Abstract
Cryptococcus neoformans causes life-threatening meningoencephalitis. Human immunodeficiency virus (HIV) infection is the most significant predisposing condition, but persons with other immunodeficiency states as well as phenotypically normal persons develop cryptococcosis. We retrospectively reviewed medical records of all patients with a diagnosis of cryptococcosis between 2005 and 2017 at our inner-city medical center in the Bronx, an epicenter of AIDS in New York City, and analyzed demographic data, clinical manifestations, laboratory findings, treatment, and mortality for these patients. In sum, 63% of the cases over this 12-year period occurred in HIV-infected patients. And 61% of the HIV-infected patients were non-adherent with antiretroviral therapy, 10% were newly diagnosed with AIDS, and 4% had unmasking cryptococcus-associated immune reconstitution inflammatory syndrome. The majority were Hispanic or black in ethnicity/race. HIV-uninfected patients (47/126) were older (P < .0001), and the majority had an immunocompromising condition. They were less likely to have a headache (P = .0004) or fever (P = .03), had prolonged time to diagnosis (P = .04), higher cerebrospinal fluid (CSF) glucose levels (P = .001), less CSF culture positivity (P = .03), and a higher 30-day mortality (P = .03). Cases in HIV-uninfected patients were often unsuspected during their initial evaluation, leading to a delay in infectious diseases consultation, which was associated with mortality (P = .03). Our study indicates that HIV infection remains the most important predisposing factor for cryptococcosis despite availability of antiretroviral therapy and highlights potential missed opportunities for earlier diagnosis and differences in clinical and prognostic factors between HIV-infected and HIV-uninfected patients.
Keywords: Cryptococcus neoformans infection, HIV infection, Cryptococcus-associated immune reconstitution inflammatory syndrome, solid organ transplant recipients, infectious diseases consultation, mortality
Introduction
Cryptococcus neoformans is an environmentally ubiquitous yeast that can cause life-threatening infection, especially cryptococcal meningitis. Those at the highest risk for cryptococcosis are persons with human immunodeficiency virus (HIV) infection due to Cryptococcus neoformans var. grubii; however, those with other conditions of immune deficiency, as well as those with no apparent immune deficiency also develop disease.1–4 Despite significant improvements in access to combination antiretroviral therapy (cART) and retention in HIV care, the main predisposing condition for cryptococcosis is still HIV infection, which continues to affect racial and ethnic minorities disproportionately in areas of poverty in the United States.5–8 Recent reports highlight a proportional increase in non-HIV associated cryptococcosis as the number of persons receiving solid organ transplants, immune modulating drugs, and biological response modifiers such as tumor necrosis factor alpha and Bruton's tyrosine kinase inhibitors9–12 has increased. There are also sporadic cases of cryptococcosis in patients with hypogammaglobulinemia, idiopathic CD4 lymphopenia, and sarcoidosis.1–4,13 In this report, we review cases of cryptococcosis at our urban safety net hospital located in the Bronx from 2005 to 2017 and compare the clinical manifestations, laboratory findings and mortality of patients with and without HIV infection.
Methods
Ethics statement
This study was approved by the Albert Einstein College of Medicine Institutional Review Board for human subjects with a waiver of informed consent.
Data collection
A retrospective cohort study was conducted by reviewing the medical records (electronic and/or paper chart) of all patients ≥18 years old with the diagnosis of cryptococcosis at Montefiore Medical Center (MMC), an inner-city tertiary care medical center composed of 1490 beds, from January 2005 through December 2017 in the Bronx, New York, USA. Patients with International Classification of Disease, 9th revision diagnosis code of cryptococcosis were identified by using Looking GlassTM Clinical Analytics (Streamline Health, Atlanta, GA, USA). Patients were categorized as HIV-infected or HIV-uninfected according to the documented HIV status. All cases were reviewed but only the first cryptococcosis episode during the examined period was used for the analysis of variables concerning the patients. The following were reviewed: patient demographics, clinical presentation, underlying conditions, laboratory findings including cryptococcal antigen (CALAS®; Meridian Bioscience, Inc. Cincinnati, OH, USA), blood cultures, initial cerebrospinal fluid (CSF) cell count, chemistry, culture, and opening pressure (OP) when available, time from symptom onset to diagnosis, time from admission to evaluation by the infectious diseases (ID) consultant, treatment regimen(s), occurrence of cryptococcus-associated immune reconstitution inflammatory syndrome (C-IRIS), and mortality at 30 days and 1 year. CD4 T cell count, HIV viral load were reviewed in all patients when available and HIV-infected patients were followed up to 1 year to evaluate CD4 T cell count and HIV viral load. Factors associated with mortality at 30 days were evaluated.
Definitions
The diagnosis of cryptococcosis was based on the following: a positive culture for Cryptococcus neoformans from blood, CSF and/or lung, a positive histopathological study or a positive blood/CSF cryptococcal antigen (CrAg) with a compatible clinical and/or radiographic presentation. Sites of involvement included: (1) central nervous system (CNS), which included meningeal and parenchymal brain involvement, including patients with infection of the CNS with and without extra-neural disease; (2) pulmonary, which included the lungs, pleura, and/or pleural fluid involvement but not the CNS; (3) blood, which involved isolation of C. neoformans in blood culture; and (4) other. A single patient may have had involvement of more than one site. Isolates from the cultures were speciated upon request. Cryptococcus gattii has not been isolated at our institution. Time from symptom onset to diagnosis was defined by the number of days between documented time of symptom onset and meeting the case definition. Time from admission to diagnosis was defined by the number of days between admission and meeting the case definition.
Statistical analysis
Categorical variables were compared by Fisher exact test and reported by absolute number (percentages). Continuous variables were compared by Mann-Whitney test and reported by the median and range. Factors associated with all-cause mortality at 30 days were evaluated using a logistic regression model. Factors with P-value less than .05 in univariate analysis were used for further multivariate analysis. Odds ratios (OR) and corresponding 95% confidence intervals were calculated. A P-value of < .05 was considered statistically significant. R was used for analysis.
