemented in culture media to increase transduction efficiency did not cause any observable differences in cell morphology or proliferation. The successful transduction of a GFP-containing vector in NP cells was demonstrated using immunofluorescence with a greater intensity of green color in the transduced cells at the MOI of five versus one (Fig. 1B). Our qPCR results showed that both PS supplementation and GFP transduction did not result in WNT gene expression and transduction of one WNT gene did not cause a significant change in the expression levels of another WNT gene (Fig. 1C). An MOI of two was chosen for this study given the notable success in WNT gene expression in the MOI of one group and a potential reduction of cell death. A successful transduction of three WNT genes (WNT3A, WNT5A, and WNT11) was verified at a protein level using western blot in expanded cells (Fig. 1D). Overexpression of WNT genes was also tracked at an mRNA level using TaqMan® real-time PCR in subsequent chondrogenically induced pellets for up to 28 days (Fig. 1E). WNT3A-OE was the only group which expressed any detectable WNT3A mRNA at each timepoint (Fig. 1E). Similarly, WNT5A-OE induced 500–2800-fold greater WNT5A mRNA expression compared to controls during redifferentiation (Fig. 1E), and WNT11-OE expressed upward of 25,000-fold greater WNT11 mRNA (Fig. 1E) at each timepoint during redifferentiation induction in pellet culture versus GFP control.
Figure 1.

Overexpression of WNT genes during redifferentiation. Herniated discs indicated by two arrows in MRI T2 images from a representative patient (A). Following transduction, GFP-expression was visualized by immunofluorescence (B) and by qPCR (C) in cell samples. Successful transduction was demonstrated by western blot for overexpression (OE) of Wnt3A, Wnt5A, and Wnt11 (D). GAPDH served as an internal control. After pellet formation, samples were analyzed at each timepoint (Day 0, 11, and 28) of redifferentiation via qPCR for WNT3A, WNT5A, and WNT11 mRNA expression (E). GAPDH was carried out as the endogenous control gene. Data are shown as bar charts. * indicates a significant difference compared with all other groups at a specific timepoint (p<0.05).
Overexpression of WNT3A enhanced NP cell proliferation
Proliferation Index (P-I) and generational analysis of stained NP cells by flow cytometry (Fig. 2) revealed that WNT3A-OE (P-I=10.65) caused the greatest increase in proliferation versus the GFP control (P-I=6.70). WNT5A-OE (P-I=3.74) proliferated significantly less than the GFP control and WNT11-OE (P-I=7.48) was slightly elevated compared to the GFP control. Also, GFP control, WNT5A-OE, and WNT11-OE groups predominantly proliferated to the third generation, whereas WNT3A-OE produced a peak in the fourth generation.
Figure 2.

Proliferation of WNT-OE cells. Flow cytometry generational analysis of claret red proliferation for GFP-CTRL, WNT3A-OE, WNT5A-OE, and WNT11-OE after five days in culture. N≥20,000 events for each sample.
Overexpression of non-canonical WNT signals promoted NP cell redifferentiation
After viral transduction and cell expansion, all WNT-OE groups significantly decreased COL1A1 expression versus the GFP control; however, in the condensation at day 0 of redifferentiation, all WNT transduced groups exhibited a significant increase in COL1A1 expression, which later attenuated for up to 28 days (Fig. 3A). For NP cell markers, despite a lower expression in the cell expansion phase, PAX1 expression significantly increased in day 0 pellets of all WNT-OE cells but not in the subsequent chondrogenic induced pellets except the WNT11-OE group at day 11 (Fig. 3B). Other marker genes, such as FOXF1 (Fig. 3C) and COL2A1 (Fig. 3D), were detected with a significant increase in both WNT5A-OE and WNT11-OE groups at day 11 but not at day 28. Interestingly, the WNT11-OE group also exhibited a significant increase of FOXF1 and COL2A1 in day 0 pellets (Fig. 3C/D), indicating that non-canonical WNT signals, particularly WNT11, play a critical role in the early stage of NP cell redifferentiation. This finding was further corroborated by ACAN expression in NP cells, which significantly increased in all WNT-OE groups at all pellet culture timepoints except there was no significant change at day 28 in the WNT11-OE group (Fig. 3E).
