Abstract
Disparities in organ acceptance practices exacerbate donor heart non-use and lead to increased waiting times and mortality for heart transplant candidates. We studied disparities in donor heart acceptance among US transplant centers and their relations to post-transplant outcomes. Candidate, potential transplant recipient match run, and deceased donor data were obtained from the United Network for Organ Sharing. We analyzed donor, candidate, and transplant center characteristics with respect to organ acceptance, offer acceptance, number of offers before acceptance (organ sequence number), and association with post-transplant mortality. A total of 693,420 donor heart offers made between April 2007 and December 2015 were included. We identified great variability in donor heart acceptance practices among US heart transplant centers. We identified donor and recipient characteristics that were strongly associated with heart organ and offer acceptance, and organ sequence number, and identified inconsistencies among centers with respect to how these characteristics influenced acceptance decisions. Finally, we identified characteristics that were highly predictive of donor heart non-use and were not associated with increased recipient mortality, which may guide future efforts aimed at increasing use of available hearts for transplantation.
INTRODUCTION
The great shortage of donor hearts deemed acceptable for transplantation continues to restrict heart transplantation to a minority of patients with end-stage heart disease who could benefit from this life-saving procedure. Despite this shortage, an analysis of the United Network for Organ Sharing (UNOS) heart transplant registry from 1995-2010 revealed disparities in donor heart acceptance rates across the United States (US), even after adjusting for key donor characteristics that may influence heart acceptance decisions.(1) This variability in donor heart acceptance practices among geographical regions, transplant centers, and even individual physicians likely exacerbates the current problem of donor heart non-use for transplantation, which ultimately increases transplant waiting times and mortality for critically ill patients.(2)
Recent efforts have been made to develop consensus guidelines for donor heart acceptance, with the hope of standardizing practices across the US and ultimately improving use of available donor hearts.(3) These efforts, however, are greatly hampered by a lack of systematic studies on this topic and analyses that could provide evidence-based recommendations for donor heart selection.
Prior work by our group and others have identified predictors of donor heart non-acceptance for transplantation in various cohorts.(4–7) Of great interest, however, was the observation that many of these donor characteristics did not necessarily portend increased recipient mortality after transplantation, suggesting that these donor risk factors are not absolute contraindications to transplantation.
The first step in systematically studying donor heart under-utilization is to identify where the greatest disparities exist—specifically: (1) Which donor and candidate characteristics are most predictive of offer acceptance, organ acceptance, and organ sequence number in the US?, (2) Which of these highly predictive donor and candidate characteristics are inconsistently applied across transplant centers to guide offer acceptance and organ acceptance decisions?, (3) Which of these inconsistently applied donor and candidate characteristics are actually predictive of post-transplant mortality or re-transplantation?, and (4) Can we identify donor and candidate characteristics that can be targeted to increase consistency in offer and organ acceptance decisions across heart transplant centers? The identification of disparities in donor heart use across the country will inform and guide future research and policy efforts focused on this issue.
METHODS
Study design
This is a retrospective study of heart transplant candidates, potential donors, and transplant centers in the US. The study was approved by the Stanford University Institutional Review Board. All transplant services in the country are joined under the nationwide Organ Procurement and Transplantation Network (OPTN), which is managed by UNOS. We requested and received the following data: thoracic candidate data (09/1985-12/2015), potential transplant recipient (PTR) match run data for thoracic transplant candidates (record of each time a donor heart offer is made to a transplant candidate, 04/2007-12/2015), and thoracic deceased donor data (09/1987-12/2015). These analyses were based on OPTN data as of February 10, 2017.
Waitlist identifiers were used to link candidates awaiting heart transplantation to transplant centers and potential donors. Exclusion criteria for candidates, donors, and offers are presented in Figure 1. Supplemental Table 1 summarizes the list of donor heart refusal codes included in the study, the percent of donors with each refusal code, and the list of refusal codes that were excluded from this analysis.
Figure 1:
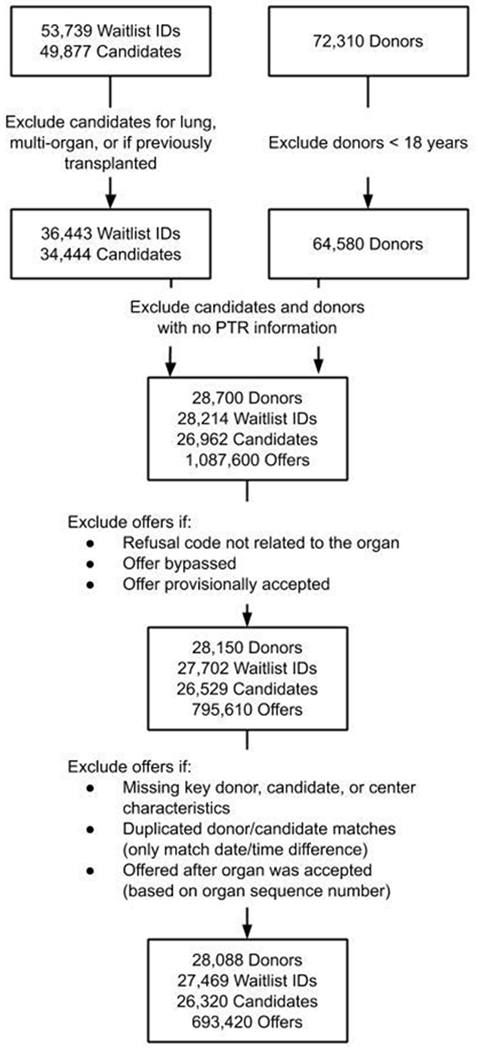
Exclusion criteria for transplant candidates, donors, and donor heart offers from April 2007 to December 2015
Outcomes
The primary objective of this study was to identify and examine the donor, candidate, and transplant center characteristics that were inconsistently used to guide offer acceptance, organ acceptance, and organ sequence number decisions across transplant centers in the US. There are two ways to analyze the decision process to accept or reject a donor heart offer. An organ may be deemed acceptable or unacceptable for all candidates listed at a transplant center based on the characteristics of the organ, or it may be evaluated on an offer-by-offer basis for each eligible candidate at a program (by position on the match run), based on the characteristics of each donor and candidate pair. These two approaches require separate analyses. We therefore analyzed donor, candidate, and transplant center characteristics with regard to (1) organ acceptance, (2) offer acceptance, (3) the number of offers before acceptance (organ sequence number), and (4) the association with post-transplant mortality or re-transplantation. Finally, we identified characteristics that are inconsistently applied across transplant centers and determined whether these characteristics predict mortality or re-transplantation within 30 days and 1 year (see Statistical Analyses section).
Candidate and donor characteristics
Based on previous literature, we identified key candidate and donor characteristics to consider,(4–11) as summarized in Tables 1 and 2. Categorical characteristics that had missing values or were coded as “unknown” were grouped together and treated as “unknown”.
Table 1.
