Abstract
We investigated if previously demonstrated inhibition of fluciclovine (18F) in vitro could be replicated in a PC3-Luc xenograft mouse model. Following intratumoral injection of 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid (BCH), alpha-(methylamino)isobutyric acid (MeAIB) or saline, fluciclovine PET tumor-to-background activity was 43.6 (± 5.4)% and 25.3 (± 5.2)% lower in BCH (n = 6) and MeAIB (n = 5) injected PC3 Luc xenografts, respectively, compared to saline-injected controls (n = 2). Partial inhibition of fluciclovine uptake by BCH and MeAIB can be demonstrated in vivo similar to previous in vitro modeling.
Keywords: Fluciclovine, Prostate cancer, PC3, Amino acid transport, Axumin
Introduction
Anti-1-amino-3–18F-fluorocyclobutane-1-carboxylic acid [FACBC or fluciclovine (18F)] is a newly FDA approved synthetic amino acid analogue PET radiotracer (Axumin®) that has demonstrated utility for the detection of recurrent prostate cancer (Schuster et al. 2014b, 2016a, b; Odewole et al. 2016). Similar to glutamine, fluciclovine (18F) (fluciclovine) is believed to be transported primarily via ASCT2, with lesser contributions by LAT1, SNAT2, and possibly LAT3 (Oka et al. 2012, 2014; Ono et al. 2015; Okudaira et al. 2011, 2013, 2014). The studies establishing transport mechanisms of fluciclovine involved in vitro experiments utilizing competitive inhibition of carbon-14 labeled FACBC uptake in prostate cancer cell lines using synthetic and natural amino acids (Oka et al. 2012; Okudaira et al. 2011, 2013). We believed it would be valuable to determine if similar selective uptake inhibition could be replicated in a more complex in vivo animal model using fluciclovine (18F).
Materials and methods
PC3–Luc cell culture
PC3 human prostate cancer cells (Augusta University, Augusta, GA, USA) expressing Luciferase (PC3-Luc) were maintained in RPMI-1640 medium supplemented with 10% FBS (Atlanta Biologicals, Lawrenceville, GA, USA), 2 mmol/L l-glutamine, 50 U/mL penicillin—50 mg/mL streptomycin, and 500 ng/mL G-418 selective antibiotic. Cells were cultured in a 37 °C incubator with humidified atmosphere of 5% CO2.
Establishment of human tumor xenografts in mice and intratumoral injection of substrates
After approval from the Institutional Animal Care and Use Committee, eighteen (18) 6-week-old male outbred homozygous nude mice (J:Nu) were purchased from Jackson Laboratory (Sacramento, CA, USA). Using a cold syringe and 27-gauge needle, 1 × 106 PC3-Luc cells in a total volume of 0.1 mL serum-free medium containing 50% matrigel (BD Biosciences, Palo Alto, CA, USA) were injected subcutaneously in the flank under isoflurane anesthesia. Mice were monitored for about 4 weeks till tumor grew to approximately 5 mm by caliper measurement. Intratumoral inhibitor injections of approximately 5 mM (0.2 mL) of BCH, MeAIB or saline (control) were then completed in the three groups of six mice each. All applicable institutional and/or national guidelines for the care and use of animals were followed in this process.
Fluciclovine micro–PET/CT imaging and analysis
60 mins post-intratumoral injection of amino acid transport inhibitors, approximately 4.7 ± 0.4 MBq of fluciclovine was injected into the tail vein of each mouse and each mouse underwent 60-min dynamic micro-PET/CT imaging (Inveon, Siemens Medical Solutions USA, Inc.) under continuous isoflurane anesthesia. Images were reconstructed and thereafter transferred to Mim 6.6 workstation (MIM Software; Cleveland, OH, USA) for interpretation. Using three-dimensional PET-edge/brush tool, regions of interests were drawn around the tumors carefully excluding the central photopenic matrigel core. Fluciclovine activity parameters were also measured within 10 mm region of interest (ROI) in the liver as a background structure. SUVmax tumor to background (T/B) ratio time activity curves were computed using specialized workflows accounting for each mouse’s body weight, activity injected and image onset time.