Figure 3.

Redifferentiation gene expression in WNT-OE. Expression of COL1A1 (A), PAX1 (B), FOXF1 (C), COL2A1 (D), and ACAN (E) mRNAs in expanded cells and chondrogenically induced pellets overexpressing WNT genes across each timepoint (Day 0, 11, and 28). GAPDH was carried out as the endogenous control gene. Data are shown as bar charts. * indicates a significant difference compared with the corresponding GFP control at a specific timepoint (p<0.05).
Despite no visible difference in the size of all pellet groups (Fig. 4A), the overexpression of non-canonical signals, WNT5A-OE and WNT11-OE, exhibited significantly increased GAG/DNA ratios at day 28 versus the GFP control, but not in canonical WNT3A-OE (Fig. 4B). Similarly, WNT5A-OE exhibited the greatest raw GAG content per pellet at day 28 (Fig. 4C) versus the GFP control. Interestingly, WNT3A-OE NP cells increased their DNA ratio versus the GFP control at days 11 and 28 and, conversely, WNT11-OE significantly decreased DNA content at these same timepoints (Fig. 4D).
Figure 4.

Redifferentiation of WNT-OE pellets. Representative pellets for each group at Day 11 and Day 28 of redifferentiation (A), GAG/DNA (B), GAG content (C), and DNA ratio (D) were analyzed. Data are shown as bar charts. * indicates a significant difference compared with the corresponding GFP control at a specific timepoint (p<0.05).
Cas9/sgRNA lentiviral transduction knocked out WNT genes
After viral transduction of NP cells with CRISPR-Cas9 vectors, guided by sgRNA sequences targeting WNT3A, WNT5A, and WNT11 exons, selection was conducted via puromycin supplementation for the knockout (KO) groups. Three different lentiviral vectors designed in this study for each WNT gene, targeting different exon sequences, were compared for the most effective KO effect in NP cells. For the selected WNT-KO groups (WNT3A-1, WNT5A-3, and WNT11–2), each respective KO target was found most significantly diminished in both expanded cells and chondrogenically induced pellets compared to the GFP control throughout the study (Fig. 5). WNT3A expression was significantly decreased (nondetectable, ND) in WNT3A-KO versus the GFP control in NP cells following transduction in cell samples and redifferentiation pellets (Fig. 5A). WNT5A-KO expressed a nearly 10- to 40-fold decrease of WNT5A mRNA versus the GFP control at each timepoint during redifferentiation (Fig. 5B). Similarly, WNT11-KO exhibited five-fold or greater decrease of WNT11 expression compared to the GFP control at day 0, day 11, and day 28 of redifferentiation induction (Fig. 5C).
Figure 5.

Knockout of WNT genes. Following transduction, cell expansion, and pellet formation, samples were analyzed in cell samples and at each timepoint (Day 0, 11, and 28) of redifferentiation for WNT3A (A), WNT5A (B), and WNT11 (C) mRNA expression for each set of respective knockout vectors. GAPDH was carried out as the endogenous control gene. For the selected WNT-KO groups (WNT3A-1, WNT5A-3, and WNT11–2), each respective KO target (*) was found most significantly diminished (p<0.05) in both expanded cells and chondrogenically induced pellets compared to GFP controls throughout the study. Data are shown as bar charts.
Knockout of non-canonical WNT signals decreased redifferentiation capacity
All WNT-KO groups exhibited increased COL1A1 expression in cell samples following expansion (Fig. 6A). WNT5A-KO and WNT11-KO most significantly increased COL1A1 mRNA expression at day 0 versus the GFP control; however, this expression was significantly attenuated at day 11 of redifferentiation but not in WNT3A-KO (Fig. 6A). Although the knockout of each respective WNT gene led to significant decreases in PAX1 (Fig. 6B) and FOXF1 (Fig. 6C) expression in all groups, with the exception of an increase of PAX1 in WNT5A-KO at day 11 (Fig. 6B), COL2A1 expression was significantly decreased versus the GFP control in both WNT5A-KO and WNT11-KO groups in cell samples and day 28 pellets, but not with knockout of the WNT3A gene (Fig. 6D). A similar trend was observed in ACAN expression, with both WNT5A-KO and WNT11-KO most significantly (approximately five-fold) decreased versus the GFP control (Fig. 6E).