Candidate characteristics
| Candidate characteristics | Overall | Not transplanted | Transplanted |
|---|---|---|---|
| N | 26320 | 9706 (36.9) | 16614 (63.1) |
| Waitlist organ = Heart only vs Heart-Lung (%) | 26180 (99.5) | 9566 (98.6) | 16614 (100.0) |
| Male (%) | 19425 (73.8) | 7230 (74.5) | 12195 (73.4) |
| Blood type (%) | |||
| A | 9903 (37.6) | 3176 (32.7) | 6727 (40.5) |
| AB | 1186 (4.5) | 229 (2.4) | 957 (5.8) |
| B | 3568 (13.6) | 1110 (11.4) | 2458 (14.8) |
| O | 11663 (44.3) | 5191 (53.5) | 6472 (39.0) |
| Diabetes (%) | |||
| None | 19036 (72.3) | 6776 (69.8) | 12260 (73.8) |
| History of diabetes 0-5 years | 559 (2.1) | 208 (2.1) | 351 (2.1) |
| History of diabetes 6-10 years | 6171 (23.4) | 2455 (25.3) | 3716 (22.4) |
| History of diabetes > 10 years | 62 (0.2) | 31 (0.3) | 31 (0.2) |
| History of diabetes unknown years | 382 (1.5) | 176 (1.8) | 206 (1.2) |
| Unknown history of diabetes | 110 (0.4) | 60 (0.6) | 50 (0.3) |
| History of cigarette use (%) | |||
| Unknown | 237 (0.9) | 165 (1.7) | 72 (0.4) |
| No | 14035 (53.3) | 4988 (51.4) | 9047 (54.5) |
| Yes | 12048 (45.8) | 4553 (46.9) | 7495 (45.1) |
| Prior cardiac surgery at listing (%) | |||
| No | 14761 (56.1) | 5397 (55.6) | 9364 (56.4) |
| Unknown | 1567 (6.0) | 474 (4.9) | 1093 (6.6) |
| Yes | 9992 (38.0) | 3835 (39.5) | 6157 (37.1) |
| Days as status 1A (median [IQR]) | 7.00 [0.00, 31.00] | 0.00 [0.00, 25.00] | 12.00 [0.00, 33.00] |
| Days as status 1B (median [IQR]) | 21.00 [0.00, 130.00] | 29.00 [0.00, 210.00] | 19.00 [0.00, 98.00] |
| Days as status 2 (median [IQR]) | 0.00 [0.00, 60.00] | 1.00 [0.00, 247.00] | 0.00 [0.00, 13.00] |
| Total days on waiting list (median [IQR]) | 154.00 [43.00, 423.00] | 357.00 [139.00, 771.75] | 90.00 [27.00, 247.00] |
| Age at listing (median [IQR]) | 54.00 [43.00, 62.00] | 54.00 [44.00, 62.00] | 54.00 [43.00, 62.00] |
| Race (%) | |||
| White | 17412 (66.2) | 6344 (65.4) | 11068 (66.6) |
| Black | 5693 (21.6) | 2286 (23.6) | 3407 (20.5) |
| Hispanic | 2130 (8.1) | 762 (7.9) | 1368 (8.2) |
| Asian | 768 (2.9) | 212 (2.2) | 556 (3.3) |
| Native American/Alaska Native | 87 (0.3) | 35 (0.4) | 52 (0.3) |
| Native Hawaiian/other Pacific Islander | 81 (0.3) | 23 (0.2) | 58 (0.3) |
| Multiracial | 149 (0.6) | 44 (0.5) | 105 (0.6) |
| Height (in cm) at removal/current time (median [IQR]) | 175.00 [167.60, 180.30] | 175.30 [167.60, 182.90] | 172.70 [165.10, 180.30] |
| Weight (in kg) at removal/current time (median [IQR]) | 82.10 [69.00, 95.30] | 85.70 [72.60, 99.80] | 80.30 [67.60, 93.00] |
| BMI at removal/current time (median [IQR]) | 27.10 [23.60, 30.80] | 28.10 [24.40, 32.00] | 26.50 [23.10, 30.20] |
| Hypertension (%) | |||
| No | 16611 (63.1) | 6046 (62.3) | 10565 (63.6) |
| Unknown | 3662 (13.9) | 1326 (13.7) | 2336 (14.1) |
| Yes | 6047 (23.0) | 2334 (24.0) | 3713 (22.3) |
BMI: body mass index; IQR: interquartile range
Table 2:
Donor characteristics
| Donor characteristic | Overall | Not transplanted | Transplanted |
|---|---|---|---|
| N | 28083 | 12403 (44.2) | 15680 (55.8) |
| Number of offers (median [IQR]) | 9.00 [2.00, 31.00] | 26.00 [10.00, 59.00] | 3.00 [1.00, 10.00] |
| BMI (median [IQR]) | 26.56 [23.37, 30.68] | 26.79 [23.40, 31.39] | 26.35 [23.33, 30.17] |
| Age in years (median [IQR]) | 36.00 [25.00, 47.00] | 44.00 [32.00, 52.00] | 31.00 [23.00, 42.00] |
| Height in cm (median [IQR]) | 172.70 [165.10, 180.00] | 170.00 [162.60, 177.80] | 175.00 [167.60, 182.00] |
| Weight in kg (median [IQR]) | 80.00 [68.30, 93.00] | 78.30 [66.55, 92.40] | 80.35 [70.00, 93.00] |
| Serum sodium (median [IQR]) | 148.00 [142.00, 154.00] | 148.00 [142.00, 153.00] | 148.00 [142.00, 154.00] |
| BUN (median [IQR]) | 15.00 [10.00, 23.00] | 16.00 [11.00, 24.00] | 14.00 [10.00, 22.00] |
| Creatinine (median [IQR]) | 1.00 [0.80, 1.40] | 1.00 [0.80, 1.50] | 1.00 [0.80, 1.40] |
| Total bilirubin (median [IQR]) | 0.70 [0.50, 1.10] | 0.70 [0.40, 1.10] | 0.70 [0.50, 1.20] |
| Male (%) | 17470 (62.2) | 6414 (51.7) | 11056 (70.5) |
| Cause of death (%) | |||
| Anoxia | 6482 (23.1) | 3156 (25.4) | 3326 (21.2) |
| Other | 658 (2.3) | 307 (2.5) | 351 (2.2) |
| Cerebrovascular/stroke | 8804 (31.3) | 5302 (42.7) | 3502 (22.3) |
| Head trauma | 11951 (42.6) | 3551 (28.6) | 8400 (53.6) |
| CNS tumor | 188 (0.7) | 87 (0.7) | 101 (0.6) |
| Race (%) | |||
| White | 18136 (64.6) | 8053 (64.9) | 10083 (64.3) |
| Black | 4505 (16.0) | 2000 (16.1) | 2505 (16.0) |
| Hispanic | 4395 (15.7) | 1793 (14.5) | 2602 (16.6) |
| Asian | 645 (2.3) | 358 (2.9) | 287 (1.8) |
| Native American/Alaska Native | 144 (0.5) | 61 (0.5) | 83 (0.5) |
| Native Hawaiian/other Pacific Islander | 43 (0.2) | 23 (0.2) | 20 (0.1) |
| Multiracial | 215 (0.8) | 115 (0.9) | 100 (0.6) |
| Blood type (%) | |||
| O | 13684 (48.7) | 5543 (44.7) | 8141 (51.9) |
| A | 10073 (35.9) | 4564 (36.8) | 5509 (35.1) |
| AB | 928 (3.3) | 591 (4.8) | 337 (2.1) |
| B | 3398 (12.1) | 1705 (13.7) | 1693 (10.8) |
| History of cigarette use (%) | |||
| No | 22132 (78.8) | 8819 (71.1) | 13313 (84.9) |
| Unknown | 375 (1.3) | 204 (1.6) | 171 (1.1) |
| Yes | 5576 (19.9) | 3380 (27.3) | 2196 (14.0) |
| Hypertension (%) | |||
| No | 20961 (74.6) | 7844 (63.2) | 13117 (83.7) |
| Unknown | 198 (0.7) | 107 (0.9) | 91 (0.6) |
| Yes | 6924 (24.7) | 4452 (35.9) | 2472 (15.8) |
| Diabetes (%) | |||
| None | 26093 (92.9) | 11047 (89.1) | 15046 (96.0) |
| History of diabetes 0-5 years | 802 (2.9) | 505 (4.1) | 297 (1.9) |
| History of diabetes 6-10 years | 341 (1.2) | 250 (2.0) | 91 (0.6) |
| History of diabetes > 10 years | 501 (1.8) | 367 (3.0) | 134 (0.9) |
| History of diabetes unknown years | 188 (0.7) | 142 (1.1) | 46 (0.3) |
