Abstract
Tumor-associated carbohydrate antigens Lewis X (Lex), Lewis Y (Ley), and KH-1 are useful targets for cancer immunotherapy. In this regard, an insight into the structure-immunogenicity relationships of these antigens is important but this has not been systematically investigated yet. In the current study, Lex, Ley, and KH-1 antigens with a lactose unit at the reducing end as a spacer were synthesized and coupled with keyhole limpet hemocyanin (KLH) protein. Immunological evaluations of the resultant conjugates revealed that they all could elicit robust immune responses whilst the Ley conjugate could provoke the highest titers of total and IgG antibodies. The binding assays of their antisera to each antigen and to cancer cells showed that each antiserum had extensive cross-reaction with all three antigens as protein conjugates and strong but somewhat antigen-selective binding towards MCF-7 cancer cell. Moreover, none of these antisera had obvious binding to SKMEL-28 cancer cell that does not express Lex, Ley and KH-1. The results of assays of these antisera to mediate complement-dependent cytotoxicity (CDC) to MCF-7 and SKMEL-28 cancer cells were very similar to the results of binding assays. Thus, it was concluded that all three antigens could form effective conjugate vaccines whereas the Ley conjugate induced the most robust immune responses and the antiserum of Lex had the highest binding and cytotoxicity to target cancer cells. In addition, as the antibodies induced by each antigen had extensive cross-reaction with other two antigens, either Lex or Ley or the two combined can be utilized to formulate effective conjugate vaccines for cancer immunotherapy. Another paradigm-shifting discovery of this study is that the presentation of Lex, Ley, and KH-1 antigens on cancer cell can be different from that in synthetic conjugates, which should be taken into consideration during the design and optimization of related cancer vaccines or immunotherapies.
Keywords: carbohydrate, cancer antigen, Lewis X, Lewis Y, KH-1, glycoconjugate, cancer vaccine
Graphical Abstract
Introduction
Cancer cells display unusual glycosylation patterns in the oncogenic process; the abnormal glycans thus generated are called tumor-associated carbohydrate antigens (TACAs) [1–3]. TACAs are promising targets for the development of therapeutic cancer vaccines or cancer immunotherapies [4–7] that aim at activating the patients’ immune system to cure cancer and are regarded as the ideal therapies due to their potentially high therapeutic efficacy and specificity [8,9]. Despite the great effort and progress in TACA-based cancer vaccine development [10–14], researches to gain a deeper understanding of the structure-immunogenicity relationships of TACAs and their immunorecognition are still difficult and largely deficient, whilst related information is especially important for the design of effective cancer immunotherapies. Systematic study and comparison of the immunological properties of TACAs and their analogs are arguably the most direct method to examine the structure-immunogenicity relationships of TACAs. For this purpose, it is necessary to have access to appropriate TACA derivatives and analogs to conduct comparative studies.
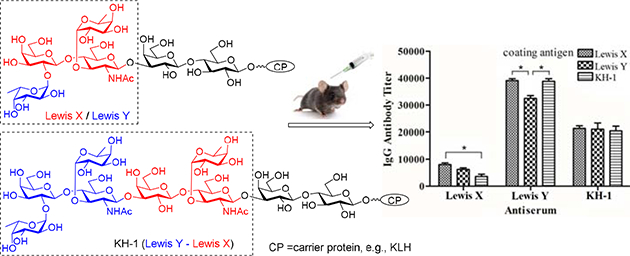
Recently, our group has developed some convergent strategies for efficient synthesis of Lewis type of TACAs, including Lewis X (Lex), Lewis Y (Ley) and KH-1 [15,16]. These structurally related antigens are expressed by several tumors [17,18] and thus have been extensively explored both as synthetic targets and as antigenic epitopes for cancer vaccine development [19–24]. Structurally, Ley is different from Lex only in that the former has an additional L-fucose (Fuc) α-linked to the 3-O-position of the galactose (Gal) residue at the non-reducing end of the latter antigen, whereas KH-1 can be regarded as the heterodimer of Lex and Ley (Figure 1). Easy access to these antigens by the new synthetic strategies had provided us the opportunity to conduct unprecedented comparative study on their immunological properties, including the relationships between the structure of Lewis antigens and their immunogenicity, the significance of a specific structural epitope in these oligosaccharide antigens for immune recognitions, etc. Consequently, in this work, the native form of Lex, Ley, and KH-1 antigens that contained the common and natural lactose spacer, i.e., having these antigens linked to the 3′-O- position of lactose, were synthesized and conjugated with keyhole limpet hemocyanin (KLH) protein. The inclusion of the lactose spacer was intended to more closely mimic the natural form of these TACAs than in literature and, in the meantime, buffer any potential influence of direct conjugation of these antigens with carrier proteins on their structure and conformation. Furthermore, lactose is one of the most common disaccharide motifs in nature, thus we would not expect it to cause immune reactions, which was eventually proved by this study. The resultant conjugates 1a, 1b, and 1c were immunologically evaluated in mouse, employing the corresponding human serum albumin (HSA) conjugates 2a, 2b, and 2c, respectively, as coating antigens for the detection of TACA-specific antibodies in the mouse antisera by enzyme-linked immunosorbent assay (ELISA).
Figure 1.
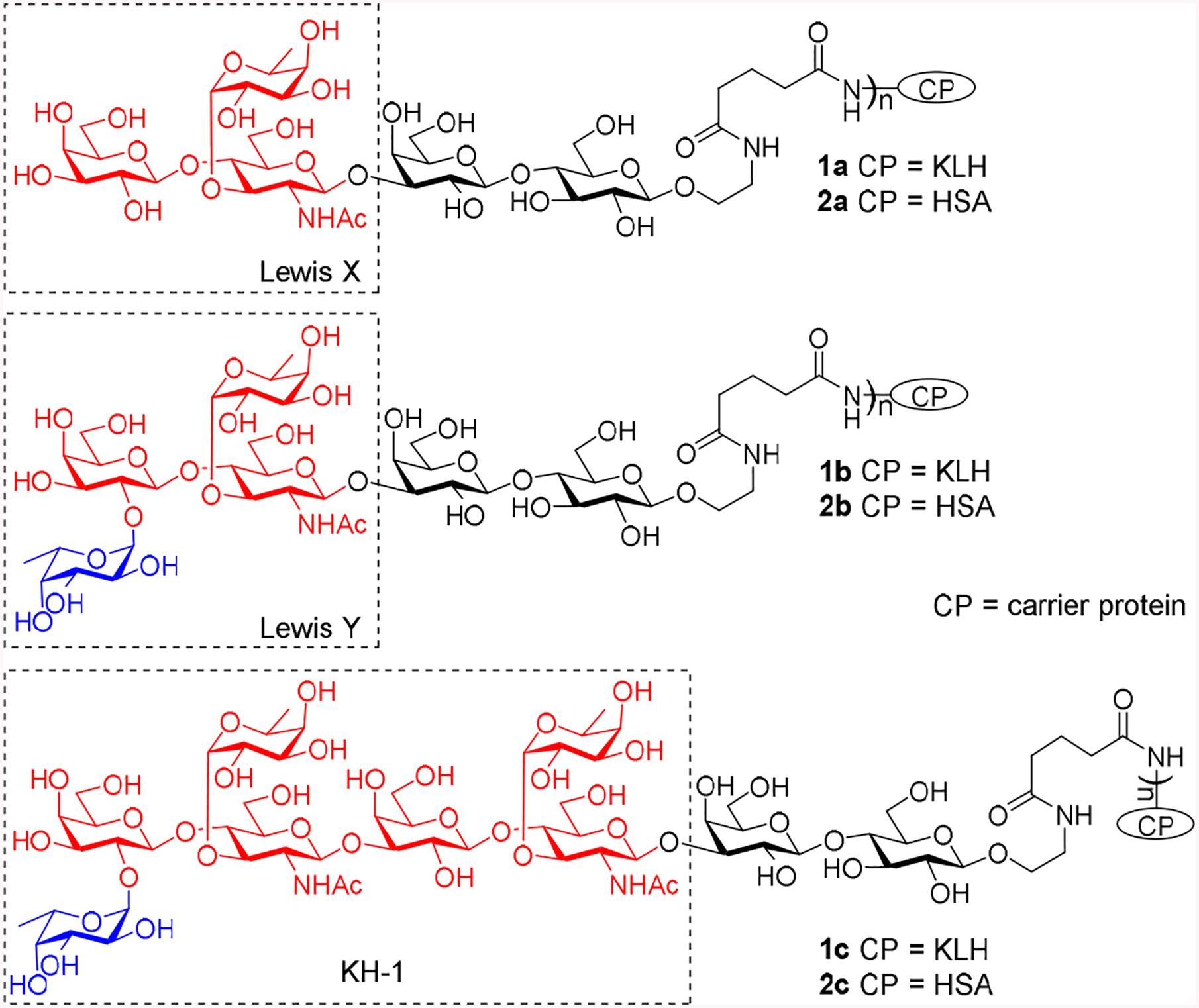
Structures of Lex, Ley, and KH-1 and their KLH and HSA conjugates 1a-c and 2a–c
Results
Lex, Ley, and KH-1 derivatives 3, 4, and 5 (Scheme 1) were synthesized according to reported methods [15,16] and were coupled with KLH and HSA via the bifunctional glutaryl group. This linker was selected as it was proved efficient for carbohydrate-protein conjugations and exhibit no obvious immunogenicity or other adverse immunological properties by itself [25]. First, the linker was attached to glycans 3–5 by a reaction with the dually activated disuccinimidal glutarate 6. The resultant activated esters 7–9 were then reacted with KLH and HSA in phosphate-buffered saline (PBS) to give conjugates 1a–c and 2a–c, which were purified by gel filtration chromatography on a Biogel A 0.5 column. The carbohydrate loadings of these conjugates were analyzed [26] and were shown to be in the desired range (Table 1).