Figure 6.

Redifferentiation gene expression in WNT-KO. Expression of COL1A1 (A), PAX1 (B), FOXF1 (C), COL2A1 (D), and ACAN (E) mRNA in expanded cells and chondrogenically induced pellets knocked out for WNT genes across each timepoint (Day 0, 11, and 28). GAPDH was carried out as the endogenous control gene. Data are shown as bar charts. * indicates a significant difference compared with the corresponding GFP control at a specific timepoint (p<0.05).
Both WNT5A-KO and WNT11-KO exhibited small pellet size compared to the GFP control (Fig. 7A). This finding was corroborated with biochemical analysis data, in which both WNT5A-KO and WNT11-KO displayed dramatically decreased GAG/DNA ratios (Fig. 7B) and GAG contents per pellet (Fig. 7C) as well as lower DNA ratios with the exception of no significant difference of WNT11-KO versus the GFP control at day 28 (Fig. 7D). Intriguingly, WNT3A-KO exhibited a significant decrease of GAG/DNA ratio at day 28 rather than day 11 (Fig. 7B); however, both GAG content per pellet (Fig. 7C) and DNA ratio (Fig. 7D) of WNT3A-KO had no significant difference compared to the GFP control.
Figure 7.
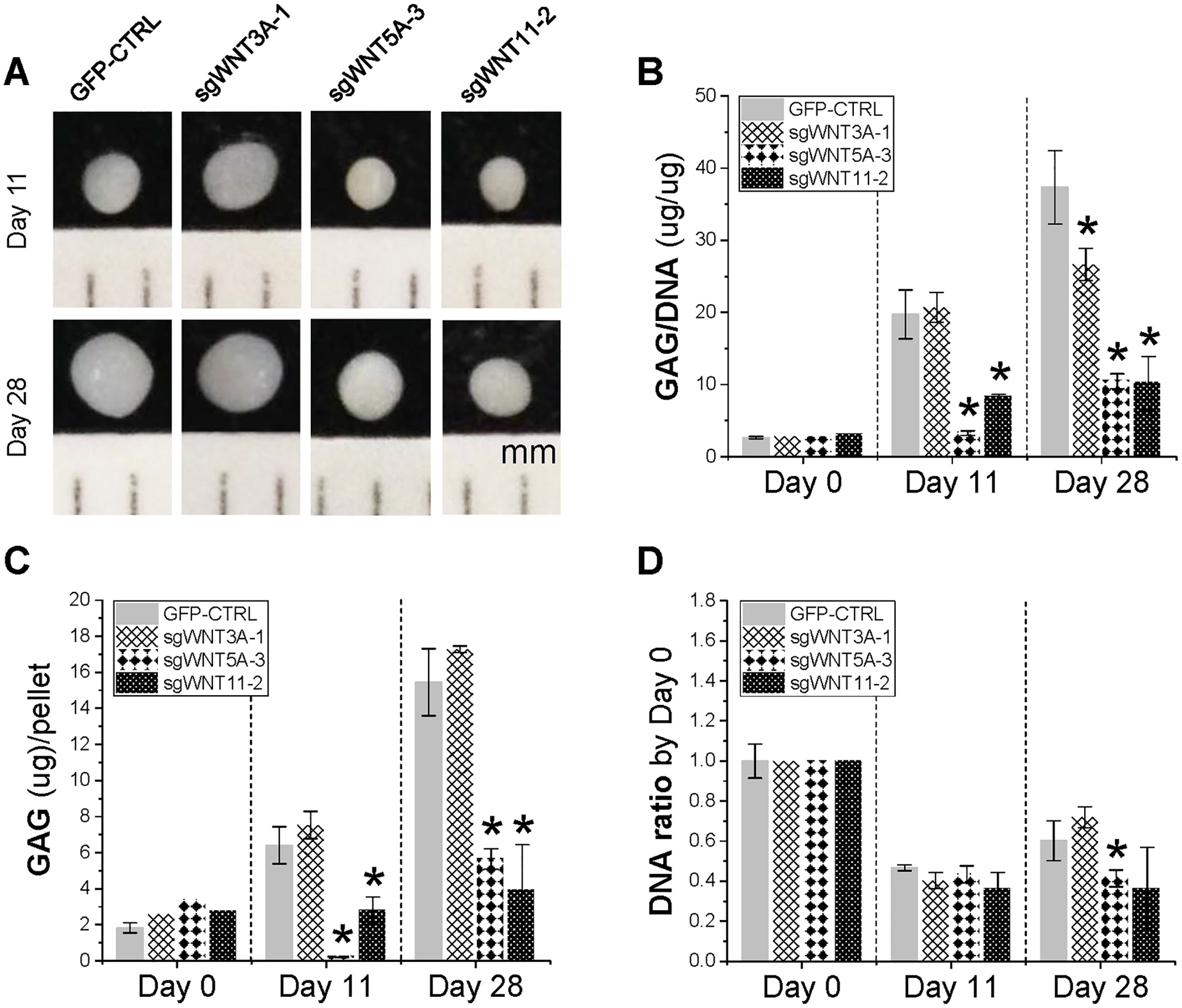
Redifferentiation of WNT-KO pellets. Representative pellets for each group at Day 11 and Day 28 of redifferentiation (A), GAG/DNA (B), GAG content (C), and DNA ratio (D) were analyzed. Data are shown as bar charts. * indicates a significant difference compared with the corresponding GFP control at a specific timepoint (p<0.05).
DISCUSSION
Although current literature has suggested involvement of Wnt signaling in IVD degeneration, few human studies have determined roles for specific Wnt signals, including both canonical and non-canonical pathways, in NP cell proliferation and redifferentiation. In this study, we sought to directly compare the expression of specific Wnt signals to the redifferentiation of human NP cells through lentiviral-mediated transduction. The data collected from this study indicated that the virally-mediated overexpression or knockout of non-canonical Wnt signals, Wnt5A and Wnt11, were much more influential in their collective impact on the redifferentiation gene expression than the effect of Cas9-mediated knockout of canonical (WNT3A) expression in human NP cells. To our knowledge, this study is the first to directly compare the influences of WNT3A, WNT5A, and WNT11 gene expression in the redifferentiation of human NP cells.