Results
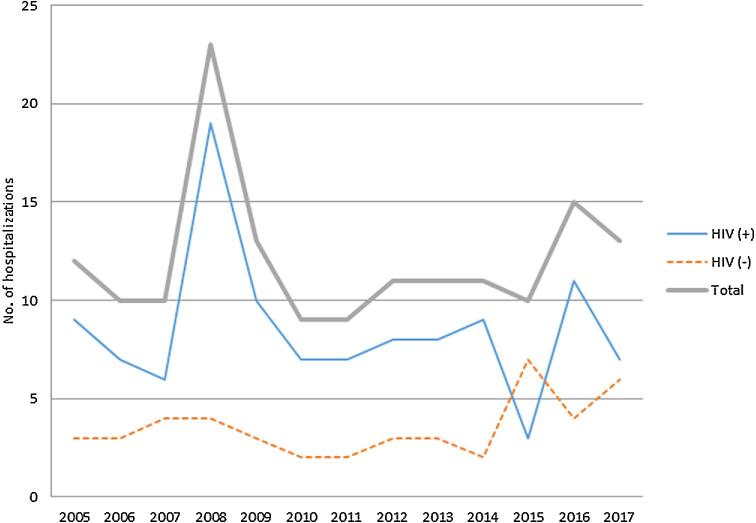
During the 12-year period from 2005 to 2017, there were 126 patients diagnosed with cryptococcosis: 79 (59 male, 20 female) were HIV-infected, and 47 (28 male, 19 female) were HIV-uninfected (Table 1). The total number of hospitalizations for cryptococcosis during this period was 114 in the HIV-infected group and 50 in the HIV-uninfected group. On average, there were 11 hospitalizations for cryptococcosis per year (Fig. 1).
Table 1.
Demographic, clinical and laboratory findings of HIV-infected and HIV-uninfected patients with cryptococcosis.
| HIV-infected (n = 79)1,2 | HIV-uninfected (n = 47) | P value | |
|---|---|---|---|
| Male sex | 59/79 (74%) | 28/47 (59%) | .11 |
| Age, median years (range) | 44 (14–70) | 57 (20–87) | <.0001 |
| Race Hispanic Black White Other | 26/79 (32%)47/79 (59%)1/79 (1%)5/79 (6%) | 13/47 (27%)19/47 (40%)5/47 (10%)10/47 (21%) | .55.04.02.02 |
| History of substance abuse | 26/74 (35%) | 19/44 (43%) | .43 |
| On cARTNot on cART cART naive cART experienced (nonadherent) | 15/74 (20%)359/74 (80%)14/74 (19%)45/74 (61%) | N/A | |
| CD4 T-cell count (cells/mm3), median (range) | 27 (1–341) (n = 75) | 427 (8–1044) (n = 14) | <.0001 |
| HIV RNA VL (copies/mL), median (range) | 73,545 (75–7,500,000) (n = 70) | N/A |
Data presented as no. (%) unless otherwise indicated.
cART, combination antiretroviral therapy; HIV, human immunodeficiency virus; N/A, not applicable; VL, viral load.
18 patients were newly diagnosed with HIV when they presented with cryptococcosis.
21 patient from West Africa was infected with HIV-2; 4 patients had congenital HIV infection
34 patients started cART within the past 30 days prior to diagnosis of cryptococcosis.
Figure 1.
Number of hospitalizations for cryptococcosis in HIV-infected and HIV-uninfected individuals during the 12 years at Montefiore Medical Center (2005–2017). The total number of hospitalizations for cryptocccosis is shown on the Y axis for HIV-infected and HIV-uninfected individuals as indicated in the legend for the years shown on the X axis. This Figure is reproduced in color in the online version of Medical Mycology.
Baseline demographics, underlying conditions, and sites of involvement
The male sex was predominant in both groups (Table 1). Compared to HIV-uninfected patients, HIV-infected patients were younger (44 vs 57 years, P < .0001), and more were Hispanic or black in ethnicity/race (92% vs 68%, P = .0009). There was no significant difference in rate of substance use between the two groups (35% vs 43%, P = .43) (Table 1). In sum, 59/74 (80%) of the patients were not on combination antiretroviral therapy (cART), among whom 45/74 (61%) were nonadherent to cART and 14/74 (19%) were cART-naive. Of the 15 patients on cART, four (27%) were started on therapy within 30 days prior to presentation. The diagnosis of HIV was made in 8/79 (10%) of the HIV-infected patients at the time of presentation with cryptococcosis. The median CD4 cell count of HIV-infected patients was 27 (range, 1–341) cells/mm3 and their median HIV viral load (VL) was 73,545 (range, 75–7,500,000) copies/mL (Table 1). The median CD4 cell count of those who were receiving cART at the time of presentation was 45 cells/mm3 (n = 15).
Of the HIV-uninfected patients, 33/46 (72%) were on chronic prednisone, 24/47 (51%) were solid organ transplant (SOT) recipients all of whom were on chronic steroids, 8/47 (17%) had an autoimmune condition, 7/47 (14%) had malignancy, 4/47 (8%) had cirrhosis, 2/47 (4%) had chronic obstructive pulmonary disease (COPD) on inhaled steroids, and 2/47 (4%) had no apparent immunocompromising condition (Table 2). Three of the non-HIV infected patients had CD4 T cell count <300 cells/mm3 (one with cirrhosis, one with possible sarcoidosis, and one with no immunocompromising condition).
Table 2.