Scheme 1.
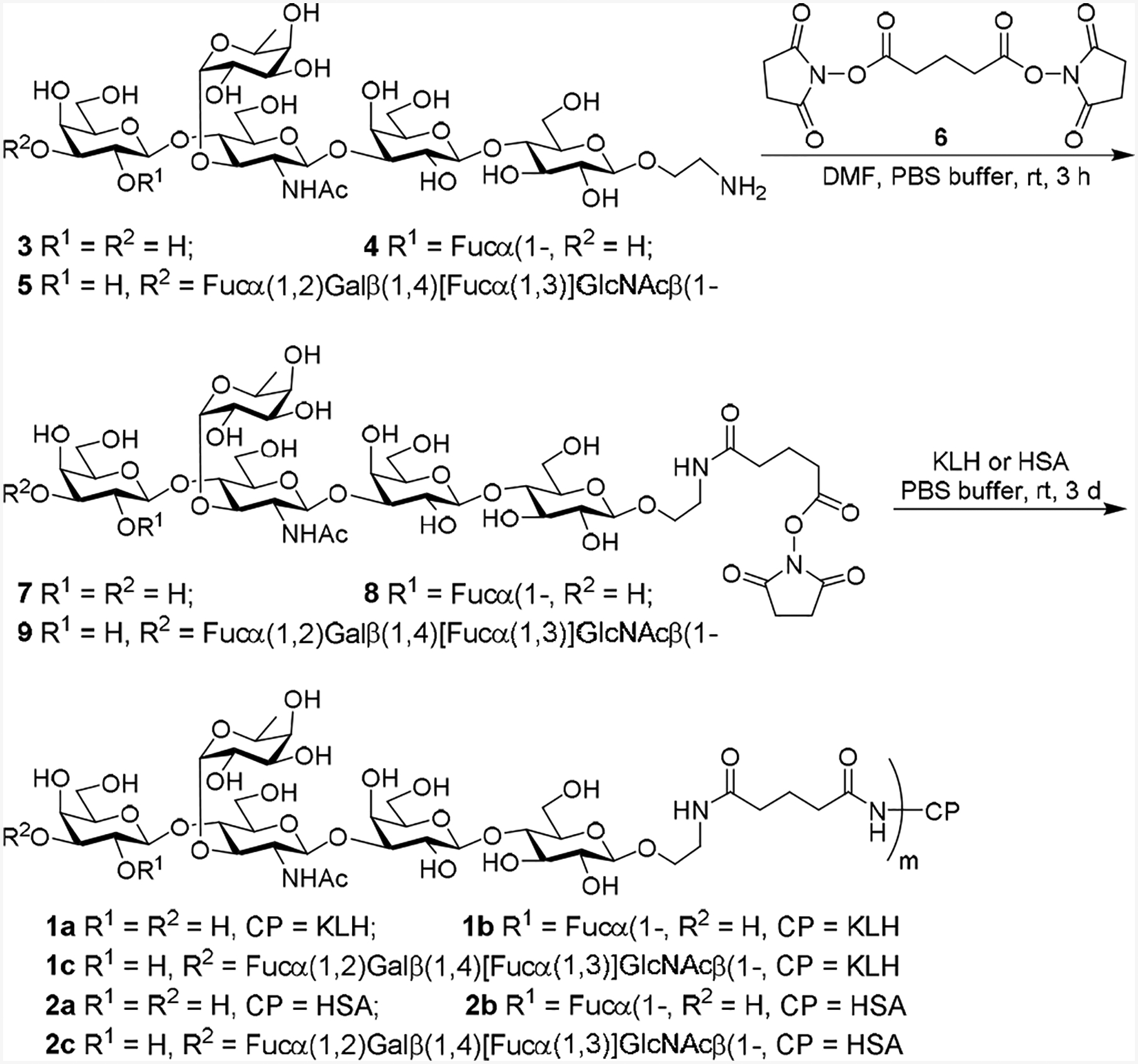
Synthesis of conjugates 1a–c and 2a–c
Table 1.
Carbohydrate loadings of conjugates 1a–cand 2a–c
| Glycoconjugate | 1a | 1b | 1c | 2a | 2b | 2c |
|---|---|---|---|---|---|---|
| Carbohydrate Loading (%) | 18.3 | 13.9 | 11.4 | 11.1 | 7.9 | 14.1 |
| Glycans/Glycoconjugate | 105.0 | 64.6 | 33.8 | 10.3 | 6.0 | 7.5 |
Immunological evaluations of the KLH conjugates 1a, 1b, and 1c were performed in female C57BL/6J mice (6–8 weeks of age). The immunization protocol was the same as reported [15], i.e., to subcutaneously (s.c.) inject each conjugate as an emulsion with complete Freund’s adjuvant (CFA) to a group of 5 mice on day 1 for initial immunization and then intraperitoneally (i.p.) inject the same conjugate as an emulsion with incomplete Freund’s adjuvant (IFA) on day 8, 15, and 36 for boost immunizations. Blood samples were collected from mice on day 0 before initial immunization (as blank controls) and on day 21 and 43 after boost immunizations. The blood samples were clotted to obtain antisera by standard protocols, which were subjected to antibody titer analysis by ELISA. For ELISA, conjugates 2a–c were utilized as capture antigens and 1:1,000 PBS-diluted alkaline phosphatase (AP)-linked goat anti-mouse kappa, IgG, and IgM antibodies were employed as secondary antibodies to detect total, IgG, and IgM antibodies, respectively. Antibody titers were defined as the dilution numbers of sera at which an optical density (OD) value of 0.2 was achieved at 405 nm wavelength, and each experiment was repeated three times.
ELISA results of the total antibodies (Figure 2A) indicated that after the second boost immunization (day 21 antisera) all three conjugates 1a, 1b, and 1c had already elicited the production of antigen-specific antibodies, whilst the total antibody titers of day 43 antisera obtained after the third boost immunization were significantly higher than that of day 21 sera. The results proved not only the efficiency of conjugates 1a, 1b, and 1c to elicit robust immune responses but also the progress and strengthening of the induced immune responses as a result of boost immunizations. Furthermore, the total antibody titers of the antisera of conjugates 1a, 1b, and 1c were significantly different from each other. The antiserum of conjugate 1b exhibited the highest antibody titer; in turn, the antibody titer of antiserum 1a was significantly higher than that of antiserum 1c. Therefore, it seemed that the order of overall immunogenicity of the conjugates or related TACAs was 1b (Ley) > 1a (Lex) > 1c (KH-1).
Figure 2.
