Abstract
There are limited data on arrhythmias in acute myocardial infarction with cardiogenic shock (AMI-CS). Using a 17-year AMI-CS population from the National Inpatient Sample, we identified common arrhythmias – atrial fibrillation (AF), atrial flutter, supraventricular tachycardia, ventricular tachycardia (VT), ventricular fibrillation (VF), and atrioventricular blocks (AVB). Admissions with concomitant cardiac surgery were excluded. Outcomes of interest included temporal trends, predictors, in-hospital mortality, and resource utilization in cohorts with and without arrhythmias. Of the 420,319 admissions with AMI-CS during 2000–2016, arrhythmias were noted in 213,718 (51%). AF (45%), VT (35%) and VF (30%) were the most common arrhythmias. Compared to those without, the cohort with arrhythmias was more often male, of white race, with ST-segment elevation AMI-CS presentation, and had higher rates of cardiac arrest and acute organ failure (all p<0.001). Temporal trends of prevalence revealed a stable trend of atrial and ventricular arrhythmias and declining trend in AVB. The cohort with arrhythmias had higher unadjusted (42% vs. 41%; odds ratio [OR] 1.03 [95% confidence interval 1.02–1.05]; p<0.001), but not adjusted (OR 1.01 [95% CI 0.99–1.03]; p=0.22) in-hospital mortality compared to those without. The cohort with arrhythmias had longer hospital stay (9±10 vs 7±9 days; p<0.001) and higher hospitalization costs ($124,000±146,000 vs $91,000±115,000; p<0.001). In the cohort with arrhythmias, older age, female sex, non-white race, higher comorbidity, presence of acute organ failure, and cardiac arrest, predicted higher in-hospital mortality. In conclusion, cardiac arrhythmias in AMI-CS are a marker of higher illness severity and are associated with greater resource utilization.
Keywords: Acute myocardial infarction, cardiogenic shock, atrial fibrillation, ventricular tachycardia, outcomes research
INTRODUCTION
Arrhythmias are a frequent complication in patients with acute myocardial infarction (AMI).1–4 Multiple studies have associated various arrhythmias with poor prognosis and a higher risk of mortality in patients with AMI.1–9 Patients with cardiogenic shock (CS) constitute the sickest spectrum of AMI, and have nearly 30% in-hospital mortality.10–23 The ischemic changes and metabolic derangements resulting from AMI-CS can precipitate arrhythmias, which is probably a reflection of LV dysfunction, higher filling pressures and worsening heart failure.2 Conversely, high ventricular rates can result in hemodynamic compromise, further worsening myocardial function and hemodynamic instability.4,24,25 The use of vasopressors and inotropes in this population is associated with a pro-arrhythmic state resulting in higher atrial and potentially ventricular arrhythmias.26 Despite this known risk of arrhythmias, there are limited contemporary data related to arrhythmias in patients with AMI-CS.7–9 Using a nationally-representative population, we sought to assess the burden of arrhythmias in admissions with AMI-CS. In addition, we evaluated the temporal trends, prevalence, clinical outcomes of cohorts with and without arrhythmias with further sub-group analyses of atrial and ventricular arrhythmias.
METHODS
The National (Nationwide) Inpatient Sample (NIS) is the largest all-payer database of hospital inpatient stays in the United States. NIS contains discharge data from a 20% stratified sample of community hospitals and is a part of the Healthcare Quality and Utilization Project (HCUP), sponsored by the Agency for Healthcare Research and Quality.27 Information regarding each discharge includes patient demographics, primary payer, hospital characteristics, principal diagnosis, up to 24 secondary diagnoses, and procedural diagnoses. The HCUP-NIS does not capture individual patients but captures all information for a given admission. Institutional Review Board approval was not sought due to the publicly available nature of this de-identified database. These data are available to other authors via the HCUP-NIS database with the Agency for Healthcare Research and Quality.16
Using the HCUP-NIS data from 2000–2016, a retrospective cohort study of admissions with AMI in the primary diagnosis field (International Classification of Diseases 9.0 Clinical Modification [ICD–9CM] 410.x and ICD–10CM I21.x–22.x) and a secondary diagnosis of CS (ICD–9CM 785.51, ICD–10CM R57.0) were identified. Similar to prior literature, we identified AF, atrial flutter (AFlut), supraventricular tachycardia (SVT), VT, VF, 1° atrioventricular block (AVB), 2° AVB (type II), 3° AVB and permanent pacemaker (PPM) implantation.28 We excluded admissions that received cardiac surgery (coronary artery bypass grafting, valve repair/replacement, durable left ventricular assist device placement and orthotopic heart transplant) since they have a distinctly different arrhythmic profile. The Deyo’s modification of the Charlson Comorbidity Index was used to identify the burden of co-morbid diseases (Supplementary Table 1).29 Demographic characteristics, hospital characteristics, acute organ failure, mechanical circulatory support, cardiac procedures, and non-cardiac organ support use were identified for all admissions using previously used methodologies from our group.10–16,20–22,28,30–36
The primary outcome was the in-hospital mortality and resource utilization in AMI-CS admissions with and without arrhythmias. Secondary outcomes included temporal trends, predictors of arrhythmias in AMI-CS, and clinical outcomes, resource utilization and predictors of in-hospital mortality in the arrhythmia cohort.
