Abstract
Purpose:
To evaluate the in vitro efficacy of rose bengal and riboflavin photodynamic antimicrobial therapy for inhibition the growth of four Pseudomonas aeruginosa (P. aeruginosa) isolates.
Methods:
Four different clinical P. aeruginosa isolates were collected from patients with confirmed keratitis. Each strain was mixed with either sterile water, 0.1% riboflavin solution, or 0.1% rose bengal solution to yield a final bacteria concentration of 1.5 × 107 CFU/mL. Aliquots from each suspension were plated onto nutrient agar in triplicate. Plates were separated into two groups: (1) no irradiation and (2) 5.4 J/cm2 of radiant exposure with custom-made LED irradiation sources. Separate irradiation sources were used for each photosensitizer. The riboflavin groups used a UV-A light source (375nm) and rose bengal groups used a green light source (525 nm). Plates were photographed at 72 hours and custom software measured bacterial growth inhibition.
Results:
Growth inhibition to riboflavin and rose bengal PDAT showed strain dependent variability. All four strains of P. aeruginosa showed greatest growth inhibition (89%–99%) in the green irradiated-rose bengal group. The UV-A-irradiated riboflavin showed inhibition of 24%–44%. UV-A irradiation only showed minimal inhibition (7%–14%). There was little inhibitory effect in the non-irradiated photosensitizer groups.
Conclusions:
Rose bengal PDAT had the greatest inhibitory effect on all four P. aeruginosa isolates. In the UV-A irradiated riboflavin group, there was moderate inhibition within the irradiation zone; however, there was no inhibition in the non-irradiated groups. These results suggest that rose bengal PDAT may be an effective alternative treatment for Pseudomonas aeruginosa infections.
Keywords: Photodynamic antimicrobial therapy, Pseudomonas aeruginosa, Keratitis, Rose bengal
INTRODUCTION
Pseudomonas aeruginosa, an opportunistic gram-negative bacteria, can cause rapidly progressive keratitis [1–3]. Risk factors for P. aeruginosa keratitis include a breach of corneal epithelium, contact lens use, chronic inflammation of the ocular surface, ocular trauma, use of topical or systemic immunosuppressive medications, and ocular surgery [4,2]. Most cases of P. aeruginosa keratitis respond to treatment with appropriate antibiotics when started in the early stages of the infection. However, complications may arise despite standard medical treatment and can progress to corneal necrosis and corneal perforation [2]. In the past 10 years, the increasing prevalence of multidrug resistant P. aeruginosa highlights the need to develop alternative treatments [5–7].
Photodynamic antimicrobial therapy (PDAT), an alternative treatment for microbial keratitis, uses a photosensitizer activated by light of a specific wavelength. Previous in vitro studies have demonstrated that rose bengal PDAT is effective against several microbial species [8–10]. More effective in vitro results were achieved with rose bengal PDAT compared to riboflavin PDAT against methicillin-resistant Staphylococcus aureus, Fusarium solani, Aspergillus fumigatus, and Candida albicans organisms [11–13]. These results demonstrate that potential utility of rose bengal PDAT as an alternative, adjunct treatment for infectious keratitis. The first reported clinical case of rose bengal PDAT was demonstrated in a patient with severe Fusarium keratopiasticum keratitis who was successfully treated and had no complications during 16 months of follow-up [11,14].
We report results of in vitro experiments with rose bengal PDAT to inhibit the growth of four clinical P. aeruginosa isolates.
MATERIALS AND METHODS
Four P. aeruginosa isolates were cultured from corneal scrapings of patients with infectious keratitis at the Ocular Microbiology Laboratory of the Bascom Palmer Eye Institute, University of Miami Miller School of Medicine, Miami, FL, USA. Isolates were selected based on Type III secretion profiles and included two invasive (strains with “S” positive, denoted S+U−) and two cytotoxic (strains with “U” positive, denoted S−U+) subtypes. The isolates were cultured at log phase, and standard inocula (1.5 × 108 CFU/mL) were prepared with a spectrophotometer and 0.5 McFarland turbidity standard (Thermo Fisher Scientific, Waltham, MA, USA).