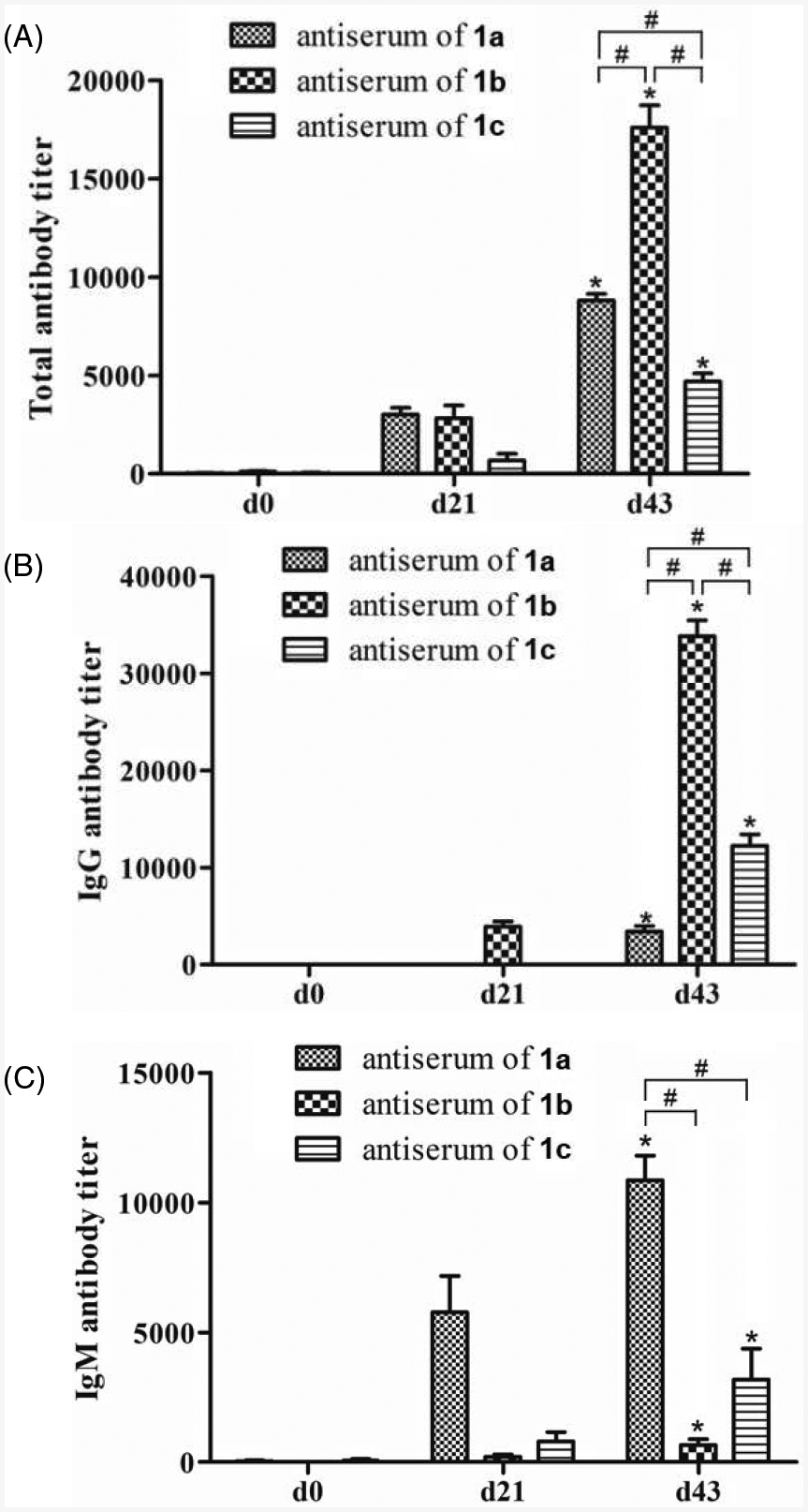
Total antibody (A), IgG antibody (B), and IgM antibody (C) titers of pooled day 0, 21, and 43 sera from mice before and after immunization with conjugates 1a, 1b, and 1c as determined by ELISA with conjugates 2a–c as coating antigens and 1:1,000 diluted AP-linked goat anti-mouse kappa (for total antibody detection), IgG and IgM antibodies as the secondary antibodies, respectively. Antibody titers were defined as the dilution numbers of sera at which the OD405 value was 0.2. The mean antibody titer of three parallel experiments was presented for each sample, and the error bar showed the standard error of mean (SEM). *Statistically different (p < 0.05) as compared to the day 0 serum; #statistically different (p < 0.05) between the two compared groups.
We also analyzed the isotypes of antibodies induced by conjugates 1a, 1b, and 1c. The results (Figures 2B and 2C) revealed that the provoked antibody responses in both day 21 and 43 antisera were mainly of IgG type for conjugate 1b, which was consistent with our previous observations that glycoproteins induce usually IgG antibodies against carbohydrate antigen [25,27,28]. On the other hand, the antibody responses were mainly of IgM type for conjugate 1a and both IgG and IgM types for conjugate 1c. It was interesting to find that although Lex, Ley and KH-1 are structurally related, they had different immunogenicity and different immunological properties as IgG and IgM antibodies are produced through different pathways and mechanisms. For example, IgM antibodies are produced by plasma cells during the initial response to a specific antigen [29,30], whilst IgG antibodies are produced later to participate in primarily secondary immune responses. Usually, IgG antibody production indicates T cell-dependent immunity, antibody class switch and affinity maturation, and long-term immune memory [31], which are the properties desirable for cancer therapy. Therefore, compared to Lex and KH-1 conjugates 1a and 1c, Ley conjugate 1b seemed to elicit more promising therapeutic immune responses.
Encouraged by the above discoveries, we investigated next the cross-reactivity of each antiserum with the other two antigens. For this purpose, we coated ELISA plates with HSA-Lex, Ley and KH-1 conjugates 2a, 2b and 2c, respectively, and used them to test cross-reactive total and IgG antibodies in each pooled antiserum by ELISA as described above. ELISA results of total antibodies shown in Figure 3A suggested that the antiserum of Ley (conjugate 1b), which gave the highest total antibody titer as compared to other two antisera (Figure 2A), also showed the highest reactivity with Lex (2a) and KH-1 (2c) antigens (Figure 3A). This was consistent with the conclusion that 1b was more immunogenic than 1a and 1c to elicit the production of a higher level of antibodies. Most significantly, the antiserum of 1b had similar reactivity with all three antigens, indicating that the antibodies elicited by 1b could recognize Lex, Ley, and KH-1 without significant discrimination. In addition, each of the antisera of conjugates 1a and 1c also exhibited essentially the same reactivity with all three antigens (Figure 3A).
Figure 3.
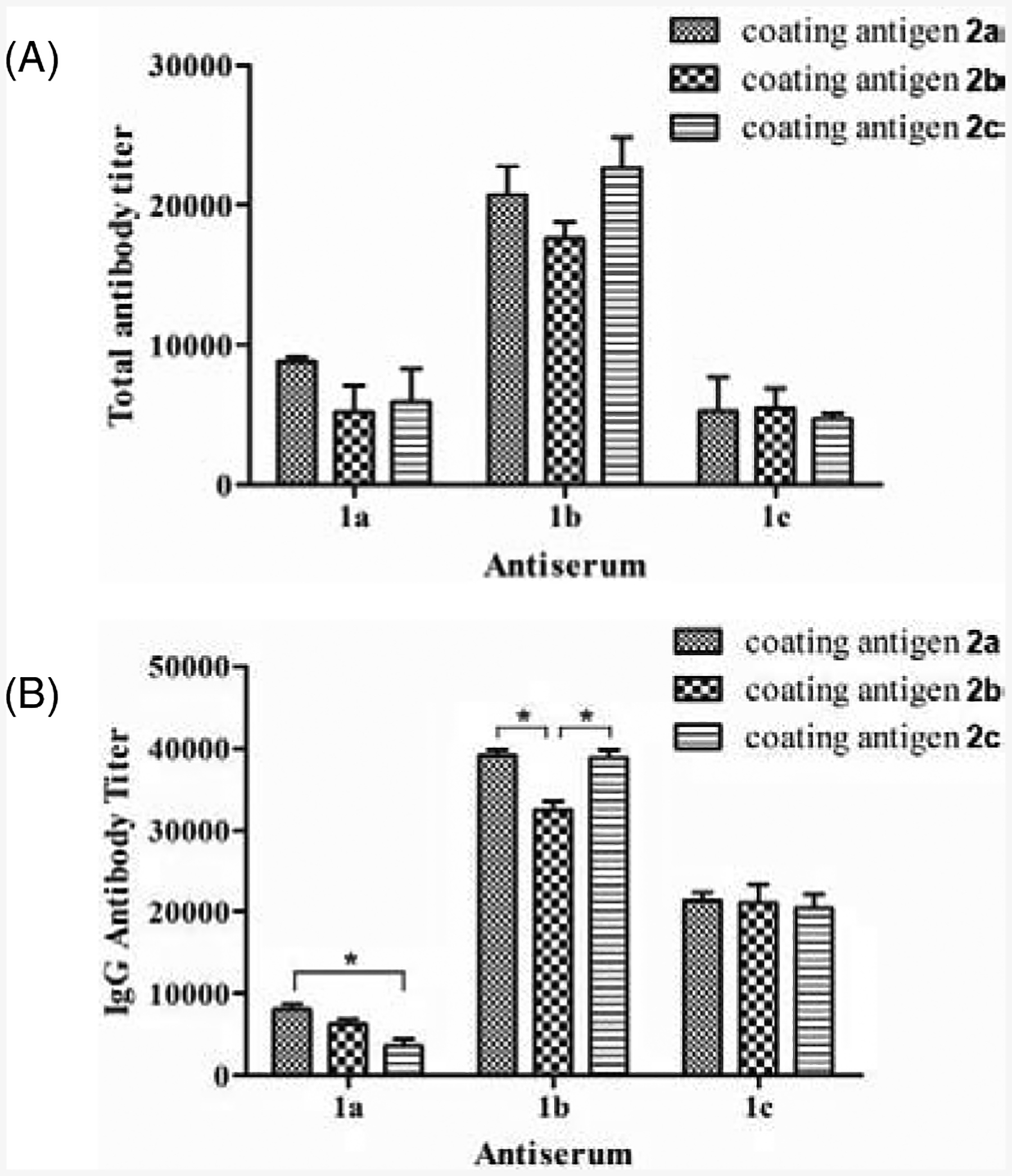
ELISA results showing the reactivity of each day 43 antiserum with all three different antigens. After ELISA plates were coated with HSA conjugates 2a, 2b and 2c, the pooled day 43 serum from each group of mice immunized with conjugates 1a, 1b or 1c was added to the plates for ELISA using 1:1,000 diluted AP-linked goat anti-mouse kappa and IgG secondary antibodies to detect total and IgG antibodies. Antibody titers were defined as the dilution numbers of sera at which an OD405 value of 0.2 was reached. The mean of antibody titers from three parallel experiments was presented for each sample, and the error bar showed the SEM. *Statistically different (p < 0.05) between the two compared groups.