As recommended by HCUP-NIS, survey procedures using discharge weights provided with HCUP-NIS database were used to generate national estimates.37 Using the trend weights provided by the HCUP-NIS, samples from 2000–2011 were re-weighted to adjust for the 2012 HCUP-NIS re-design.37 Chi-square/one-way analysis of variance and t-tests were used to compare 2/≥2 categorical and continuous variables, respectively. Multivariable logistic regression was used to analyze trends over time (referent year 2000). The inherent restrictions of the HCUP-NIS database related to research design, data interpretation, and data analysis were reviewed and addressed.37,38 Pertinent considerations include not assessing individual hospital-level volumes (due to changes to sampling design detailed above), treating each entry as an ‘admission’ as opposed to individual patients, restricting the study details to inpatient factors since the HCUP-NIS does not include outpatient data, and limiting administrative codes to those previously validated and used for similar studies. Univariable analysis for trends and outcomes was performed and was represented as odds ratio (OR) with 95% confidence interval (CI). Multivariable logistic regression analysis incorporating age, sex, race, primary payer status, socio-economic stratum, hospital characteristics, comorbidities, acute organ failure, AMI-type, cardiac procedures, and non-cardiac procedures was performed for assessing temporal trends of prevalence and in-hospital mortality. For the multivariable modeling, regression analysis with purposeful selection of statistically (liberal threshold of p<0.20 in univariate analysis) and clinically relevant variables was conducted. Two-tailed p<0.05 was considered statistically significant. All statistical analyses were performed using SPSS v25.0 (IBM Corp, Armonk NY).
RESULTS
In the period from January 1, 2000 to December 31, 2016, there were over 10 million AMI admissions, of which 513,288 had concomitant CS (5.1%). Of these, 92,969 (18.5%) received concomitant cardiac surgery and were excluded. In the final cohort of 420,319, arrhythmias were noted in 213,718 (50.8%) of the admissions, with AF, VT and VF being the most common arrhythmias (Figure 1). The cohort with arrhythmias was more often male, of white race, with higher rates of acute organ failure, cardiac arrest, and greater use of cardiac and non-cardiac organ support devices (Tables 1 and 2).
Figure 1. Arrhythmias in AMI-CS.

Cumulative arrhythmias in AMI-CS showing overlap and relative percentages between different arrhythmia categories and types in the arrhythmia cohort (N=213,718)
Abbreviations: AF: atrial fibrillation; AFlut: atrial flutter; AMI: acute myocardial infarction; AVB: atrio-ventricular block; CS: cardiogenic shock; SVT: supraventricular tachycardia; VF: ventricular fibrillation; VT: ventricular tachycardia
Table 1.
Baseline characteristics of AMI-CS stratified by arrhythmias
| Variable | AMI-CS cohort (N=420,319) |
Arrhythmia cohort (N=213,718) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Arrhythmia (N=213,718) |
No Arrhythmias (N=206,601) |
P | Atrial (N=72,264) |
Ventricular (N=80,388) |
AVB (N=18,123) |
≥2 types (N=42,943) |
P | ||
| Age (years) | 69.8±13.1 | 69.8±13.4 | 0.27 | 75.7±11.4 | 64.4±12.9 | 69.6±13.0 | 70.0±12.1 | <0.001 | |
| Women | 37.1% | 43.4% | <0.001 | 43.7% | 30.3% | 46.5% | 34.8% | <0.001 | |
| White | 79.6% | 75.5% | <0.001 | 81.7% | 77.1% | 76.1% | 82.1% | <0.001 | |
| Black | 6.8% | 8.2% | 5.6% | 8.0% | 8.2% | 6.1% | |||
| Othera | 13.6% | 16.3% | 12.71% | 14.90% | 15.67% | 11.77% | |||
| Primary payer | Medicare | 62.9% | 63.7% | <0.001 | 78.1% | 48.8% | 61.8% | 64.1% | <0.001 |
| Medicaid | 6.1% | 6.8% | 3.8% | 8.1% | 6.9% | 6.0% | |||
| Private | 23.5% | 21.6% | 14.0% | 32.4% | 23.0% | 22.9% | |||
| Othersb | 7.5% | 7.9% | 4.1% | 10.8% | 8.3% | 7.1% | |||
| Hospital teaching status and location | Rural | 6.7% | 9.5% | <0.001 | 8.4% | 5.7% | 7.2% | 5.7% | <0.001 |
| Urban non-teaching | 38.7% | 40.6% | 39.7% | 37.6% | 40.2% | 38.5% | |||
| Urban teaching | 54.5% | 49.9% | 52.0% | 56.7% | 52.5% | 55.8% | |||
| Hospital bed-size | Small | 8.4% | 9.4% | <0.001 | 9.3% | 8.1% | 8.5% | 7.5% | <0.001 |
| Medium | 23.7% | 24.0% | 24.5% | 22.9% | 24.1% | 23.6% | |||
| Large | 67.9% | 66.6% | 66.2% | 69.1% | 67.4% | 68.9% | |||
| Hospital region | Northeast | 18.5% | 18.6% | <0.001 | 20.4% | 17.9% | 17.5% | 17.1% | <0.001 |
| Midwest | 23.1% | 22.7% | 22.4% | 22.9% | 23.6% | 24.7% | |||
| South | 37.5% | 39.1% | 35.9% | 38.9% | 38.4% | 37.0% | |||
| West | 20.8% | 19.7% | 21.3% | 20.3% | 20.5% | 21.3% | |||
| Charlson Comorbidity Index | 0–3 | 24.5% | 23.2% | <0.001 | 9.6% | 38.2% | 26.7% | 23.0% | <0.001 |
| 4–6 | 53.3% | 53.7% | 56.2% | 48.8% | 55.5% | 55.7% | |||
| ≥7 | 22.2% | 23.1 % | 34.2% | 12.9% | 17.8% | 21.2% | |||
| Prior pacemaker | 2.1% | 1.7% | <0.001 | 3.7% | 1.0% | 1.4% | 1.7% | <0.001 | |
| Prior implantable cardioverter-defibrillator | 1.4% | 1.1% | <0.001 | 1.5% | 1.5% | 0.4% | 1.6% | <0.001 | |
Represented as percentage or mean ± standard deviation;
Hispanic, Asian, Native American, Others;
Uninsured, No Charge, Others
Abbreviations: AMI: acute myocardial infarction; AVB: atrioventricular block; CS: cardiogenic shock
Table 2.