| Unknown history of diabetes | 158 (0.6) | 92 (0.7) | 66 (0.4) |
| Cocaine use (%) | |||
| No | 22634 (80.6) | 9821 (79.2) | 12813 (81.7) |
| Unknown | 611 (2.2) | 281 (2.3) | 330 (2.1) |
| Yes | 4838 (17.2) | 2301 (18.6) | 2537 (16.2) |
| History of myocardial infarction (%) | |||
| No | 27612 (98.3) | 12079 (97.4) | 15533 (99.1) |
| Unknown | 276 (1.0) | 185 (1.5) | 91 (0.6) |
| Yes | 195 (0.7) | 139 (1.1) | 56 (0.4) |
| Pre-recovery steroids (%) | |||
| No | 7030 (25.0) | 3164 (25.5) | 3866 (24.7) |
| Unknown | 34 (0.1) | 21 (0.2) | 13 (0.1) |
| Yes | 21019 (74.8) | 9218 (74.3) | 11801 (75.3) |
| Pre-recovery thyroxine-T4 (%) | |||
| No | 7377 (26.3) | 3269 (26.4) | 4108 (26.2) |
| Unknown | 20 (0.1) | 11 (0.1) | 9 (0.1) |
| Yes | 20686 (73.7) | 9123 (73.6) | 11563 (73.7) |
| Public Health Service high risk (%) | |||
| No | 23786 (84.7) | 10370 (83.6) | 13416 (85.6) |
| Unknown | 50 (0.2) | 23 (0.2) | 27 (0.2) |
| Yes | 4247 (15.1) | 2010 (16.2) | 2237 (14.3) |
| History of heavy alcohol use (2+ drinks/day) (%) | |||
| No | 22534 (80.2) | 9719 (78.4) | 12815 (81.7) |
| Unknown | 488 (1.7) | 215 (1.7) | 273 (1.7) |
| Yes | 5061 (18.0) | 2469 (19.9) | 2592 (16.5) |
| Left ventricular ejection fraction (%) | |||
| > 50 | 24397 (86.9) | 9706 (78.3) | 14691 (93.7) |
| 50 or less | 3374 (12.0) | 2410 (19.4) | 964 (6.1) |
| Unknown | 312 (1.1) | 287 (2.3) | 25 (0.2) |
| Donor/Recipient weight ratio (median [IQR]) | - | - | 0.99 [0.87, 1.16] |
| Donor/Recipient weight mismatch (%) | - | - | 348 (2.2) |
| Donor/Recipient identical blood type (%) | - | - | 13330 (85.0) |
| Donor/Recipient sex mismatch (%) | - | - | 3969 (25.3) |
| Cold ischemic time (%) | |||
| 4 or more hours | - | - | 3355 (21.4) |
| < 4 hours | - | - | 11960 (76.3) |
| Unknown | - | - | 365 (2.3) |
BMI: body mass index, BUN: blood urea nitrogen, CNS: central nervous system; IQR: interquartile range
Transplant center volume and access to transplantation
Center volume was included as a covariate in all models and was calculated according to the convention described by Shuhaiber et al.(12) Centers were characterized as very low-, low-, medium-, or high-volume centers (0-12, 13-21, 22-33, and >33, respectively) based on the average number of transplants per year from 2007-2015. The access rate for each transplant center was calculated as the number of candidates at the center divided by the sum of the proportion of the time that each candidate spent waiting. Access rate is similar in calculation to the “person-years” waiting metric reported by the Scientific Registry of Transplant Recipients (SRTR), but is calculated per center and over the entire time period for which data was available (see Detailed Methods in the Supplemental Material).
Statistical analyses
Figure 2 illustrates the statistical analyses performed for this study. For donor heart offer acceptance and organ sequence number, we identified candidate, donor, and transplant center characteristics predictive of the outcome using elastic net regularized regression.(13) Donor and transplant center characteristics, but not candidate characteristics, were included in models of organ acceptance. Similar to the LASSO (Least Absolute Shrinkage and Selection Operator) method,(14) elastic net uses the penalized likelihood to select characteristics that are most predictive of the outcome; however, unlike LASSO, elastic net allows highly correlated characteristics to be included in the model. We chose this method because we aimed to identify characteristics that were most predictive of the outcomes of interest, while also allowing for correlation among these characteristics.
Figure 2:
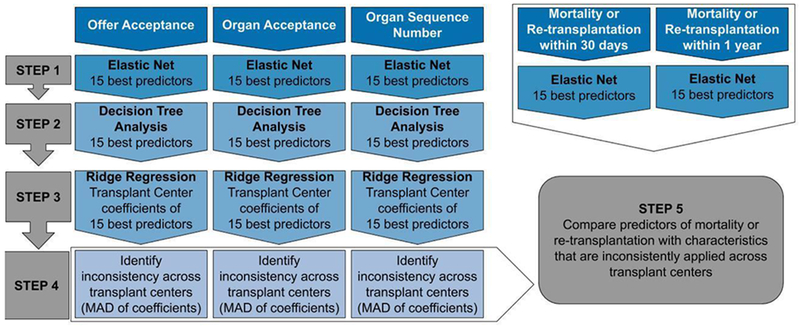
Overview of statistical analyses performed (MAD: median absolute deviation)
Logistic regression models were used for the dichotomous outcomes (offer acceptance, organ acceptance, and mortality/re-transplantation) while a Poisson regression model was used to model organ sequence number for organs that were ultimately accepted. Since not all candidates have equal access to transplantation and since access is known to influence mortality/re-transplantation, we included access rate as a covariate in the mortality/re-transplantation models. All analyses were completed in the R statistical computing environment,(15) utilizing the glmnet package.(16)
To gain a better understanding of the interactions among candidate and donor characteristics on the outcomes of interest, we used conditional inference trees (17) with the 15 most predictive characteristics as input. The conditional inference trees were implemented with the partykit (18) package in R.(15)
Identification of characteristics that are inconsistently applied across transplant centers
For each outcome of interest, we identified the 15 most predictive donor, candidate, and transplant center characteristics, based on the rank of the characteristic in the elastic net model results. Next, using these top 15 characteristics, we independently fit ridge regression models to data from each center, such that a separate model was fit for each center. Ridge regression includes all characteristics in the model, but also accounts for correlation among characteristics.(19)
For each outcome and characteristic, we calculated the median absolute deviation (MAD) of the centers’ ridge regression coefficients. Characteristics with MAD in the lowest quartile were considered to vary the least. Characteristics with MAD in the highest quartile were considered to vary the most and represented characteristics that were inconsistently applied across centers.