Clinical variables associated with cryptococcosis in HIV-uninfected patients.
| Variable | No. (%) (n = 47) |
|---|---|
| Receipt of immunosuppressive agents Prednisone only Prednisone + MMF + tacrolimus Prednisone + MMF Prednisone + others (tocilizumab) Inhaled corticosteroids Others (methotrexate, interferon, thalidomide, blinatumomab) | 38/46 (82%)5/46 (11%)25/46 (54%)2/46 (4%)1/46 (2%)2/46 (4%)3/46 (7%) |
| Immunocompromising condition | |
| Solid organ transplantation | 24/47 (51%) |
| Autoimmune disease | 8/47 (17%) |
| Malignancy | 7/47 (14%) |
| Cirrhosis | 4/47 (8%) |
| Asthma/COPD on inhaled steroids | 2/47 (4%) |
| Normal host | 2/47 (4%) |
| Type of transplant | |
| Liver | 2/24 (8%) |
| Heart | 1/24 (4%) |
| Kidney | 20/24 (83%) |
| Liver/Kidney | 1/24 (4%) |
| Weaning of immunosuppressive agents after diagnosis of cryptococcosis | 31/38 (82%) |
| High dose corticosteroid use for C-IRIS | 5/47 (11%) |
Data presented as no. (%) unless indicated otherwise.
C-IRIS, cryptococcus-associated immune reconstitution inflammatory syndrome; COPD, chronic obstructive pulmonary disease; MMF, mycophenolate mofetil.
The CNS was the most common site of infection in HIV-infected and HIV-uninfected patients (64 vs 63%, P > .99), followed by blood stream infection (47 vs 27%, P = .04) (Table 3). HIV-uninfected patients were more likely to have non-bloodstream extra-neural involvement, including pulmonary, skin, soft tissue, larynx, or osteo-articular sites than HIV-infected patients although this difference was not statistically significant (36 vs 20%, P = .06) (Table 3).
Table 3.
Clinical and cerebral spinal fluid (CSF) findings, time to infectious diseases (ID) consult/diagnosis, treatment and outcomes in HIV-infected and HIV-uninfected patients with cryptococcosis.
| HIV-infected patients (n = 79, CM in 51) | HIV-uninfected patients (n = 47, CM in 30) | P value | |
|---|---|---|---|
| Sites of infection CNS Bloodstream Pulmonary Skin, soft-tissue, or osteoarticular Others (stomach, placenta, larynx) | 51/79 (64%)34/72 (47%)11/79 (13%)3/79 (3%)2/79 (2%) | 30/47 (63%)11/40 (27%)13/47 (27%)3/47 (6%)1/47 (2%) | >.99.04.06.67>.99 |
| Serum cryptococcal antigen titer, median (range) | 512 (2–65,536) (n = 73) | 64 (2–16,384) (n = 41) | .0002 |
| Presenting symptoms and CSF findings in those with CM | |||
| Headache | 45/51 (88%) | 15/30 (50%) | .0004 |
| Fever | 48/51 (94%) | 23/30 (76%) | .03 |
| Altered mental status | 27/51 (52%) | 17/30 (56%) | .81 |
| Photophobia | 15/51 (29%) | 4/30 (13%) | .11 |
| Blurred vision | 6/51 (11%) | 0/30 (0%) | .07 |
| Seizures | 6/51 (11%) | 2/30 (6%) | .70 |
| Dizziness | 7/51 (13%) | 3/30 (10%) | .73 |
| Nausea and/or vomiting | 18/51 (35%) | 11/30 (36%) | >.99 |
| Fatigue, lethargy | 9/51 (17%) | 9/30 (30%) | .26 |
| Fall | 3/51 (20%) | 3/30 (10%) | .66 |
| Opening pressure ≥ 25 cmH2O | 21/46 (45%) | 9/18 (50%) | .78 |
| Positive CSF culture | 28/46 (60%) | 10/30 (33%) | .03 |
| CSF glucose, g/l, median (range) | 52 (5–683) (n = 51) | 70 (8–175) (n = 30) | .001 |
| CSF WBC, cells/mm3, median (range) | 6 (0–590) (n = 51) | 17 (0–1550) (n = 30) | .06 |
| CSF protein, g/l, median (range) | 47 (6–249) (n = 51) | 56 (8–622) (n = 30) | .12 |
| Time to diagnosis from symptom onset, days, median (range) | 8 (1–36) (n = 71) | 13 (1–240) (n = 43) | .047 |
| Hospital day ID consult was requested, days, median (range) | 1 (1–19) (n = 68) | 2 (1–30) (n = 43) | .24 |
| Hospital day diagnosis was made, days, median (range) | 2 (1–20) (n = 69) | 6 (1–60) (n = 44) | <.0001 |
| Induction treatment for CM Amphotericin + flucytosine Amphotericin Fluconazole | 43/51 (84%)7/51 (14%)1/51 (2%) | 23/29 (79%)3/29 (10%)3/29 (10%) | .76>.99.12 |
| Paradoxical C-IRISUnmasking C-IRIS | 5/79 (6%)3/79 (4%)1 | 5/47 (10%) | .49 |
| Readmission 6 month 1 year | 13/79 (16%)21/79 (26%) | 0/47 (0%)1/47 (2%) | .001.0002 |
| Lost to follow-up 30 days 1 year | 11/79 (14%)24/79 (30%) | 3/47 (6%)5/47 (11%) | .24.02 |
| Mortality 30-day mortality 1-year mortality | 4/68 (6%)7/55 (13%) | 9/44 (20%)10/42 (24%) | .03.18 |
| Cause of death Cryptococcosis | 3/7 (43%) | 6/10 (60%) | .63 |
Data presented as no. (%) unless indicated otherwise.
C-IRIS, cryptococcus-associated immune reconstitution inflammatory syndrome; CM, cryptococcal meningitis; CNS, central nervous system; CSF, cerebrospinal fluid; ID, infectious diseases; WBC, white blood cell.
11 case was diagnosed prior to 2012 when serum cryptococcal antigen screening was being routinely recommended in the United States.
