Abstract
The incidence of invasive fungal diseases (IFDs) with central nervous system (CNS) involvement is increasing due to the rising numbers of immunocompromised individuals, such as patients receiving chemotherapy, transplantation procedures, or immune-modulating therapies. CNS IFDs cause significant morbidity and mortality, and treatments are complicated by difficulties in identifying fungal pathogens and delivering antifungal agents to the CNS. Isavuconazole is a novel triazole with broad-spectrum activity that has shown good blood–brain barrier penetration in animal models. We present a retrospective analysis of isavuconazole in the treatment of patients with CNS IFDs and who either participated in the phase III VITAL or SECURE clinical trials, or were included in a named-patient program. A total of 36 patients were identified, including 27 patients from the clinical trials. Of these patients, 47.2% had hematologic malignancies, while 13.9% had no identifiable underlying conditions. Mucorales, Aspergillus species, and Cryptococcus species accounted for 30.6%, 22.2%, and 13.9% of infections, respectively. The overall survival rate was 80.6% at day 42 and 69.4% at day 84, and at the end of treatment, a complete or partial clinical response was achieved in 58.3% of patients. Isavuconazole exhibited clinical activity in a variety of CNS IFDs.
Keywords: Aspergillus, central nervous system, isavuconazole, invasive fungal diseases, Mucorales
Introduction
Invasive fungal diseases (IFDs) are significant causes of morbidity and mortality in patients with decreased immune function.1-3 IFDs of the central nervous system (CNS) typically originate from a primary site outside of the nervous system or occur after invasive procedures such as neurosurgical or vascular intervention.1 CNS involvement is common in Cryptococcus infections,4 but rare for most other fungal species. However, the incidence is increasing with more widespread use of novel antineoplastic agents.5-7 In addition, some fungi (e.g., Cladophialophora bantiana) exhibit neurotropism, causing CNS infections in healthy individuals.8-10
Diagnostic confirmation of IFD of the CNS is frequently challenging, as cerebrospinal fluid often has a low yield for infecting pathogens,11 while tissue biopsy is invasive and frequently not feasible. Furthermore, treatment with antifungal agents is frequently compromised by resistance and limited CNS penetration.1 As such, CNS IFDs frequently progress rapidly and are associated with high mortality rates.1,12
Combination therapy with amphotericin B and flucytosine remains the optimal initial approach in cryptococcal meningitis.3,13 However, amphotericin B displays limited CNS penetration, is associated with toxicity, including renal adverse events, and is largely ineffective in CNS mould infections.14-16 Among azoles, fluconazole is effective against Cryptococcus and Candida spp., whereas voriconazole has good CNS penetration and has been shown to be effective against a broad spectrum of fungal pathogens but is ineffective against Mucorales.12,17
Isavuconazole is a novel triazole with a similar mode of action to voriconazole but with broad-spectrum activity encompassing a wider range of fungal species, including Mucorales. Isavuconazole demonstrates blood–brain barrier penetration in animal models,18 and clinical efficacy has been reported in a single patient with disseminated mucormycosis and CNS involvement, who was treated with isavuconazole under the Emergency Investigational New Drug program.19 The phase III VITAL trial (NCT00634049) evaluated isavuconazole as primary therapy in patients infected with rare fungi, principally mucormycosis, or renally impaired patients with aspergillosis.20 A subgroup analysis of this trial evaluated the efficacy of isavuconazole against Cryptococcus species and dimorphic fungi.21 The phase III SECURE trial (NCT00412893) compared isavuconazole with voriconazole as a primary treatment for infections caused by Aspergillus species or other filamentous fungi.22 Isavuconazole was also made available as part of a named patient program (NPP), ahead of country-specific approvals for use in IFD.23,24
The aim of this analysis was to assess the effectiveness of isavuconazole in the treatment of CNS IFDs, by examining relevant cases from the two isavuconazole phase III clinical trials and the NPP.
Methods
Data were collated from the VITAL20 and SECURE trials22 as well as from the NPP. Disseminated IFD or CNS IFD were regarded as proven by histopathology or culture of fungal species from sterile body sites including cerebrospinal fluid, brain fine-needle aspiration, or brain biopsy using EORTC/(Mycoses Study Group [MSG] criteria.25 In both trials, patients with probable IFD were also included. For cases of probable disseminated IFD, confirmation by CNS imaging, either by magnetic resonance imaging (MRI) or computed tomography (CT), in conjunction with a positive direct or indirect mycology test from another site (blood, sinus, lower respiratory tract), was regarded as evidence of CNS involvement. Both trials and the NPP excluded patients with Candida infections.
Adult dosages were consistent with the prescribing information. In pediatric patients, the dosages varied and were adjusted to maintain a target trough plasma level of 2000–4000 ng/ml (the approximate average adult trough plasma level observed in phase III studies).23,24
Data regarding patient demographics, treatments, and outcomes were recorded and summarized. The primary outcome measure was the overall rate of survival at day 42 and day 84. Additionally, overall response based on clinical, mycological, and radiological response was examined, as assessed by a data review committee for the clinical trials,20,22 or assessed by the treating physicians for NPP patients.