Similarly, the antiserum of 1b exhibited the highest titers of IgG antibodies for all three antigens (Figure 3B), which was also consistent with the above results that conjugate 1b induced the strongest IgG antibody response (Figure 2B). Furthermore, each of the antisera of conjugates 1a, 1b, and 1c showed comparable IgG antibody titers for all three different antigens. The slightly higher reactivity of antiserum 1b with 2a and 2c as compared to the reactivity with 2b may be attributed to the higher carbohydrate loadings of 2a and 2c (10.3 and 7.5 vs 6.0 glycans/conjugate). The slightly decreased reactivity of the IgG antibodies in antiserum 1a with conjugate 2c (Figure 3B) may indicate that some of the IgG antibodies induced by Lex could not recognize KH-1. The antiserum of 1a showed the similar trend of decreased reactivity, but less significantly, with conjugate 2b.
The linker used in conjugates 1a, 1b, and 1c was proved in other glycoconjugates to exhibit no obvious immunogenicity or other adverse immunological properties by itself [25]. Here, the reactivity of antisera 1a, 1b, and 1c with the linker and the common lactose motif was assessed to exclude their contribution to the observed cross-reaction. In these experiments, mannose- and lactose-HSA conjugates containing the same linker as in conjugates 1a, 1b, and 1c were used as the capturing antigen for ELISA. Both conjugates were proved to exhibit similarly low reactivity with all three antisera (Tables S5 and S6, Figures S1 and S2 of SI), suggesting that the observed different antibody titers shown in Figures 2 and 3 were indeed due to different carbohydrate antigen-antibody binding or cross-reaction.
Subsequently, we analyzed whether the carbohydrate hapten-specific antibodies induced by conjugates 1a, 1b, and 1c could recognize and bind to Lex, Ley, and KH-1 antigens present on the cancer cell surface by flow cytometry (FACS). In this study, MCF-7 cell, a human breast cancer cell line that was confirmed to express Lex, Ley and KH-1 antigens [17], was used as the positive control, whereas SKMEL-28 cell, a human melanoma cell line that was shown to not express Lex, Ley or KH-1 [20,32], was utilized as the negative control. Both cell lines were incubated with the normal mouse serum or a day 43 antiserum and then with FITC-linked goat anti-mouse kappa antibody. The cells were finally subjected to FACS study. As shown in Figure 4A, the fluorescent intensities of MCF-7 cells treated with the antisera of conjugates 1a, 1b, and 1c were all much higher than that of cells treated with the normal mouse serum, and the increases in median fluorescence intensity (MFI) value were statistically significant (P < 0.05). On the other hand, treating SKMEL-28 cells with antisera 1a, 1b, and 1c caused little changes (statistically insignificant) in the fluorescent intensity when compared to cells treated with normal mouse serum (Figure 4B). The FACS results indicated that antibodies provoked by conjugates 1a, 1b, and 1c could specifically bind to MCF-7 cell which expresses Lex, Ley and KH-1 antigens. Furthermore, the antisera of 1a and 1b exhibited similar binding activity to MCF-7 cell, and the activity was higher than that of the antiserum of 1c.
Figure 4.
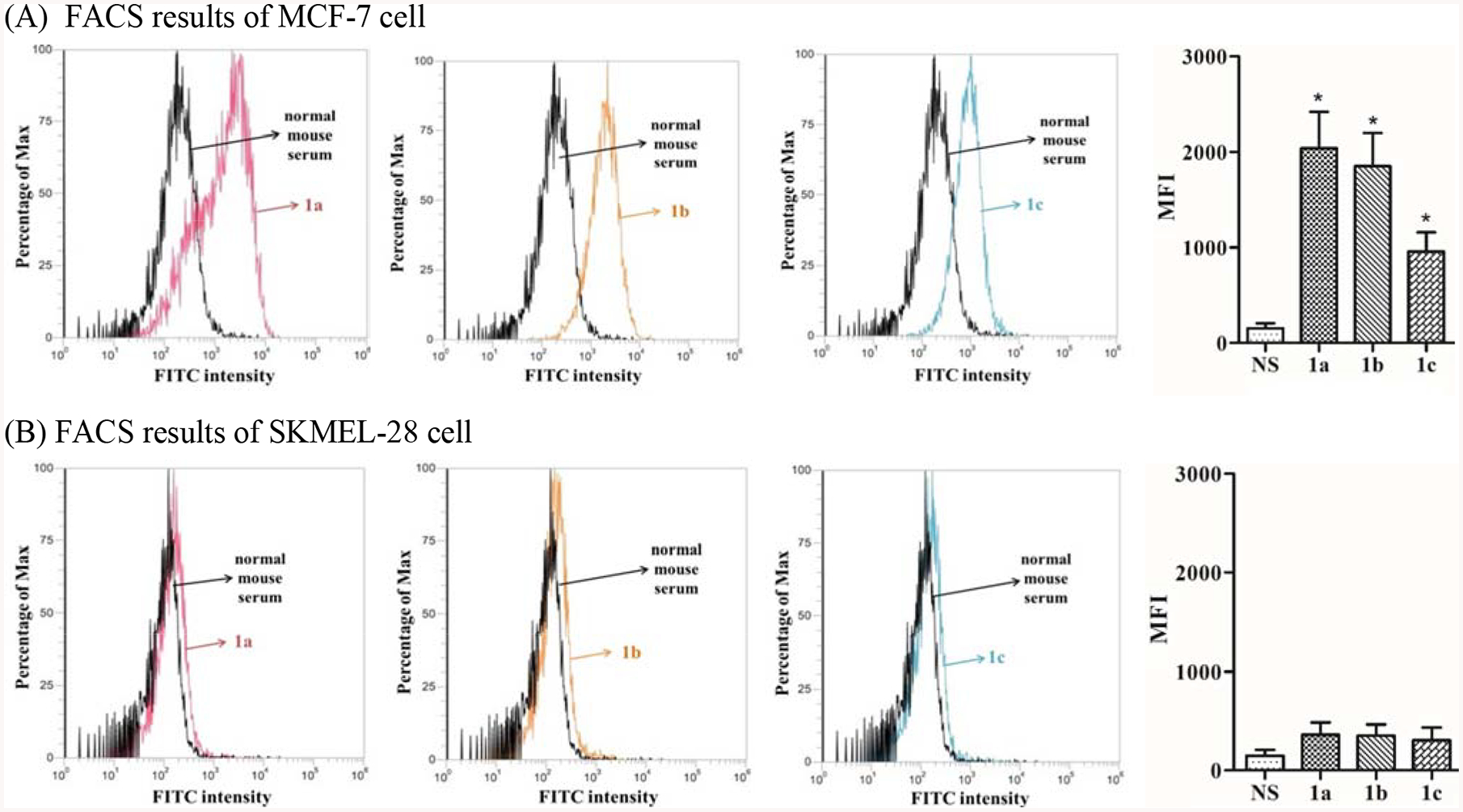
FACS results about the binding of antibodies in normal mouse serum (black) or in the pooled antiserum of conjugate 1a (red), conjugate 1b (orange) and conjugate 1c (blue) with MCF-7 cell (A) and SKMEL-28 cell (B), respectively, as well as the calculated MFI values. The error bar represents standard error of the mean (SEM) of three independent experiments. *Statistically different (P < 0.05) compared to that of cells treated with normal mouse serum.