Serological results
HIV-infected patients had higher median serum CrAg titers than HIV-uninfected patients (512 versus 64, P = .0002) (Table 3).
Signs and symptoms
Among patients with cryptococcal meningitis, HIV-infected patients were more likely to present with a headache (88% vs 50%, P = .0004) and a fever (94% vs 76%, P = .03) than HIV-uninfected patients (Table 3). There was no significant difference between the two groups in regards to altered mental status, photophobia, blurred vision, seizures, dizziness, nausea and vomiting, fatigue, or fall as part of their initial presenting symptoms (Table 3).
CSF findings
CSF findings for the 51 HIV-infected and 30 HIV-uninfected patients with cryptococcal meningitis are shown in Table 3. The CSF CrAg was positive in all patients with cryptococcal meningitis. An OP ≥ 25 cmH2O was common in both groups (45% vs 50%, P = .78). HIV-uninfected patients had lower CSF culture positivity (33% vs 60%, P = .03) and higher glucose levels (70 vs 52 g/l, P = .001) than HIV-infected patients. There was no significant difference in CSF leukocyte counts and protein levels between the two groups (Table 3).
Diagnosis
The median time to diagnosis from documented symptom onset for HIV-infected and HIV-uninfected patients were 8 days (range, 1–36 days) and 13 days (range, 1–240 days), respectively (P = .047) (Table 3). For the SOT recipients, the median time from transplant to cryptococcosis was 11 months (n = 20; range, 6 days–16 years). The median number of hospital days until an ID consultation was requested for HIV-infected and HIV-uninfected were 1 day (range, 1–19 days) and 2 days (range, 1–30 days), respectively (P = .24), and the median number of hospital days until the diagnosis of cryptococcosis was made were 2 days (range, 1–20 days) and 6 days (1–60 days), respectively (P < .0001) (Table 3).
Among HIV-uninfected patients, cryptococcosis was in the initial differential diagnosis of either the ID consultant or the primary team in 53% (24/45) of the cases. In 46% (21/45) of cases when cryptococcosis was not in the initial differential diagnosis, cryptococcosis was diagnosed after cultures from the blood, CSF, lung tissue, or histopathology unexpectedly yielded C. neoformans.
Antifungal therapy
The main induction treatment in both groups was an amphotericin B-based regimen administered per Infectious Diseases Society of America (IDSA) guidelines. Among the HIV-uninfected group with cryptococcal meningitis, 23/29 (79%) of the patients were treated with 2 weeks of amphotericin and flucytosine and 3/29 (10%) received 4–6 weeks of induction therapy with amphotericin (Table 3). Among HIV-uninfected patients with cryptococcosis, 23/42 (55%) developed acute renal insufficiency and 2/42 (5%) developed cytopenia during treatment with antifungal therapy.
Complications and mortality
In HIV-infected patients, five patients had clinical presentations suggestive of paradoxical C-IRIS and three had unmasking C-IRIS based on the case definitions outlined by Haddow et al.14 In non-HIV infected patients, there were five cases of paradoxical C-IRIS as defined by Singh et al.15,16 in the setting of antifungal therapy and reduction of immunosuppression (Table 3). Four cases occurred in SOT recipients and one in a patient with rheumatoid arthritis on chronic prednisone. All five patients had CNS involvement with persistently elevated intracranial pressure, had higher initial median serum CrAg titer compared to those who did not develop C-IRIS (1024 vs 64, P = .15), and had worsening mass lesion in the CNS (2/5) or lungs (1/5) concerning for cryptococcomas during anti-fungal treatment. All cases occurred within 2 to 6 weeks of anti-fungal therapy and reduction in immunosuppression. All SOT recipients were on tacrolimus, mycophenolate mofetil (MMF), and prednisone. Once diagnosis of cryptococcosis was made, MMF was held and tacrolimus and prednisone were given at a lower dose; 75% (3/4) of the transplant recipient with C-IRIS developed graft failure. All five patients required serial lumbar punctures or lumbar drain placement and were started on a course of high-dose corticosteroids with clinical improvement and survival at 1 year.
HIV-infected patients were significantly more likely to be readmitted in 6 months (16 vs 0%, P = .001), in 1 year (26 vs 2%, P = .0002) and more likely to be lost to follow up in 1 year (30 vs 11%, P = .02) (Table 3). At 1 year follow-up, 20/44 (45%) patients had detectable viral load.
Overall, 1-year mortality was 18% (17/97). HIV-uninfected patients had significantly higher 30-day mortality (20 vs 6%, P = .03) and higher 1-year mortality that was not significant (24 vs 13%, P = .18) (Table 3). Death was attributable to cryptococcosis in 43% of HIV-infected group and 60% of HIV-uninfected group. In univariate analysis, factors associated with mortality at 30 days included: older age (P = .001), elevated CSF white blood cell count (WBC) (P = .04), longer time to ID consultation (P = .0009), and longer time to diagnosis of cryptococcosis (P = .006) (Table 4). In multivariate analysis, older age (OR 5.03, P = .01) and longer time to ID consultation (OR 2.49, P = .03) were significantly associated with mortality at 30 days (Table 4).
Table 4.