In-hospital characteristics of AMI-CS stratified by arrhythmias
| Variable | AMI-CS cohort (N=420,319) |
Arrhythmia cohort (N=213,718) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Arrhythmia (N=213,718) |
No Arrhythmias (N=206,601) |
P | Atrial (N=72,264) |
Ventricular (N=80,388) |
AVB (N=18,123) |
≥2 types (N=42,943) |
P | ||
| AMI type | STEMI | 71.8% | 67.0% | <0.001% | 58.3% | 79.3% | 81.8% | 76.2% | <0.001 |
| NSTEMI | 28.2% | 33.0% | 41.7% | 20.7% | 18.2% | 23.8% | |||
| Acute organ failure | Respiratory | 51.9% | 42.6% | <0.001 | 46.6% | 57.2% | 39.8% | 55.9% | <0.001 |
| Renal | 38.2% | 34.1% | <0.001 | 43.5% | 34.0% | 33.8% | 39.3% | <0.001 | |
| Hepatic | 10.6% | 6.9% | <0.001 | 9.0% | 11.9% | 8.5% | 11.7% | <0.001 | |
| Hematologic | 9.9% | 7.8% | <0.001 | 10.0% | 9.7% | 7.4% | 11.0% | <0.001 | |
| Neurologic | 18.9 % | 11.1% | <0.001 | 11.6% | 26.6% | 10.0% | 20.4% | <0.001 | |
| Out of hospital cardiac arrest | 40.6% | 13.3% | <0.001 | 11.9% | 65.5% | 15.7% | 53.0% | <0.001 | |
| Coronary angiography | 72.8% | 64.7% | <0.001 | 58.7% | 80.9% | 77.8% | 79.1% | <0.001 | |
| Percutaneous coronary intervention | 58.8% | 49.6% | <0.001 | 43.9% | 67.4% | 64.7% | 65.1% | <0.001 | |
| Invasive hemodynamic monitoringa | 19.8% | 17.8% | <0.001 | 19.5% | 20.5% | 15.6% | 20.8% | <0.001 | |
| Mechanical circulatory support | Total | 43.0% | 36.9% | <0.001 | 31.7% | 52.4% | 39.0% | 46.2% | <0.001 |
| IABP | 40.6% | 35.3% | <0.001 | 30.1% | 49.2% | 37.4% | 43.7% | <0.001 | |
| pLVAD | 2.8% | 1.8% | <0.001 | 1.9 % | 3.6% | 2.0% | 3.0% | <0.001 | |
| ECMO | 0.7% | 0.3% | <0.001 | 0.3% | 1.1% | 0.4% | 0.9% | <0.001 | |
| Invasive mechanical ventilation | 49.1% | 39.2% | <0.001 | 40.8% | 56.4% | 37.4% | 54.6% | <0.001 | |
| Hemodialysis | 3.2% | 2.6% | <0.001 | 3.8% | 2.6% | 2.9% | 3.4% | <0.001 | |
Represented as percentage or mean ± standard deviation;
pulmonary artery catheterization or right heart catheterization
Abbreviations: AMI: acute myocardial infarction; AVB: atrioventricular block; CS: cardiogenic shock; ECMO: extracorporeal membrane oxygenation; IABP: intra-aortic balloon pump; NSTEMI: non-ST-segment elevation myocardial infarction; pLVAD: percutaneous left ventricular assist device; STEMI: ST-segment elevation myocardial infarction
Among the 213,718 admissions with arrhythmias, the temporal trends of atrial arrhythmias (AF, AFlut, and SVT), ventricular arrhythmias (VT, VF) and AVB–related admissions are presented in Figure 2. There was a temporal increase of all arrhythmia sub-groups in the unadjusted analysis; however the adjusted analysis showed relatively stable trends of atrial and ventricular arrhythmias and decreasing trend in AVB. The cohort with ventricular arrhythmias on average had higher rates of ST-segment elevation myocardial infarction with cardiogenic shock (STEMI-CS), cardiac arrest, neurological failure, and mechanical circulatory support use (Table 2). PPM was implanted in 6,837 (1.6%) of the admissions and the temporal trends showed a relatively steady rate of implantation during the study period (Figure 2C).
Figure 2. Trends in the prevalence of atrial, ventricular and AVB arrhythmias in AMI-CS.