We found that WNT3A-OE was the only group during WNT-gene overexpression which expressed any detectable WNT3A mRNA at each timepoint. Despite generally less robust expression when compared to non-canonical signals, in this study, WNT3A overexpression increased both proliferation index and peak generation number compared to other groups and was the only group to significantly increase DNA content during redifferentiation. These results seem to be consistent with other reports. Narcisi and colleagues reported that Wnt3A has increased proliferation in MSCs;24 similar results have been reported in which Wnt3A has been shown to promote the proliferation of pancreatic stem cells,37 neural stem cells,38 bone sarcoma cells,39 and HEK293T cells.40
As expected, the knockout of all WNT genes led to significantly decreased PAX1 and FOXF1 expression, markers suggested to be representative of the healthy, mature NP cell phenotype.34,41 Interestingly, the overexpression of WNT5A and WNT11 genes increased PAX1 expression by five-fold and nine-fold, respectively, versus the GFP control at day 0 of redifferentiation; however, this effect was attenuated by day 28. Similarly, WNT11-OE was the only group to significantly increase FOXF1 expression (6.5-fold) at day 0, while the other groups were significantly decreased versus the GFP control, further suggesting WNT11’s role as an early regulator of NP cell redifferentiation. At day 11, non-canonical WNT overexpression (WNT5A-OE and WNT11-OE) both significantly increased versus the GFP control for FOXF1 expression, but not WNT3A-OE. By day 28, these effects were abolished, and in fact, significantly decreased for the non-canonical WNT overexpression groups. As expected, WNT5A-OE and WNT11-OE produced more dramatic changes in ACAN mRNA versus the GFP control than WNT3A-OE in the early to middle stage of redifferentiation. These findings support the premise that non-canonical Wnt signals in human NP cell regeneration follow a spatiotemporal pattern.
