Abstract
Experimental studies support a link between activation of the renin-angiotensin-aldosterone system (RAAS) and cardiovascular disease (CVD). The relationship with subclinical atherosclerosis is uncertain. Among 1,699 individuals without prevalent CVD from the Multi-Ethnic Study of Atherosclerosis (MESA), we measured plasma renin activity (PRA) and aldosterone. Using multivariable logistic regression with restricted cubic splines, we assessed continuous log-transformed PRA and aldosterone associations with the ankle-brachial index (ABI) and coronary artery calcium (CAC) scores (Agatston) with adjustment for CVD risk factors, kidney function, and inflammatory biomarkers. In fully adjusted models mutually adjusting for PRA and aldosterone, higher PRA was associated with an ABI <1.0 (p-overall <0.001, p-non-linear=0.02) and CAC Agatston score >300 (p-overall=0.02, p-non-linear=0.22), while aldosterone was not associated with either outcome. For example, compared to the 10th percentile (0.16ng/mL/hr) of PRA, the 90th percentile (2.68ng/mL/hr) had 3.6 times (OR 3.62; 95%CI: 2.13-6.13) and 1.7 times higher odds (OR 1.67; 95%CI: 1.13-2.48) of ABI <1.0 and CAC >300, respectively. These associations persisted after adjustment for levels of C-reactive protein, IL-6 and TNF-α. There were no significant differences in these associations by race/ethnicity or antihypertensive medication status. In conclusion, in a multi-ethnic cohort of community-living adults without prevalent clinical CVD, PRA was associated with greater burden of subclinical peripheral artery and coronary artery disease. These findings provide additional evidence that PRA may have deleterious effects on cardiovascular health through an atherosclerotic pathway.
Keywords: ankle brachial index, coronary artery calcium, hypertension, primary prevention
Introduction
Although there is a pathologic basis and growing clinical evidence to support a link between plasma renin activity (PRA) and cardiovascular events,1,2 the association of PRA and aldosterone (ALDO) with markers of subclinical CVD in a population free of known clinical CVD has not been previously examined. Moreover, understanding the role of PRA and ALDO levels earlier in the course of development of atherosclerotic disease could inform future preventive interventions, such as the use of medications directed at blockade of this system. Given this, we conducted a study using data from a multi-ethnic population without clinical CVD to test the hypothesis that both PRA and ALDO would be significantly associated with the following markers of subclinical peripheral artery disease (PAD) and coronary artery disease: the ankle-brachial index (ABI) and coronary artery calcium (CAC), respectively. Both PAD3 and CAC4 are associated with an increased risk for CVD events and mortality.
Methods
The Multi-Ethnic Study of Atherosclerosis (MESA) is a prospective observational cohort of 6,814 men and women without clinical CVD at baseline.5 In brief, participants aged 45-84 years, who identified themselves as African-American, Chinese-American, Hispanic-American and non-Hispanic White (based on the 2000 U.S. census questionnaire) were recruited between August 1, 2000, and July 30, 2002, from 6 United States communities. Examination 2 (2002-2004) and Examination 3 (2004-2005) occurred about 1.5 and 3 years after baseline, respectively. Participants provided informed consent and the MESA was Institutional Review Board approved at all field centers.
At Examination 2 and 3, a subsample of 1,772 MESA participants had both PRA and ADLO levels measured as part of an ancillary study investigating kidney disease. Participants underwent these measurements at either visit 2 or 3 based upon when they participated in the ancillary study; no individual participated in the ancillary study at both visits. Of the 1,772 subjects with both PRA and ALDO measured, participants were excluded if they experienced or may have experienced a cardiovascular event between baseline and the PRA/ALDO measurement (n=53), or their PRA/ALDO ratio >500 (n=20). This resulted in a final sample size of 1,699.
Age, sex, race/ethnicity and smoking history were self-reported. Cigarette smoking was defined as current, former, or never. Resting seated blood pressure was measured three times using a Dinamap model Pro100 automated oscillometric sphygmomanometer (Critikon, Tampa, FL), and the average of the second and third readings was used in analysis. Trained staff recorded participant use of blood pressure, anti-lipid/statin and diabetes medications.
At each visit, 12-hour fasting venous blood samples were obtained and processed using standard methods.5,6 Participant samples were originally collected in the morning, aliquoted into approximately 65 aliquots per participant and immediately placed on ice, flash frozen and stored at −80°C after processing.