Factors associated with 30-day mortality in patients with cryptococcosis.
| Univariate logistic regression1 | Multiple logistic regression | ||||
|---|---|---|---|---|---|
| Variable | Odds ratio | P value | P trend2 | Odds ratio | P value |
| Age, years | |||||
| 1–37 | Reference | ||||
| 38–48 | 0.87 | .92 | 0.001 | 5.03 | .01 |
| 49–58 | 1.13 | .93 | |||
| 59–87 | 15.9 | .01 | |||
| Positive blood culture | 0.79 | .72 | |||
| Positive CSF culture | 1.53 | .51 | |||
| Cryptococcal meningitis | 3.58 | .11 | |||
| CSF WBC, cells/mm3 | |||||
| 0–1 | Reference | ||||
| 2–7 | 4.00 | .27 | 0.04 | 1.75 | .13 |
| 8–38 | 6.10 | .12 | |||
| 39–1550 | 8.42 | .06 | |||
| CSF CrAg reactive | 6.74 × 107 | .99 | |||
| Time to ID consultation, days | |||||
| 1–2 | Reference | ||||
| 2–3 | 4.24 × 10−7 | .99 | 0.0009 | 2.49 | .03 |
| 3–4 | 12.3 | .05 | |||
| 4–30 | 25.9 | .003 | |||
| Time to diagnosis, days | |||||
| 1–2 | Reference | ||||
| 2–3 | 1 | 1 | 0.006 | 1.56 | .5 |
| 3–7 | 6.50 × 107 | .99 | |||
| 7–60 | 1.05 × 108 | .99 | |||
| Amphotericin and flucytosine | 1.23 | .73 | |||
| Amphotericin alone | 1.82 | .4 | |||
| Fluconazole alone | 0.22 | .16 | |||
Note: The continuous variables were categorized by quartiles for the regression analysis. R was used for analysis. CSF, cerebrospinal fluid; ID, infectious diseases; WBC, white blood cell.
1The reference group is group 1. All other groups were compared with group 1.
2The groups were considered as an ordeal variable.
Discussion
In our center, 63% of cryptococcosis cases over the 12-year period from 2005 to 2017 occurred in HIV-infected persons. Most cases occurred in patients who were nonadherent with or not on cART; the remainder were newly diagnosed with HIV and/or exhibited unmasking C-IRIS. Consistent with earlier reports that pre-date the cART era,6,17,18 HIV-infected patients with cryptococcosis were younger, had a shorter time from symptom onset to diagnosis, higher serum CrAg titer, more positive blood and CSF cultures, and were more likely to present with a headache and a fever than HIV-uninfected patients. The majority of cases occurred in racial and ethnic minorities with a history of substance abuse. The latter is closely linked to poor medical compliance and a break in linkage to care.19
Others have reported a declining incidence of cryptococcosis in HIV-infected compared to non-HIV individuals in the cART era.7,17,20–24 In the Bronx, there has been a decrease in HIV diagnoses and an increase in people living with HIV/AIDS, among whom 69% had a suppressed viral load in year 2017 (New York City Department of Health HIV/AIDS Annual Surveillance Statistics, 2001–2017). Nevertheless, our data show a consistent absolute number of cases of cryptococcosis in HIV-infected individuals in each year of this study, greater than that of HIV-uninfected individuals, reflecting failure to engage and retain marginalized group of individuals most at risk. This is becoming a challenge in other African and Latin American cohorts as well where over 50% of cryptococcosis cases are now being diagnosed in cART-experienced patients with universal access to cART.25–29 Few cases of unmasking C-IRIS that were noted in our cohort which represent missed opportunities to screen with CrAg prior to initiation of cART may also have occurred due to inconsistent medical follow-up and/or providers’ unfamiliarity with the guidelines.30 As “treatment as prevention” and “test and treat”31 strategies allow earlier diagnosis and treatment in cART-naive individuals, it would be important to place extra effort in engaging key at-risk population who remain vulnerable despite improving screening and treatment strategies.
Among HIV-uninfected patients with cryptococcosis, 24/47 (51%) were SOT recipients, and 33/46 (72%) were on chronic prednisone. This is reflective of SOT volume at MMC, where there were 270 SOTs performed in adults between July 2016 and June 2017 (Scientific Registry of Transplant Recipients). Cirrhosis, which is associated with high risk of disseminated cryptococcosis,32–34 was present in 4/47 (8%) of HIV-uninfected patients. This is a lower rate than in other centers, where 21 to 36% of HIV-uninfected cases occurred in patients with cirrhosis.2,32–35 Our rate of cases in those with no immunocompromising condition, 2/47 (4%), was also lower than that reported in other centers; up to 20% in the United States, Australia, and New Zealand,2,36,37 and 64% of CN cases in China38 occurred in non-immunocompromised patients.
The risk factors associated with cryptococcosis in otherwise phenotypically “normal” patients are not fully understood. One patient in our study with no obvious risk factors had CD4 T-cell count of 237 cells/μl concerning for undiagnosed idiopathic CD4 lymphopenia. While not tested in our patient, studies have shown presence of anti-granulocyte-macrophage colony-stimulating factor (GM-CSF) as a risk factor for CNS infection by Cryptococcus gattii in immunocompetent patients.39 Of note, two patients had inhaled corticosteroids use as their only risk factors and developed laryngeal and pulmonary cryptococcosis respectively. Inhaled corticosteroid use, while not generally recognized as a risk factor for disseminated cryptococcosis, has been reported to be associated with localized laryngeal infection with cryptococcosis.40,41
The lower rate of cryptococcosis in our HIV-uninfected patients may reflect a failure to pursue the diagnosis of cryptococcosis due to nonspecific presenting symptoms noted in our cohort, unfamiliarity of the disease by the clinicians, and/or limited sensitivity of the cultures in the setting of low fungal burden as reflected in our low blood/CSF culture positivity. In fact, cryptococcosis was in the initial differential diagnosis of HIV-uninfected patients in only 24/45 (53%) of the cases. In many cases, this led to a delay in evaluation by an infectious diseases specialist, which was significantly associated with 30-day mortality in our multivariate analysis. While involvement of ID consultant did not always lead to an earlier diagnosis, we speculate that it had facilitated adherence to standards of care and improved management of patients. Spec and others reported a higher 90-day mortality in patients with cryptococcosis who did not have an infectious diseases consultant involved in their care compared to those who did.42 Growing evidence supports early involvement of infectious diseases consultants for management of resistant and difficult to treat infections, which has been linked to improved patient care, mortality, and morbidity.43–45