Results
In total, 36 patient cases were identified from the relevant studies. This included 23 of 146 patients from the VITAL study, four of 516 patients from the SECURE study who were randomized to the isavuconazole arm, and nine of 101 patients from the NPP. From the NPP, three pediatric patients were included.23,24 The demographic and baseline clinical characteristics of the included patients are provided in Table 1.
Table 1.
Demographic and baseline clinical characteristics, and associated survival.
| Survival | |||
|---|---|---|---|
| Characteristic | N = 36 n (%) | Day 42 n (%)a | Day 84 n (%)a |
| Age, years: | |||
| Median (range) | 51 (3–82) | NA | NA |
| <18 | 3 (8.3) | 3 (100) | 3 (100) |
| Male sex | 21 (58.3) | 14 (66.7) | 13 (61.9) |
| Underlying conditions: | |||
| Hematologic malignancy | 17 (47.2) | 12 (70.6) | 9 (52.9) |
| Allogeneic HCT | 5 (13.9) | 3 (60.0) | 2 (40.0) |
| Use of corticosteroids | 9 (25.0) | 9 (100) | 8 (88.9) |
| Neutropenia | 9 (25.0) | 7 (77.8) | 6 (66.7) |
| Diabetes mellitus | 5 (13.9) | 4 (80.0) | 4 (80.0) |
| Solid organ transplant | 3 (8.3) | 3 (100) | 2 (66.7) |
| Penetrating trauma | 2 (5.6) | 2 (100) | 2 (100) |
| Otherb | 4 (11.1) | 4 (100) | 4 (100) |
| None (at time of initial infection)c | 5 (13.9) | 4 (80.0) | 4 (80.0) |
Data are proportion (%) of patients, unless otherwise specified. Underlying condition counts total to more than 36, because patients may have more than one underlying condition.
HCT = hematopoietic stem cell transplantation, NA = not applicable.
aPercentages are numbers of patients with the characteristic who were alive at day 42 or day 84/total numbers with the characteristic.
bIncludes one each of: cell-mediated immune deficiency (uncontrolled), Crohn's disease, interstitial lung disease, and systemic lupus erythematosus.
cIncludes patients who had infections caused by Cryptococcus gattii (three), Exophiala species (one), and Paracoccidioides brasiliensis (one).
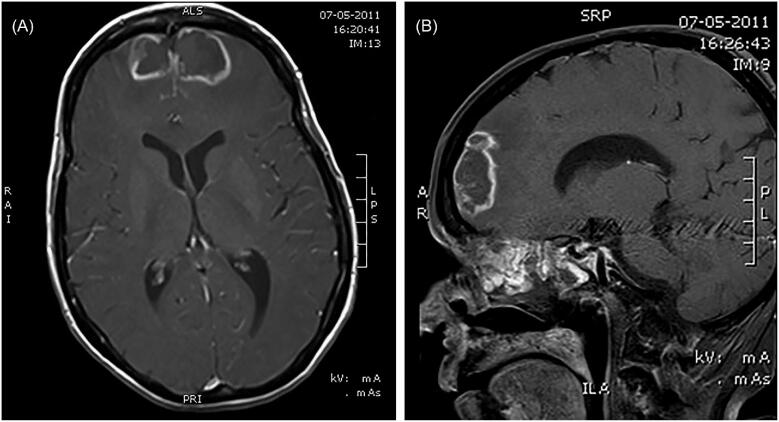
Eight patients had meningitis, caused by Cryptococcus species in five patients (C. gattii in three, C. neoformans in two), Exserohilum rostratum in two patients, and Exophiala species in one patient. Twenty-eight patients had brain abscesses. Proven CNS IFD was documented in 33 patients (91.7%), with probable infections in the remaining three patients (Table 2). All three patients with probable infections had CNS involvement (rhinocerebral disease) with positive culture from paranasal sinuses. Two of these patients were subsequently assessed using brain MRI; one of these patients had extensive sinusitis combined with bilateral frontal intracranial abscesses with extension into the brain parenchyma (Fig. 1); the other patient had pansinusitis, with fluid and fat stranding in the extraocular space, abnormal hyperintensity involving the extraocular muscles, and acute infarct in the right medial temporal region. The third patient's infection was subsequently confirmed by brain CT, which showed sinusitis combined with intracranial bleeding. Lower respiratory tract disease (LRTD) was identified in 12 patients (33.3%), with a difference in proportions between patients receiving primary therapy (9/20, 45.0%) compared with salvage therapy (3/16, 23.1%).
Table 2.
Disease categorization, lower respiratory tract involvement, therapy status, and associated survival.
| Survival | |||
|---|---|---|---|
| Characteristic | N = 36 n (%) | Day 42 n (%)a | Day 84 n (%)a |
| Disease categorization: | |||
| Proven | 33 (91.7) | 27 (81.8) | 24 (72.7) |
| Probable | 3 (8.3) | 2 (66.7) | 1 (33.3) |
| Lower respiratory tract involvement: | |||
| Yes | 12 (33.3) | 7 (58.3) | 6 (50.0) |
| No | 24 (66.7) | 22 (91.7) | 19 (79.2) |
| Therapy status: | |||
| Primary | 20 (55.6) | 14 (70.0) | 11 (55.0) |
| Salvage (refractory) | 11 (30.6) | 10 (90.9) | 9 (81.8) |
| Salvage (intolerant) | 2 (5.6) | 2 (100) | 2 (100) |
| Salvage (refractory and intolerant) | 3 (8.3) | 3 (100) | 3 (100) |
aPercentages are numbers of patients with the site of infection who were alive at day 42 or day 84/total numbers with the site of infection.