Serum glucose was measured by rate reflectance spectrophotometry using thin film adaptation of the glucose oxidase method on the Vitros analyzer (Johnson & Johnson Clinical Diagnostics, Rochester, NY). Glucose levels were classified into normal (< 100 mg/dL), impaired fasting glucose (100-126 mg/dL) and diabetes (≥ 126 mg/dL) or use of diabetes medication.7 Fasting total cholesterol (TC) was measured in plasma on the Hitachi 911 using a cholesterol esterase, cholesterol oxidase reaction (Chol R1, Roche Diagnostics). The same reaction was also used to measure HDL-cholesterol (HDL-C) after precipitation of non-HDL-cholesterol (non-HDL-C) with magnesium/dextran.
Serum creatinine was measured by rate reflectance spectrophotometry using thin-film adaptation of the creatinine amidinohydrolase method on the VITROS analyzer (Johnson & Johnson Clinical Diagnostics) and calibrated to Cleveland Clinic. Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.8 Urine albumin (g/mL)-to-creatinine (mg/mL) ratios (UACR) were calculated from urine samples measured by nephelometry and the rate Jaffe reaction, respectively.
C-reactive protein (CRP), Interleukin-6 (IL-6) and Tumor Necrosis Factor-α (TNF-alpha) were measured by Human Serum Adipokine Panel B LINCOplex Kit (Linco Research, Inc., St. Charles, MO). All assays were performed at the Laboratory for Clinical Biochemistry Research (University of Vermont, Burlington, VT).
Both PRA and aldosterone assays were run in duplicate and averaged. PRA was measured after 3 freeze–thaw cycles; aldosterone was measured after 2 cycles. Angiotensin I levels directly correlate with PRA. Therefore, PRA was measured using a radioimmunoassay of generated angiotensin I (GammaCoat Plasma Renin Activity 125I Kit; DiaSorin, Stillwater, MN). Plasma renin activity was defined as nanograms of angiotensin I generated per milliliter of sample per hour (ng/ml/hr). The assay range reference was 0.05–5.0ng/ml/hour. Intra-assay coefficients of variation ranged 6.9%–18.4%. Aldosterone was measured using a competition-based radioimmunoassay (ALDOCTK-2; Diasorin, Stillwater, MN). Intra-assay coefficients of variation ranged 6.3%–8.9%. All assays were performed at the Laboratory for Clinical Biochemistry Research (University of Vermont, Burlington, VT).
The ABI was measured at Exam 1 and Exam 3. We used the Exam 1 ABI for individuals with PRA and ALDO levels measured at Visit 2. The ABI was calculated from systolic blood pressure measurements made in the bilateral brachial, dorsalis pedis and posterior tibial arteries. These were obtained in the supine position after 5 minutes of rest, using a 5-mHz continuous wave Doppler probe. ABIs were calculated for each leg as the ratio of higher of the two ankle pressures divided by the highest of the two arm blood pressures. The leg with the lower ABI was used to classify the severity of PAD in the participant. We used and ABI <1.0 as a marker for significant PAD as ABI values below this level are associated with increased risk for CVD events and mortality.9,10 Individuals with an ABI of 1.0-1.4 were considered the normal referent group. Individuals with missing ABI (n=9) or an ABI >1.4 (n=19) were excluded as not to mask the presence of PAD by stiff arteries.
CAC was measured at Examination 2 and 3 in participants through use of either electron-beam or multidetector row helical computed tomography. Each participant was scanned twice, consecutively. These scans were read independently and the results of the 2 scans averaged. CAC was quantified via the Agatston scoring method,11,12 We considered Agatston scores > 300 as a subclinical marker of high risk in our analyses.13
We began by examining PRA and ALDO levels with histograms to assess their distributions. Both PRA and aldosterone were log-transformed to improve normality. Means and standard deviations were used to summarize the characteristics of the various covariates across quartiles. Categorical and normally distributed continuous variables were compared with χ2 and ANOVA tests, respectively.