Overall 30-day mortality in our cohort was 12% (13/112). Our analysis did not reveal associations between mortality and CrAg titer or blood/CSF culture positivity as reported in studies conducted in resource-limited countries.23,46 We did find direct associations between mortality and CSF pleocytosis in our univariate analysis, mortality, and older age in multivariate analysis and significantly higher 30-day mortality in HIV-uninfected than HIV-infected patients (20 vs 6%, P = .03). These findings stress the role of host immunity in mediating the balance between microbial clearance and damaging inflammatory process.47 The hypothesized factors contributing to higher mortality in HIV-uninfected patients include delayed diagnosis, poor tolerance to induction therapy, and/or paradoxical clinical worsening in the setting of relatively intact host immune system.12,23,33,36,47,48 While we did not find any association between induction regimen and mortality, frequent occurrence of acute renal insufficiency and cytopenia made treatment course challenging and the regimen was often based on expert opinion extrapolated from studies done in HIV-infected patients and retrospective studies.49–51
Of interest, 4/24 (17%) of SOT recipients with cryptococcosis developed paradoxical C-IRIS, which is a rate higher than 5–14% that is reported in the literature.16,52,53 We suspect that this may in part be due to use of potent immunosuppressive regimen and aggressive reduction in immunosuppression with the diagnosis of cryptococcosis. In our institution, management of immunosuppression was mostly guided by the organ transplant team and not necessarily based on a guideline that can take into account the degree of host immunity and/or fungal burden as is done with cART initiation in HIV-infected individuals.54 All of our transplant recipients had immunosuppression reduced with the diagnosis of cryptococcosis while some institutions reported only one-quarter of the transplant recipients to have their immunosuppressive regimen changed with very low prevalence of IRIS.21 Others have reported favorable outcome in SOT recipient with CNS cryptococcosis who had C-IRIS than those who did not which may be due to the pro-inflammatory responses55,56 or perhaps due to the aggressive medical intervention that follows after the diagnosis of C-IRIS to lower the intracranial pressure as was the case in our cohort. Long-term neurological morbidities of these patients are not well understood.57,58 These findings highlight the complicated interplay of host and fungal factors in susceptibility to cryptococcosis and C-IRIS.47 There are no predictive markers of C-IRIS in this cohort. Antibody repertoire differences have been identified in HIV-infected patients with C-IRIS compared to those without C-IRIS.59
There were several limitations to our study. It is retrospective and limited to a single tertiary care hospital with a relatively small cohort. Duration of symptoms reported by the patients is subject to recall bias. Time to ID consultation is subject to selection bias as those with solid organ transplant, HIV, and/or obvious clinical symptoms are more likely to have an ID evaluation requested earlier in the hospital course. Correlation does not necessarily prove causality and given high association between presenting symptoms and timing of ID consultation, it may be the subclinical manifestation that drove delayed diagnosis and mortality. Finally, we combined two or more different patient populations in the regression model with very different pathophysiology and may have confounding variables that were not taken into account.
Our findings underscore that cryptococcal disease is associated with a wide spectrum of clinical manifestations that likely depends on both pathogen and host factors. As a single center study in an area of high HIV prevalence with a majority of cryptococcosis cases in those with HIV infection, our data highlight the need for expanded efforts in HIV prevention, eradication, and linkage to routine HIV care for socioeconomically disadvantaged HIV-infected persons. Cryptococcosis continues to be an important disease with a poor prognosis that poses diagnostic and therapeutic challenges in HIV-uninfected patients that contribute to high mortality. Further study is needed to understand the factors that determine clinical expression and prognosis of disease in patients who are either immunosuppressed or immunocompetent.
Acknowledgements
We thank Dr. Jonathan Shuter and Dr. Joshua D. Nosanchuk for critical reading and comments.
Funding
This work was supported by the National Institutes of Health (R01-AI097096 to L.P.).
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and the writing of the paper.
References
- 1. Mitchell TG, Perfect JR. Cryptococcosis in the era of AIDS—100 years after the discovery of Cryptococcus neoformans. Clin Microbiol Rev. 1995; 8: 515–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pappas PG, Perfect JR, Cloud GA et al.. Cryptococcosis in human immunodeficiency virus-negative patients in the era of effective azole therapy. Clin Infect Dis. 2001; 33: 690–699. [DOI] [PubMed] [Google Scholar]
- 3. Perfect JR, Casadevall A. Cryptococcosis. Infect DisClin North Am. 2002; 16: 837–874, v–vi. [DOI] [PubMed] [Google Scholar]
- 4. Gupta S, Ellis M, Cesario T, Ruhling M, Vayuvegula B. Disseminated cryptococcal infection in a patient with hypogammaglobulinemia and normal T cell functions. Am J Med. 1987; 82: 129–131. [DOI] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prvention (CDC) HIV surveillance supplemental report. CDC; Atlanta, GA, USA: 2016; available online https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2016-vol-28.pdf. [Google Scholar]