In vivo bioluminescence imaging
Bioluminescence imaging to assess for tumor viability was carried out on an IVIS Spectrum Bioluminescence Imaging System (PerkinElmer) following intraperitoneal injection of 1.5 mg of d-luciferin (PerkinElmer). Measurements were performed when signal reached maximal plateau, with a ROI defined for each tumor, and the average radiance (p/s/cm2/sr) values extracted for quantification. Each mouse was scanned separately in supine position, using high-exposure parameters.
Statistical analysis
Dynamic PET/CT time activity curves were plotted over 60 min for each mouse. The trend of mean ± standard deviation (SD) of fluciclovine uptake in groups of mice injected with BCH, saline and MeAIB as well as tumors to background (liver) ratios were analyzed. Uptake ratios were also calculated as a percent of control (saline) to quantify the effects of intratumoral injections of BCH and MeAIB on fluciclovine uptake. Statistical analysis was done using Microsoft Excel 2010.
Results
After exclusion of five mice due to either non-viability of tumors on bioluminescence or difficulty with visualization on PET imaging, six BCH-treated, five MeAIB-treated and two saline-treated mice were analyzed. There was no significant difference in tumor size across all groups of mice (P = 0.07). Details of mice body weight, tumor size, administered dose and bioluminescence are shown in Table 1.
Table 1.
Demographics, bioluminescence and fluciclovine uptake in PC3-Luc xenografts injected with BCH, MeAIB or saline
| Substrate injected (mouse) |
Mouse size (gm) | Tumor size (mm) | Fluciclovine dose injected (μci) | Bioluminescence | |
|---|---|---|---|---|---|
| Total flux (p/s) | Average radiance (p/s/cm2/ sr) | ||||
| BCHa (1) | 28 | 12 × 9.0 | 128 | 5.43E+08 | 1.38E+07 |
| BCH (2) | 27.4 | 7.6 × 9.0 | 131 | 1.75E+08 | 4.50E+06 |
| BCH (3) | 27.5 | 4.8 × 5.2 | 141 | 2.47E+07 | 5.12E+05 |
| BCH (4) | 27 | 5.2 × 5.7 | 130 | 3.15E+07 | 1.21E+06 |
| BCH (5) | 30.3 | 6.7 × 6.3 | 128 | 2.61E+08 | 6.36E+06 |
| BCH (6) | 30 | 6.0 × 6.3 | 123 | 1.45E+07 | 4.83E+05 |
| MeAIBb (1) | 26.3 | 5.6 × 5.0 | 113 | 1.15E+08 | 2.93E+06 |
| MeAIB (2) | 26.9 | 6.1 × 8.9 | 110 | 9.37E+07 | 2.37E+06 |
| MeAIB (3) | 28.2 | 6.0 × 5.0 | 141 | 7.80E+06 | 3.27E+05 |
| MeAIB (4) | 28.8 | 6.5 × 7.2 | 122 | 8.62E+07 | 3.31E+06 |
| MeAIB (5) | 30 | 8.2 × 7.9 | 122 | 2.48E+08 | 5.66E+06 |
| Saline (1) | 25 | 7.6 × 6.6 | 137 | 1.32E+08 | 3.35E+06 |
| Saline (2) | 26.6 | 6.6 × 7.1 | 130 | 3.90E+07 | 1.41E+06 |
BCH-2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid
MeAIB-alpha-(methylamino)isobutyric acid
Tumor to background time activity curves
As demonstrated in Fig. 1a, tumor to background activity increased over time in all tumor groups. When compared to saline controls (n = 2), T/B ratios were most suppressed in prostate cancer xenografts injected with BCH (n = 6) with less of an effect in those injected with MeAIB (n = 5). Figure 1b normalizes the activity ratio at each time point to that of saline controls and shows that BCH and MeAIB injections, respectively, led to a 44.8 (± 6.9) and 26.4 (± 7.4)% inhibition of fluciclovine uptake across all 10 time points. Due to small sample size, especially in the control group, test of statistical significance in differences could not be computed.
Fig. 1.