We constructed a series of progressively adjusted multivariable logistic regression models to assess the associations of PRA and ALDO with both ABI <1.0 and CAC score >300. Our preliminary data exploration suggested a possible non-linear relationship of both PRA and ALDO with both outcomes. As a result, we modeled PRA and ALDO using restricted cubic splines with 3 knots (10th, 50th, and 90th percentiles) to allow model flexibility using the SAS macro by Desquilbet.14 In model 1, we assessed associations of PRA with adjustment for age, sex, race/ethnicity and ALDO. In model 2 (final model), we further adjusted for traditional CVD risk factors (systolic BP, antihypertensive use, total and HDL cholesterol, statin use, diabetes, and smoking) and kidney function (urine albumin-creatinine ratio and eGFR). As an exploratory analysis, in model 3 we further adjusted for three inflammatory markers (CRP, IL-6 and TNF-alpha) to assess if the association of PRA and ALDO with the outcomes are possibly mediated through an inflammatory pathway. While both the ABI and CAC are continuous variables, we choose to model the association using well-established clinical cutoffs that provide an interpretable measure of relative risk.
We performed a series of sensitivity analyses and tests for potential interaction. As antihypertensive medications are known to alter the RAAS system, we re-examined associations of PRA and ALDO with ABI <1.0 and CAC score >300 in individuals not taking any antihypertensive medications. Furthermore, as PRA and ALDO levels are known to differ by race/ethnicity,15 we initially tested each model for interactions by race/ethnic group by creating a multiplicative term between the continuous predictor variable and race/ethnic categories. Given that final overall models showed significant non-linear relationships, we further explored interaction by examining continuous dose-response associations of PRA and ALDO with both outcomes by plotting stratified analyses by race/ethnic group. Lastly, we reran multivariable linear regression treating ABI and log-transformed CAC as continuous measures to assess if the shape of the relationship differed to using a binary cutoff. All analyses were performed in SAS (v9.4, Cary, NC) or R (R Foundation for Statistical Computing). A two-sided p-value <0.05 was used for statistical significance for all analyses including interaction terms.
Results
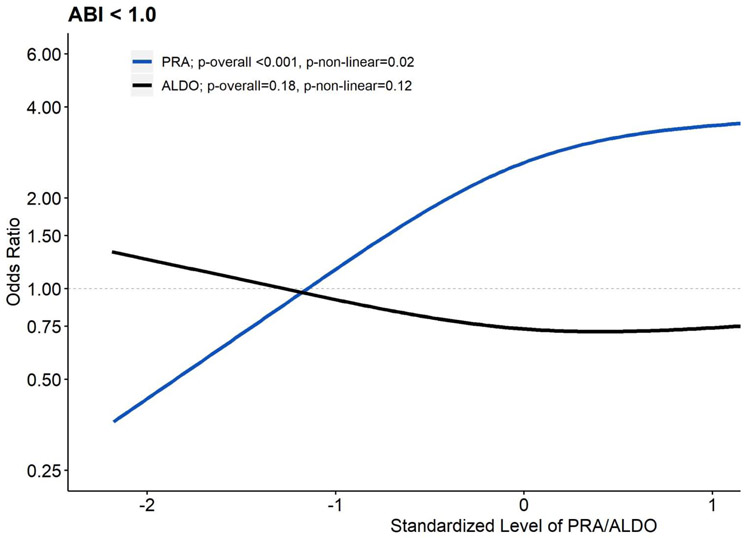
Table 1 displays overall study population characteristics and Supplemental Table 1 displays characteristics by quartiles of PRA. Table 2 displays the associations of PRA with an ABI <1.0. In model 1 that adjusted for demographics and ALDO, higher levels of PRA were associated with higher odds of an ABI <1.0 (p-overall <0.001, p-non-linear=0.06). Further adjustment for CVD risk factors and kidney function (model 2) strengthened the associations of PRA with an ABI <1.0. In model 3, adjustment for potential inflammatory mediators strengthened, not attenuated, the associations of PRA with an ABI <1.0. Levels of ALDO (p=0.21) were not associated (Supplemental Table 2) with an ABI <1.0. Figure 1 displays the mutually adjusted relationship of PRA and ALDO with an ABI <1.0.
Table 1.