Photosensitizer solutions of 0.1% riboflavin and 0.1% rose bengal, were prepared by dissolving 10 mg of riboflavin (R7774; Sigma-Aldrich, St. Louis, MO, USA) and 10 mg of rose bengal (198250; Sigma-Aldrich) in 10 mL of sterile water, respectively. Riboflavin and rose bengal have different molecular weights, so the molarities of the riboflavin and rose bengal solutions were 2.7 × 10−3and 1.0 × 10−3 M, respectively. Each P. aeruginosa strain was mixed with either sterile water (control), 0.1% riboflavin solution, or 0.1% rose bengal solution to yield a bacteria to photosensitizer ratio of 1:9 and a final bacteria concentration of 1.5 × 107 CFU/mL. A 1 mL aliquot from each solution was plated onto a 15 mm × 100 mm nutrient agar plate (W31; Hardy Diagnostics, Santa Maria, CA, USA) in triplicate.
Plates were then separated into two groups, one received no irradiation and another received 15 minutes of irradiation at 6 mW/cm2 with a radiant exposure of 5.4 J/cm2. The plates were irradiated with custom-made LED irradiation sources composed of 54 LEDs (Figure 1). Separate irradiation sources were made for the two different photosensitizers. For the riboflavin group, the central wavelength for the ultraviolet-A (UV-A) irradiation source was 375 nm, and for the rose bengal group, the green irradiation sources had a central wavelength of 525 nm. The spectral output of the two irradiation sources was measured with a spectrometer (SM442, Spectral Products, Putnam, CT, USA) (Figure 2). Previous experiments, not presented here, showed that there was no increase in temperature of the agar plates during the 15 minutes of continuous irradiation.
Figure 1.
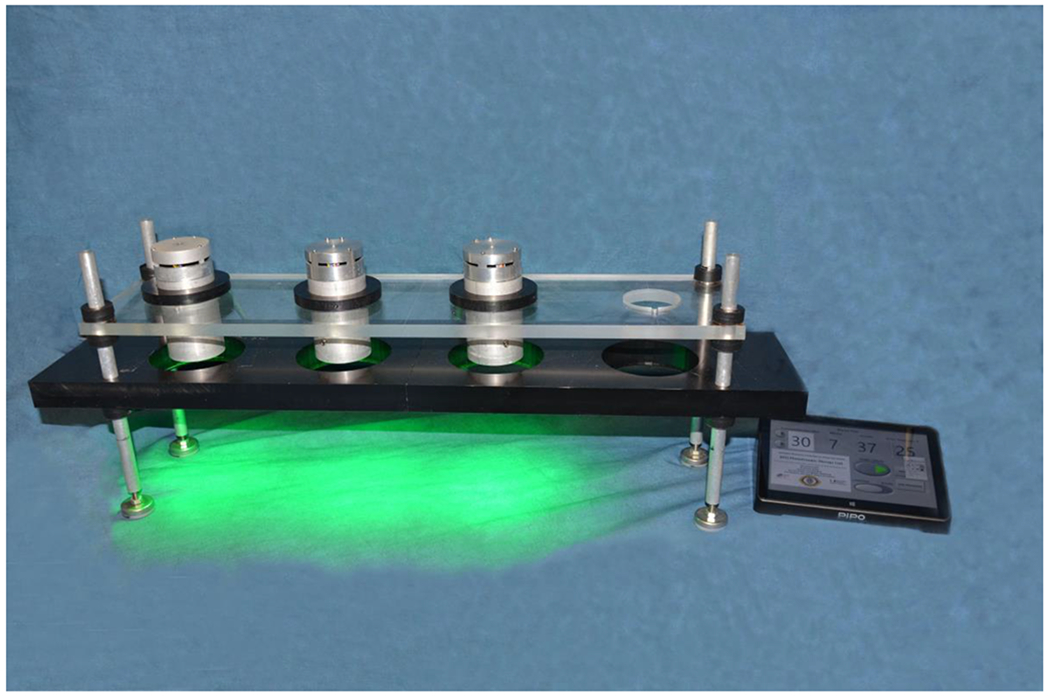
Custom-made green irradiation source console for in vitro testing of rose bengal PDAT. Three identical green irradiation sources were created using 54 light emitting diodes (LEDs) of central wavelength of 525 nm. Irradiation sources are computer controlled with custom LabVIEW software. Agar plates are placed in the black custom-made plate pictured to ensure appropriate positioning. Not pictured are the UV-A irradiation sources which have similar design.
Figure 2.