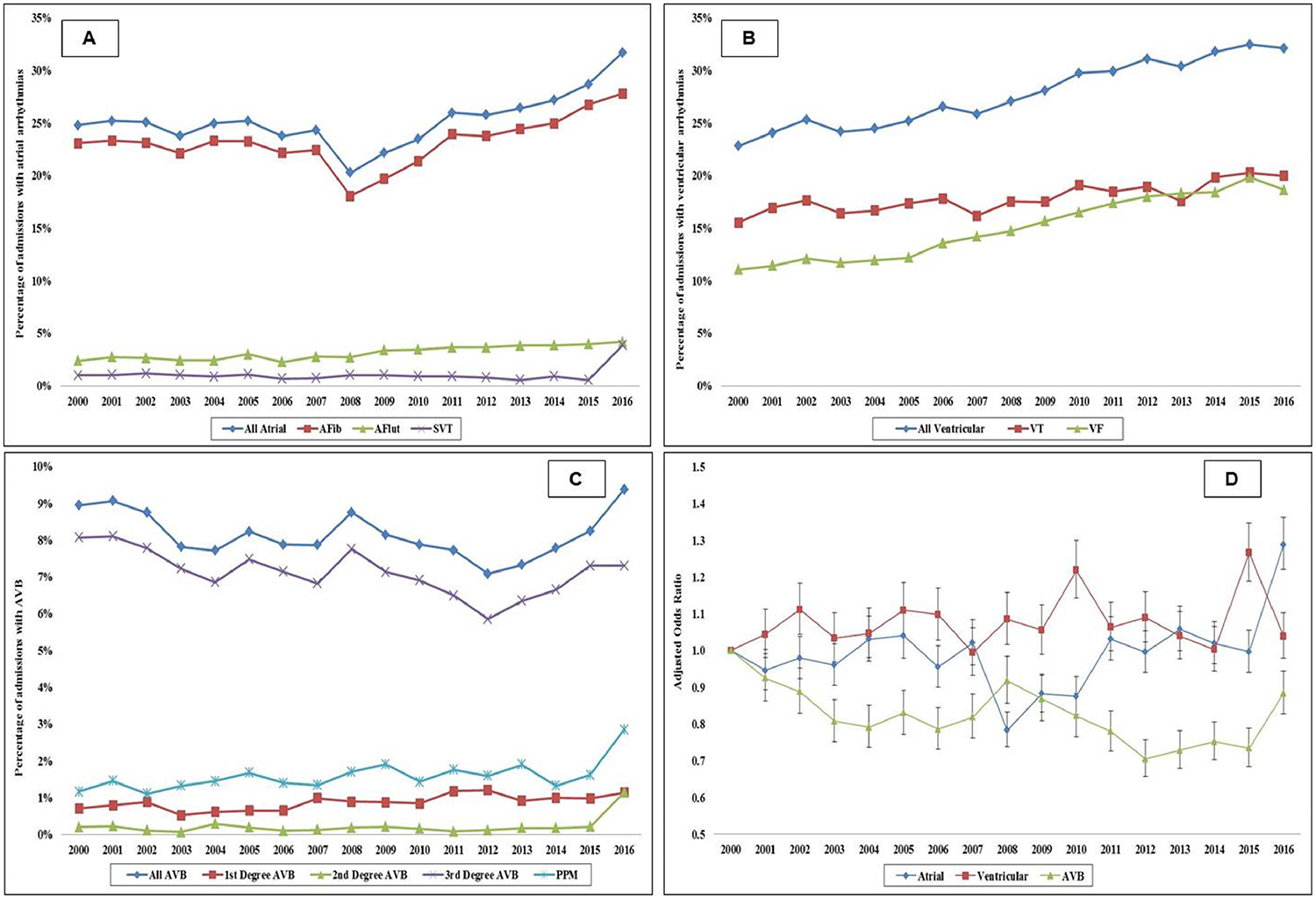
17-year unadjusted trends in the prevalence of atrial arrhythmias (A), ventricular arrhythmias (B), AVB and PPM (C); all p<0.001 for trend over time; D: Adjusted multivariate logistic regression for prevalence of atrial, ventricular and AVB arrhythmias temporal trends with 2000 as referent year; adjusted for age, sex, race, comorbidity, primary payer, socio-economic stratum, hospital characteristics, comorbidities, AMI type, acute organ failure, cardiac arrest, cardiac and non-cardiac procedures (p<0.001 for trend over time).
Abbreviations: AF: atrial fibrillation; AFlut: atrial flutter; AMI: acute myocardial infarction; AVB: atrio-ventricular block; CS: cardiogenic shock; PPM: permanent pacemaker; SVT: supraventricular tachycardia; VF: ventricular fibrillation; VT: ventricular tachycardia
The all-cause in-hospital mortality in admissions with arrhythmias was higher than those without (42.1% vs. 41.3%; OR 1.03 [95% CI 1.02–1.05]; p<0.001). There was a consistent decrease in in-hospital mortality across both categories during this 17-year study period (Figure 3). In a multivariable logistic regression for in-hospital mortality, the cohort arrhythmias had comparable mortality to those without (OR 1.01 [95% CI 0.99–1.03]; p=0.22) (Supplementary Table 2). The cohort with arrhythmias had longer hospital stay, higher hospitalization costs, and fewer discharges to home (Table 3).
Figure 3. Trends of in-hospital mortality in AMI-CS admissions with and without arrhythmias.

A: Unadjusted in-hospital mortality in AMI-CS by year of admission, stratified by arrhythmias (p<0.001 for trend over time); D: Adjusted multivariate logistic regression for in-hospital mortality temporal trends stratified by arrhythmias with 2000 as referent year; adjusted for age, sex, race, comorbidity, primary payer, socio-economic stratum, hospital characteristics, comorbidities, AMI type, acute organ failure, cardiac arrest, cardiac and non-cardiac procedures (p<0.001 for trend over time).
Abbreviations: AMI: acute myocardial infarction; CS: cardiogenic shock
Table 3.