Participant Sociodemographic, Health and Laboratory Characteristics: Multi-Ethnic Study of Atherosclerosis (2002-2005)
| Variable | Overall (n=1,699) |
|---|---|
| Plasma Renin Activity [ng/mL/hr] | 1.45 ± 3.3 |
| Plasma Aldosterone [ng/dL] | 15.0 ± 8.6 |
| Age [years] | 64.4 ± 9.6 |
| Women | 848 (49.9%) |
| Non-Hispanic White | 696 (41.0%) |
| Chinese | 228 (13.4%) |
| Black | 337 (19.8%) |
| Hispanic | 438 (25.8%) |
| Systolic Blood Pressure [mmHg] | 124.6 ± 21.0 |
| Diastolic Blood Pressure [mmHg] | 70.8 ± 9.9 |
| Antihypertensive Use | 680 (40.0%) |
| Total Cholesterol [mg/dL] | 191.9 ± 34.8 |
| High Density Lipoprotein Cholesterol [mg/dL] | 51.1 ± 15.1 |
| Statin Use | 336 (19.8%) |
| Diabetes Mellitus | |
| Normal | 1,231 (72.5%) |
| Impaired Fasting Glucose | 233 (13.7%) |
| Diabetes | 235 (13.8%) |
| Cigarette Use | |
| Never | 821 (48.3%) |
| Former | 681 (40.1%) |
| Current | 197 (11.6%) |
| Estimated glomerular filtration rate [mL/min/1.73m2] | 79.3 ± 17.4 |
| Urine Albumin-Creatinine Ratio | 27.1 ± 127.0 |
| C-Reactive Protein [mg/L] | 3.1 ± 6.1 |
| Interleukin-6 [pg/mL] | 2.3 ± 1.8 |
| Tumor Necrosis Factor-α [pg/mL] | 5.8 ± 9.8 |
| Outcomes | |
| Ankle Brachial Index | 1.13 ± 0.12 |
| Ankle Brachial Index <1.0 | 173 (10.4%) |
| Coronary Artery Calcium [Agatston] if >0 | 296 ± 504 |
| Calcium [Agatston] >300 | 247 (15.0%) |
Table 2.
Associations of Plasma Renin Activity with Ankle Brachial Index < 1.0: MESA (2002-2005)
| Percentile [Value]* | Model 1† | Model 2† | Model 3† |
|---|---|---|---|
| 10th [0.16]* | 1 (ref) | 1 (ref) | 1 (ref) |
| 25th [0.30]* | 1.54 (1.21-1.94) | 1.63 (1.27-2.09) | 1.68 (1.30-2.17) |
| 50th [0.57]* | 2.22 (1.48-3.34) | 2.46 (1.59-3.80) | 2.59 (1.65-4.05) |
| 75th [1.16]* | 2.84 (1.78-4.52) | 3.16 (1.92-5.21) | 3.38 (2.02-5.66) |
| 90th [2.68]* | 3.33 (2.06-5.38) | 3.62 (2.13-6.13) | 3.91 (2.27-6.72) |
| 95th [5.34]* | 3.73 (2.18-6.38) | 3.94 (2.15-7.21) | 4.29 (2.31-7.96) |
| p-overall‡ | <0.001 | <0.001 | <0.001 |
| p-non-linear‡ | 0.06 | 0.03 | 0.02 |
Plasma Renin Activity values in [ng/mL/hr]
Reported are odds ratio (95% confidence interval) from logistic regression models evaluating log-transformed plasma renin using restricted cubic splines with 3 knots (10th, 50th, 90th percentiles)
Results for Wald X2 test
Model 1 [n=1,691, 174 cases] adjusts for age, sex, race/ethnicity and aldosterone
Model 2 [n=1,667, 169 cases] adjusts for Model 1 + systolic blood pressure, antihypertensive use, total cholesterol, HDL cholesterol, statin use, diabetes, smoking history, UACR and eGFR
Model 3 [n=1,603, 163 cases] adjusts for Model 2 + TNF-alpha, IL-6 and CRP
Abbreviations: MESA, Multi-Ethnic Study of Atherosclerosis; HDL, high density lipoprotein; UACR, urine albumin to creatinine ratio; eGFR, estimated glomerular filtration rate; TNF, tumor necrosis factor; IL, interleukin; CRP, c-reactive protein.
Figure 1. Associations of Plasma Renin Activity and Aldosterone Levels with Subclinical Peripheral Artery Disease (n=1,667): MESA (2002-2005).