Interestingly, the results of antibody binding to MCF-7 cells (Figure 4A) did not agree completely with the results of antibody titers (Figure 2A) and antibody-antigen cross-reactivity (Figure 3A) of the antisera. For example, the total antibody titer of antiserum 1b was significantly higher than that of antisera 1a and 1c, but antiserum 1b showed similar or even slightly lower binding to MCF-7 cell than antiserum 1a. This result was also contradictory to the observation that antibodies in the antiserum of 1b had extensive cross-reactivity with all synthetic antigens or conjugates (Figure 3A). Consequently, we propose that (1) at least a part of the antibodies in the antiserum of 1b were specific to the Ley antigen on MCF-7 cells, i.e., they did not recognize and bind to Lex antigens on cancer cell, and (2) MCF-7 cell expresses a higher level of Lex antigen than Ley antigen and/or antibodies in the antiserum of 1a had more extensive cross-reaction with Ley and KH-1 antigens on the MCF-7 cell surface to result in elevated binding for the antiserum of 1a. Similarly, the difference in binding of the antisera of 1b and 1c to MCF-7 cell was obviously less significant than the difference in their total antibody titers, which also suggested a higher expression level of KH-1 than Ley and/or more cross-reactivity of the antibodies in the antiserum of 1c with Lex and Ley antigens on MCF-7 cell. Currently, there is no reported qualitative analysis of the expression levels of Lex, Ley, and KH-1 antigens on MCF-7 cell, thus we cannot make conclusions about the cross-reactivity of antibodies raised by conjugates 1a and 1c with Ley on the MCF-7 cell surface. However, the results may suggest that at least some antibodies raised by conjugate 1b could not recognize and bind to Lex and KH-1 antigens on the cancer cell surface although antibodies against 1b showed extensive cross-reactivity with the protein conjugates of synthetic Lex and KH-1 in ELISA. Previously, Kitamura et al. also observed that the antibodies raised with synthetic Ley conjugate could not effectively recognize natural Ley antigens expressed on cancer cells [33].
To investigate the correlation between the cell-binding abilities of antibodies raised by conjugate 1a, 1b or 1c and their potential anticancer activities, we evaluated further the activities of antisera 1a, 1b, and 1c to mediate complement-dependent cytotoxicity (CDC) against MCF-7 and SKMEL-28 cells. Therefore, after the cells were treated with normal mouse serum (negative control) or the antiserum of 1a, 1b or 1c, as described above, they were incubated with rabbit complements and then subjected to cell lysis analysis by the lactate dehydrogenase (LDH) test. The positive control of 100% cell lysis was obtained by treating cells with 1% triton X-100. As indicated in Figure 5, all three antisera could mediate significant lysis of MCF-7 cell that expresses Lex, Ley, and KH-1 antigens, and the results correlated well with the antibody-cell binding results described above (Figure 4A). In contrast, under the same conditions none of the three antisera induced significant lysis of SKMEL-28 cell that does not express Lex, Ley and KH-1 antigens and showed no binding with the antisera (Figure 4B).
Figure 5.
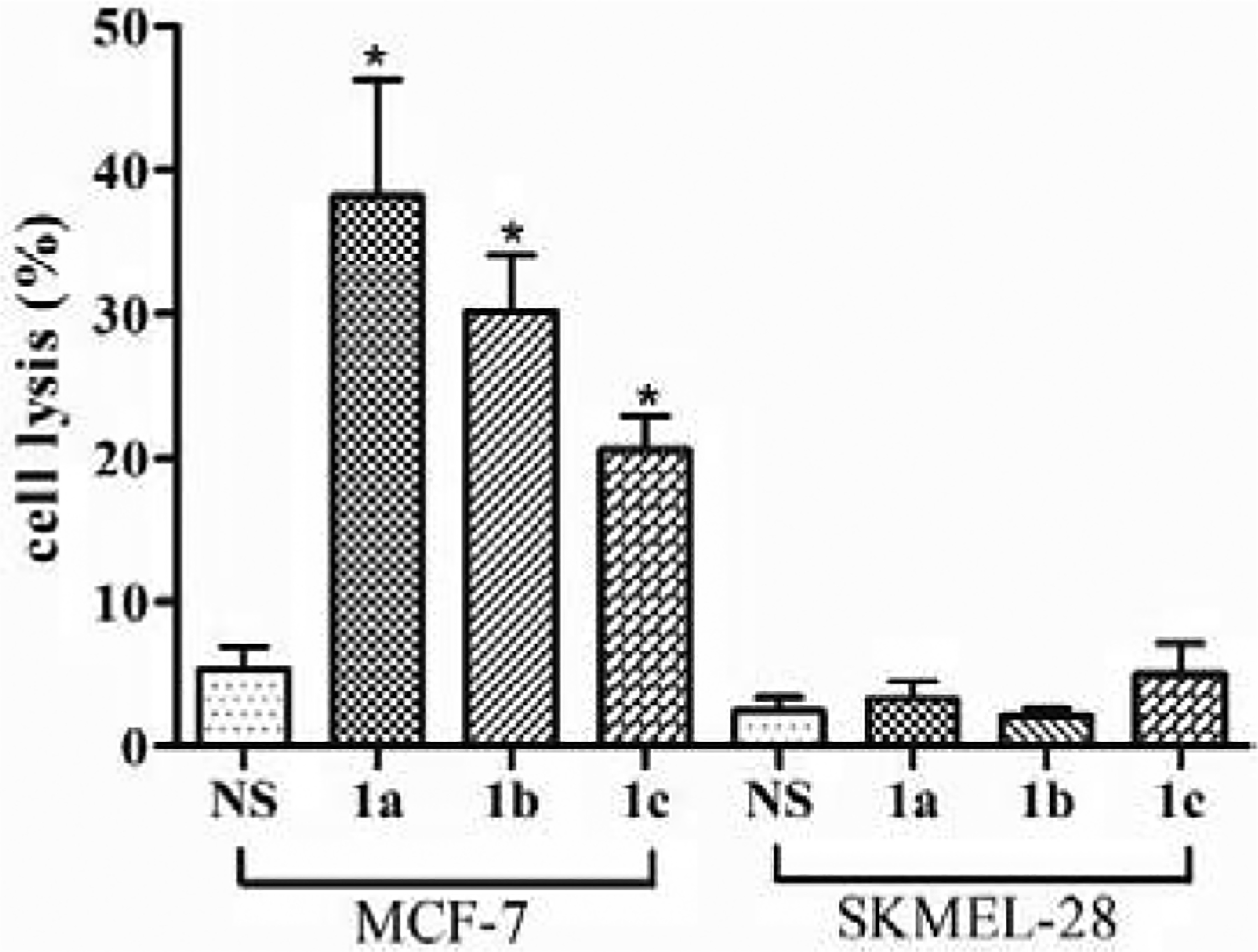
The results of antibody-mediated CDC to MCF-7 and SKMEL-28 cells presented as cell lysis caused by treatment with normal mouse serum (NS) or a pooled antiserum of conjugate 1a, 1b or 1c and then with rabbit complements. The error bar shows the SEM of 3 independent experiments. *Significantly different (P < 0.05) compared to the NS group.
Discussion
TACAs expressed on cancer cells are useful targets for the development of new cancer therapies, such as therapeutic cancer vaccines or cancer immunotherapies. In this regard, it is critical to appreciate the relationship between the structure of TACAs and their immunogenicity, the correlation between synthetic TACA conjugates and natural ones on cancer cells, the interaction between TACAs and their antibodies, etc. To gain a better understanding of Lewis type of TACAs, Lex, Ley and KH-1, we prepared their KLH conjugates and studied their immunological properties, the interaction of their antisera with synthetic and natural antigens and the efficacy of their antisera to mediate CDC to cancer cells.
Lex antigen is remarkably upregulated in colon cancer and several other tumors [34] and was shown to be associated with cancer progression. Ley antigen is an oncodevelopment-associated TACA, which has been detected in almost all colonic carcinoma tissues. Notably, Ley is a specific marker for malignancy [35]. KH-1 antigen is relatively cancer-specific, which has not been observed in normal tissues yet [36]. Structurally, these three antigens are related. Ley and Lex are different in that the former has an additional fucosyl residue at the glycan non-reducing end, whereas KH-1 is a heterodimer of Ley and Lex (Figure 1). Consequently, a systematic and comparative study on these antigens may provide interesting insights into their immunology.
Our immunological evaluation of the KLH-Lex, Ley and KH-1 conjugates in mice revealed that they all provoked robust immune responses but had indeed different immunogenicity. The Ley conjugate induced significantly higher total antibody titers than the Lex and KH-1 conjugates, and the Lex conjugate elicited stronger immune responses than the KH-1 conjugate. Furthermore, it was shown that Ley induced mainly IgG antibody responses while Lex induced mainly IgM antibody responses. These results indicated that the structure of TACAs had a major impact on their immunogenicity and other immunological properties, although they are structurally similar. On the other hand, the Boons group found that the protein conjugate of a Ley/Lex hetereodimer elicited a higher antibody titer than the Ley conjugate [37]. A major difference between Boons conjugate and our KH-1 conjugate was that the former had Ley and Lex antigens stitched together by an artificial linker whilst in our conjugate, Ley and Lex antigens were directly coupled together without any spacer. Therefore, it seems that in addition to the principal structure of haptens, other factors such as linkage form, conjugation method, immunization protocol, etc. may also have a significant impact on the immunogenicity of resultant glycoconjugate vaccines [38].