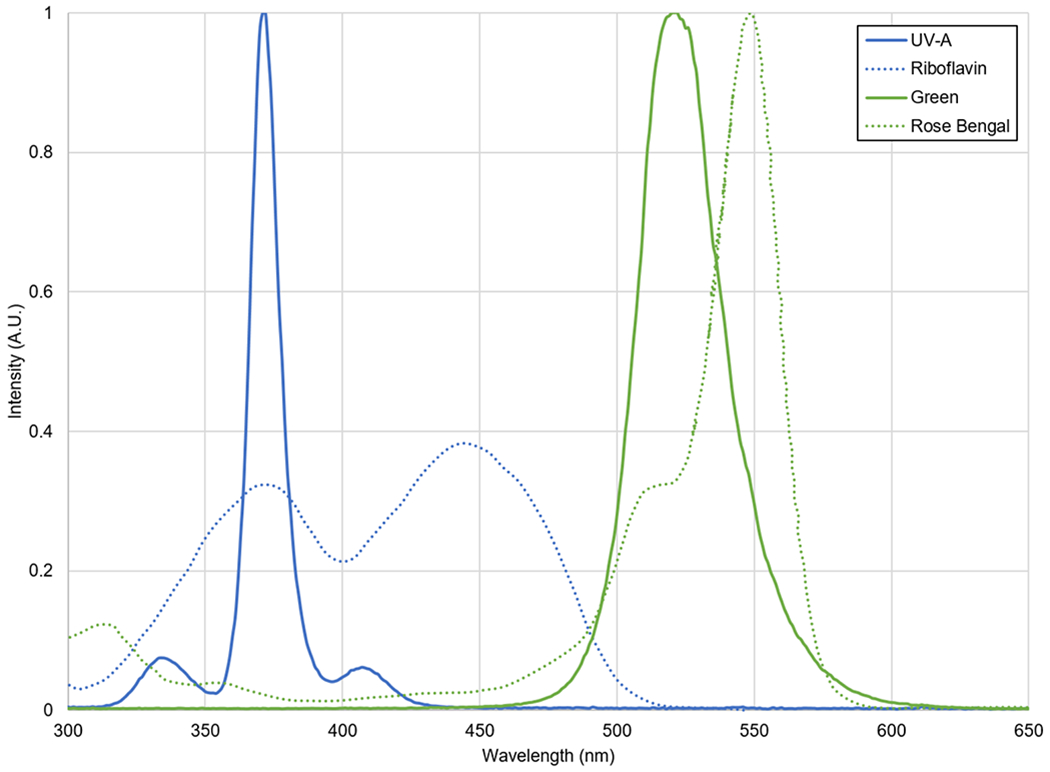
Normalized emission spectra of the UV-A and Green irradiation sources as well as absorption spectra of the riboflavin and rose bengal.
After irradiation, three plates for each group were incubated at 37°C for 72 hours. They were then photographed with a digital camera (16.2 MP, Nikon D7000; Nikon Inc, Melville, NY, USA) and percent growth measurement was calculated on the photographs with custom-made software written in LabVIEW 6 (National Instruments, Austin, TX, USA), as described by Arboleda and associates [12]. For each plate, a central zone was selected for measurement corresponding to the diameter of the irradiation source (47 mm). The images were converted to grayscale and the background illumination was normalized; the image contrast was increased and the images were segmented by applying a series of binary threshold filters. The resulting black and white image utilized white pixels for bacterial growth and black pixels for no growth. We calculated the area of growth by summing the total number of white pixels. Percent growth was determined by dividing total growth by the total pixel area of the irradiation zone:
Percent growth values were validated by comparison with manual segmentation methods and found to be within the same range of precision.
The control group was the dark condition (non-irradiated) inoculated with P. aeruginosa and corresponded to 100% growth. Values in the other experimental groups were compared with those in this group. To calculate the percent inhibition, the percent growth was subtracted from 100%, as the control group demonstrated 0% inhibition:
An analysis of variance (ANOVA) followed by post hoc test for group-wise comparisons were performed using Statistical Package for Social Sciences (SPSS) version 22.0 software (IBM Corporation, Armonk, NY, USA). The data exhibited a homogeneous distribution; thus, zone of inhibition (in mm) was analyzed using the mean of all the readings obtained; p-values 0.05 were considered to be statistically significant.