All associations were estimated with logistic regression models mutually adjusting for log-transformed PRA and log-transformed ALDO using restricted cubic splines with 3 knots controlling for age, race/ethnicity, systolic blood pressure, antihypertensive use, total cholesterol, HDL cholesterol, statin use, diabetes, smoking history, UACR and GFR. Results were trimmed at the 1st and 99th percentiles. The referent was set to the 10th percentile for both PRA and ALDO. PRA and ALDO were converted to z-scores to allow visual overlay. Compared to the 10th percentile of PRA (0.16 ng/mL/hr), respective ORs and 95% CIs relative to the 25th (0.30 ng/mL/hr), 50th (0.57 ng/mL/hr), 75th (1.16 ng/mL/hr), and 90th (2.68 ng/mL/hr) were 1.63 (1.27-2.09), 2.46 (1.59-3.80), 3.16 (1.92-5.21), and 3.62 (2.13-6.13). Relative to the 10th percentile of ALDO (6.57 ng/dL), respective ORs and 95% CIs relative to the 25th (9.34 ng/dL), 50th (13.02 ng/dL), 75th (18.51 ng/dL), and 90th (25.14 ng/dL) percentile were 0.84 (0.68-1.02), 0.75 (0.55-1.03), 0.74 (0.52-1.06), and 0.78 (0.51-1.18).
Abbreviations: MESA, Multi-Ethnic Study of Atherosclerosis; PRA, plasma renin activity; ALDO, aldosterone; HDL, high density lipoprotein; UACR, urine albumin creatinine ratio; GFR, glomerular filtration rate; OR, odds ratio; CI, confidence interval.
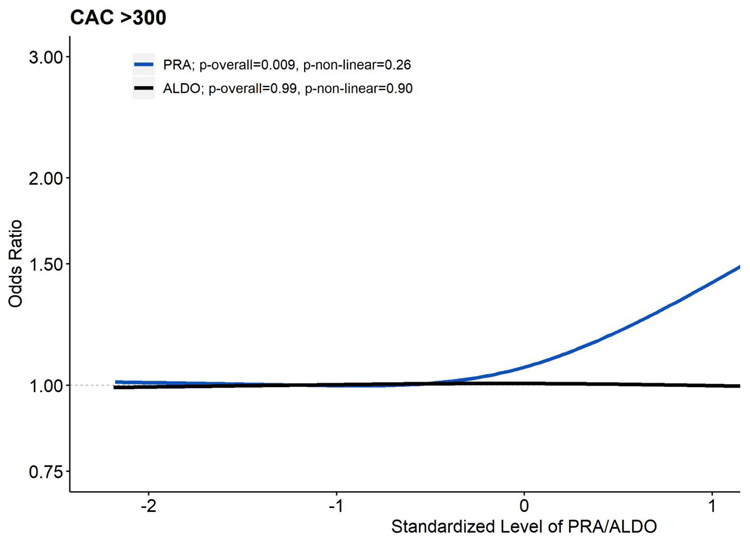
Table 3 displays the associations of PRA with a CAC score >300. In the demographic and ALDO adjusted model, higher PRA was associated with a higher odds of CAC score >300, which showed a graded relationship across quartiles (p-overall <0.001, p-non-linear=0.16). Adjustment for CVD risk factors and kidney function slightly attenuated the strength of the association, but the overall pattern persisted. Similarly, in model 3, adjustment for potential inflammatory mediators slightly attenuated the association, but the overall pattern and significance of the association of PRA and CAC score >300 persisted. Levels of ALDO were not associated with a CAC score >300 (p=0.99; Supplemental Table 3). Figure 2 displays the mutually adjusted relationship of PRA and ALDO with a CAC score >300.
Table 3.