Theoretically, a complex carbohydrate antigen can induce a diversity of antibodies against not only its intact structure but also its partial sequences. Therefore, the induced antibodies may have cross-reactions with other closely related antigens [39]. Our results about the cross-reactivity between different antisera and antigens have revealed that antibodies in each antiserum could recognize all three antigens without significant difference, thus the cross-reactivity was strong and extensive. Furthermore, for each antiserum, the titers of cross-reactive total and IgG antibodies in Figure 3 corroborated the ELISA results of antigen-specific antibodies in Figure 2. The Boons group also reported that antibodies elicited by a Ley/Lex dimer could recognize both Ley and Lex although their titers were significantly lower than that of the binding to the Ley/Lex dimer [37]. Since all of the Lex, Ley and KH-1 antigens contain the fucosyl residue, we propose that the observed cross-reactivity may be at least partially related to this epitope, which is worthy further investigation in the future. On the other hand, because over-fucosylation is common among different tumors and is closely related to carcinogenesis [40,41], immunity centered on the fucose epitope may be of general significance for cancer immunotherapy.
Interestingly, the results of antibodies in the antisera to bind with Lex, Ley and KH-1 antigens expressed on cancer cells were significantly different from the ELISA results of antibody-antigen binding or cross-reactivity obtained with synthetic conjugates. In this study, first, we proved that the induced antibodies were specific to Lewis type of TACAs as Lex, Ley and KH-1 antigen negative cells did not have obvious binding with the antisera. Next, we disclosed that the antiserum of Lex, which contained a significantly lower concentration of antibodies than the antiserum of Ley (Figure 2A), showed similar or even slightly higher binding to Lex, Ley and KH-1 antigen-expressing MCF-7 cell (Figure 4A). These results suggested that at least some of the antibodies induced by the KLH-Ley conjugate were specific to Ley antigen and did not recognize or bind to Lex and KH-1 antigens on the cancer cell surface; otherwise, we would have observed the highest binding of anti-Ley serum to MCF-7 cell. These results were further corroborated by antibody-mediated CDC study (Figure 5). The CDC study also confirmed the feasibility of Lewis antigen-based cancer immunotherapy.
There are two potential explanations for the increased binding of anti-Lex serum to cancer cells, which are: (1) a higher expression level of Lex antigen than Ley antigen and (2) stronger antibody-antigen binding and/or more cross-reaction of the antibodies in the anti-Lex serum with Ley and KH-1 antigens on cancer cells. If the differences between ELISA and antibody-cell binding assays (Figures 3A and 4A) were not simply because of the different expression level of antigens, which is likely to be the case, our results may indicate the potential discrepancies of these antisera to bind and cross-react with synthetic and natural Ley antigens. This should be perceivable because TACAs may have different interactions with molecules in artificial environment on ELISA plate and in the cancer cell matrix to affect the structure and organization of TACAs, namely that the antigens may have adopted different conformations on ELISA plates and cells. This conclusion was in agreement with the seminal discovery that antibodies raised by a specific TACA could distinguish the same antigen in different expression levels on cancer and normal cells [41–43], which has been the molecular foundation for the specificity and the therapeutic efficacy of TACA-based cancer vaccine or immunotherapy. In general, carbohydrates are flexible molecules that can adjust their structure to accommodate the environment [44,45]. Therefore, it should be easily perceivable that TACAs may adopt different forms of organization and presentation under different conditions, which can thereby affect the molecular recognition process [46].
Conclusion
In conclusion, the KLH conjugates of Lex, Ley, and KH-1 antigens were synthesized, immunologically evaluated, and compared. Whereas all of the conjugates could induce robust immune responses, they had indeed different immunogenicity and the KLH-Ley conjugate elicited the highest titers of IgG antibodies. Therefore, Ley seemed to be a more promising antigen for the development of cancer vaccines. Intriguingly, whereas the antiserum of each antigen had extensive cross-reactivity with the remaining two antigens in the form of synthetic HSA conjugates, these antisera exhibited some selectivity toward natural antigens on cancer cells. The antiserum-cell binding and the antibody-mediated CDC studies further indicated that MCF-7 cell may express different levels of Lex, Ley and KH-1 antigens, which is a research topic worthy further investigation. Overall, our findings combined with literature reports suggested that for the design of new TACA-based therapeutic cancer vaccine, in addition to the target antigen, we should be also careful about the selection of carrier proteins, conjugation methods, and so on, as these factors may affect TACA presentation and recognition and thereby the therapeutic efficacy of resultant vaccines.
Experimental Section
Synthesis of TACA-protein conjugates 1a-c and 2a-c (Scheme 1).
A mixture of oligosaccharide 3, 4 or 5 (6.0 mg) and disuccinimidal glutarate 6 (15 equiv) in DMF and PBS buffer (0.1x) (4:1, 0.5 mL) was stirred at rt for 4 h. The solution was condensed in vacuum, and then EtOAc (9 volumes) was added. The precipitates were isolated and washed with EtOAc 10 times and dried in vacuum to result in crude active ester 7, 8 or 9, respectively, which was directly used for the next step. Each product was mixed with HSA or KLH at a ratio of 1:1 (W/W) in 0.1 M PBS buffer (0.4 mL). The solution was stirred at rt for 3 days and then applied to a Superdex® 200 column, which was eluted with 0.1 M PBS buffer (I = 0.1, pH = 7.8). The glycoprotein-containing fractions, which were characterized by the bicinchoninic acid (BCA) assay for protein and charring with 15% (v/v) H2SO4 in EtOH for carbohydrates, were combined, dialyzed against distilled water for 2 days, and finally lyophilized to afford the desirable glycoconjugate 1a, 1b, 1c, 2a, 2b or 2c as a white solid, respectively.
Analysis of the carbohydrate loading of glycoconjugates 1a–c and 2a–c.
Chemical analysis to determine the carbohydrate loadings of glycoconjugates was performed according to a literature method [26]. First, a calibration curve was prepared for sugars utilizing a mixture of fucose, galactose, N-acetylglucosamine, and glucose in molar ratios equal to that in each antigen. Aliquots of the standards dissolved in distilled water (1 mg/mL) were transferred into 10 dry 10-ml tubes in 5 μL increments ranging from 5 to 50 μL. To the tubes were sequentially added 500 μL of 4% phenol and 2.5 mL of 96% sulfuric acid. The glycosyl bonds were cleaved and a colored complex was developed in this step. Solutions were transferred from the test tubes to cuvettes and measured at the 490 nm wavelength. The calibration curve was obtained by plotting A490 absorbance against the total weight (μg) of sugars in each standard sample. To analyze the carbohydrate loadings of 1a–c and 2a–c, three accurately weighed samples of each glycoconjugate (final concentration of the sugar should be in the range of the calibration curve) were dissolved in distilled water in 10-mL test tubes and then examined by the exactly same protocol, whereas free KLH and HSA proteins were used as the blank controls, respectively. The amount of sugars present in each sample was calculated based on its A490 against the calibration curve, and the carbohydrate loading of each glycoconjugate was calculated according to the following equation:
The carbohydrate loadings of HSA conjugates 2a–c were confirmed by MALDI-TOF mass spectrometry (Supporting Information), which were calculated according to the following equation:
Apical points of the MS peaks were used for estimation of the average molecule weights of HSA and its conjugates 2a–c.
Immunization of animals.
Fifteen female C57BL/6J mice (6–8 weeks of age) were assigned into three groups randomly (five mice per group). On day 1, mice were subcutaneously injected with 0.1 mL of the CFA emulsion of a specific glycoconjugate containing 3 μg of the carbohydrate antigen. Thereafter, mice were immunologically boosted three times on day 8, 15 and 36, respectively. For boosting immunization, mice were intraperitoneally injected with 0.1 mL of the IFA emulsion of the same glycoconjugate at the same dose. Blood samples were collected from the mice via the saphenous vein on day 0 before the initial immunization and day 21 and 43 after immunization to prepare blank sera and antisera after clotting and centrifugation at 5000 rpm for 7 min at 4 °C. The animal use protocol for this research (#201609560) was approved by the Institutional Animal Care and Use Committee of University of Florida.
ELISA.
ELISA was performed according to a previously report protocol with minor modifications [47]. ELISA plates were incubated with conjugate 2a, 2b or 2c (2 μg/mL, 100 μL per well) in coating buffer (0.1 M bicarbonate, pH 9.6) at 4 °C overnight and then at 37 °C for 1 h. After washing with PBST (PBS containing 0.05% of Tween-20) 3 times, the plates were treated with blocking buffer (1% BSA in PBST) at rt for 1 h. Pooled antisera diluted from 1:300 to 1:72900 in PBS in serial half-log manner were added to the plates (100 μL/well), which was followed by incubation at 37 °C for 2 h. Then, AP-linked goat anti-mouse kappa, IgG and IgM antibodies (1:1,000 dilution in PBS) were added and the plates were incubated at rt for 1 h. After washing with PBST 3 times, 100 μL of a p-nitrophenylphosphate (PNPP) solution (1.67 mg/mL in PBS) were added and the plates were then incubated at rt for 30 min. The optical density (OD) values of the ELISA plates at 405 nm wavelength were read using a microplate reader (Cytation 1, Bio-Tek instruments Inc.). OD values were plotted against the serum dilution numbers to obtain a best-fit logarithm line and the equation of this line was used to calculate the antibody titer. The dilution number at which the OD value of 0.2 was achieved was taken as the antibody titer.