Finally, we separately identified donor and recipient characteristics that were highly predictive of mortality or re-transplantation within 30 days or 1 year, adjusting for transplant center access rates. We compared these characteristics to those that were inconsistently applied across transplant centers, as determined previously. We thereby identified characteristics that could be targeted for more consistent donor heart utilization across centers.
For additional details, please see Detailed Methods in the Supplemental Material.
RESULTS
A total of 28,088 potential donors, 26,320 heart transplant candidates, and 693,420 donor heart offers made in the US between April 2007 and December 2015 were included in this study (Figure 1). As shown in Supplemental Table 1, most donor hearts (67%) were turned down by transplant centers due to concerns about donor heart “quality” (code 830). The majority of candidates included in this study (63.1%) received a heart transplant, although some received a heart from a donor that was excluded from this analysis, most commonly due to donor age less than 18 years. Only data on candidates and donors who met study inclusion criteria were used for subsequent analyses. Of the donor hearts included in this study, 55.8% were ultimately transplanted (this is higher than the average national donor heart utilization rate of 30-35%,(1) as only donor hearts that were offered for transplantation were included). Candidate and donor characteristics are summarized in Tables 1 and 2.
Most transplant centers were very low- or low-volume centers: 55.9% were very low (0-12 transplants/year), 26.2% were low (13-21 transplants/year), 9.0% were medium (22-33 transplants/year), and 9.0% were high volume centers (>33 transplants/year). Centers had a median access rate of 8.62 (IQR 5.17-11.74). Not surprisingly, median access rate generally increased as transplant center volume increased (Figure 3) but there was considerable variability among very low-volume centers, with a median access rate of 7.52 (IQR 3.96-11.16). For this reason, access rate was added as a covariate to the mortality models.
Figure 3:
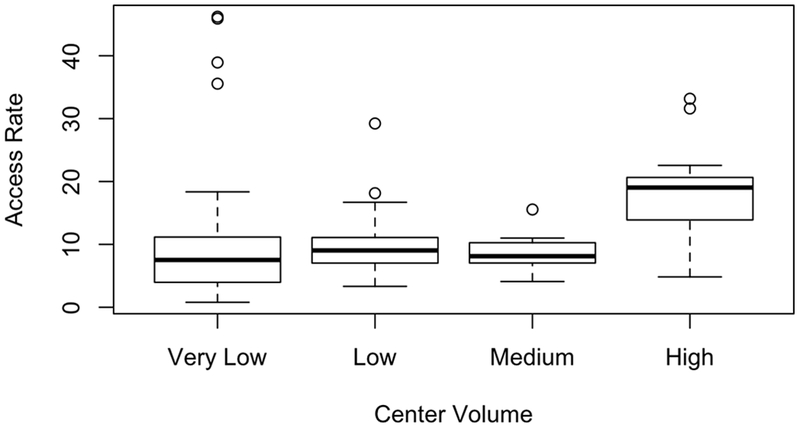
Access rate, by transplant center volume (very low=0-12/year, low=13-21/year, medium=22-33/year, high>33/year)
Offer acceptance
The characteristics that were most predictive of donor heart offer acceptance, by ranked order, were: candidate total days waiting, candidate days as status 1B, donor age, candidate days as status 2, candidate days as status 1A, candidate BMI, donor/candidate weight ratio, donor LVEF>50%, candidate blood type O, and donor weight. Standardized coefficients (which indicate the direction and magnitude of the effect) and rankings for the 15 most predictive characteristics are shown in Table 3A and for all characteristics can be seen in Supplemental Table 2A. Note that negative coefficients indicate that the offer was less likely to be accepted, while positive coefficients indicate that the offer was more likely to be accepted. For example, heart offers were less likely to be accepted for candidates who were waiting longer or if the donors were older, had LVEF≤50%, or if there was a donor/candidate weight mismatch. Conversely, heart offers were more likely to be accepted if the candidate had blood type O or if the donor was larger (higher weight and height).
Table 3A:
Top 15 characteristics most predictive of offer acceptance
| Characteristic | Standardized Coefficient | Rank (importance) |
|---|---|---|
| Candidate days waiting | −1.130 | 1 |
| Candidate days on status 1B | −1.065 | 2 |
| Donor age | −0.785 | 3 |
| Candidate days on status 2 | −0.606 | 4 |
| Candidate days on status 1A | −0.501 | 5 |
| Candidate BMI | −0.386 | 6 |
| Donor/Candidate weight ratio | −0.359 | 7 |
| Donor LV EF | 8 | |
| >50% | 0.341 | |
| ≤50% | −0.270 | |
| Unknown | −0.061 | |
| Candidate blood type | 9 | |
| O | 0.330 | |
| A | −0.164 | |
| AB | −0.124 | |
| B | 0.000 | |
| Donor weight | 0.327 | 10 |
| Candidate cigarette use | 11 | |
| Yes | −0.005 | |
| No | 0.000 | |
| Unknown | 0.312 | |
| Candidate weight | 0.293 | 12 |
| Candidate height | −0.290 | 13 |
| Candidate/Donor weight mismatch | −0.275 | 14 |
| Donor height | 0.260 | 15 |
LV: left ventricular, EF: ejection fraction, BMI: body mass index
The conditional inference tree analysis indicated that donor age and LVEF were the primary determinants of offer acceptance, followed by the length of time that the candidate was waiting, candidate blood type, and donor height (Supplemental Figure 1).
Organ acceptance
The donor characteristics that were most predictive of donor heart organ acceptance were, by ranked order: age, LVEF, height, cause of death, PHS high risk designation, hypertension, blood type, cigarette use, race, BUN, history of myocardial infarction, diabetes, sex, and BMI. Standardized coefficients and rankings for the 15 most predictive donor characteristics are shown in Table 3B and for all donor characteristics can be seen in Supplemental Table 2B. These results show that donor hearts were more likely to be accepted if the donor’s LVEF was >50%, if the cause of death was head trauma, or if the donor was blood type O, and were less likely to be accepted if the donor was older, had blood type AB, or was Public Health Service (PHS) high risk, for example.