Clinical outcomes of AMI-CS stratified by arrhythmias
| Clinical Outcomes | AMI-CS cohort (N=420,319) |
Arrhythmia cohort (N=213,718) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Arrhythmia (N=213,718) |
No Arrhythmias (N=206,601) |
P | Atrial (N=72,264) |
Ventricular (N=80,388) |
AVB (N=18,123) |
≥2 types (N=42,943) |
P | ||
| In-hospital mortality | 42.1% | 41.3% | <0.001 | 43.0% | 42.7% | 38.7% | 40.8% | <0.001 | |
| Length of stay (days) | 9.1±9.9 | 7.4±8.7 | <0.001 | 9.0±9.2 | 9.1±10.9 | 7.1±7.7 | 9.9±9.9 | <0.001 | |
| Hospitalization costs (x1000 USD) | 124±146 | 91±115 | <0.001 | 107±135 | 134±156 | 101±110 | 142±156 | <0.001 | |
| Disposition | Home | 44.3% | 46.8 % | <0.001 | 32.8% | 52.2% | 54.1% | 44.2% | <0.001 |
| Transfer | 11.5% | 15.5% | 12.4% | 11.5% | 10.8% | 10.1% | |||
| SNF | 29.4 % | 24.0% | 37.5% | 23.3% | 22.2% | 30.7% | |||
| Home with HHC | 14.3% | 13.1% | 16.9% | 12.3% | 12.6% | 14.7% | |||
| AMA | 0.4% | 0.6% | 0.3% | 0.7% | 0.3% | 0.3% | |||
Represented as percentage or mean ± standard deviation
Abbreviations: AMA: against medical advice; AMI: acute myocardial infarction; AVB: atrio-ventricular block; CS: cardiogenic shock; HHC: home health care; SNF: skilled nursing facility; USD: United States Dollars
In the cohort with arrhythmias, the cohort with atrial (43.0%) and ventricular (42.7%) arrhythmias had higher in-hospital mortality compared to those with AVB (38.7%) and with more than one category of arrhythmias (40.8%), p<0.001. In the cohort with arrhythmias, older age, female sex, lack of insurance, non-white race, higher comorbidity, presence of acute organ failure, cardiac arrest, and non-cardiac organ support were predictive of higher in-hospital mortality (Figure 4).
Figure 4. Adjusted odds ratio for in-hospital mortality in AMI admissions with arrhythmias.

Odds ratio with 95% confidence interval using multivariable regression analysis for prediction of in-hospital mortality; for cohorts with multiple categories (i.e. age, sex, race, primary payer, CCI, SES, AMI type) the first category was used as reference category for calculating odds ratios
Abbreviations: AMI: acute myocardial infarction; CCI: Charlson comorbidity index; CS: cardiogenic shock; IHDM: invasive hemodynamic monitoring; IMV: invasive mechanical ventilation; MCS: mechanical circulatory support; NSTEMI: non-ST-elevation myocardial infarction; PCI: percutaneous coronary intervention; SES: socio-economic status; STEMI: ST-elevation myocardial infarction
DISCUSSION
In this nationally representative study of over 400,000 AMI-CS admissions, nearly half the admissions had arrhythmias, with AF, VT and VF being the most commonly noted ones. During this 17-year study period, there was a steady temporal trend across all arrhythmic categories after adjusting for comorbidity and illness severity. Though the cohort with arrhythmias had longer hospital stay and greater resource utilization, they had comparable in-hospital mortality to the cohort without arrhythmias. The cohort with arrhythmias expectedly had greater acuity of illness, and higher use of cardiac and non-cardiac organ support.
Previous studies looking at the impact of arrhythmias in the AMI-CS population have limited their evaluation to a single type of arrhythmia.7,39 Sub-studies from large clinical trials in the AMI-CS population have looked at the prognostic impact of AF in AMI-CS,39 and others have evaluated incidence and burden of ventricular arrhythmias in this population.7 Unlike these studies, we evaluated the prognostic impact of all types of arrhythmias among in-hospital admissions of AMI-CS. We found higher rates and a relatively stable trend for ventricular arrhythmias in contrast to other contemporary studies. A single-center study evaluating trends over 25 years reported a decline in the incidence of VT and VF from 1986 to 2011.7 In comparison to this prior study, our cohort was a national sample, included only AMI-CS patients and spanned a greater time period during which early revascularization techniques and vasoactive medications were increasingly used for the AMI-CS population. Therefore it is possible to surmise that arrhythmias associated with reperfusion and use of vasopressors could have contributed to the higher and stable incidence rates of ventricular arrhythmias.9 A decline in the incidence of atrioventricular block is in line with evidence from the reperfusion era.40
In our study STEMI-CS, acute organ failure, and use of circulatory support devices were independently associated with arrhythmias. Patients with STEMI have a higher likelihood of developing arrhythmias, especially ventricular arrhythmias due to the acuity of onset, larger myocardial involvement and higher rates of coronary revascularization.41 We also noted a significant percentage of acute organ failure in this population with a greater incidence in the arrhythmia cohort.11 Arrhythmias rapidly alter atrial and ventricular hemodynamics affecting loading conditions thereby inducing further damage to an already compromised cardiac status in AMI-CS and worsening end-organ perfusion.11 Therefore acute organ failure bears a bidirectional relationship to arrhythmias in AMI-CS. The use of contemporary mechanical circulatory support devices to provide higher hemodynamic support and reduce transmyocardial strain,10,12,14,17–19 could result in electrolyte imbalances in the myocardium and mechanical irritation potentially increasing incidence of arrhythmias in patients using these devices.42 In addition, patients with AMI-CS are subject to a greater utilization of inotropes and vasoactive medications that potentially precipitate arrhythmias.12,26
Similar to our results, studies from the IABP-SHOCK (Intraaortic Balloon Pump in Cardiogenic Shock) trial and CULPRIT-SHOCK (Culprit Lesion Only PCI versus Multivessel PCI in Cardiogenic Shock) trial also reported no association of AF with mortality.43,44 Nonetheless, results from the Bremen STEMI registry showed that AF was associated with a higher one-year mortality in STEMI-CS.45 The Bremen STEMI registry had patients that were less sick and less likely to have utilized vasoactive medications, circulatory support devices compared to former studies, which could explain the differences. Ventricular arrhythmias, both VT and VF were also found to not have any impact on the mortality in AMI-CS.7 The increase in understanding of the pathophysiology associated with AMI-CS and the advancement of care for these patients, early identification of arrhythmias and prompt access to defibrillation has likely resulted in earlier termination of malignant arrhythmias.