Figure 1.
Axial (A) and sagittal (B) MRI scans of extensive sinusitis combined with bilateral frontal intracranial abscesses in a single patient. MRI = magnetic resonance imaging.
Isavuconazole was administered as the primary therapy to 20 patients (55.6%), and as salvage therapy to 16 patients (44.4%). The median duration of isavuconazole therapy was 103.5 days (range, 2–882 days). Patients were switched to isavuconazole oral therapy after a median duration of 13.5 days (range, 2–91 days) of isavuconazole intravenous therapy.
Mucorales (11 patients; 30.6%) and Aspergillus species (8 patients; 22.2%) were the most commonly isolated types of fungi (Table 3). Cryptococcus species were observed in five patients (13.9%), and dimorphic fungi (Histoplasma capsulatum and Paracoccidioides brasiliensis) accounted for infections in one patient each (5.6% in total). Mixed infections were observed in three patients (8.3%), with Aspergillus species involved in each case as one of the IFD-causing organisms. Other rare moulds (C. bantiana, E. rostratum, Exophiala species, and other nonspeciated moulds) were observed in 16.7% of patients. At a species level, Aspergillus fumigatus and Rhizopus oryzae were the most common fungi identified, both appearing in five patients (13.9%). For A. fumigatus, four cases were monoinfections, and one case was a mixed infection accompanied by Verticillium tricorpus; all of the R. oryzae cases were monoinfections.
Table 3.
Mycological findings.
| Survival | |||
|---|---|---|---|
| Fungal organism | N = 36 n (%) | Day 42 n (%)a | Day 84 n (%)a |
| Aspergillus species | 8 (22.2) | 7 (87.5) | 5 (62.5) |
| Aspergillus fumigatus | 4 (11.1) | 4 (100) | 4 (100) |
| Aspergillus flavus | 2 (5.6) | 2 (100) | 1 (50.0) |
| Aspergillus flavus and Aspergillus terreus | 1 (2.8) | 0 (0) | 0 (0) |
| Not speciated | 1 (2.8) | 1 (100) | 0 (0) |
| Cryptococcus species | 5 (13.9) | 5 (100) | 5 (100) |
| Cryptococcus gattii | 3 (8.3) | 3 (100) | 3 (100) |
| Cryptococcus neoformans | 2 (5.6) | 2 (100) | 2 (100) |
| Dimorphic fungi | 2 (5.6) | 1 (50.0) | 1 (50.0) |
| Histoplasma capsulatum | 1 (2.8) | 1 (100) | 1 (100) |
| Paracoccidioides brasiliensis | 1 (2.8) | 0 (0) | 0 (0) |
| Mucorales | 11 (30.6) | 7 (63.6) | 7 (63.6) |
| Rhizopus oryzae | 5 (13.9) | 3 (60.0) | 3 (60.0) |
| Rhizomucor pusillus | 1 (2.8) | 0 (0) | 0 (0) |
| Mucor species (not speciated) | 1 (2.8) | 0 (0) | 0 (0) |
| Lichtheimia species (not speciated) | 1 (2.8) | 1 (100) | 1 (100) |
| Mucorales (not otherwise specified) | 3 (8.3) | 3 (100) | 3 (100) |
| Mixed infection | 3 (8.3) | 2 (66.7) | 1 (33.3) |
| Aspergillus terreus and Lichtheimia corymbifera | 1 (2.8) | 0 (0) | 0 (0) |
| Aspergillus fumigatus and Verticillium tricorpus | 1 (2.8) | 1 (100) | 0 (0) |
| Aspergillus niger and Mucorales (not otherwise specified) | 1 (2.8) | 1 (100) | 1 (100) |
| Other moulds | 6 (16.7) | 6 (100) | 5 (83.3) |
| Cladophialophora bantiana | 2 (5.6) | 2 (100) | 1 (50.0) |
| Exserohilum rostratum | 2 (5.6) | 2 (100) | 2 (100) |
| Exophiala species | 1 (2.8) | 1 (100) | 1 (100) |
| Not speciated | 1 (2.8) | 1 (100) | 1 (100) |
aPercentages are numbers of patients with the fungal organism who were alive at day 42 or day 84/total numbers with the fungal organism.
At the end of treatment, complete or partial clinical responses were achieved in 58.3% of patients (Table 4). Overall response rates were lower, with 36.1% of patients achieving a complete or partial overall response at the end of treatment. However, it should be noted that this is a composite endpoint of clinical, mycological, and radiological response, and that radiological response was previously noted to lag behind clinical improvement in both clinical trials.20,22
Table 4.