FACS.
The binding of antibodies in the antisera to cancer cells was detected by FACS assay as reported previously [48,49]. MCF-7 and SKMEL-28 cells were cultured in DMEM containing 10% of fetal bovine serum (FBS) and 1% of antibiotics. Cells were harvested, washed twice with FACS buffer (PBS containing 5% of FBS) and counted (4.0 × 105 cells for each experiment). Thereafter, cells were incubated with 50 μL of 10 time-diluted (in FACS buffer) normal mouse serum or pooled day 43 antiserum at 4 °C for 30 min. Cells were washed twice and incubated with fluorescein isothiocyanate (FITC)-linked goat anti-mouse kappa antibody (50 μL, 1:25 dilution in FACS buffer) at 4 °C for 30 min. Then, cells were washed twice and re-suspended in 400 μL of FACS buffer for detection. The fluorescence intensity of cells were analyzed with Attune Nxt Acoustic Focusing Cytometer.
Evaluation of antibody-mediated CDC.
Antibody-mediated CDC analysis was performed as reported previously [49,50]. MCF-7 and SKMEL-28 cells were cultured as described above and then plated in 96-well plates (1.0×104 cells/well for MCF-7 and 1.5×104 cells/well for SKMEL-28) to allow for attachment overnight. After washing with DMEM without FBS, attached cells were treated with normal mouse serum or a day 43 pooled antiserum (100 μL/well, 1:50 dilution in DMEM without FBS) at 37 °C for 2 h. Then, cells were washed and incubated with rabbit complement serum (100 μL/well, 1:10 dilution in DMEM without FBS) at 37 °C for 1 h. The low control was the spontaneous LDH release from cells treated with rabbit complement serum only without mouse serum. For the high control, 1% triton X-100 (100 μL/well, dilution in DMEM without FBS) was added instead of the rabbit complement serum. After incubation, 20 μL of the supernatant from each well was relocated and mixed with 80 μL of PBS in each well on another 96-well plate. Thereafter, the LDH activity in each well was detected with LDH Cytotoxicity Detection Kit (Pierce LDH Cytotoxicity Assay Kit, Thermo Scientific Co.) according to manufacturer’s instructions. LDH cytotoxicity detection reagent (100 μL/well) was added and incubated at rt in the dark for 1 h. The OD value of each well at 490 nm and 680 nm (instrument background signal) wavelengths was read with a microplate reader. The antibody-mediated CDC was indicated by the percentage of cell lysis, calculated according to the equation below:
where “experimental A” is the OD490-OD680 value of cells treated with normal mouse serum or a pooled day 43 antiserum, “low control A” is the OD490-OD680 value of cells treated without any serum, and “high control A” is the OD490-OD680 value of cells completely lysed with 1% triton X-100.
Statistical analysis.
All data were analyzed by one-way analysis of variance (more than 2 groups) or independent t test (between 2 groups) using SPSS software. P < 0.05 was considered as statistically significant.
Supplementary Material
Funding
This work was financially supported by a grant of the National Institute of Cancer of National Institutes of Health (R01 CA095142).
Abbreviations
- AP
alkaline phosphatase
- BCA
bicinchoninic acid
- CDC
complement-dependent cytotoxicity
- CFA
complete Freund’s adjuvant
- DMEM
Dulbecco’s modified eagle medium
- DMF
N,N-dimethyl formaldehyde
- ELISA
enzyme-linked immunosorbent assay
- FACS
flow cytometry
- FBS
fetal bovine serum
- FITC
fluorescein isothiocyanate
- Fuc
fucose
- Gal
galactose
- HSA
human serum albumin
- IFA
incomplete Freund’s adjuvant
- i.p.
intraperitoneal
- KLH
keyhole limpet hemocyanin
- LDH
lactate dehydrogenase
- Lex
Lewis X
- Ley
Lewis Y
- OD
optical density
- PBS
phosphate-buffered saline
- PBST
PBS containing 0.05% of Tween-20
- PNPP
p-nitrophenylphosphate
- s.c.
subcutaneous
- SEM
standard error of mean
- TACA
Tumor-associated carbohydrate antigen
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Approval of animal use
The animal use protocol for this research (#201609560) was approved by the Institutional Animal Care and Use Committee of University of Florida.
Consent for publication
All of the authors have read agreed to publish this article and declare that it is original, has never been published before, and has not been submitted to other journals.
Competing interests
All of the authors disclose no competing interests.
Additional information
An additional file containing original ELISA, FACS and antibody-mediated CDC data and the MALDI-TOF mass spectra of glycoconjugates 2a, 2b and 2c is available via the Internet at the journal’s website.
References
- [1].Feizi T, Carbohydrate antigens in human cancer. Cancer Surv. 4 (1985) 245–269. [PubMed] [Google Scholar]
- [2].Hakomori S.-i., Aberrant glycosylation in cancer cell membranes as focused on glycolipids: overview and perspectives. Cancer Res. 45 (1985) 2405–2414. [PubMed] [Google Scholar]
- [3].Lloyd KO, Humoral immune responses to tumor-associated carbohydrate antigens. Semin. Cancer Biol 2 (1991) 421–431. [PubMed] [Google Scholar]
- [4].Hossain F and Andreana PR, Developments in carbohydrate-based cancer therapeutics. Pharmaceuticals 12 (84) (2019) 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Feng D, Shaikh AS, and Wang F, Recent advance in tumor-associated carbohydrate antigens (TACAs)-based antitumor vaccines. ACS Chem. Biol 11 (2016) 850–863. [DOI] [PubMed] [Google Scholar]
- [6].Yin Z and Huang X, Recent development in carbohydrate based anti-cancer vaccines. J Carbohydr Chem 31 (2012) 143–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Guo Z and Wang Q, Recent development in carbohydrate-based cancer vaccines. Curr. Opin. Chem. Biol 13 (2009) 608–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yang Y, Cancer immunotherapy: harnessing the immune system to battle cancer. J. Clin. Invest 125 (2015) 3335–3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chen C-H and Wu T-C, Experimental vaccine strategies for cancer immunotherapy. J. Biomed. Sci 5 (1998) 231–252. [DOI] [PubMed] [Google Scholar]
- [10].Jin K-T, Lan H-R, Chen X-Y, Wang S-B, Ying X-J, Lin Y, and Mou X-Z, Recent advances in carbohydrate-based cancer vaccines. Biotechnol. Lett (2019) 1–10. [DOI] [PubMed] [Google Scholar]
- [11].Wei MM, Wang YS, and Ye XS, Carbohydrate-based vaccines for oncotherapy. Med. Res. Rev 38 (2018) 1003–1026. [DOI] [PubMed] [Google Scholar]
- [12].Buskas T, Thompson P, and Boons G-J, Immunotherapy for cancer: synthetic carbohydrate-based vaccines. Chem. Commun (2009) 5335–5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Slovin SF, Keding SJ, and Ragupathi G, Carbohydrate vaccines as immunotherapy for cancer. Immunol. Cell Biol 83 (2005) 418–428. [DOI] [PubMed] [Google Scholar]
- [14].Danishefsky SJ and Allen JR, From the laboratory to the clinic: A retrospective on fully synthetic carbohydrate-based anticancer vaccines. Angew. Chem. Int. Ed 39 (2000) 837–863. [DOI] [PubMed] [Google Scholar]
- [15].Li Q, Jiang W, Guo J, Jaiswal M, and Guo Z, Synthesis of Lewis Y Analogs and Their Protein Conjugates for Structure-Immunogenicity Relationship Studies of Lewis Y Antigen. J. Org. Chem (2019) DOI: 10.1021/acs.joc.1029b00537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Li Q and Guo Z, Synthesis of the Cancer-Associated KH-1 Antigen by Block Assembly of Its Backbone Structure Followed by One-Step Grafting of Three Fucose Residues. Org. Lett 19 (2017) 6558–6561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sakamoto J, Furukawa K, Cordon-Cardo C, Yin BW, Rettig WJ, Oettgen HF, Old LJ, and Lloyd KO, Expression of Lewisa, Lewisb, X, and Y blood group antigens in human colonic tumors and normal tissue and in human tumor-derived cell lines. Cancer Res. 46 (1986) 1553–1561. [PubMed] [Google Scholar]
- [18].Westwood JA, Murray WK, Trivett M, Haynes NM, Solomon B, Mileshkin L, Ball D, Michael M, Burman A, and Mayura-Guru P, The Lewis-Y carbohydrate antigen is expressed by many human tumors and can serve as a target for genetically redirected T cells despite the presence of soluble antigen in serum. J. Immunother 32 (2009) 292–301. [DOI] [PubMed] [Google Scholar]