This study has several limitations, some of which are inherent to the analysis of a large administrative database. The HCUP-NIS attempts to mitigate potential errors by using internal and external quality control measures. The lack of information on the duration of arrhythmia, management strategies including rate control, defibrillation and use of anticoagulation limit the generalized ability of our findings. Though we have previously demonstrated that admission with AMI-CS have become sicker over time,11 it is possible that the increase in prevalence of arrhythmias may be attributable to improvements in recognition and coding practices.
In conclusion, development of cardiac arrhythmias during admission for AMI-CS is a marker of higher illness severity and is associated with worse acute organ failure. Though the cohort with arrhythmias did not have higher in-hospital mortality, they had greater resource utilization.
Supplementary Material
SOURCES OF FUNDING
Dr. Saraschandra Vallabhajosyula is supported by the Clinical and Translational Science Award (CTSA) Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
REFERENCES
- 1.Jons C, Jacobsen UG, Joergensen RM, Olsen NT, Dixen U, Johannessen A, Huikuri H, Messier M, McNitt S, Thomsen PE. The incidence and prognostic significance of new-onset atrial fibrillation in patients with acute myocardial infarction and left ventricular systolic dysfunction: a CARISMA substudy. Heart Rhythm 2011;8:342–348. [DOI] [PubMed] [Google Scholar]
- 2.Schmitt J, Duray G, Gersh BJ, Hohnloser SH. Atrial fibrillation in acute myocardial infarction: a systematic review of the incidence, clinical features and prognostic implications. Eur Heart J 2009;30:1038–1045. [DOI] [PubMed] [Google Scholar]
- 3.Kundu A, O’Day K, Shaikh AY, Lessard DM, Saczynski JS, Yarzebski J, Darling CE, Thabet R, Akhter MW, Floyd KC, Goldberg RJ, McManus DD. Relation of atrial fibrillation in acute myocardial infarction to in-hospital complications and early hospital readmission. Am J Cardiol 2016;117:1213–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garg L, Agrawal S, Agarwal M, Shah M, Garg A, Patel B, Agarwal N, Nanda S, Sharma A, Cox D. Influence of atrial fibrillation on outcomes in patients who underwent primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Am J Cardiol 2018;121:684–689. [DOI] [PubMed] [Google Scholar]
- 5.Stenestrand U, Tabrizi F, Lindback J, Englund A, Rosenqvist M, Wallentin L. Comorbidity and myocardial dysfunction are the main explanations for the higher 1-year mortality in acute myocardial infarction with left bundle-branch block. Circulation 2004;110:1896–1902. [DOI] [PubMed] [Google Scholar]
- 6.Meine TJ, Al-Khatib SM, Alexander JH, Granger CB, White HD, Kilaru R, Williams K, Ohman EM, Topol E, Califf RM. Incidence, predictors, and outcomes of high-degree atrioventricular block complicating acute myocardial infarction treated with thrombolytic therapy. Am Heart J 2005;149:670–674. [DOI] [PubMed] [Google Scholar]
- 7.Tran HV, Ash AS, Gore JM, Darling CE, Kiefe CI, Goldberg RJ. Twenty-five year trends (1986–2011) in hospital incidence and case-fatality rates of ventricular tachycardia and ventricular fibrillation complicating acute myocardial infarction. Am Heart J 2019;208:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia-Garcia C, Oliveras T, Rueda F, Perez-Fernandez S, Ferrer M, Serra J, Labata C, Vila J, Carrillo X, Rodriguez-Leor O, Fernandez-Nofrerias E, Faixedas MT, Jimenez J, Mauri J, Lupon J, Bayes-Genis A. Primary ventricular fibrillation in the primary percutaneous coronary intervention ST-segment elevation myocardial infarction era (from the “codi IAM” multicenter registry). Am J Cardiol 2018;122:529–536. [DOI] [PubMed] [Google Scholar]
- 9.Mehta RH, Starr AZ, Lopes RD, Hochman JS, Widimsky P, Pieper KS, Armstrong PW, Granger CB. Incidence of and outcomes associated with ventricular tachycardia or fibrillation in patients undergoing primary percutaneous coronary intervention. JAMA 2009;301:1779–1789. [DOI] [PubMed] [Google Scholar]
- 10.Vallabhajosyula S, Arora S, Lahewala S, Kumar V, Shantha GPS, Jentzer JC, Stulak JM, Gersh BJ, Gulati R, Rihal CS, Prasad A, Deshmukh AJ. Temporary mechanical circulatory support for refractory cardiogenic shock before left ventricular assist device surgery. J Am Heart Assoc 2018;7:e010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vallabhajosyula S, Dunlay SM, Prasad A, Kashani K, Sakhuja A, Gersh BJ, Jaffe AS, Holmes DR Jr., Barsness GW. Acute noncardiac organ failure in acute myocardial infarction with cardiogenic shock. J Am Coll Cardiol 2019;73:1781–1791. [DOI] [PubMed] [Google Scholar]
- 12.Vallabhajosyula S, Dunlay SM, Kashani K, Vallabhajosyula S, Vallabhajosyula S, Sundaragiri PR, Jaffe AS, Barsness GW. Temporal trends and outcomes of prolonged invasive mechanical ventilation and tracheostomy use in acute myocardial infarction with cardiogenic shock in the United States. Int J Cardiol 2019;285:6–10. [DOI] [PubMed] [Google Scholar]