Table 3B:
Top 15 characteristics most predictive of organ acceptance
| Characteristic | Standardized Coefficient | Rank (importance) |
|---|---|---|
| Donor age | −0.710 | 1 |
| Donor LV EF | 2 | |
| > 50% | 0.588 | |
| ≤50% | 0.000 | |
| Unknown | −0.108 | |
| Donor height | 0.262 | 3 |
| Donor cause of death | 4 | |
| Head trauma | 0.179 | |
| Anoxia | −0.018 | |
| Other | −0.006 | |
| Cerebrovascular/stroke | 0.000 | |
| CNS tumor | 0.005 | |
| Donor PHS risk factors | 5 | |
| Yes | −0.166 | |
| No | 0.000 | |
| Unknown | 0.000 | |
| Donor hypertension | 6 | |
| Yes | −0.166 | |
| No | 0.010 | |
| Unknown | 0.000 | |
| Donor blood type | 7 | |
| O | 0.139 | |
| A | 0.000 | |
| AB | −0.161 | |
| B | −0.071 | |
| Donor history of cigarette use | 8 | |
| Yes | −0.103 | |
| No | 0.022 | |
| Unknown | 0.000 | |
| Donor race | 9 | |
| Multiracial | −0.087 | |
| White | 0.012 | |
| Native Hawaiian/Pacific Islander | −0.024 | |
| Asian | −0.014 | |
| Native American/Alaska Native | −0.009 | |
| Black | −0.010 | |
| Hispanic | 0.000 | |
| Donor BUN | −0.071 | 10 |
| Donor history of MI | 11 | |
| Yes | −0.003 | |
| No | 0.066 | |
| Unknown | 0.000 | |
| Donor history of diabetes | 12 | |
| Yes, > 10 years | −0.065 | |
| Yes, 6-10 years | −0.057 | |
| Yes, 0-5 years | 0.000 | |
| Yes, unknown years | −0.029 | |
| Unknown | 0.030 | |
| No | 0.061 | |
| Donor sex | 13 | |
| Male | 0.062 | |
| Female | −0.044 | |
| Donor BMI | 0.054 | 14 |
| Donor total bilirubin | −0.033 | 15 |
LV: left ventricular, EF: ejection fraction, MI: myocardial infarction, CNS: central nervous system; BUN: blood urea nitrogen, PHS: public health service; BMI: body mass index
The conditional inference tree analysis (Supplemental Figure 2) indicated that donor age was again the primary determinant of organ acceptance, followed by donor LVEF and donor height. Donor cause of death and history of hypertension were important, but lesser factors, in organ acceptance decisions.
Organ sequence number
The median organ sequence number of hearts accepted for transplantation was 3 (IQR 1-10). Figure 4 illustrates the variability in median sequence numbers across the US, by transplant centers’ donation service areas. The characteristics that were most predictive of the number of offers before acceptance of a donor heart were, in order of importance: recipient days waiting, recipient days as status 2, transplant center volume, donor blood type, ischemic time, recipient age, donor age, recipient blood type, donor height, donor BMI, donor cause of death, and donor race. Standardized coefficients and rankings for the 15 most predictive characteristics are shown in Table 3C, and for all characteristics can be seen in Supplemental Table 2C. Negative coefficients indicate that fewer offers were made prior to heart acceptance (lower sequence number), while positive coefficients indicate that more offers were made prior to acceptance (higher sequence number). These results indicate that high volume transplant centers had higher sequence numbers. This is most likely due to the fact that high volume centers were more likely to accept hearts that were turned down by other centers; however, there may be other reasons for this finding, including the possibility that high volume centers were turning down offers for the first candidate on their waitlist in favor of candidates who were lower down on their list. Blood type O donors also had higher sequence numbers (hearts were likely to be accepted after more offers), while sequence numbers were lower (hearts were likely to be accepted after fewer offers) if the candidate had been waiting longer, if the candidate had blood type AB, or if the donor was taller.
Figure 4:
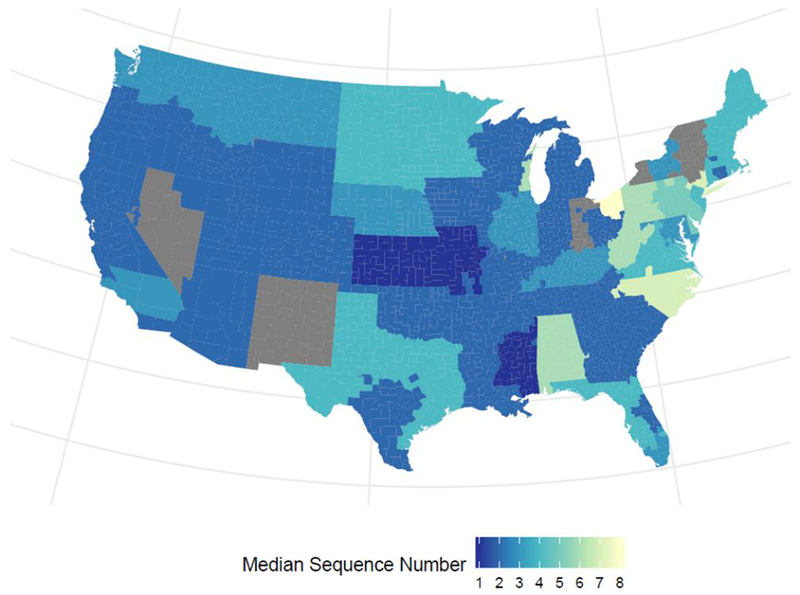
Median organ sequence number, by transplant centers’ donation service area. Grey areas indicate areas excluded due to lack of data (see Detailed Methods in the Supplemental Material)
Table 3C:
Top 15 characteristics most predictive of organ sequence number at acceptance
| Characteristic | Standardized Coefficient | Rank (importance) |
|---|---|---|
| Recipient days waiting | −0.329 | 1 |
| Recipient days on status 2 | 0.316 | 2 |
| Center volume | 3 | |
| Very low | 0.000 | |
| Low | −0.044 | |
| Medium | −0.002 | |
| High | 0.316 | |
| Donor blood type | 4 | |
| O | 0.208 | |
| AB | −0.031 | |
| A | 0.000 | |
| B | 0.000 | |
| Cold ischemic time | 5 | |
| ≥4 hours | 0.182 | |
| < 4 hours | −0.131 | |
| Unknown | 0.000 | |
| Recipient age at listing | 0.175 | 6 |
| Donor age | 0.158 | 7 |
| Recipient blood type | 8 | |
| O | 0.000 | |
| AB | −0.155 | |
| A | 0.000 | |
| B | −0.104 | |
| Donor height | −0.137 | 9 |
| Donor BMI | 0.128 | 10 |
| Donor cause of death | 11 | |
| Head trauma | −0.127 | |
| CNS tumor | 0.045 | |
| Anoxia | 0.022 | |
| Cerebrovascular/stroke | 0.000 | |
| Other | 0.000 | |
| Donor race | 12 | |
| Hispanic | −0.117 | |
| Asian | −0.030 | |
| White | 0.000 | |
| Native Hawaiian/Pacific Islander | −0.010 | |
| Black | 0.000 | |
| Multiracial | −0.010 | |
| Native American/Alaska Native | −0.013 | |
| Recipient hypertension | 13 | |
| Yes | 0.000 | |
| No | 0.000 | |
| Unknown | −0.113 | |
| Recipient and Donor identical blood type | 14 | |
| Yes | −0.084 | |
| No | 0.070 | |
| Donor cocaine use | 15 | |
| Yes | 0.082 | |
| No | 0.000 | |
| Unknown | 0.000 | |
BMI: body mass index; CNS: central nervous system
The conditional inference tree analysis (Supplemental Figure 3) indicated that transplant center volume was the primary determinant of the organ sequence number. Ischemic time, donor age, and donor cause of death were also important contributing factors.