- 6. Mirza SA, Phelan M, Rimland D et al.. The changing epidemiology of cryptococcosis: an update from population-based active surveillance in 2 large metropolitan areas, 1992–2000. Clin Infect Dis. 2003; 36: 789–794. [DOI] [PubMed] [Google Scholar]
- 7. Pyrgos V, Seitz AE, Steiner CA, Prevots DR, Williamson PR. Epidemiology of cryptococcal meningitis in the US: 1997–2009. PloS One. 2013; 8: e56269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. El Bcheraoui C, Mokdad AH, Dwyer-Lindgren L et al.. Trends and patterns of differences in infectious disease mortality among US counties, 1980–2014. JAMA. 2018; 319: 1248–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mourad A, Perfect JR. The war on cryptococcosis: a review of the antifungal arsenal. Mem Inst Oswaldo Cruz. 2018; 113: e170391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Messina JA, Maziarz EK, Spec A, Kontoyiannis DP, Perfect JR. Disseminated cryptococcosis with brain involvement in patients with chronic lymphoid malignancies on ibrutinib. Open Forum Infect Dis. 2017; 4: ofw261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Munoz P, Giannella M, Valerio M et al.. Cryptococcal meningitis in a patient treated with infliximab. DiagncMmicrobiol Infect Dis. 2007; 57: 443–446. [DOI] [PubMed] [Google Scholar]
- 12. George IA, Spec A, Powderly WG, Santos CAQ. Comparative epidemiology and outcomes of human immunodeficiency virus (HIV), non-HIV non-transplant, and solid organ transplant associated cryptococcosis: a population-based study. Clin Infect Dis. 2018; 66: 608–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zonios DI, Falloon J, Huang CY, Chaitt D, Bennett JE. Cryptococcosis and idiopathic CD4 lymphocytopenia. Medicine. 2007; 86: 78–92. [DOI] [PubMed] [Google Scholar]
- 14. Haddow LJ, Colebunders R, Meintjes G et al.. Cryptococcal immune reconstitution inflammatory syndrome in HIV-1-infected individuals: proposed clinical case definitions. Lancet Infect Dis. 2010; 10: 791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Singh N, Lortholary O, Alexander BD et al.. An immune reconstitution syndrome-like illness associated with Cryptococcus neoformans infection in organ transplant recipients. Clin Infect Dis. 2005; 40: 1756–1761. [DOI] [PubMed] [Google Scholar]
- 16. Baddley JW, Forrest GN. Cryptococcosis in solid organ transplantation: guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019: e13543. [DOI] [PubMed] [Google Scholar]
- 17. Dromer F, Mathoulin S, Dupont B, Laporte A. Epidemiology of cryptococcosis in France: a 9-year survey (1985–1993). French Cryptococcosis Study Group. Clin Infect Dis. 1996; 23: 82–90. [DOI] [PubMed] [Google Scholar]
- 18. Powderly WG. Cryptococcal meningitis and AIDS. Clin Infect Dis. 1993; 17: 837–842. [DOI] [PubMed] [Google Scholar]
- 19. Bulsara SM, Wainberg ML, Newton-John TRO. Predictors of adult retention in HIV care: a systematic review. AIDS Behav. 2018; 22: 752–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shaheen AA, Somayaji R, Myers R, Mody CH. Epidemiology and trends of cryptococcosis in the United States from 2000 to 2007: a population-based study. Int J STD AIDS. 2018; 29: 453–460. [DOI] [PubMed] [Google Scholar]
- 21. Bratton EW, El Husseini N, Chastain CA et al.. Comparison and temporal trends of three groups with cryptococcosis: HIV-infected, solid organ transplant, and HIV-negative/non-transplant. PloS One. 2012; 7: e43582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dromer F, Mathoulin-Pelissier S, Fontanet A, Ronin O, Dupont B, Lortholary O. Epidemiology of HIV-associated cryptococcosis in France (1985–2001): comparison of the pre- and post-HAART eras. AIDS. 2004; 18: 555–562. [DOI] [PubMed] [Google Scholar]
- 23. Brizendine KD, Baddley JW, Pappas PG. Predictors of mortality and differences in clinical features among patients with Cryptococcosis according to immune status. PloS One. 2013; 8: e60431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hevey M, George IA, Raval K, Powderly WG, Spec A. Presentation and mortality of cryptococcal infection varies by predisposing illness, a retrospective cohort study. Am J Med. 2019. doi: 10.1016/j.amjmed.2019.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tenforde MW, Mokomane M, Leeme T et al.. Advanced human immunodeficiency virus disease in Botswana following successful antiretroviral therapy rollout: incidence of and temporal trends in cryptococcal meningitis. Clin Infect Dis. 2017; 65: 779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jarvis JN, Boulle A, Loyse A et al.. High ongoing burden of cryptococcal disease in Africa despite antiretroviral roll out. AIDS. 2009; 23: 1182–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Azambuja AZ, Wissmann Neto G, Watte G, Antoniolli L, Goldani LZ. Cryptococcal meningitis: a retrospective cohort of a Brazilian reference hospital in the post-HAART era of universal access. Can J Infect Dis Med Microbiol 2018; 2018: 6512468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lortholary O, Poizat G, Zeller V et al.. Long-term outcome of AIDS-associated cryptococcosis in the era of combination antiretroviral therapy. AIDS. 2006; 20: 2183–2191. [DOI] [PubMed] [Google Scholar]
- 29. Crabtree Ramirez B, Caro Vega Y, Shepherd BE et al.. Outcomes of HIV-positive patients with cryptococcal meningitis in the Americas. Int J Infect Dis. 2017; 63: 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Spec A, Mejia-Chew C, Powderly WG, Cornely OA. EQUAL Cryptococcus ccore 2018: a European confederation of medical mycology score derived from current guidelines to measure QUALity of clinical cryptococcosis management. Open Forum Infect Dis. 2018; 5: ofy299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Smith K, Powers KA, Kashuba AD, Cohen MS. HIV-1 treatment as prevention: the good, the bad, and the challenges. Curr Opin HIV AIDS. 2011; 6: 315–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Singh N, Sifri CD, Silveira FP et al.. Cryptococcosis in patients with cirrhosis of the liver and posttransplant outcomes. Transplantation. 2015; 99: 2132–2141. [DOI] [PubMed] [Google Scholar]