Cumulative trends in these redifferentiation data seem to suggest that, early in redifferentiation, non-canonical signals can increase markers representative of mature NP cells. This trend does not remain consistent through day 28, likely due to the persistent overexpression of these Wnt signals, as they are more tightly regulated to increase or decrease appropriately in non-transduced cells. This same early to middle-stage expression trend is apparent in COL2A1 expression of WNT-OE NP cells, particularly notable for WNT5A and WNT11 signals. WNT11-OE seems most influential in early NP cell redifferentiation. This notion of stage-specific Wnt regulation of differentiation is supported by evidence from a study by Sinha and colleagues where they implicated WNT11 as an early expression gene in tissue development and differentiation.42 In another study investigating mouse limb development, both Wnt5A and Wnt11 were implicated in early limb development and chondrogenesis, whereas Wnt3A was undetectable throughout.43 A recent study investigating the role of miRNA-410 in MSCs found that miRNA-410, a promoter of chondrogenesis, slightly increased at day 15 of differentiation and was significantly elevated at day 25. They report that this miRNA also pairs with WNT3A mRNA and that increases in miRNA-410 negatively correlate with Wnt3A protein expression.44 Further studies are needed to investigate the stage-specific expression of Wnt signals and Wnt agonists/regulators, which could provide more detailed evidence for Wnt regulation in differentiation.
Despite the positive results in redifferentiation gene expression, we recognize the limitations of this study. It remains to be determined whether WNT5A or WNT11 are individually superior to one another or to combination gene therapy, which was not clearly distinguishable in our results. Future studies which more thoroughly evaluate other downstream targets of both canonical and non-canonical Wnt signals, such as Axin, Cyclin D1, Adenomatous polyposis coli (APC), and β-catenin could provide further evidence to support the findings of this study and in the redifferentiation of human NP cells. Furthermore, a study which uses both overexpression and targeted knockout of WNT genes at various stages of differentiation (early, middle, and late) in the same NP cells could more accurately and completely elucidate the stage-specificity of Wnt signals in NP cell redifferentiation, rather than the relatively static methods of genetic manipulation used in our study. This design is especially important when consideration is given to the natural expression patterns of both canonical and non-canonical Wnt signals during differentiation.
Although many studies aim to highlight the apparent differences between canonical and non-canonical Wnt signals for cell differentiation, it is important to recognize the interplay, crosstalk, and potential advantageous utilization of each of the Wnt signaling pathways. Interestingly, Hsu and Huang reported that Wnt5A-mediated and Wnt3A-mediated MSC differentiation could each be directed based on distinct, three-dimensional substrate and culture environments45 which raises new concerns for NP cell expansion and IVD tissue engineering. A recent study by Alok and colleagues has suggested that Wnt signals, particularly those of the non-canonical variety, can synergistically activate β-catenin in several cell types as a combinatory mechanism regulating Wnt signaling.46 It is possible that non-canonical signals in this study, WNT5A and WNT11, can work together in a similar, potentially stage-specific manner during redifferentiation of NP cells. This concept of stage-specific expression has been recently suggested in human SDSCs during their expansion in the presence of fibroblast growth factor 2 during cell expansion or chondrogenic differentiation.13 Another recent study has provided evidence that several non-canonical signals in the liver, including WNT5A, can act as antagonists to Wnt3A-induced β-catenin/T-cell factor (TCF) signaling to promote tissue differentiation, suggesting non-canonical signals can “fine-tune” the effects of canonical Wnt signaling.47 Additionally, studies which can uncover further regulatory intricacies and the crosstalk between canonical and non-canonical Wnt pathways would be beneficial; for example, recent studies have demonstrated that canonical suppression can occur through protein tyrosine kinase 7 (PTK7),48 as well as crosstalk with the Smad/TGF-β pathway,49 known to promote differentiation.