RESULTS
Figure 3 shows representative photographs of agar plates showing the growth inhibition of the P. aeruginosa isolates. All four strains of P. aeruginosa showed greatest growth inhibition (89%–99%) in the green irradiated rose bengal group (Figure 4). The UV-A irradiated riboflavin showed inhibition of 24%–44%. UV-A irradiation only showed minimal inhibition (7%–14%). There was little inhibitory effect (8%–18%) in the non-irradiated rose bengal group. In the non-irradiated riboflavin group, the results varied; Strain 4 was more inhibited (28%) while Strain 1 had increased growth of 9% compared to the control group.
Figure 3.
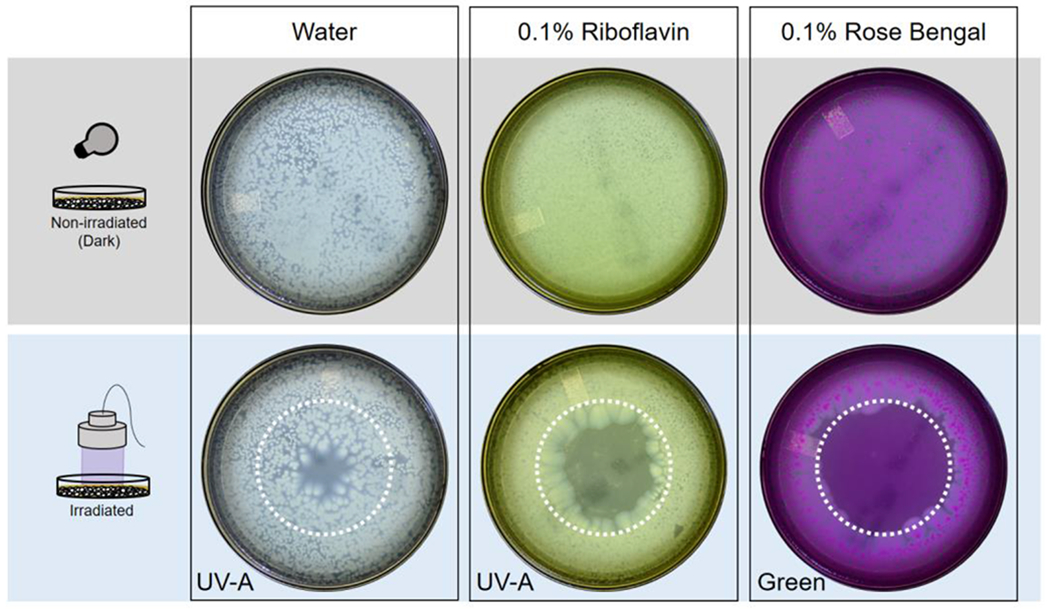
Results from in vitro experiments testing antimicrobial efficacy of photodynamic antimicrobial therapy against Pseudomonas aeruginosa isolates from patients. Photographs of agar plates at 72 hours showing isolate growth. Dotted white circle indicates the area of the agar plate irradiated by the custom-made LED sources.
Figure 4.
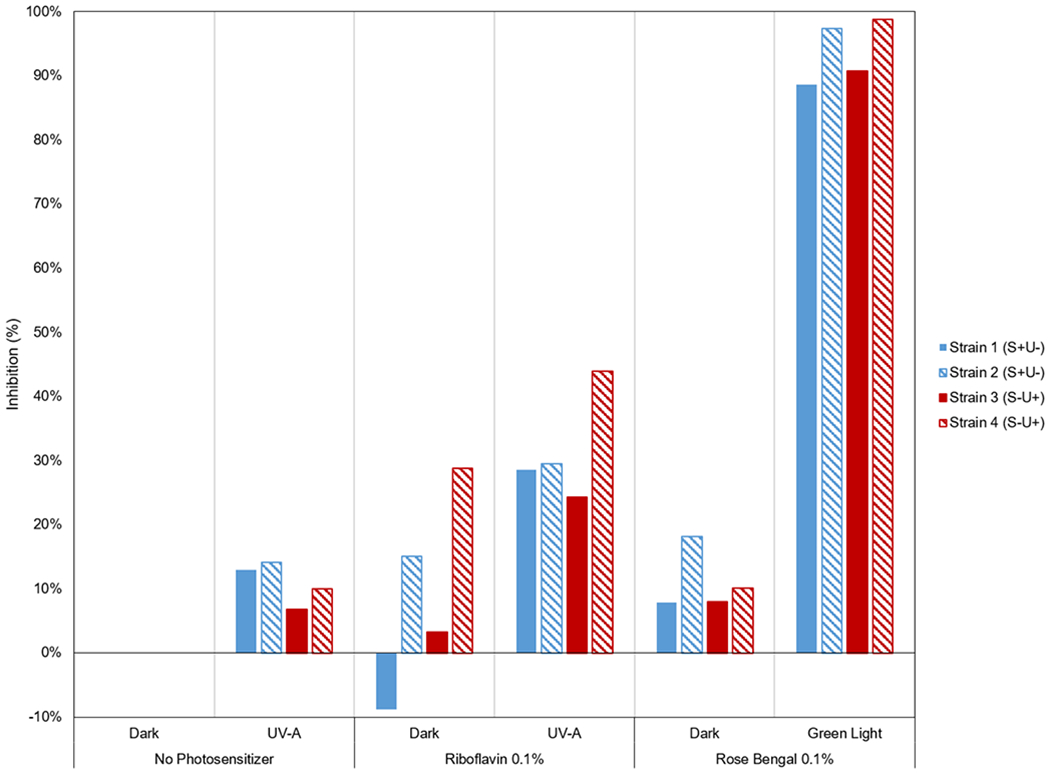
Graph of PDAT effect on four different Pseudomonas aeruginosa isolates (two strains with “S” positive, denoting S+U− [invasive] and two strains with “U” positive, denoting S−U+ [cytotoxic]). The results for each photosensitizer (none, riboflavin, rose bengal) in the irradiated groups are compared with the non-irradiated condition. The greatest inhibition of P. aeruginosa growth was observed in the green irradiated rose bengal group.
The maximum inhibition was seen in the green irradiated rose bengal group with a mean of 93.9% (SD ± 4.3) followed by the UV-A irradiated riboflavin group with a mean of 31.6 % (SD ± 7.4) and minimum in the control group with a mean of 0.0%. This difference in values was statistically significant [P < 0.001].
When subjected to post hoc test analysis, statistically significant differences were determined between the green irradiated rose bengal group compared with any other group. Strains 1 and 2 on the green irradiated rose bengal group had a mean bacterial inhibition of 93% (SD ± 6) and Strain 3 and 4 was 95% (SD ± 6) with no statistically significant difference between groups.
DISCUSSION