Associations of Plasma Renin Activity with Coronary Artery Calcium Score > 300 Agatston Units: MESA (2002-2005)
| Percentile [Value]* | Model 1† | Model 2† | Model 3† |
|---|---|---|---|
| 10th [0.16]* | 1 (ref) | 1 (ref) | 1 (ref) |
| 25th [0.30]* | 1.05 (0.90-1.24) | 1.03 (0.87-1.23) | 1.02 (0.86-1.21) |
| 50th [0.57]* | 1.16 (0.88-1.53) | 1.11 (0.83-1.50) | 1.08 (0.80-1.45) |
| 75th [1.16]* | 1.40 (1.02-1.93) | 1.30 (0.92-1.83) | 1.24 (0.88-1.75) |
| 90th [2.68]* | 1.89 (1.32-2.69) | 1.67 (1.13-2.48) | 1.57 (1.05-2.34) |
| 95th [5.34]* | 2.43 (1.59-3.73) | 2.08 (1.28-3.37) | 1.92 (1.17-3.16) |
| p-overall‡ | <0.001 | 0.009 | 0.02 |
| p-non-linear‡ | 0.16 | 0.24 | 0.22 |
Plasma Renin Activity values in [ng/mL/hr]
Reported are odds ratio (95% confidence interval) from logistic regression models evaluating log-transformed plasma renin using restricted cubic splines with 3 knots (10th, 50th, 90th percentiles)
Results for Wald X2 test
Model 1 [n=1,668, 248 cases] adjusts for age, sex, race/ethnicity and aldosterone
Model 2 [n=1,644, 242 cases] adjusts for Model 1 + systolic blood pressure, antihypertensive use, total cholesterol, HDL cholesterol, statin use, diabetes, smoking history, UACR and eGFR
Model 3 [n=1,579, 233 cases] adjusts for Model 2 + TNF-α, IL-6 and CRP
Abbreviations: MESA, Multi-Ethnic Study of Atherosclerosis; HDL, high density lipoprotein; UACR, urine albumin to creatinine ratio; eGFR, estimated glomerular filtration rate; TNF, tumor necrosis factor; IL, interleukin; CRP, c-reactive protein.
Figure 2. Associations of Plasma Renin Activity and Aldosterone Levels with Subclinical Coronary Artery Disease (n=1,668): MESA (2002-2005).
All associations were estimated with logistic regression models mutually adjusting for log-transformed PRA and log-transformed ALDO using restricted cubic splines with 3 knots controlling for age, race/ethnicity, systolic blood pressure, antihypertensive use, total cholesterol, HDL cholesterol, statin use, diabetes, smoking history, UACR and GFR. Results were trimmed at the 1st and 99th percentiles. The referent was set to the 10th percentile for both PRA and ALDO. PRA and ALDO were converted to z-scores to allow visual overlay. Compared to the 10th percentile of PRA (0.16 ng/mL/hr), respective ORs and 95% CIs relative to the 25th (0.30 ng/mL/hr), 50th (0.57 ng/mL/hr), 75th (1.16 ng/mL/hr), and 90th (2.68 ng/mL/hr) were 1.03 (0.87-1.23), 1.11 (0.83-1.50), 1.30 (0.92-1.83), and 1.67 (1.13-2.48). Relative to the 10th percentile of ALDO (6.57 ng/dL), respective ORs and 95% CIs relative to the 25th (9.34 ng/dL), 50th (13.02 ng/dL), 75th (18.51 ng/dL), and 90th (25.14 ng/dL) percentile were 0.99 (0.82-1.21), 0.99 (0.72-1.35), 0.98 (0.69-1.38), and 0.97 (0.65-1.44).
Abbreviations: MESA, Multi-Ethnic Study of Atherosclerosis; PRA, plasma renin activity; ALDO, aldosterone; HDL, high density lipoprotein; UACR, urine albumin creatinine ratio; GFR, glomerular filtration rate; OR, odds ratio; CI, confidence interval.
Supplemental Table 4 displays results from sensitivity analyses in individuals not taking antihypertensive medications. The associations of PRA with an ABI <1.0 are similar in those not taking antihypertensives. For CAC score >300, the overall pattern persisted and the associations of PRA and CAC score >300 may be slightly stronger in those not taking antihypertensives. We reran the same models using multivariable regression to assess associations of PRA and ALDO with continuous ABI and CAC. The shape and significance of the association did not differ when the outcome was treated as continuous (data not shown).
We tested for interactions by race/ethnicity for PRA and ALDO with each of the subclinical disease outcomes. We observed no significant interactions between PRA and ABI <1.0 (p=0.86) or CAC score >300 (p=0.24) by race/ethnicity. Furthermore, plotted trajectories showed similar shapes of the dose-response associations by race/ethnicity.
Discussion
We demonstrate that PRA is significantly and consistently associated with higher levels of several markers of subclinical CVD in a multiethnic cohort without clinically manifest CVD. The associations were independent of traditional CVD risk factors, kidney function, and biomarkers of inflammation. Furthermore, the associations appeared similar across race/ethnic groups. We did not observe any significant associations between ALDO and either of the subclinical CVD measures.