Clinical outcomes.
| Outcome | N = 36 n (%) |
|---|---|
| Clinical responsea at end-of-treatment: | |
| Complete/partial | 21 (58.3) |
| Complete | 11 (30.6) |
| Partial | 10 (27.8) |
| Failure | 15 (41.7) |
| Overall responseb at end-of-treatment: | |
| Complete | 7 (19.4) |
| Partial | 6 (16.7) |
| Stable | 6 (16.7) |
| Progression | 17 (47.2) |
| Survival at day 42 | 29 (80.6) |
| Survival at day 84 | 25 (69.4) |
aClinical response is defined as the resolution or partial resolution of all attributable clinical symptoms and physical findings.
bOverall response was based on a composite of clinical, mycological, and radiological response as assessed by a data review committee or the treating physicians for patients in the named-patient program.
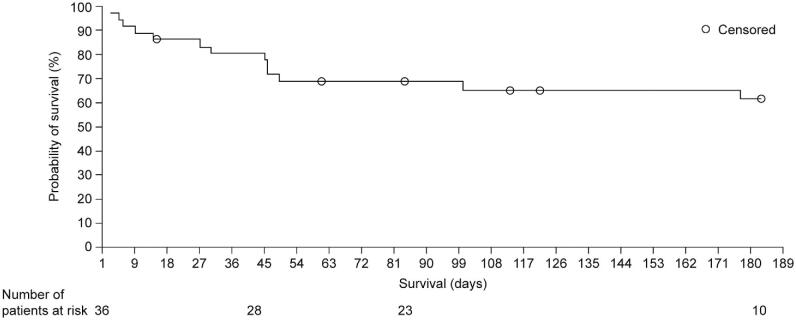
The overall survival rate was 80.6% at day 42 and 69.4% at day 84. The overall probability of survival at day 183 was 61.6% (Fig. 2). By day 42, seven patients had died: six patients died due to the consequences of progressive IFD, and one patient died from pseudomonal pneumonia. Survival varied according to patient demographics and disease characteristics. The lowest survival rate was observed in patients with hematologic malignancies (n = 17; day 42: 70.6%; day 84: 52.9%), and a survival rate of < 80% was also observed in patients with neutropenia (n = 9; day 42: 77.8%; day 84: 66.7%) or solid organ transplant (n = 3; day 42: 100%; day 84: 66.7%) (Table 1). Survival was higher in female patients than in male patients (day 42: 100% vs. 66.7%; day 84: 80.0% vs. 61.9%).
Figure 2.
Kaplan–Meier survival curve of 36 patients receiving isavuconazole treatment for fungal infections of the CNS. CNS = central nervous system.
The presence of lower respiratory tract involvement was associated with reduced survival. In patients with LRTD, survival was 58.3% at day 42 and 50.0% at day 84. In the absence of LRTD, survival was 91.7% at day 42 and 79.2% at day 84 (Table 2). Survival rates differed according to the line of therapy in which isavuconazole was used. When used as primary therapy, the overall survival rate was 70.0% at day 42 and 55.0% at day 84. In contrast, overall survival rates were higher in patients who received isavuconazole as salvage therapy (day 42: 93.8%; day 84: 87.5%).
Survival rates were also impacted by the varying types of infecting fungi (Table 3), although patient numbers were low for some genera. The highest rates of survival (100% survival at day 42 and day 84) were observed in patients with Cryptococcus infections and infections caused by the rare moulds Cladophialophora, Exserohilum, and Exophiala species, among others. Lower rates of survival were observed with Aspergillus species (day 42: 87.5%; day 84: 62.5%) and Mucorales (days 42 and 84: 63.6%).
In 25 patients (69.4%), surgical interventions other than brain biopsy were used in addition to isavuconazole treatment (Table 5). In 12 patients, these were direct neurological interventions: repeat lumbar punctures or craniotomy with or without abscess resection. The other 13 patients received sinus or nasal surgery, mastoidectomy or facial resection. Survival rates associated with surgical intervention were numerically higher compared with patients where surgical intervention was not employed (day 42: 80.0% with surgery vs. 75.0% without; day 84: 68.0% with surgery vs. 62.5% without).
Table 5.
Surgical interventions.
| Survival | |||
|---|---|---|---|
| Surgical intervention | N = 36an (%) | Day 42 n (%)b | Day 84 n (%)b |
| Any intervention | 25 (69.4) | 20 (80.0) | 17 (68.0) |
| Sinus or nasal surgery | 10 (27.8) | 7 (70.0) | 6 (60.0) |
| Repeat lumbar puncturesc | 7 (19.4) | 7 (100) | 7 (100) |
| Craniotomy ± abscess resection | 5 (13.9) | 4 (80.0) | 3 (60.0) |
| Mastoidectomy | 2 (5.6) | 2 (100) | 1 (50.0) |
| Facial resection | 1 (2.8) | 0 (0) | 0 (0) |
| None | 8 (22.2) | 6 (75.0) | 5 (62.5) |
aNo information was available for three patients.
bPercentages are numbers of patients with the fungal organism who were alive at day 42 or 84/total numbers with the intervention.
cNo information available on whether these were performed for diagnostic or therapeutic purposes.