The in vitro inhibitory effect of riboflavin and rose bengal PDAT on four clinical strains of P. aeruginosa were described in this manuscript. The organisms tested in this study were collected from patients who were unresponsive to antibiotic therapy. The rose bengal irradiated group showed the greatest inhibitory effect on all four P. aeruginosa isolates. In the UV-A irradiated riboflavin group, there was moderate inhibition within the irradiation zone; however, there was no inhibition in either the non-irradiated riboflavin or non-irradiated rose bengal groups.
These results reflect the findings from previous studies which demonstrated that rose bengal PDT inhibits gram-positive, gram-negative bacteria [8–13]. Furthermore, the inhibitory effect to the riboflavin and rose bengal PDAT showed strain dependent variability. Further testing on more strains will confirm which strains and genotypes are most susceptible to PDAT. This information will be important for clinicians when utilizing this potential treatment as the different genotypes of P. aeruginosa have different clinical behavior and outcomes [15,16].
The advantage of rose bengal PDAT as an alternative treatment is due to a combination of the following: the microbial killing of the Pseudomonas species [10], the oxidation of virulence factors [17], an increase in the tensile strength of the cornea [18], and remodeling of the extracellular matrix of the cornea [19]. Increasing the tensile strength of the cornea can halt the corneal melting process that occurs during the infection, thus allowing more time for the antimicrobial medications to take effect. Additionally, it is unlikely that microorganisms will develop resistance to this therapy because of its unique mechanism of action [20].
Another advantage of this novel treatment is that the safety of rose bengal and green light in the eye has been reported in the literature [18,19, 21]. Zhu and associates conducted an in vivo study with 0.1% rose bengal and green irradiation for a total radiant exposure of 150 J/cm2 and found the keratocytes, iris cells, and retina to be unaffected by the treatment [21]. It is of note that the light source used in this study utilized a much lower radiant exposure (5.4 J/cm2) as compared to the Zhu study.