These findings are consistent with observational data from several cohorts that demonstrate higher PRA has an association with cardiovascular mortality. Among high-risk patients in both the HOPE16 (Heart Outcomes Prevention Evaluation) and the LURIC2 (The Ludwigshafen Risk and Cardiovascular Health) studies, higher PRA was associated with incident CVD events and mortality. PRA was also linked to cardiovascular mortality among participants in the PREVEND cohort among those who were not using anti-hypertensive medications.1 Our study extends these findings further in that we found associations between PRA and subclinical atherosclerotic manifestations of CVD. This, in part, suggests elevated PRA and CVD events is mediated through atherosclerotic pathways.
The lack of association of aldosterone with subclinical CVD markers is somewhat surprising. Previous clinical studies, such as LURIC20, did show an increased risk of cardiovascular events among those in the highest quartiles of aldosterone. However, this cohort consisted of patients scheduled for coronary angiography, and thus, represented a referral population at higher risk for CVD events. A possible explanation for our findings is that the impact of aldosterone levels on CVD events becomes more apparent later in the process of myocardial remodeling, through an atherosclerosis independent pathway. In this regard, prior animal models have shown that aldosterone increases the risk of myocardial hypertrophy, fibrosis,21 and autonomic dysfunction.22 Another possibility is that aldosterone, as measured for this study, did not adequately reflect true levels (as it was measured without control of salt intake or medication use). However, we did find statistical variation in both renin and aldosterone levels across race/ethnicity as expected from prior studies, so measurement error seems less likely.
The associations we observed persisted even after adjustment for medications known to therapeutically modulate the RAAS (i.e. beta blockers, ACE inhibitor, etc.), suggesting that PRA maintains its association with subclinical CVD independent of such therapies. In addition, despite substantial variability in RAAS levels across the race/ethnic groups studied, the clinical implications of a given level of PRA seemed similar across race/ethnicity, suggesting a common biological pathway related to absolute PRA level rather than its interaction with the race/ethnicity of the individual.
The strengths of this study include the racial and ethnically diverse population of individuals, the measurement of PRA in a central laboratory, and the careful assessment of covariates. However, our study has important limitations. The PRA assay was not obtained in supine position after salt-loading, and thus the PRA value is subject to variation based on the influence of daily sodium intake and extracellular volume fluctuations. For measurement of the ABI, we carried forward visit 1 ABIs for those seen at visit two and this may result in misclassification. However, since subclinical CVD tends to progress with time rather than regress, we believe this would tend to bias our results to the null rather than lead to false positive findings. Despite adjustment for medications that impact PRA levels, it is difficult to fully assess the individual influence of each antihypertensive (or combination of antihypertensives) agent on PRA levels, given the complex feedback mechanisms, lack of 24-hour urine sodium collections, and confounding by indication to use on anti-hypertensive versus another in individual patients. Additionally, subclinical renovascular disease may have led to higher PRA. Thus, residual confounding remains a possibility. Of note, our findings were robust to adjustment for medication use, and to exclusion of those taking any antihypertensives in a sensitivity analysis, suggesting that renin retained independent associations with these subclinical markers.
In conclusion, PRA is independently associated with the presence of two important measures of subclinical CVD in a large, diverse cohort of community-living individuals without prevalent CVD. This finding is consistent with existing experimental and clinical data suggesting RAAS activation negatively impacts cardiovascular health. Further studies are necessary to determine whether PRA can serve as a target of early identification, preventive, or treatment strategies for CVD.
Supplementary Material
Acknowledgments:
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding Sources: This work was supported by the National Heart, Lung and Blood Institute at the National Institutes of Health via MESA contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169, and R01 HL098077 to SJB, and by grants UL1-TR-000040 and UL1-RR-025005 from National Center for Research Resources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have no financial disclosures.