Variation across transplant centers
The donor and candidate characteristics that varied most among transplant centers with respect to offer acceptance, organ acceptance, and organ sequence number are summarized in Supplemental Figures 4–6.
Among the characteristics that were most predictive of offer acceptance, organ acceptance, and organ sequence number, transplant centers varied the most in how they weighed donor age and height in the decision process (Figure 5). Those characteristics that were most predictive of organ acceptance and organ sequence number but were inconsistently treated across centers were donor race and donor cause of death. Candidate characteristics highly predictive of offer acceptance and organ sequence number but inconsistently treated across centers were days waiting as status 2, while for offer acceptance the greatest inconsistency across centers was related to candidate days on the waiting list.
Figure 5:
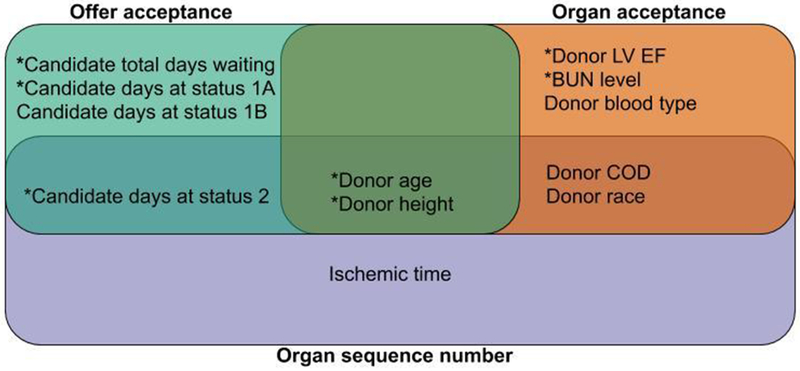
Donor and candidate characteristics that demonstrated the greatest inconsistency between transplant centers, by offer acceptance, organ acceptance, and organ sequence number. Transplant centers were most inconsistent in how they weighed donor age and height in the decision process. Characteristics that were not predictive of recipient post-transplant mortality are highlighted with an asterix (*) and represent key characteristics that can be targeted to safely increase donor heart utilization.
BUN: blood urea nitrogen, LV: left ventricular, EF: ejection fraction, COD; cause of death
Mortality
A total of 15,042 recipients were included in the 30 day mortality analysis and 12,979 recipients were included in the 1 year mortality analysis. In total, these transplants occurred across 145 centers. Two hundred two recipients died and 31 were re-transplanted (1.5%) within 30 days, while 1,862 died and 37 were re-transplanted (14.6%) within one year.
Tables 3D and 3E list the 15 donor and candidate characteristics most predictive of mortality or re-transplantation within 30 days and one year, respectively, after adjusting for access to transplantation. The standardized coefficients and rankings for all characteristics are provided in Supplemental Tables 3A and 3B. Note that positive coefficients indicate an increased risk of mortality.
Table 3D:
Top 15 characteristics most predictive of mortality or re-transplantation within 30 days
| Characteristic | Standardized Coefficient | Rank (importance) |
|---|---|---|
| Cold ischemic time | 1 | |
| < 4 hours | −0.066 | |
| ≥4 hours | 0.000 | |
| Unknown | 0.160 | |
| Recipient height | −0.127 | 2 |
| Recipient cigarette use | 3 | |
| Yes | 0.000 | |
| No | 0.000 | |
| Unknown | 0.088 | |
| Recipient prior cardiac surgery | 4 | |
| Yes | 0.082 | |
| No | −0.030 | |
| Unknown | 0.000 | |
| Donor cocaine use | 5 | |
| Yes | 0.074 | |
| No | 0.000 | |
| Unknown | −0.036 | |
| Donor PHS high risk | 6 | |
| Yes | 0.029 | |
| No | −0.049 | |
| Unknown | 0.000 | |
| Recipient race | 7 | |
| Hispanic | 0.000 | |
| Asian | 0.047 | |
| White | 0.000 | |
| Native Hawaiian/Pacific Islander | 0.000 | |
| Black | 0.000 | |
| Multiracial | 0.044 | |
| Native American/Alaska Native | 0.000 | |
| Recipient diabetes | 8 | |
| Yes, > 10 years | 0.000 | |
| Yes, 6-10 years | 0.000 | |
| Yes, 0-5 years | 0.000 | |
| Yes, unknown years | 0.000 | |
| No | 0.000 | |
| Unknown | 0.033 | |
| Donor last serum sodium | −0.029 | 9 |
| Donor hypertension | 10 | |
| Yes | 0.000 | |
| No | −0.028 | |
| Unknown | 0.013 | |
| Donor blood type | 11 | |
| O | 0.000 | |
| AB | 0.027 | |
| A | 0.000 | |
| B | 0.000 | |
| Recipient weight | −0.023 | 12 |
| Center volume | 13 | |
| Very low | 0.000 | |
| Low | 0.000 | |
| Medium | 0.000 | |
| High | −0.019 | |
| Recipient hypertension | 14 | |
| Yes | 0.000 | 15 |
| No | −0.013 | |
| Unknown | 0.000 |
PHS: public health service
Table 3E:
Top 15 characteristics most predictive of mortality or re-transplantation within 1 year
| Characteristic | Standardized Coefficient | Rank (importance) |
|---|---|---|
| Recipient days on status 1B | 0.043 | 1 |
| Donor cocaine use | 2 | |
| Yes | 0.000 | |
| No | 0.000 | |
| Unknown | −0.036 | |
| Donor hypertension | 3 | |
| Yes | 0.000 | |
| No | −0.032 | |
| Unknown | 0.001 | |
| Donor race | 4 | |
| Hispanic | 0.000 | |
| Asian | 0.000 | |
| White | 0.000 | |
| Native Hawaiian/Pacific Islander | −0.010 | |
| Black | 0.028 | |
| Multiracial | 0.000 | |
| Native American/Alaska Native | 0.000 | |
| Donor diabetes | 5 | |
| Yes, > 10 years | −0.027 | |
| Yes, 6-10 years | 0.000 | |
| Yes, 0-5 years | 0.010 | |
| Yes, un known years | 0.000 | |