These results suggest that rose bengal PDAT may be an effective alternative treatment for Pseudomonas aeruginosa infections. Further studies are required to better understand the parameters, efficacy, and utility of rose bengal PDAT as a therapeutic option in order to improve outcomes of patients with infectious keratitis secondary to P. aeruginosa.
ACKNOWLEDGMENTS/DISCLOSURES
a. Funding/Support:
This research was supported by the Edward D. and Janet K. Robson Foundation (Tulsa, OK, USA), the Florida Lions Eye Bank and the Beauty of Sight Foundation (Miami, FL, USA), Drs. K. R. Olsen and M. E. Hildebrandt, Drs. Raksha Urs and Aaron Furtado, NIH Center Grant P30EY14801, Research to Prevent Blindness, the Pan-American Association of Ophthalmology (PAAO) and Retina Research Foundation (J. D. Martinez) and the Henri and Flore Lesieur Foundation (Chicago, IL, USA) (J.-M. Parel)
b. Financial Disclosures
The authors have no financial disclosures (HD, AA, MCA, JDM, KA, NR, JMM, DM, GA, JMP)
c. Other Acknowledgments
The authors are grateful to Cornelis Rowaan, BS, Alex Gonzalez, BA, Andres Bernal, MS and Juan Silgado, BS, of the Ophthalmic Biophysics Center for participating to the design, development and construction of the irradiation sources.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Blanco C, Nunez MX (2010) Endophthalmitis by Pseudomonas aeruginosa after penetrating keratoplasty, case report with an epidemiological investigation. Biomedica 30 (3):327–331 [PubMed] [Google Scholar]
- 2.Al-Mujaini A, Al-Kharusi N, Thakral A, Wali UK (2009) Bacterial keratitis: perspective on epidemiology, clinico-pathogenesis, diagnosis and treatment. Sultan Qaboos Univ Med J 9 (2):184–195 [PMC free article] [PubMed] [Google Scholar]
- 3.Alexandrakis G, Alfonso EC, Miller D (2000) Shifting trends in bacterial keratitis in south Florida and emerging resistance to fluoroquinolones. Ophthalmology 107 (8):1497–1502 [DOI] [PubMed] [Google Scholar]
- 4.Green M, Apel A, Stapleton F (2008) Risk factors and causative organisms in microbial keratitis. Cornea 27 (1):22–27. doi: 10.1097/ICO.0b013e318156caf2 [DOI] [PubMed] [Google Scholar]
- 5.Vazirani J, Wurity S, Ali MH (2015) Multidrug-Resistant Pseudomonas aeruginosa Keratitis: Risk Factors, Clinical Characteristics, and Outcomes. Ophthalmology 122 (10):2110–2114. doi: 10.1016/j.ophtha.2015.06.007 [DOI] [PubMed] [Google Scholar]
- 6.Garg P, Sharma S, Rao GN (1999) Ciprofloxacin-resistant Pseudomonas keratitis. Ophthalmology 106 (7):1319–1323. doi: 10.1016/S0161-6420(99)00717-4 [DOI] [PubMed] [Google Scholar]
- 7.Willcox MD (2011) Review of resistance of ocular isolates of Pseudomonas aeruginosa and staphylococci from keratitis to ciprofloxacin, gentamicin and cephalosporins. Clin Exp Optom 94 (2):161–168. doi: 10.1111/j.1444-0938.2010.00536.x [DOI] [PubMed] [Google Scholar]
- 8.Costa AC, Chibebe Junior J, Pereira CA, Machado AK, Beltrame Junior M, Junqueira JC, Jorge AO (2010) Susceptibility of planktonic cultures of Streptococcus mutans to photodynamic therapy with a light-emitting diode. Braz Oral Res 24 (4):413–418 [DOI] [PubMed] [Google Scholar]
- 9.Pereira CA, Costa AC, Carreira CM, Junqueira JC, Jorge AO (2013) Photodynamic inactivation of Streptococcus mutans and Streptococcus sanguinis biofilms in vitro. Lasers Med Sci 28 (3):859–864. doi: 10.1007/s10103-012-1175-3 [DOI] [PubMed] [Google Scholar]
- 10.Banks JG, Board RG, Carter J, Dodge AD (1985) The cytotoxic and photodynamic inactivation of micro-organisms by Rose Bengal. J Appl Bacteriol 58 (4):391–400 [DOI] [PubMed] [Google Scholar]