Discussion
This analysis provides evidence of the effectiveness of isavuconazole as a treatment for IFDs with CNS involvement. Overall, survival rates were 80.6% at day 42 and 69.4% at day 84. The presence of hematologic malignancy was associated with a lower survival rate, which is consistent with previous studies of CNS IFD in patients receiving hematopoietic stem cell transplantation (HCT).2,12 Improved survival was observed in female patients compared with male patients, which might be explained by microbiological differences, given the higher proportion of Mucorales infections among males. These infections are known to have an aggressive clinical course,26 complicated by frequent resistance to some commonly used antifungal agents.27
A higher survival rate was also observed in patients receiving isavuconazole as salvage therapy compared with primary therapy, a somewhat unexpected finding as patients receiving salvage therapy would be more likely to have advanced disease than patients receiving primary therapy. This finding may be explained by a selection bias: patients who survived long enough to be switched to isavuconazole would potentially have less severe illness than those who did not survive to receive a second-line therapy. An alternative explanation is that a substantially greater proportion of patients receiving isavuconazole as primary therapy had LRTD (45.0%), and therefore a worse prognosis, compared with patients receiving salvage isavuconazole (18.8%). Involvement of the respiratory tract appeared to be associated with lower survival, although no obvious microbiological differences were noted between those with or without LRTD. This location of IFD, caused by inhalation of fungi as the first step of infection, can be the source of a disseminated and more complex course of fungal disease also including the CNS.28 The effectiveness of isavuconazole as salvage therapy for infections of the CNS was also assessed in a retrospective series of patients with coccidioidal meningitis.29 A total of nine patients were initially treated with fluconazole and switched to voriconazole. All nine patients subsequently received isavuconazole as salvage therapy, with the majority of voriconazole patients switching to isavuconazole due to adverse events (one patient switched after voriconazole treatment failure). All nine patients achieved treatment success (three patients) or stable disease (six patients) during a follow-up with isavuconazole therapy ranging from 138 to 810 days. These data further support the utility of isavuconazole in fungal infections of the CNS.
In the present retrospective study, treatment with isavuconazole was effective against CNS infections caused by a wide range of different fungi. Our data also support previous animal studies that showed effective penetration of isavuconazole across the blood–brain barrier.18 These findings have recently been augmented by a case report demonstrating adequate brain tissue penetration for isavuconazole in a patient with invasive cerebral aspergillosis, although the level in abscess fluid was low.30 Another recent study reported higher concentrations of isavuconazole in inflammatory brain tissue compared with healthy brain tissue in two patients with A. fumigatus infections. In one patient, the concentration in inflammatory brain tissue also exceeded the plasma concentration.31 No pharmacokinetic data on brain penetration are available from our current study, although trough plasma concentrations have been reported previously.20,22 An analysis comparing mean trough concentrations between participants in SECURE who received at least one dose of isavuconazole found no concentration-dependent relationships for efficacy or safety.32 Furthermore, a relationship between trough isavuconazole plasma concentrations, fungal isolate minimum inhibitory concentrations, and key outcomes could not be identified in VITAL, although this may have been due to the small number of patients with data available.20 Therefore, additional clinical data in different localizations and stages of infection are needed to better understand brain tissue and CNS fluid penetration of this azole.
Voriconazole is another azole used for the treatment of invasive fungal infections, for which effectiveness against CNS IFD was evaluated in an analysis similar to the current study.33 A total of 192 cases were accumulated from the voriconazole clinical trial database and published case reports and case series. Treatment with voriconazole resulted in complete or partial responses in 63% of cases as primary therapy and 45% of cases as salvage therapy, and outcomes improved significantly with antifungal combination therapy and with surgery. These results were largely similar to those observed in the current study, in which isavuconazole achieved a complete or partial response in 50.0% of patients as primary therapy and 68.8% as salvage therapy. However, this comparison must be interpreted with caution because of epidemiological differences across the two retrospective cohorts. In the voriconazole study, there was a higher proportion of cases involving Aspergillus species (62.5% vs. 22.2% in the current study) and no cases of mucormycosis, which is particularly relevant given the lack of activity of voriconazole against Mucorales.27 In addition, the voriconazole study included more pediatric patients (21.4% vs. 8.3%), and a lower proportion of cases with hematologic malignancy as an underlying condition (18.2% vs. 47.2%), compared with the current study. Clinical responses were more frequently observed in published versus database cases treated with voriconazole for CNS IFD suggesting a publication bias. A publication bias is unlikely true for the present analysis as we have included patients from clinical databases only.
In an earlier study that retrospectively analyzed the effectiveness of voriconazole in 81 patients with Aspergillus infections of the CNS,34 the survival rate was 31%. While this is lower than the survival rate in Aspergillus infections observed in the current analysis (day 42: 87.5%; day 84: 62.5%), comparisons may not be meaningful because of differences in study populations and the considerable time that has elapsed between the studies, which may have allowed for improvements in other aspects of care. In SECURE,22 only two patients were treated with voriconazole for infections with CNS involvement, both as primary therapy. A partial clinical response was achieved in one patient infected with Aspergillus spp. who subsequently died on day 77, whereas one patient infected with A. fumigatus experienced treatment failure but survived.