| No | 0.000 | |
| Unknown | 0.000 | |
| Recipient diabetes | 6 | |
| Yes, > 10 years | 0.000 | |
| Yes, 6-10 years | 0.025 | |
| Yes, 0-5 years | −0.022 | |
| Yes, unknown years | 0.000 | |
| No | 0.000 | |
| Unknown | 0.000 | |
| Center volume | 7 | |
| Very low | 0.000 | |
| Low | 0.000 | |
| Medium | 0.022 | |
| High | 0.000 | |
| Recipient sex | 8 | |
| Male | −0.021 | |
| Female | 0.020 | |
| Donor cause of death | 9 | |
| Head trauma | 0.000 | |
| CNS tumor | 0.000 | |
| Anoxia | 0.013 | |
| Cerebrovascular/stroke | −0.004 | |
| Other | 0.021 | |
| Recipient cigarette use | 10 | |
| Yes | 0.000 | |
| No | 0.008 | |
| Unknown | −0.013 | |
| Center access rate | 0.013 | 11 |
| Recipient hypertension | 12 | |
| Yes | 0.013 | |
| No | 0.000 | |
| Unknown | 0.000 | |
| Cold ischemic time | 13 | |
| < 4 hours | 0.000 | |
| ≥4 hours | 0.013 | |
| Unknown | −0.006 | |
| Recipient race | 14 | |
| Hispanic | 0.000 | |
| Asian | 0.000 | |
| White | 0.000 | |
| Native Hawaiian/Pacific Islander | 0.000 | |
| Black | 0.000 | |
| Multiracial | 0.011 | |
| Native American/Alaska Native | 0.000 | |
| Donor creatinine | −0.009 | 15 |
CNS: central nervous system
Transplant center donor heart acceptance practices and recipient mortality
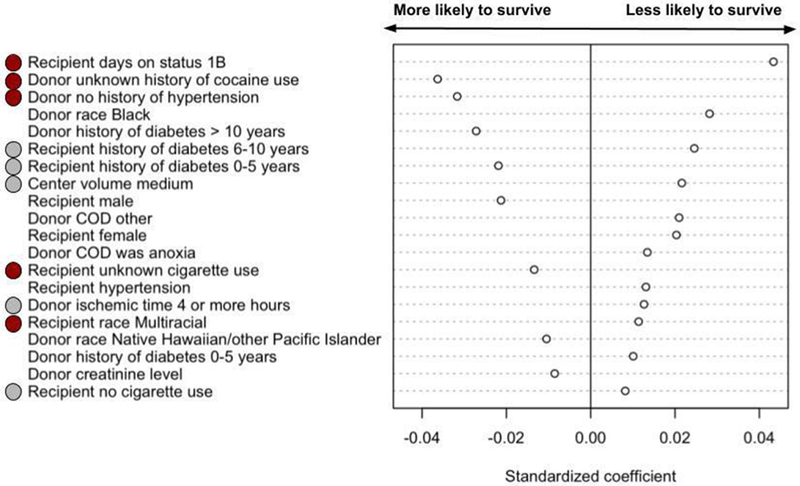
Factors predictive of both 30 day and 1 year mortality, after adjusting for access to transplantation, are shown in Figures 6A and 6B. The characteristics that were most predictive of both 30-day and 1-year mortality or retransplantation were ischemic time, recipient cigarette use, donor cocaine use, recipient race, recipient diabetes, donor hypertension, transplant center volume, and recipient hypertension.
Figure 6:
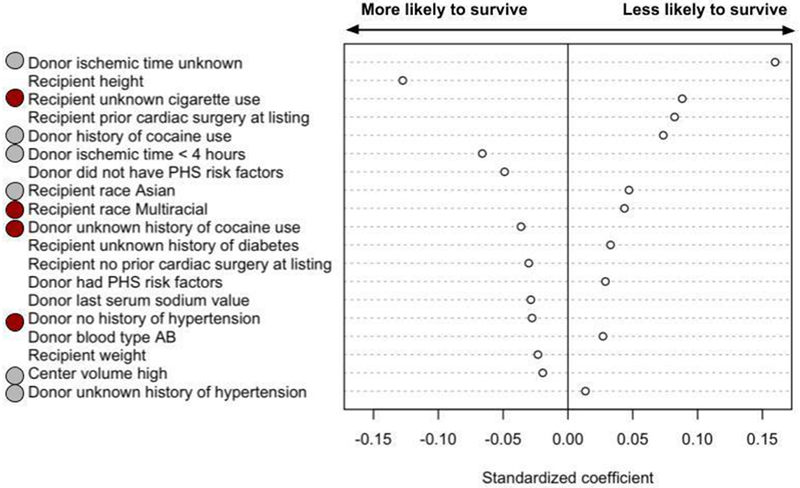
A: Donor and recipient characteristics most predictive of mortality or re-transplantation within 30 days. Red circles indicate that the characteristic (e.g. race) and the category (e.g. multiracial) were predictive of both 30-day and 1-year mortality or re-transplantation. Grey circles indicate that the characteristic is predictive of both 30-day and 1-year mortality or re-transplantation, but not the category (for example, transplant center volume is associated with both 30-day and 1-year mortality or re-transplantation, but high volume is associated with 30-day mortality and medium volume is associated with 1-year mortality).
B: Donor and recipient characteristics most predictive of mortality or re-transplantation within 1 year. Red circles indicate that the characteristic (e.g. race) and the category (e.g. multiracial) were predictive of both 30-day and 1-year mortality or re-transplantation. Grey circles indicate that the characteristic is predictive of both 30-day and 1-year mortality or re-transplantation, but not the category (for example, transplant center volume is associated with both 30-day and 1-year mortality or re-transplantation, but high volume is associated with 30-day mortality and medium volume is associated with 1-year mortality).
The characteristics that were most predictive of recipient death or retransplant within 30 days but did not predict one year mortality were recipient height, recipient prior cardiac surgery, donor PHS high risk designation, donor last serum sodium value, donor blood type, and recipient weight. The strength and direction of association are shown in Figure 6A. Thirty six percent of patients who died or were retransplanted within 30 days were from low volume centers, compared to 26% who were from high volume centers.
Characteristics most predictive of death or retransplant within 1 year, but were not predictive of mortality or retransplantation within 30 days, were recipient days at Status 1B, donor race, donor diabetes, recipient sex, donor cause of death, donor creatinine, and recipient hypertension. The strength and direction of association are shown in Figure 6B. Notably, the difference in mortality by center volume was not explained by transplant access rates.
Transplant centers demonstrated great variability with regards to the following candidate and donor characteristics that were highly predictive of recipient post-transplant mortality: candidate days at status 1B, ischemic time, donor blood type, donor cause of death, and donor race.
Transplant center practices were also highly variable with respect to other characteristics that were not predictive of recipient post-transplant mortality: candidate total days spent waiting, candidate days at status 1A or status 2; and donor age, height, BUN, and LVEF. These final results identify key opportunities to safely expand acceptance of available donor hearts (Figure 5).