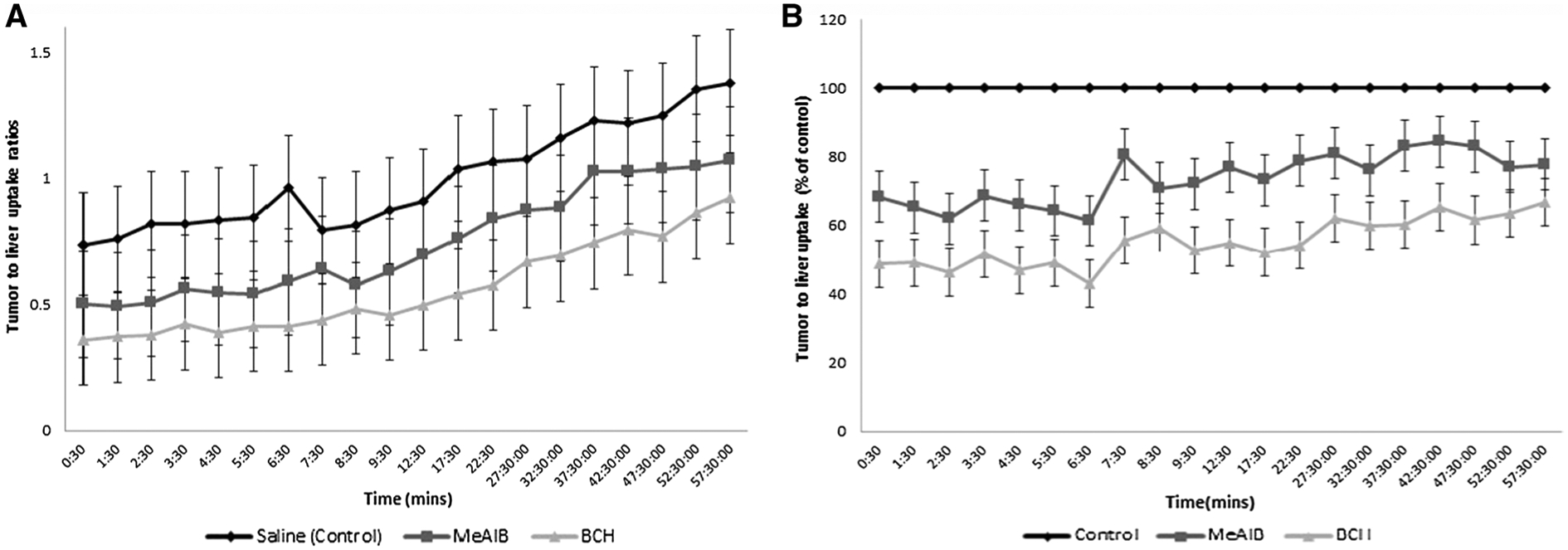
a Fluciclovine tumor to background ratio time activity curves in PC3 xenografts injected with saline (control), MeAIB and BCH (bars on chart are standard deviation bars). b Fluciclovine tumor to background uptake in tumors injected with inhibitors normalized as a percent of control (saline) uptake (bars on chart are standard deviation bars)
Discussion
Based on a series of in vitro cell uptake and inhibition studies in prostate cancer cell lines as well as siRNA knockdown experiments, transport of fluciclovine (18F) in prostate cancer cells has been postulated to occur mainly via ASCT2 and less so via LAT1 (with possibly a minor role of LAT3) with some contribution by SNAT2 (Okudaira et al. 2011, 2013, 2014; Ono et al. 2015; Oka et al. 2014). Specifically, we observed a trend that BCH exhibited partial (approximately 20%) inhibition of uptake while MeAIB had less (approximately 10%) inhibitory effect compared with controls.
Similarly, in our study, intratumoral injection of BCH, a known LAT1 substrate and system L inhibitor (Kim et al. 2008), resulted in 44% inhibition of T/B activity while MeAIB had 25% inhibition compared with controls. Due to differences in methodology between the previous in vitro study and our in vivo study, quantitative results would not be expected to be directly comparable, but overall qualitative results of both studies are similar in that system L inhibitor achieved partial transport inhibition and less so for system A. It is also possible that in the presence of an acidic dense tumor environment, there was more transport via LAT1 than would be seen from in vitro cell lines alone (Okudaira et al. 2011; Ono et al. 2013; Oka et al. 2012, 2014). The finding of overall agreement between previously published in vitro data and results of our in vivo work adds weight to the translatability of in vitro work on fluciclovine uptake mechanisms.