Posaconazole has also been studied as a salvage therapy option for CNS IFD. In a multinational, multicenter, open-label clinical trial, 39 evaluable patients received posaconazole for CNS IFD, 29 of whom had human immunodeficiency virus infection as the underlying condition. Of the evaluable patients, 14 of 29 (48%) with cryptococcal meningitis and five of 10 (50%) with other fungal pathogens had successful outcomes. Of the 10 patients with other fungal pathogens, four patients had Aspergillus infections, and one patient had mucormycosis (Apophysomyces elegans) combined with a Basidiomycetes spp. infection. Treatment was partially successful in one case (A. fumigatus), and three of the four remaining patients died within 2 weeks of posaconazole treatment initiation.35 While the results of our analysis appear more favorable, in addition to the time elapsed between studies, the differences in underlying conditions makes a meaningful comparison difficult.
In two later analyses, posaconazole treatment for patients with mucormycosis and brain lesions achieved partial or complete responses in two out of four (50%) patients in two open-label trials36 and in eight out of 11 (72.7%) patients receiving treatment under a compassionate-use program.37 More recently, a review analyzed 96 cases of mucormycosis treated with posaconazole as salvage therapy. Of the seven patients whose infections were reported to have definite CNS involvement, three had complete responses, one had a partial response, one remained stable, and two died.38 The underlying causes for infection in these summaries are consistent with our analysis, and our results are comparable for mucormycosis. However, posaconazole penetration of the CNS has been reported to be poor.39 While there is evidence to suggest that CNS penetration of posaconazole may improve with inflammatory disruption of the blood–brain barrier,40 this was not supported by a recent study in a patient with cerebral phaeohyphomycosis.41
In the current analysis, surgical intervention seemed to be associated with a modest benefit in overall survival. Previous research has shown a significant benefit of neurosurgery in the treatment of CNS IFD caused by a broad spectrum of species.33 In the current study, the conditions under which surgery was used and the types of surgery selected were highly variable, which may have impacted the overall benefit observed. In addition, seven of the patients in our analysis underwent repeated lumbar punctures, but it is not known whether these were intended therapeutically (e.g., to alleviate increased intracranial pressure) or were performed for diagnostic purposes. However, as five of these patients had Cryptococcus infections, it should be noted that controlling intracranial pressure in cryptococcal meningitis is mandatory.3,13 A further reason for differences may be that infections amenable to surgical treatment (e.g., brain abscess, rhinocerebral mucormycosis) are most probably different compared with infections without surgical options (e.g., cryptococcal meningitis). A previous study in patients undergoing HCT found that many patients were considered ineligible for surgery.2 The high proportion of patients with hematologic malignancies in our analysis may partially explain the limited use and effect of surgery.
Overall, our findings should be interpreted with caution as our analysis featured a relatively low number of highly selected patients and was retrospective in nature. Furthermore, this analysis included data from two different trials and a separate database and covered a range of fungal pathogens.
Our report suggests that isavuconazole may be a valuable option in the treatment of CNS IFDs. To improve outcomes, future research should focus on advances in diagnostic methods (e.g., molecular detection, fungus identification with susceptibility testing), CNS pharmacokinetics of antifungal compounds, development of antifungal compounds with enhanced CNS-directed efficacy, and further investigation of crucial host defense mechanisms.
Acknowledgments
We would like to thank Ben Caldwell of Spirit, a division of Spirit Medical Communications Group Ltd., for medical writing support, under the direction of the authors, and editorial assistance (funded by Basilea Pharmaceutica International Ltd.). This work was supported by Basilea Pharmaceutica International Ltd.
Declaration of interest
S.S. has received personal fees (speaker, advisory board membership honoraria, or both) and travel grants from Amgen, Basilea, BTG International Inc., Gilead, Jazz Pharmaceuticals, Merck/MSD, and Pfizer; and has served as consultant to Amgen. O.A.C. has received research grants from Actelion, Amplyx, Arsanis, Astellas, AstraZeneca, Basilea, Bayer, Cidara, F2G, Gilead, GSK, Leeds University, Matinas, Medicines Company, MedPace, Melinta, Merck/MSD, Miltenyi, Pfizer, Rempex, Roche, Sanofi Pasteur, Scynexis, and Seres; has served as a consultant to Actelion, Allecra Therapeutics, Amplyx, Astellas, Basilea, Cidara, Da Volterra, F2G, Gilead, Janssen, Matinas, Menarini, Merck/MSD, Paratek, PSI, Scynexis, Seres, Summit, Tetraphase, and Vical, and has received lecture honoraria from Astellas, Basilea, Gilead, Merck/MSD, and Pfizer. K.H. is employed by Basilea Pharmaceutica International Ltd. F.M.M. has received personal fees from Alexion, Basilea, Chimerix, Fate, Gilead, GSK, Merck, Roche Molecular Diagnostics, Shire, and United Medical; and grants from Astellas, Chimerix, Cidara, Gilead, Shire, and Brigham and Women's Hospital, outside of the submitted work. J.M. has served as consultant to Gilead MSD, Pfizer, Astellas, Basilea, F2G, Cidara, Amplyx, Scynexis, Vical, Bio-Rad, Fujisawa Healthcare Inc., Zeneus (Cephalon), Viropharma, Boehringer-Ingelheim, Amgen, Celgene and Shire; has received speakers’ fees from Gilead MSD, Pfizer, Basilea, Bio-Rad, Fujisawa Healthcare, Astellas Pharma, and Zeneus (Cephalon); and has received research funding from Bio-Rad, MSD, Gilead, and Pfizer. G.R. has served as a consultant for and received grant support from Merck/MSD and Pfizer; and has received lecture honoraria from Pfizer and Astellas. R.H. has received personal fees for advisory board membership and speakers’ fees from Basilea and MSD; personal fees for advisory board membership from Astellas and Novartis; and grant support, personal fees for advisory board membership, and speakers’ fees from Gilead and Pfizer. W.J.H. has received research grants from Merck/MSD and Pfizer; speakers’ fees from Alexion, Astellas, Basilea, Bristol-Myers Squibb, Gilead, Janssen, Merck/MSD, and Pfizer; and travel grants from Alexion, Astellas, Lilly, Merck/MSD, Novartis, and Pfizer. The authors alone are responsible for the content and the writing of the paper.