- [19].Deshpande PP and Danishefsky SJ, Total synthesis of the potential anticancer vaccine KH-1 adenocarcinoma antigen. Nature 387 (1997) 164. [DOI] [PubMed] [Google Scholar]
- [20].Ragupathi G, Deshpande PP, Coltart DM, Kim HM, Williams LJ, Danishefsky SJ, and Livingston PO, Constructing an adenocarcinoma vaccine: Immunization of mice with synthetic KH - 1 nonasaccharide stimulates anti - KH - 1 and anti - Ley antibodies. Int. J. Cancer 99 (2002) 207–212. [DOI] [PubMed] [Google Scholar]
- [21].Miermont A, Zeng Y, Jing Y, Ye X.-s., and Huang X, Syntheses of LewisX and dimeric LewisX: construction of branched oligosaccharides by a combination of preactivation and reactivity based chemoselective one-pot glycosylations. J. Org. Chem 72 (2007) 8958–8961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Spassova MK, Bornmann WG, Ragupathi G, Sukenick G, Livingston PO, and Danishefsky SJ, Synthesis of selected LeY and KH-1 analogues: a medicinal chemistry approach to vaccine optimization. J. Org. Chem 70 (2005) 3383–3395. [DOI] [PubMed] [Google Scholar]
- [23].Sabbatini PJ, Kudryashov V, Ragupathi G, Danishefsky SJ, Livingston PO, Bornmann W, Spassova M, Zatorski A, Spriggs D, and Aghajanian C, Immunization of ovarian cancer patients with a synthetic Lewisy‐protein conjugate vaccine: A phase 1 trial. Int. J. Cancer 87 (2000) 79–85. [PubMed] [Google Scholar]
- [24].Kudryashov V, Kim HM, Ragupathi G, Danishefsky SJ, Livingston PO, and Lloyd KO, Immunogenicity of synthetic conjugates of Lewis y oligosaccharide with proteins in mice: towards the design of anticancer vaccines. Cancer Immunol. Immunother 45 (1998) 281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang Q, Ekanayaka SA, Wu J, Zhang J, and Guo Z, Synthetic and immunological studies of 5′-N-phenylacetyl sTn to develop carbohydrate-based cancer vaccines and to explore the impacts of linkage between carbohydrate antigens and carrier proteins. Bioconjug. Chem 19 (2008) 2060–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wrolstad RE, Acree TE, Decker EA, Penner MH, Reid DS, Schwartz SJ, Shoemaker CF, Smith D, and Sporns P, Handbook of food analytical chemistry: pigments, colorants, flavors, texture, and bioactive food components. John Wiley and Sons, Inc: 2005. [Google Scholar]
- [27].Pan Y, Chefalo P, Nagy N, Harding CV, and Guo Z, Synthesis and immunological properties of N-modified GM3 antigens as therapeutic cancer vaccines. J. Med. Chem 48 (2005) 875–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Liao G, Zhou Z, and Guo Z, Synthesis and immunological study of α−2,9-oligosialic acid conjugates as anti-group C meningitis vaccines Chem. Commun. 51 (2015) 9647–9650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Marshall JS, Warrington R, Watson W, and Kim HL, An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol 14(Suppl 2):49 (2018) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chaplin DD, Overview of the immune response. J. Allergy Clin. Immunol 125 (2 Suppl 2) (2010) S3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Vidarsson G, Dekkers G, and Rispens T, IgG subclasses and allotypes: from structure to effector functions. Front Immunol 5:520 (2014) 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lloyd KO, Larson G, Strömberg N, Thurin J, and Karlsson K-A, Mouse monoclonal antibody F-3 recognizes the difucosyl type-2 blood group structure. Immunogenetics 17 (1983) 537–541. [DOI] [PubMed] [Google Scholar]
- [33].Kitamura K, Stockert E, Garin-Chesa P, Welt S, Lloyd KO, Armour KL, Wallace TP, Harris WJ, Carr FJ, and Old LJ, Specificity analysis of blood group Lewis-y (Le (y)) antibodies generatedagainst synthetic and natural Le (y) determinants. Proc. Natl. Acad. Sci. USA 91 (1994) 12957–12961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Itzkowitz SH, Yuan M, Fukushi Y, Palekar A, Phelps PC, Shamsuddin AM, Trump BF, Hakomori S.-i., and Kim YS, Lewisx-and sialylated Lewisx-related antigen expression in human malignant and nonmalignant colonic tissues. Cancer Res. 46 (1986) 2627–2632. [PubMed] [Google Scholar]
- [35].Kim YS, Yuan M, Itzkowitz SH, Sun Q, Kaizu T, Palekar A, Trump BF, and Hakomori S.-i., Expression of LeY and extended LeY blood group-related antigens in human malignant, premalignant, and nonmalignant colonic tissues. Cancer Res. 46 (1986) 5985–5992. [PubMed] [Google Scholar]
- [36].Nudelman E, Levery SB, Kaizu T, and S.-i. Hakomori, Novel fucolipids of human adenocarcinoma: characterization of the major Ley antigen of human adenocarcinoma as trifucosylnonaosyl Ley glycolipid (III3FucV3FucVI2FucnLc6). J. Biol. Chem 261 (1986) 11247–11253. [PubMed] [Google Scholar]
- [37].Buskas T, Li Y, and Boons GJ, Synthesis of a dimeric Lewis antigen and the evaluation of the epitope specificity of antibodies elicited in mice. Chem. Eur. J 11 (2005) 5457–5467. [DOI] [PubMed] [Google Scholar]
- [38].Vliegenthart JF, Carbohydrate based vaccines. FEBS Lett. 580 (2006) 2945–2950. [DOI] [PubMed] [Google Scholar]
- [39].Koichi F, Welt S, Yin BW, Feickert H-J, Takashi T, Ueda R, and Lloyd KO, Analysis of the fine specificities of 11 mouse monoclonal antibodies reactive with type 2 blood group determinants. Mol. Immunol 27 (1990) 723–732. [DOI] [PubMed] [Google Scholar]
- [40].Miyoshi E, Moriwaki K, Terao N, Tan C-C, Terao M, Nakagawa T, Matsumoto H, Shinzaki S, and Kamada Y, Fucosylation is a promising target for cancer diagnosis and therapy. Biomolecules 2 (2012) 34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Dingjan T, Spendlove I, Durrant LG, Scott AM, Yuriev E, and Ramsland PA, Structural biology of antibody recognition of carbohydrate epitopes and potential uses for targeted cancer immunotherapies. Mol. Immunol 67 (2015) 75–88. [DOI] [PubMed] [Google Scholar]
- [42].Zhang S, Zhang HS, Cordon - Cardo C, Reuter VE, Singhal AK, Lloyd KO, and Livingston PO, Selection of tumor antigens as targets for immune attack using immunohistochemistry: II. Blood group - related antigens. Int. J. Cancer 73 (1997) 50–56. [DOI] [PubMed] [Google Scholar]
- [43].Nores GA, Dohi T, Taniguchi M, and Hakomori S, Density dependent recognition of cell surface GM3 by a certain anti-melanoma antibody, and GM3 lactone as a possible immunogen: requirements for tumor-associated antigen and immunogen. J. Immunol 139 (1987) 3171–3176. [PubMed] [Google Scholar]
- [44].Dwek RA, Glycobiology: toward understanding the function of sugars. Chem. Rev 96 (1996) 683–720. [DOI] [PubMed] [Google Scholar]
- [45].Kiessling LL and Grim JC, Glycopolymer probes of signal transduction. Chem. Soc. Rev 42 (2013) 4476–4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hakomori S.-i., Tumor-associated carbohydrate antigens. Annu. Rev. Immunol 2 (1984) 103–126. [DOI] [PubMed] [Google Scholar]
- [47].Zhou Z, Mondal M, Liao G, and Guo Z, Synthesis and evaluation of monophosphoryl lipid A derivatives as fully synthetic self-adjuvanting glycoconjugate cancer vaccine carriers. Org. Biomol. Chem 12 (2014) 3238–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Zhou Z, Liao G, Mandal SS, Suryawanshi S, and Guo Z, A fully synthetic self-adjuvanting globo H-Based vaccine elicited strong T cell-mediated antitumor immunity. Chem. Sci 6 (2015) 7112–7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Zhou Z, Mandal SS, Liao G, Guo J, and Guo Z, Synthesis and evaluation of GM2-monophosphoryl lipid A conjugate as a fully synthetic self-adjuvant cancer vaccine. Sci. Rep 7 (2017) 11403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wang Q, Zhang J, and Guo Z, Efficient glycoengineering of GM3 on melanoma cell and monoclonal antibody-mediated selective killing of the glycoengineered cancer cell. Bioorg. Med. Chem 15 (2007) 7561–7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