Overall, it seems likely that both canonical and non-canonical Wnt signals are significantly involved in the redifferentiation of human NP cells; however, based on our results, it seems that WNT3A can potentially contribute to NP cell proliferation, whereas non-canonical signals promote early redifferentiation. In conclusion, changes in Wnt signal expression can modulate the redifferentiation of human NP cells. It may be most effective to promote non-canonical Wnt pathway activation through WNT5A and WNT11 than canonical WNT3A. Each signal represents potential targets for NP cell redifferentiation and for future therapeutic strategies aiming to improve NP tissue regeneration.
ACKNOWLEDGEMENTS
We thank Suzanne Danley for editing the manuscript and Dr. Gerald Hobbs for assistance with statistics. Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number (AR062763-01A1 and AR067747-01A1) and the Musculoskeletal Transplant Foundation to Ming Pei, and the National Natural Science Foundation of China (31771063 and 31570978) to Fan He.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
REFERENCES
- 1.Urban JP, Roberts S. 1995. Development and degeneration of the intervertebral discs. Mol Med Today 1:329–335. [DOI] [PubMed] [Google Scholar]
- 2.Dieleman JL, Baral R, Birger M, et al. 2016. US Spending on Personal Health Care and Public Health, 1996–2013. JAMA 316:2627–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hegewald AA, Endres M, Abbushi A, et al. 2011. Adequacy of herniated disc tissue as a cell source for nucleus pulposus regeneration. J Neurosurg Spine 14:273–280. [DOI] [PubMed] [Google Scholar]
- 4.McCann MR, Séguin CA. 2016. Notochord Cells in Intervertebral Disc Development and Degeneration. J Dev Biol 4:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Twomey LT, Taylor JR. 1987. Age changes in lumbar vertebrae and intervertebral discs. Clin Orthop Relat Res (224):97–104. [PubMed] [Google Scholar]
- 6.Pei M 2017. Environmental preconditioning rejuvenates adult stem cells’ proliferation and chondrogenic potential. Biomaterials 117:10–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shoukry M, Li J, Pei M. 2013. Reconstruction of an in vitro niche for the transition from intervertebral disc development to nucleus pulposus regeneration. Stem Cells Dev 22:1162–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srivastava A, Isa IL, Rooney P, Pandit A. 2017. Bioengineered three-dimensional diseased intervertebral disc model revealed inflammatory crosstalk. Biomaterials 123:127–141. [DOI] [PubMed] [Google Scholar]
- 9.Arai F, Hiyama A, Sakai D, et al. 2012. The expression and role of non-canonical (PKC) signaling in nucleus pulposus cell metabolism. J Orthop Res 30:1478–1485. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Chen H, Cao P, et al. 2016. Inflammatory cytokines induce caveolin-1/β-catenin signalling in rat nucleus pulposus cell apoptosis through the p38 MAPK pathway. Cell Prolif 49:362–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcin CL, Habib SJ. 2017. A Comparative Perspective on Wnt/β-Catenin Signalling in Cell Fate Determination. Results Probl Cell Differ 61:323–350. [DOI] [PubMed] [Google Scholar]
- 12.Green JD, Tollemar V, Dougherty M, et al. 2015. Multifaceted signaling regulators of chondrogenesis: Implications in cartilage regeneration and tissue engineering. Genes Dis 2:307–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pizzute T, Li J, Zhang Y, et al. 2016. Fibroblast Growth Factor Ligand Dependent Proliferation and Chondrogenic Differentiation of Synovium-Derived Stem Cells and Concomitant Adaptation of Wnt/Mitogen-Activated Protein Kinase Signals. Tissue Eng Part A 22:1036–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhong L, Huang X, Karperien M, Post JN. 2015. The Regulatory Role of Signaling Crosstalk in Hypertrophy of MSCs and Human Articular Chondrocytes. Int J Mol Sci 16:19225–19247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohammed MK, Shao C, Wang J, et al. 2016. Wnt/β-catenin signaling plays an ever-expanding role in stem cell self-renewal, tumorigenesis and cancer chemoresistance. Genes Dis 3:11–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Pizzute T, Pei M. 2014. A review of crosstalk between MAPK and Wnt signals and its impact on cartilage regeneration. Cell Tissue Res 358:633–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiyama A, Yokoyama K, Nukaga T, Sakai D, Mochida J. 2013. A complex interaction between Wnt signaling and TNF-α in nucleus pulposus cells. Arthritis Res Ther 15:R189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiyama A, Yokoyama K, Nukaga T, Sakai D, Mochida J. 2015. Response to tumor necrosis factor-α mediated inflammation involving activation of prostaglandin E2 and Wnt signaling in nucleus pulposus cells. J Orthop Res 33:1756–1768. [DOI] [PubMed] [Google Scholar]
- 19.Iwata M, Aikawa T, Hakozaki T, et al. 2015. Enhancement of Runx2 expression is potentially linked to β-catenin accumulation in canine intervertebral disc degeneration. J Cell Physiol 230:180–190. [DOI] [PubMed] [Google Scholar]
- 20.Hiyama A, Sakai D, Risbud MV, et al. 2010. Enhancement of intervertebral disc cell senescence by WNT/β-catenin signaling-induced matrix metalloproteinase expression. Arthritis Rheum 62:3036–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hiyama A, Sakai D, Tanaka M, et al. 2011. The relationship between the Wnt/β-catenin and TGF-β/BMP signals in the intervertebral disc cell. J Cell Physiol 226:1139–1148. [DOI] [PubMed] [Google Scholar]
- 22.Buchtova M, Oralova V, Aklian A, et al. 2015. Fibroblast growth factor and canonical WNT/β-catenin signaling cooperate in suppression of chondrocyte differentiation in experimental models of FGFR signaling in cartilage. Biochim Biophys Acta 1852:839–850. [DOI] [PubMed] [Google Scholar]
- 23.Narcisi R, Arikan OH, Lehmann J, et al. 2016. Differential Effects of Small Molecule WNT Agonists on the Multilineage Differentiation Capacity of Human Mesenchymal Stem Cells. Tissue Eng Part A 22:1264–1273. [DOI] [PubMed] [Google Scholar]
- 24.Narcisi R, Cleary MA, Brama PA, et al. 2015. Long-term expansion, enhanced chondrogenic potential, and suppression of endochondral ossification of adult human MSCs via WNT signaling modulation. Stem Cell Rep 4:459–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hosseini-Farahabadi S, Geetha-Loganathan P, Fu K, et al. 2013. Dual functions for WNT5A during cartilage development and in disease. Matrix Biol 32:252–264. [DOI] [PubMed] [Google Scholar]
- 26.Liu S, Zhang E, Yang M, Lu L. 2014. Overexpression of Wnt11 promotes chondrogenic differentiation of bone marrow-derived mesenchymal stem cells in synergism with TGF-β. Mol Cell Biochem 390:123–131. [DOI] [PubMed] [Google Scholar]
- 27.Thorfve A, Dehne T, Lindahl A, et al. 2012. Characteristic markers of the WNT signaling pathways are differentially expressed in osteoarthritic cartilage. Cartilage 3:43–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Usami Y, Gunawardena AT, Iwamoto M, Enomoto-Iwamoto M. 2016. Wnt signaling in cartilage development and diseases: lessons from animal studies. Lab Invest 96:186–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu X, Zhu H, Zhang L, et al. 2012. Wls-mediated Wnts differentially regulate distal limb patterning and tissue morphogenesis. Dev Biol 365:328–338. [DOI] [PubMed] [Google Scholar]