Our study findings are important because LAT1 expression intensity has been found to correlate with poor patient survival, and could serve as additional prognostic biomarker compared to Ki-67 proliferation index and Gleason score alone (Sakata et al. 2009). As fluciclovine uptake has been described in several tumor types (Schuster et al. 2014a), delineation of the underlying tumor-specific transport mechanisms, and thus the associated biological processes, is necessary to inform how it can be used to validate and direct the use of current and emerging cancer therapies, particularly those targeting tumor metabolism.
Limitations of this study include small sample size. Also, uneven distribution of inhibitors within the tumors may have occurred, but we believed the use of SUVmax for tumor to background ratios, mitigates the effect of this variability. Our ability to directly confirm the contribution of ASCT2 to fluciclovine transport was also limited since highly selective ASCT2 inhibitors were not commercially available at the time of this study. Due to size, fluciclovine uptake in the other background organs could not be easily measured using a 10 mm ROI except in the liver. However, we believed that the liver better reflects biodistribution and is in fact used clinically to aid in interpretation of both fluciclovine and FDG PET-CT (Nye et al. 2007; Schuster et al. 2011). Finally, there could be an imbalance in the magnitude of intravenous fluciclovine reaching the tumor versus directly injected inhibitor; yet, as an initial effort, we sought to determine if there would be any effect even under these extreme conditions.
Conclusion
The results of our in vivo study with PC3-Luc xenografts suggest similar findings compared with earlier in vitro studies: that fluciclovine transport is only partially inhibited by BCH and less so by MeAIB, reinforcing the data suggesting lesser roles for system L (LAT1) and system A (SNAT2) transporters. Further studies with a larger sample size and also utilizing stable shRNA knockdown prostate cancer cell lines with lentivirally encoded shRNA constructs that target specific amino acid transporters are recommended.
Acknowledgements
Research reported in this publication was supported in part by the Cancer Animal Models Shared Resource, a core supported by the Winship Cancer Institute of Emory University and Cancer Center Support Grant P30 CA138292.
Funding This study was otherwise internally funded.
Footnotes
Conflict of interest Emory University licensed and approved the 18F-fluciclovine patent technology in accordance with the Emory Conflict of Interest Committee policies. M. M. Goodman and Emory University are eligible to receive royalties derived from the sale of 18F-fluciclovine. H. Okudaira and S. Oka are employees of Nihon Medi-Physics Co., Ltd. (NMP) and collaborate with M. M. Goodman and D. M. Schuster on preclinical and clinical studies involving 18F-fluciclovine. F. I. Tade, O. Akin-Akintayo, and D. M. Schuster receive funding/resources from Blue Earth Diagnostics Ltd. and Nihon Medi-Physics Co., Ltd. through the Emory University Office of Sponsored Projects for research outside that reported in this manuscript. All other co-authors declared no conflicts of interest.
Research involving human participants and/or animals This study was performed with the approval of the Institutional Animal Care and Use Committee. All procedures performed in this study using animals were in accordance with institutional and/or national guidelines and ethical standards for the care and use of animals. No human studies were conducted.
Informed consent No human studies were conducted.