References
- 1. Schwartz S, Kontoyiannis DP, Harrison T, Ruhnke M. Advances in the diagnosis and treatment of fungal infections of the CNS. Lancet Neurology. 2018; 17: 362–372. [DOI] [PubMed] [Google Scholar]
- 2. Sun YQ, Liu ZY, Huang XJ et al. A retrospective study of central nervous system invasive fungal disease after allogeneic stem cell transplantation: risk factors, clinical characteristics, and outcomes. Biol Blood Marrow Transplant. 2017; 23: 1158–1164. [DOI] [PubMed] [Google Scholar]
- 3. Williamson PR, Jarvis JN, Panackal AA et al. Cryptococcal meningitis: epidemiology, immunology, diagnosis and therapy. Nat Rev Neurol. 2017; 13: 13–24. [DOI] [PubMed] [Google Scholar]
- 4. Rajasingham R, Smith RM, Park BJ et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis. 2017; 17: 873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ghez D, Calleja A, Protin C et al. Early-onset invasive aspergillosis and other fungal infections in patients treated with ibrutinib. Blood. 2018; 131: 1955–1959. [DOI] [PubMed] [Google Scholar]
- 6. Lionakis MS, Dunleavy K, Roschewski M et al. Inhibition of B cell receptor signaling by ibrutinib in primary CNS lymphoma. Cancer Cell. 2017; 31: 833–843.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chamilos G, Lionakis MS, Kontoyiannis DP. Call for action: invasive fungal infections associated with ibrutinib and other small molecule kinase inhibitors targeting immune signaling pathways. Clin Infect Dis. 2018; 66: 140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kantarcioglu AS, de Hoog GS. Infections of the central nervous system by melanized fungi: a review of cases presented between 1999 and 2004. Mycoses. 2004; 47: 4–13. [DOI] [PubMed] [Google Scholar]
- 9. Kantarcioglu AS, Guarro J, De Hoog S, Apaydin H, Kiraz N. An updated comprehensive systematic review of Cladophialophora bantiana and analysis of epidemiology, clinical characteristics, and outcome of cerebral cases. Med Mycol. 2017; 55: 579–604. [DOI] [PubMed] [Google Scholar]
- 10. Li DM, de Hoog GS. Cerebral phaeohyphomycosis: a cure at what lengths? Lancet Infect Dis. 2009; 9: 376–383. [DOI] [PubMed] [Google Scholar]
- 11. Lyons JL, Zhang SX.. Current laboratory approaches to diagnosis of CNS fungal infections. Future Microbiol. 2016; 11: 175–177. [DOI] [PubMed] [Google Scholar]
- 12. McCarthy M, Rosengart A, Schuetz AN, Kontoyiannis DP, Walsh TJ. Mold infections of the central nervous system. N Engl J Med. 2014; 371: 150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Molloy SF, Kanyama C, Heyderman RS et al. Antifungal combinations for treatment of cryptococcal meningitis in Africa. N Engl J Med. 2018; 378: 1004–1017. [DOI] [PubMed] [Google Scholar]
- 14. Mourad A, Perfect JR.. Tolerability profile of the current antifungal armoury. J Antimicrob Chemother. 2018; 73: i26–i32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Patterson TF. Treatment of invasive aspergillosis: Polyenes, echinocandins, or azoles? Med Mycol. 2006; 44: S357–S362. [DOI] [PubMed] [Google Scholar]
- 16. Schwartz S, Ruhnke M, Ribaud P et al. Poor efficacy of amphotericin B-based therapy in CNS aspergillosis. Mycoses. 2007; 50: 196–200. [DOI] [PubMed] [Google Scholar]
- 17. Goralska K, Blaszkowska J, Dzikowiec M. Neuroinfections caused by fungi. Infection. 2018; 46: 443–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schmitt-Hoffmann A-N, Kato K, Townsend R et al. Tissue distribution and elimination of isavuconazole following single and repeat oral-dose administration of isavuconazonium sulfate to rats. Antimicrob. Agents Chemother. 2017; 61: e01292-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peixoto D, Gagne LS, Hammond SP et al. Isavuconazole treatment of a patient with disseminated mucormycosis. J Clin Microbiol. 2014; 52: 1016–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marty FM, Ostrosky-Zeichner L, Cornely OA et al. Isavuconazole treatment for mucormycosis: a single-arm open-label trial and case-control analysis. Lancet Infect Dis. 2016; 16: 828–837. [DOI] [PubMed] [Google Scholar]