- 11.Amescua G, Arboleda A, Nikpoor N, Durkee H, Relhan N, Aguilar MC, Flynn HW, Miller D, Parel JM (2017) Rose Bengal Photodynamic Antimicrobial Therapy: A Novel Treatment for Resistant Fusarium Keratitis. Cornea 36 (9):1141–1144. doi: 10.1097/ICO.0000000000001265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arboleda A, Miller D, Cabot F, Taneja M, Aguilar MC, Alawa K, Amescua G, Yoo SH, Parel JM (2014) Assessment of rose bengal versus riboflavin photodynamic therapy for inhibition of fungal keratitis isolates. Am J Ophthalmol 158 (1):64–70 e62. doi: 10.1016/j.ajo.2014.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halili F, Arboleda A, Durkee H, Taneja M, Miller D, Alawa KA, Aguilar MC, Amescua G, Flynn HW Jr., Parel JM (2016) Rose Bengal- and Riboflavin-Mediated Photodynamic Therapy to Inhibit Methicillin-Resistant Staphylococcus aureus Keratitis Isolates. Am J Ophthalmol 166:194–202. doi: 10.1016/j.ajo.2016.03.014 [DOI] [PubMed] [Google Scholar]
- 14.Martinez JD, Naranjo A, Amescua G, Dubovy SR, Arboleda A, Durkee H, Aguilar MC, Flynn HW, Miller D, Parel J-M (2018) Human Corneal Changes After Rose Bengal Photodynamic Antimicrobial Therapy for Treatment of Fungal Keratitis. Cornea 37 (10):e46–e48. doi: 10.1097/ico.0000000000001701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borkar DS, Fleiszig SM, Leong C, Lalitha P, Srinivasan M, Ghanekar AA, Tam C, Li WY, Zegans ME, McLeod SD, Lietman TM, Acharya NR (2013) Association between cytotoxic and invasive Pseudomonas aeruginosa and clinical outcomes in bacterial keratitis. JAMA Ophthalmol 131 (2):147–153. doi: 10.1001/jamaophthalmol.2013.778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen EP, Hsieh Y-T, Chu H-S, Chang S-C, Hu F-R (2015) Correlation of Pseudomonas aeruginosa Genotype With Antibiotic Susceptibility and Clinical Features of Induced Central Keratitis Association of T3SS With Pseudomonal Keratitis. Investigative Ophthalmology & Visual Science 56 (1):365–371. doi: 10.1167/iovs.14-15241 [DOI] [PubMed] [Google Scholar]
- 17.Reszka KJ, Denning GM, Britigan BE (2006) Photosensitized oxidation and inactivation of pyocyanin, a virulence factor of Pseudomonas aeruginosa. Photochem Photobiol 82 (2):466–473. doi: 10.1562/2005-07-29-RA-626 [DOI] [PubMed] [Google Scholar]
- 18.Cherfan D, Verter EE, Melki S, Gisel TE, Doyle FJ Jr., Scarcelli G, Yun SH, Redmond RW, Kochevar IE (2013) Collagen cross-linking using rose bengal and green light to increase corneal stiffness. Invest Ophthalmol Vis Sci 54 (5):3426–3433. doi: 10.1167/iovs.12-11509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallego-Munoz P, Ibares-Frias L, Lorenzo E, Marcos S, Perez-Merino P, Bekesi N, Kochevar IE, Martinez-Garcia MC (2017) Corneal Wound Repair After Rose Bengal and Green Light Crosslinking: Clinical and Histologic Study. Invest Ophthalmol Vis Sci 58 (9): 3471–3480. doi: 10.1167/iovs.16-21365 [DOI] [PubMed] [Google Scholar]
- 20.Kashef N, Hamblin MR (2017) Can microbial cells develop resistance to oxidative stress in antimicrobial photodynamic inactivation? Drug Resist Updat 31:31–42. doi: 10.1016/j.drup.2017.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu H, Alt C, Webb RH, Melki S, Kochevar IE (2016) Corneal Crosslinking With Rose Bengal and Green Light: Efficacy and Safety Evaluation. Cornea 35 (9):1234–1241. doi: 10.1097/ICO.0000000000000916 [DOI] [PubMed] [Google Scholar]