References
- 1.de Boer RA, Schroten NF, Bakker SJ, Mahmud H, Szymanski MK, van der Harst P, Gansevoort RT, van Veldhuisen DJ, van Gilst WH, Hillege HL. Plasma renin and outcome in the community: data from PREVEND. Eur Heart J 2012;33:2351–2359. [DOI] [PubMed] [Google Scholar]
- 2.Tomaschitz A, Pilz S, Ritz E, Morganti A, Grammer T, Amrein K, Boehm BO, Marz W. Associations of plasma renin with 10-year cardiovascular mortality, sudden cardiac death, and death due to heart failure. Eur Heart J 2011;32:2642–2649. [DOI] [PubMed] [Google Scholar]
- 3.Shu J, Santulli G. Update on peripheral artery disease: Epidemiology and evidence-based facts. Atherosclerosis 2018;275:379–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Budoff MJ, Hokanson JE, Nasir K, Shaw LJ, Kinney GL, Chow D, Demoss D, Nuguri V, Nabavi V, Ratakonda R, Berman DS, Raggi P. Progression of coronary artery calcium predicts all-cause mortality. JACC Cardiovasc Imaging 2010;3:1229–1236. [DOI] [PubMed] [Google Scholar]
- 5.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr.,Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 6.Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem 1995;41:264–270. [PubMed] [Google Scholar]
- 7.American Diabetes A Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33 Suppl 1:S62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS, Investigators C-E. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012;367:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Criqui MH, McClelland RL, McDermott MM, Allison MA, Blumenthal RS, Aboyans V, Ix JH, Burke GL, Liu K, Shea S. The ankle-brachial index and incident cardiovascular events in the MESA (Multi-Ethnic Study of Atherosclerosis). Journal of the American College of Cardiology 2010;56:1506–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ankle Brachial Index Collaboration, Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, Folsom AR, Hirsch AT, Dramaix M, deBacker G, Wautrecht JC, Kornitzer M, Newman AB, Cushman M, Sutton-Tyrrell K, Fowkes FG, Lee AJ, Price JF, d'Agostino RB, Murabito JM, Norman PE, Jamrozik K, Curb JD, Masaki KH, Rodriguez BL, Dekker JM, Bouter LM, Heine RJ, Nijpels G, Stehouwer CD, Ferrucci L, McDermott MM, Stoffers HE, Hooi JD, Knottnerus JA, Ogren M, Hedblad B, Witteman JC, Breteler MM, Hunink MG, Hofman A, Criqui MH, Langer RD, Fronek A, Hiatt WR, Hamman R, Resnick HE, Guralnik J, McDermott MM. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA 2008;300:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR Jr., Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology 2005;234:35–43. [DOI] [PubMed] [Google Scholar]
- 12.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15:827–832. [DOI] [PubMed] [Google Scholar]
- 13.Goff DC Jr., Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB Sr., Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC Jr., Sorlie P, Stone NJ, Wilson PW, American College of Cardiology/American Heart Association Task Force on Practice G. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2935–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med 2010;29:1037–1057. [DOI] [PubMed] [Google Scholar]
- 15.Rifkin DE, Khaki AR, Jenny NS, McClelland RL, Budoff M, Watson K, Ix JH, Allison MA. Association of renin and aldosterone with ethnicity and blood pressure: the Multi-Ethnic Study of Atherosclerosis. Am J Hypertens 2014;27:801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verma S, Gupta M, Holmes DT, Xu L, Teoh H, Gupta S, Yusuf S, Lonn EM. Plasma renin activity predicts cardiovascular mortality in the Heart Outcomes Prevention Evaluation (HOPE) study. Eur Heart J 2011;32:2135–2142. [DOI] [PubMed] [Google Scholar]
- 17.Nickenig G, Harrison DG. The AT(1)-type angiotensin receptor in oxidative stress and atherogenesis: part I: oxidative stress and atherogenesis. Circulation 2002;105:393–396. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt-Ott KM, Kagiyama S, Phillips MI. The multiple actions of angiotensin II in atherosclerosis. Regul Pept 2000;93:65–77. [DOI] [PubMed] [Google Scholar]
- 19.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res 2002;90:251–262. [PubMed] [Google Scholar]
- 20.Tomaschitz A, Pilz S, Ritz E, Meinitzer A, Boehm BO, Marz W. Plasma aldosterone levels are associated with increased cardiovascular mortality: the Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Eur Heart J 2010;31:1237–1247. [DOI] [PubMed] [Google Scholar]
- 21.Brilla CG, Matsubara LS, Weber KT. Anti-aldosterone treatment and the prevention of myocardial fibrosis in primary and secondary hyperaldosteronism. J Mol Cell Cardiol 1993;25:563–575. [DOI] [PubMed] [Google Scholar]
- 22.Barr CS, Lang CC, Hanson J, Arnott M, Kennedy N, Struthers AD. Effects of adding spironolactone to an angiotensin-converting enzyme inhibitor in chronic congestive heart failure secondary to coronary artery disease. Am J Cardiol 1995;76:1259–1265. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.