- 13.Vallabhajosyula S, Dunlay SM, Barsness GW, Rihal CS, Holmes DR Jr., Prasad A. Hospital-level disparities in the outcomes of acute myocardial infarction with cardiogenic shock. Am J Cardiol 2019;124:491–498. [DOI] [PubMed] [Google Scholar]
- 14.Vallabhajosyula S, Arora S, Sakhuja A, Lahewala S, Kumar V, Shantha GPS, Egbe AC, Stulak JM, Gersh BJ, Gulati R, Rihal CS, Prasad A, Deshmukh AJ. Trends, predictors, and outcomes of temporary mechanical circulatory support for postcardiac surgery cardiogenic shock. Am J Cardiol 2019;123:489–497. [DOI] [PubMed] [Google Scholar]
- 15.Vallabhajosyula S, Dunlay SM, Murphree DH Jr., Barsness GW, Sandhu GS, Lerman A, Prasad A. Cardiogenic shock in takotsubo cardiomyopathy versus acute myocardial infarction: An 8-year national perspective on clinical characteristics, management, and outcomes. JACC Heart Fail 2019;7:469–476. [DOI] [PubMed] [Google Scholar]
- 16.Vallabhajosyula S, Kashani K, Dunlay SM, Vallabhajosyula S, Vallabhajosyula S, Sundaragiri PR, Gersh BJ, Jaffe AS, Barsness GW. Acute respiratory failure and mechanical ventilation in cardiogenic shock complicating acute myocardial infarction in the USA, 2000–2014. Ann Intensive Care 2019;9:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vallabhajosyula S, O’Horo JC, Antharam P, Ananthaneni S, Vallabhajosyula S, Stulak JM, Dunlay SM, Holmes DR Jr., Barsness GW. Venoarterial extracorporeal membrane oxygenation with concomitant Impella versus venoarterial extracorporeal membrane oxygenation for cardiogenic shock. ASAIO J 2019; doi: 10.1097/MAT.0000000000001039. [DOI] [PubMed] [Google Scholar]
- 18.Vallabhajosyula S, O’Horo JC, Antharam P, Ananthaneni S, Vallabhajosyula S, Stulak JM, Eleid MF, Dunlay SM, Gersh BJ, Rihal CS, Barsness GW. Concomitant intra-aortic balloon pump use in cardiogenic shock requiring veno-arterial extracorporeal membrane oxygenation. Circ Cardiovasc Interv 2018;11:e006930. [DOI] [PubMed] [Google Scholar]
- 19.Vallabhajosyula S, Patlolla SH, Sandhyavenu H, Vallabhajosyula S, Barsness GW, Dunlay SM, Greason KL, Holmes DR, Jr., Eleid MF. Periprocedural cardiopulmonary bypass or venoarterial extracorporeal membrane oxygenation during transcatheter aortic valve replacement: A systematic review. J Am Heart Assoc 2018;7:e009608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vallabhajosyula S, Prasad A, Dunlay SM, Murphree DH Jr., Ingram C, Mueller PS, Gersh BJ, Holmes DR Jr., Barsness GW. Utilization of palliative care for cardiogenic shock complicating acute myocardial infarction: A 15-year national perspective on trends, disparities, predictors, and outcomes. J Am Heart Assoc 2019;8:e011954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vallabhajosyula S, Prasad A, Gulati R, Barsness GW. Contemporary prevalence, trends, and outcomes of coronary chronic total occlusions in acute myocardial infarction with cardiogenic shock. Int J Cardiol Heart Vasc 2019;24:100414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vallabhajosyula S, Ya’Qoub L, Dunlay SM, Vallabhajosyula S, Vallabhajosyula S, Sundaragiri PR, Jaffe AS, Gersh BJ, Kashani K. Sex disparities in acute kidney injury complicating acute myocardial infarction with cardiogenic shock. ESC Heart Fail 2019;6:874–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vallabhajosyula S, Vallabhajosyula S, Vaidya VR, Patlolla SH, Desai V, Mulpuru SK, Noseworthy PA, Kapa S, Egbe AC, Gersh BJ, Deshmukh AJ. Venoarterial extracorporeal membrane oxygenation support for ventricular tachycardia ablation: A systematic review. ASAIO J 2020; doi: 10.1097/MAT.0000000000001125. [DOI] [PubMed] [Google Scholar]
- 24.Jeger RV, Assmann SF, Yehudai L, Ramanathan K, Farkouh ME, Hochman JS. Causes of death and re-hospitalization in cardiogenic shock. Acute Cardiac Care 2007;9:25–33. [DOI] [PubMed] [Google Scholar]
- 25.DiMarco John P. Atrial fibrillation and acute decompensated heart failure. Circ Heart Fail 2009;2:72–73. [DOI] [PubMed] [Google Scholar]
- 26.Levy B, Clere-Jehl R, Legras A, Morichau-Beauchant T, Leone M, Frederique G, Quenot JP, Kimmoun A, Cariou A, Lassus J, Harjola VP, Meziani F, Louis G, Rossignol P, Duarte K, Girerd N, Mebazaa A, Vignon P. Epinephrine versus norepinephrine for cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol 2018;72:173–182. [DOI] [PubMed] [Google Scholar]
- 27.Introduction to the HCUP Nationwide Inpatient Sample 2009. http://www.hcup-us.ahrq.gov/db/nation/nis/NIS_2009_INTRODUCTION.pdf. Accessed Jan 18, 2015: HCUP. [Google Scholar]
- 28.Egbe AC, Vallabhajosyula S, Vojjini R, Banala K, Najam M, Faizee F, Khalil F, Ullah MW, Deshmukh AJ. Prevalence and in-hospital mortality during arrhythmia-related admissions in adults with tetralogy of Fallot. Int J Cardiol 2019;297:49–54. [DOI] [PubMed] [Google Scholar]
- 29.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130–1139. [DOI] [PubMed] [Google Scholar]