- 21. Thompson GR 3rd, Rendon A, Ribeiro Dos Santos R et al. Isavuconazole treatment of cryptococcosis and dimorphic mycoses. Clin Infect Dis. 2016; 63: 356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maertens JA, Raad II, Marr KA et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet. 2016; 387: 760–769. [DOI] [PubMed] [Google Scholar]
- 23. Barg AA, Malkiel S, Bartuv M et al. Successful treatment of invasive mucormycosis with isavuconazole in pediatric patients. Pediatr Blood Cancer. 2018; 65: e27281. [DOI] [PubMed] [Google Scholar]
- 24. Cornu M, Bruno B, Loridant S et al. Successful outcome of disseminated mucormycosis in a 3-year-old child suffering from acute leukaemia: the role of isavuconazole? A case report. BMC Pharmacol Toxicol. 2018; 19: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. De Pauw B, Walsh TJ, Donnelly JP et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008; 46: 1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Skiada A, Lanternier F, Groll AH et al. Diagnosis and treatment of mucormycosis in patients with hematological malignancies: guidelines from the 3rd European Conference on Infections in Leukemia (ECIL 3). Haematologica. 2013; 98: 492–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vitale RG, de Hoog GS, Schwarz P et al. Antifungal susceptibility and phylogeny of opportunistic members of the order mucorales. J Clin Microbiol. 2012; 50: 66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smith JA, Kauffman CA. Pulmonary fungal infections. Respirology. 2012; 17: 913–926. [DOI] [PubMed] [Google Scholar]
- 29. Heidari A, Quinlan M, Benjamin DJ et al. Isavuconazole in the treatment of coccidioidal meningitis. Antimicrob Agents Chemother. 2019; 63: e02232-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lamoth F, Mercier T, Andre P et al. Isavuconazole brain penetration in cerebral aspergillosis. J Antimicrob Chemother. 2019;74:1751–1753. [DOI] [PubMed] [Google Scholar]
- 31. Rouzaud C, Jullien V, Herbrecht A et al. Isavuconazole diffusion in infected human brain. Antimicrob Agents Chemother. 2019; 63: e02474-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kaindl T, Andes D, Engelhardt M et al. Variability and exposure-response relationships of isavuconazole plasma concentrations in the Phase 3 SECURE trial of patients with invasive mould diseases. J Antimicrob Chemother. 2019; 74: 761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schwartz S, Reisman A, Troke PF. The efficacy of voriconazole in the treatment of 192 fungal central nervous system infections: a retrospective analysis. Infection. 2011; 39: 201–210. [DOI] [PubMed] [Google Scholar]
- 34. Schwartz S, Ruhnke M, Ribaud P et al. Improved outcome in central nervous system aspergillosis, using voriconazole treatment. Blood. 2005; 106: 2641–2645. [DOI] [PubMed] [Google Scholar]
- 35. Pitisuttithum P, Negroni R, Graybill JR et al. Activity of posaconazole in the treatment of central nervous system fungal infections. J Antimicrob Chemother. 2005; 56: 745–755. [DOI] [PubMed] [Google Scholar]
- 36. Greenberg RN, Mullane K, van Burik JA et al. Posaconazole as salvage therapy for zygomycosis. Antimicrob Agents Chemother. 2006; 50: 126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van Burik JA, Hare RS, Solomon HF, Corrado ML, Kontoyiannis DP. Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases. Clin Infect Dis. 2006; 42: e61–65. [DOI] [PubMed] [Google Scholar]
- 38. Vehreschild JJ, Birtel A, Vehreschild MJ et al. Mucormycosis treated with posaconazole: review of 96 case reports. Crit Rev Microbiol. 2013; 39: 310–324. [DOI] [PubMed] [Google Scholar]
- 39. Reinwald M, Uharek L, Lampe D et al. Limited penetration of posaconazole into cerebrospinal fluid in an allogeneic stem cell recipient with invasive pulmonary aspergillosis. Bone Marrow Transplant. 2009; 44: 269–270. [DOI] [PubMed] [Google Scholar]
- 40. Ruping MJ, Albermann N, Ebinger F et al. Posaconazole concentrations in the central nervous system. J Antimicrob Chemother. 2008; 62: 1468–1470. [DOI] [PubMed] [Google Scholar]
- 41. Barde F, Billaud E, Goldwirt L et al. Low central nervous system posaconazole concentrations during cerebral phaeohyphomycosis. Antimicrob Agents Chemother. 2019; pii:AAC.01184-19. [DOI] [PMC free article] [PubMed] [Google Scholar]