- 33. Spec A, Raval K, Powderly WG. End-stage liver disease is a strong predictor of early mortality in cryptococcosis. Open Forum Infect Dis. 2016; 3: ofv197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Singh N, Husain S, De Vera M, Gayowski T, Cacciarelli TV. Cryptococcus neoformans infection in patients with cirrhosis, including liver transplant candidates. Medicine. 2004; 83: 188–192. [DOI] [PubMed] [Google Scholar]
- 35. Chuang YM, Ho YC, Chang HT, Yu CJ, Yang PC, Hsueh PR. Disseminated cryptococcosis in HIV-uninfected patients. Eur Jf Clin Microbiol Iinfect Dis. 2008; 27: 307–310. [DOI] [PubMed] [Google Scholar]
- 36. Pappas PG. Cryptococcal infections in non-HIV-infected patients. Trans Am Clin Climatol Assoc. 2013; 124: 61–79. [PMC free article] [PubMed] [Google Scholar]
- 37. Chen S, Sorrell T, Nimmo G et al.. Epidemiology and host- and variety-dependent characteristics of infection due to Cryptococcus neoformans in Australia and New Zealand: Australasian Cryptococcal Study Group. Clin Infect Dis. 2000; 31: 499–508. [DOI] [PubMed] [Google Scholar]
- 38. Liu Y, Kang M, Wu SY et al.. Different characteristics of cryptococcal meningitis between HIV-infected and HIV-uninfected patients in the Southwest of China. Med Mycol. 2017; 55: 255–261. [DOI] [PubMed] [Google Scholar]
- 39. Saijo T, Chen J, Chen SC et al.. Anti-granulocyte-macrophage colony-stimulating factor autoantibodies are a risk factor for central nervous system infection by Cryptococcus gattii in otherwise immunocompetent patients. mBio. 2014; 5: e00912–00914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wong DJY, Stanley P, Paddle P. Laryngeal cryptococcosis associated with inhaled corticosteroid use: case reports and literature review. Front Surg. 2017; 4: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Quintero O, Trachuk P, Lerner MZ, Sarungbam J, Pirofski LA, Park SO. Risk factors of laryngeal cryptococcosis: a case report. Med Mycol Case Rep. 2019; 24: 82–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Spec A, Olsen MA, Raval K, Powderly WG. Impact of infectious diseases consultation on mortality of cryptococcal infection in patients without HIV. Clin Infect Dis. 2017; 64: 558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bai AD, Showler A, Burry L et al.. Impact of infectious disease consultation on quality of care, mortality, and length of stay in Staphylococcus aureus bacteremia: results from a large multicenter cohort study. Clin Infect Dis. 2015; 60: 1451–1461. [DOI] [PubMed] [Google Scholar]
- 44. Burnham JP, Olsen MA, Stwalley D, Kwon JH, Babcock HM, Kollef MH. Infectious diseases consultation reduces 30-day and 1-year all-cause mortality for multidrug-resistant organism infections. Open Forum Infect Dis. 2018; 5: ofy026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hamandi B, Husain S, Humar A, Papadimitropoulos EA. Impact of infectious disease consultation on the clinical and economic outcomes of solid organ transplant recipients admitted for infectious complications. Clin Infect Dis. 2014; 59: 1074–1082. [DOI] [PubMed] [Google Scholar]
- 46. Jarvis JN, Bicanic T, Loyse A et al.. Determinants of mortality in a combined cohort of 501 patients with HIV-associated cryptococcal meningitis: implications for improving outcomes. Clin Infect Dis. 2014; 58: 736–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pirofski LA, Casadevall A. Immune-mediated damage completes the parabola: Cryptococcus neoformans pathogenesis can reflect the outcome of a weak or strong immune response. mBio. 2017; 8: e02063–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Neal LM, Xing E, Xu J et al.. CD4+ T cells orchestrate lethal immune pathology despite fungal clearance during Cryptococcus neoformans meningoencephalitis. mBio. 2017; 8: e01415–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ecevit IZ, Clancy CJ, Schmalfuss IM, Nguyen MH. The poor prognosis of central nervous system cryptococcosis among nonimmunosuppressed patients: a call for better disease recognition and evaluation of adjuncts to antifungal therapy. Clin Infect Dis. 2006; 42: 1443–1447. [DOI] [PubMed] [Google Scholar]
- 50. Dromer F, Bernede-Bauduin C, Guillemot D, Lortholary O. Major role for amphotericin B-flucytosine combination in severe cryptococcosis. PloS One. 2008; 3: e2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Perfect JR, Dismukes WE, Dromer F et al.. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2010; 50: 291–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sun HY, Alexander BD, Huprikar S et al.. Predictors of immune reconstitution syndrome in organ transplant recipients with cryptococcosis: implications for the management of immunosuppression. Clin Infect Dis. 2015; 60: 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sun HY, Singh N. Immune reconstitution inflammatory syndrome in non-HIV immunocompromised patients. Curr Opin Infect Dis. 2009; 22: 394–402. [DOI] [PubMed] [Google Scholar]
- 54. Boulware DR, Meya DB, Muzoora C et al.. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. New Engl J Med. 2014; 370: 2487–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Singh N, Lortholary O, Dromer F et al.. Central nervous system cryptococcosis in solid organ transplant recipients: clinical relevance of abnormal neuroimaging findings. Transplantation. 2008; 86: 647–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shelburne SA, Darcourt J 3rd, White AC Jr et al.. The role of immune reconstitution inflammatory syndrome in AIDS-related Cryptococcus neoformans disease in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005; 40: 1049–1052. [DOI] [PubMed] [Google Scholar]
- 57. Marr KA, Sun Y, Spec A et al.. A multicenter, longitudinal cohort study of cryptococcosis in HIV-negative people in the United States. Clin Infect Dis. 2019. doi: 10.1093/cid/ciz193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pasquier E, Kunda J, De Beaudrap P et al.. Long-term mortality and disability in cryptococcal meningitis: a systematic literature review. Clin Infect Dis. 2018; 66: 1122–1132. [DOI] [PubMed] [Google Scholar]
- 59. Yoon HA, Nakouzi A, Chang CC et al.. Association between plasma antibody responses and risk for Cryptococcus-associated immune reconstitution inflammatory syndrome. J Infect Dis. 2019; 219: 420–428. [DOI] [PMC free article] [PubMed] [Google Scholar]