- 30.Vallabhajosyula S, Deshmukh AJ, Kashani K, Prasad A, Sakhuja A. Tako-tsubo cardiomyopathy in severe sepsis: nationwide trends, predictors, and outcomes. J Am Heart Assoc 2018;7:e009160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vallabhajosyula S, Prasad A, Sandhu GS, Bell MR, Gulati R, Eleid MF, Best PJM, Gersh BJ, Singh M, Lerman A, Holmes DR Jr., Rihal CS, Barsness GW. Mechanical circulatory support-assisted early percutaneous coronary intervention in acute myocardial infarction with cardiogenic shock: 10-year national temporal trends, predictors and outcomes. EuroIntervention 2019; doi: 10.4244/EIJ-D-19-00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vallabhajosyula S, Prasad A, Bell MR, Sandhu GS, Eleid MF, Dunlay SM, Schears GJ, Stulak JM, Singh M, Gersh BJ, Jaffe AS, Holmes DR Jr., Rihal CS, Barsness GW. Extracorporeal membrane oxygenation use in acute myocardial infarction in the United States, 2000 to 2014. Circ Heart Fail 2019;12:e005929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vallabhajosyula S, Vallabhajosyula S, Burstein B, Ternus BW, Sundaragiri PR, White RD, Barsness GW, Jentzer JC. Epidemiology of in-hospital cardiac arrest complicating non-ST-segment elevation myocardial infarction receiving early coronary angiography. Am Heart J 2020;223:59–64. [DOI] [PubMed] [Google Scholar]
- 34.Vallabhajosyula S, Jentzer JC, Zack CJ. Cardiac arrest definition using administrative codes and outcomes in acute myocardial infarction. Mayo Clin Proc 2020;95:611–613. [DOI] [PubMed] [Google Scholar]
- 35.Vallabhajosyula S, Kumar V, Vallabhajosyula S, Subramaniam AV, Patlolla SH, Verghese D, Ya’Qoub L, Stulak JM, Sandhu GS, Prasad A, Holmes DR Jr., Barsness GW. Acute myocardial infarction-cardiogenic shock in patients with prior coronary artery bypass grafting: A 16-year national cohort analysis of temporal trends, management and outcomes. Int J Cardiol 2020; doi: 10.1016/j.ijcard.2020.02.033. [DOI] [PubMed] [Google Scholar]
- 36.Vallabhajosyula S, Patlolla SH, Dunlay SM, Prasad A, Bell MR, Jaffe AS, Gersh BJ, Rihal CS, Holmes DR Jr., Barsness GW. Regional variation in the management and outcomes of acute myocardial infarction with cardiogenic shock in the United States. Circ Heart Fail 2020;13:e006661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khera R, Krumholz HM. With great power comes great responsibility: big data research from the National Inpatient Sample. Circ Cardiovasc Qual Outcomes 2017;10:e003846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khera R, Angraal S, Couch T, Welsh JW, Nallamothu BK, Girotra S, Chan PS, Krumholz HM. Adherence to Methodological Standards in Research Using the National Inpatient Sample. JAMA 2017;318:2011–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Waha S, Schoene K, Fuernau G, Desch S, Eitel I, Poss J, Meyer-Saraei R, Eitel C, Tilz R, Schuler G, Werdan K, Schneider S, Ouarrak T, Zeymer U, Thiele H. Prognostic impact of atrial fibrillation in cardiogenic shock complicating acute myocardial infarction: a substudy of the IABP-SHOCK II trial. Clin Res Cardiol 2018;107:233–240. [DOI] [PubMed] [Google Scholar]
- 40.Wong CK, Stewart RA, Gao W, French JK, Raffel C, White HD. Prognostic differences between different types of bundle branch block during the early phase of acute myocardial infarction: insights from the Hirulog and Early Reperfusion or Occlusion (HERO)-2 trial. Eur Heart J 2006;27:21–28. [DOI] [PubMed] [Google Scholar]
- 41.Liang JJ, Fender EA, Cha YM, Lennon RJ, Prasad A, Barsness GW. Long-term outcomes in survivors of early ventricular arrhythmias after acute ST-elevation and non-ST-elevation myocardial infarction treated with percutaneous coronary intervention. Am J Cardiol 2016;117:709–713. [DOI] [PubMed] [Google Scholar]
- 42.Kadado AJ, Akar JG, Hummel JP. Arrhythmias after left ventricular assist device implantation: Incidence and management. Trends Cardiovasc Med 2018;28:41–50. [DOI] [PubMed] [Google Scholar]
- 43.de Waha S, Schoene K, Fuernau G, Desch S, Eitel I, Pöss J, Meyer-Saraei R, Eitel C, Tilz R, Schuler G, Werdan K, Schneider S, Ouarrak T, Zeymer U, Thiele H. Prognostic impact of atrial fibrillation in cardiogenic shock complicating acute myocardial infarction: a substudy of the IABP-SHOCK II trial. Clin Res Cardiol 2018;107:233–240. [DOI] [PubMed] [Google Scholar]
- 44.Feistritzer H-J, Desch S, Zeymer U, Fuernau G, de Waha-Thiele S, Dudek D, Huber K, Stepinska J, Schneider S, Ouarrak T, Thiele H. Prognostic impact of atrial fibrillation in acute myocardial infarction and cardiogenic shock. Circ Cardiovasc Interv 2019;12:e007661. [DOI] [PubMed] [Google Scholar]
- 45.Backhaus T, Fach A, Schmucker J, Fiehn E, Garstka D, Stehmeier J, Hambrecht R, Wienbergen H. Management and predictors of outcome in unselected patients with cardiogenic shock complicating acute ST-segment elevation myocardial infarction: results from the Bremen STEMI Registry. Clin Res Cardiol 2018;107:371–379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