- 30.Chen S, Fu P, Wu H, Pei M. 2017. Meniscus, articular cartilage and nucleus pulposus: a comparative review of cartilage-like tissues in anatomy, development and function. Cell Tissue Res 370:53–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang JP, Li XL, Li GH, et al. 2017. Efficient precise knockin with a double cut HDR donor after CRISPR/Cas9-mediated double-stranded DNA cleavage. Genome Biol 18:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang JP, Li XL, Neises A, et al. 2016. Different Effects of sgRNA Length on CRISPR-mediated Gene Knockout Efficiency. Sci Rep 6:28566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meng X, Baylink DJ, Sheng M, et al. 2012. Erythroid promoter confines FGF2 expression to the marrow after hematopoietic stem cell gene therapy and leads to enhanced endosteal bone formation. PLoS One 7:e37569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Minogue BM, Richardson SM, Zeef LA, et al. 2010. Characterization of the human nucleus pulposus cell phenotype and evaluation of novel marker gene expression to define adult stem cell differentiation. Arthritis Rheum 62:3695–3705. [DOI] [PubMed] [Google Scholar]
- 35.Jacobs LJ, Chen AF, Kang JD, Lee JY. 2016. Reliable Magnetic Resonance Imaging Based Grading System for Cervical Intervertebral Disc Degeneration. Asian Spine J 10:70–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watanabe A, Benneker LM, Boesch C, et al. 2007. Classification of intervertebral disk degeneration with axial T2 mapping. AJR Am J Roentgenol 189:936–942. [DOI] [PubMed] [Google Scholar]
- 37.He X, Han W, Hu SX, et al. 2015. Canonical Wnt signaling pathway contributes to the proliferation and survival in porcine pancreatic stem cells (PSCs). Cell Tissue Res 362:379–388. [DOI] [PubMed] [Google Scholar]
- 38.Yang XT, Bi YY, Chen ET, Feng DF. 2014. Overexpression of Wnt3a facilitates the proliferation and neural differentiation of neural stem cells in vitro and after transplantation into an injured rat retina. J Neurosci Res 92:148–161. [DOI] [PubMed] [Google Scholar]
- 39.Chen C, Zhao M, Tian A, et al. 2015. Aberrant activation of Wnt/β-catenin signaling drives proliferation of bone sarcoma cells. Oncotarget 6:17570–17583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reischmann P, Fiebeck J, von der Weiden N, Müller O. 2015. Measured Effects of Wnt3a on Proliferation of HEK293T Cells Depend on the Applied Assay. Int J Cell Biol 2015:928502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thorpe AA, Binch AL, Creemers LB, et al. 2016. Nucleus pulposus phenotypic markers to determine stem cell differentiation: fact or fiction? Oncotarget 7:2189–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sinha T, Lin L, Li D, et al. 2015. Mapping the dynamic expression of Wnt11 and the lineage contribution of Wnt11-expressing cells during early mouse development. Dev Biol 398:177–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Witte F, Dokas J, Neuendorf F, et al. 2009. Comprehensive expression analysis of all Wnt genes and their major secreted antagonists during mouse limb development and cartilage differentiation. Gene Expr Patterns 9:215–223. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Huang X, Yuan Y. 2017. MicroRNA-410 promotes chondrogenic differentiation of human bone marrow mesenchymal stem cells through down-regulating Wnt3a. Am J Transl Res 9:136–145. [PMC free article] [PubMed] [Google Scholar]
- 45.Hsu SH, Huang GS. 2013. Substrate-dependent Wnt signaling in MSC differentiation within biomaterial-derived 3D spheroids. Biomaterials 34:4725–4738. [DOI] [PubMed] [Google Scholar]
- 46.Alok A, Lei Z, Jagannathan NS, et al. 2017. Wnts synergize to activate β-catenin signaling. J Cell Sci 130:1532–1544. [DOI] [PubMed] [Google Scholar]
- 47.Fan J, Wei Q, Liao J, et al. 2017. Noncanonical Wnt signaling plays an important role in modulating canonical Wnt-regulated stemness, proliferation and terminal differentiation of hepatic progenitors. Oncotarget 8:27105–27119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berger H, Breuer M, Peradziryi H, et al. 2017. PTK7 localization and protein stability is affected by canonical Wnt ligands. J Cell Sci 130:1890–1903. [DOI] [PubMed] [Google Scholar]
- 49.Webber HC, Bermudez JY, Sethi A, et al. 2016. Crosstalk between TGFβ and Wnt signaling pathways in the human trabecular meshwork. Exp Eye Res 148:97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]