References
- Kim CS, Cho SH, Chun HS et al. (2008) BCH, an inhibitor of system L amino acid transporters, induces apoptosis in cancer cells. Biol Pharm Bull 31(6):1096–1100 [DOI] [PubMed] [Google Scholar]
- Nye JA, Schuster DM, Yu W et al. (2007) Biodistribution and radiation dosimetry of the synthetic nonmetabolized amino acid analogue anti-18F-FACBC in humans. J Nucl Med Off Publ Soc Nucl Med 48(6):1017–1020. 10.2967/jnumed.107.040097 [DOI] [PubMed] [Google Scholar]
- Odewole OA, Tade FI, Nieh PT et al. (2016) Recurrent prostate cancer detection with anti-3-[(18)F]FACBC PET/CT: comparison with CT. Eur J Nucl Med Mol Imaging 43(10):1773–1783. 10.1007/s00259-016-3383-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka S, Okudaira H, Yoshida Y et al. (2012) Transport mechanisms of trans-1-amino-3-fluoro[1-(14)C]cyclobutanecarboxylic acid in prostate cancer cells. Nucl Med Biol 39(1):109–119. 10.1016/j.nucmedbio.2011.06.008 [DOI] [PubMed] [Google Scholar]
- Oka S, Okudaira H, Ono M et al. (2014) Differences in transport mechanisms of trans-1-amino-3-[18F]fluorocyclobutanecarboxylic acid in inflammation, prostate cancer, and glioma cells: comparison with l-[methyl-11C]methionine and 2-deoxy-2-[18F]fluoro-d-glucose. Mol Imaging Biol 16(3):322–329. 10.1007/s11307-013-0693-0 [DOI] [PubMed] [Google Scholar]
- Okudaira H, Shikano N, Nishii R et al. (2011) Putative transport mechanism and intracellular fate of trans-1-amino-3-18F-fluorocyclobutanecarboxylic acid in human prostate cancer. J Nucl Med Off Publ Soc Nucl Med 52(5):822–829. 10.2967/jnumed.110.086074 [DOI] [PubMed] [Google Scholar]
- Okudaira H, Nakanishi T, Oka S et al. (2013) Kinetic analyses of trans-1-amino-3-[18F]fluorocyclobutanecarboxylic acid transport in Xenopus laevis oocytes expressing human ASCT2 and SNAT2. Nucl Med Biol 40(5):670–675. 10.1016/j.nucmedbio.2013.03.009 [DOI] [PubMed] [Google Scholar]
- Okudaira H, Oka S, Ono M et al. (2014) Accumulation of trans-1-amino-3-[(18)F]fluorocyclobutanecarboxylic acid in prostate cancer due to androgen-induced expression of amino acid transporters. Mol Imaging Biol 16(6):756–764. 10.1007/s11307-014-0756-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M, Oka S, Okudaira H et al. (2013) Comparative evaluation of transport mechanisms of trans-1-amino-3-[(1)(8)F]fluorocyclobutanecarboxylic acid and l-[methyl-(1)(1)C]methionine in human glioma cell lines. Brain Res 1535:24–37. 10.1016/j.brainres.2013.08.037 [DOI] [PubMed] [Google Scholar]
- Ono M, Oka S, Okudaira H et al. (2015) [(14)C]Fluciclovine (alias anti-[(14)C]FACBC) uptake and ASCT2 expression in castration-resistant prostate cancer cells. Nucl Med Biol 42(11):887–892. 10.1016/j.nucmedbio.2015.07.005 [DOI] [PubMed] [Google Scholar]
- Sakata T, Ferdous G, Tsuruta T et al. (2009) l-Type amino-acid transporter 1 as a novel biomarker for high-grade malignancy in prostate cancer. Pathol Int 59(1):7–18. 10.1111/j.1440-1827.2008.02319.x [DOI] [PubMed] [Google Scholar]
- Schuster DM, Savir-Baruch B, Nieh PT et al. (2011) Detection of recurrent prostate carcinoma with anti-1-amino-3–18F-fluorocyclobutane-1-carboxylic acid PET/CT and 111In-capromab pendetide SPECT/CT. Radiology 259(3):852–861. 10.1148/radiol.11102023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster DM, Nanni C, Fanti S et al. (2014a) Anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid: physiologic uptake patterns, incidental findings, and variants that may simulate disease. J Nucl Med Off Publ Soc Nucl Med 55(12):1986–1992. 10.2967/jnumed.114.143628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster DM, Nieh PT, Jani AB et al. (2014b) Anti-3-[(18)F]FACBC positron emission tomography-computerized tomography and (111)In-capromab pendetide single photon emission computerized tomography-computerized tomography for recurrent prostate carcinoma: results of a prospective clinical trial. J Urol 191(5):1446–1453. 10.1016/j.juro.2013.10.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster DM, Nanni C, Fanti S (2016a) Evaluation of prostate cancer with radiolabeled amino acid analogs. J Nucl Med Off Publ Soc Nucl Med 57(Suppl 3):61S–66S. 10.2967/jnumed.115.170209 [DOI] [PubMed] [Google Scholar]
- Schuster DM, Nanni C, Fanti S (2016b) PET tracers beyond FDG in prostate cancer. Semin Nucl Med 46(6):507–521. 10.1053/j.semnuclmed.2016.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]