DISCUSSION
Much of the recent discussion surrounding the processes by which hearts are distributed for transplantation in the US has focused on allocation policies that determine the order in which offers are made to candidates on the waiting list. Indeed, the US heart allocation system was extensively reviewed and a revised allocation system was implemented in October 2018.(20, 21) Comparatively little attention, however, has been paid to assessing whether donor heart offers are accepted in an efficient manner that best serves the needs of the growing number of patients on the waiting list. Low organ and offer acceptance leads to inefficiency, longer waiting times (and subsequent higher candidate mortality), and under-utilization of available organs. As shown previously by Wey et. al, donor heart offer acceptance practices contribute significantly to program-level variability in the probability of waitlist mortality.(22)
Various reasons for low heart acceptance rates have been proposed, including expectations of poor post-transplant survival based upon perceived donor risk factors, anticipated interactions between donor and recipient characteristics, and a reluctance to accept a “high risk” donor heart for a clinically stable recipient or a recipient with uncomplicated, durable mechanical circulatory support. A single-center effort to liberalize donor heart acceptance criteria at the University of Washington, however, led to an increase in use of available donor hearts within the region from 46% to 75%, with an accompanying decrease in waitlist mortality from 17% to 12%, while maintaining 90% recipient 1-year survival.(23)
Although the effects of individual donor and candidate characteristics on recipient post-transplant survival have been well studied, we sought to advance the field by analyzing the complex relationship between donor and candidate characteristics in combination, to better understand which characteristics are viewed inconsistently across centers during donor heart acceptance decisions. In other words, we hypothesized that variation in acceptance practices, beyond that which can be explained by isolated donor and recipient characteristics, exists among transplant centers. We hope that our efforts will help standardize (and thereby improve) donor heart acceptance.
The likelihood of donor heart acceptance varied greatly among transplant centers and across the US. This variation in sequence numbers (as shown in Figure 4) reflects differential organ availability across regions of the country, differential willingness to accept organs that are perceived to be “high risk”, and differential transplant center waiting list sizes, clinical practices, and candidate characteristics.
We found great variation between transplant centers when examining their likelihood of accepting a donor heart, according to donor and candidate characteristics. As shown in Table 3A, the length of time that a candidate spent as status 1B varied the most across centers for offer acceptance. This observation may be related to the extended waiting times for stable patients bridged with left ventricular assist device support—a practice that is often center-specific.(24, 25) Donor age, on the other hand, was highly variable between centers when examining organ acceptance, reflecting great variability in centers’ willingness to accept older donor hearts. Finally, candidate waiting time, transplant center volume, and ischemic time varied the most across centers in terms of organ sequence number. This finding suggests that there is great variability in centers’ willingness to accept less desirable (e.g. higher sequence number) donor hearts for critically ill candidates, especially those with prolonged waiting times.
It is important to recognize that acceptance decisions are made by evaluating donor and candidate characteristics in combination. This decision-making process is illustrated by the results of the decision tree analyses presented in Supplemental Figures 1–3.
The great heterogeneity identified in transplant centers’ acceptance practices should help to focus future research efforts. Studies focused on the optimal timing for transplantation, and the trade-offs between donor risk and candidate waiting time should inform these decisions. Similarly, rigorous studies focused on defining acceptable donor and recipient age and size combinations are required, as donor age, height, and BMI were variable across centers with respect to offer acceptance, organ acceptance, and organ sequence number. Finally, transplant centers continue to demonstrate variability in their willingness to accept donor hearts with LV dysfunction (EF≤50%), despite several recent studies showing that recipients of carefully-selected donors with LV dysfunction have excellent post-transplant outcomes.(26, 27)
A key finding of this study is that acceptance of hearts for transplant based on donor age, BMI, BUN, and LVEF varied greatly between centers, and yet was not predictive of recipient post-transplant mortality. This finding thereby identifies opportunities for centers to safely liberalize their donor heart acceptance practices in order to increase access to transplantation for candidates on their waiting lists.
In summary, this study demonstrated that transplant centers across the US vary greatly with respect to donor heart acceptance practices. Currently, regulatory efforts in transplantation focus on monitoring post-transplant survival, such that centers with lower-than-expected survival may be placed on probation or otherwise penalized. Another approach could be to flag centers with low donor heart acceptance rates. Such data are now available via the SRTR online program-specific reports. Indeed, we observed great variability between centers in access rates for heart transplantation.
This study has several limitations. Our analyses were limited by the availability and quality of data submitted by OPOs and transplant centers to UNOS. While some variables (such as mortality) are mandatory, other variables had missing values and/or quality may have been affected by accuracy of entry by OPO and transplant center personnel. As is seen in the results, “unknown” characteristics were often strongly associated with the outcomes of interest. We cannot determine whether these features were truly unknown, were simply not entered, or if the characteristic was not present. We chose to keep these “unknown” characteristics in order to present our results in the most accurate way possible. As with any study that relies on “turndown” codes for donor heart declines, we relied on the single code entered into DonorNet. In reality, however, the decision to decline a heart is often based on a combination of donor and candidate factors. Moreover, there are unmeasured variables that an experienced clinician incorporates into the evaluation of a potential donor that may not be captured. Certainly, organ acceptance may be influenced by variability in organ donation and recovery, population density, and the number of OPOs and transplant centers within a given region. Organ acceptance may also be influenced by the particular transplant center personnel evaluating the offer, and whether centers use standardized criteria and decision-making tools to guide offer acceptance. In addition, organ offers are based upon the donor inclusion/exclusion criteria listed by individual transplant centers. While some centers may list very few exclusions, other centers may be more selective and may therefore receive fewer offers. Organ selection may also be influenced by the practices of the local OPO. Transplant centers served by under-performing OPOs may be more likely to accept “marginal” donor organs due to fewer offers. Conversely, transplant centers served by high-performing OPOs may decline donor hearts with elevated perceived risk, due to their expectation of receiving a “better” offer. In addition, there may have been small changes in donor heart acceptance practices after December 2015 that are not captured in this data set.
Finally, a new donor heart allocation system was introduced in the United States in October 2018, which changed the 3-status candidate prioritization system to a more granular 6-status system. The impact of the new allocation system cannot be wholly known from this current analysis; however, we can still conclude that, all other factors remaining the same, the results regarding Status 1A roughly translate to the new Statuses 1-3, the results regarding Status 1B roughly translate to the new Status 4, and the findings related to Status 2 roughly translate to the new Statuses 5-6. While it remains to be seen the extent to which our findings related to Status 1A, 1B, and 2 map to the new allocation system, our central findings about donor heart selection practices and strategies to safely expand donor heart utilization remain unchanged. Despite these limitations, we present the results of rigorous analyses involving a very large sample size in the contemporary transplant era.
CONCLUSIONS
Low donor heart acceptance rates continue to result in allocation inefficiency, inequity in access to transplantation, and the discard of potentially suitable organs in the US. We demonstrated great variability in donor heart acceptance decisions among transplant centers. We identified donor and recipient characteristics associated with offer and organ acceptance, and identified inconsistencies among centers with respect to how these characteristics influenced acceptance decisions. Finally, we demonstrated that several key risk factors that were highly predictive of donor heart non-use (and variability in acceptance across centers) were not associated with increased recipient mortality. These findings may help focus efforts on increasing use of available donor hearts for transplantation.
Supplementary Material
ACKNOWLEDGMENTS
Kiran Khush is the Principal Investigator of an NIH-sponsored study: “Evidence Based Evaluation and Acceptance of Donor Hearts for Transplantation”, grant R01HL125303. This work was supported in part by Health Resources and Services Administration contract 234-2005-370011C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. There are no relationships with industry.
Abbreviations:
- BMI
body mass index
- BUN
blood urea nitrogen
- LV
left ventricular
- LVEF
left ventricular ejection fraction
- MAD
median absolute deviation
- OPO
organ procurement organization
- OPTN
organ procurement and transplantation network
- PHS
public health service
- PTR
potential transplant recipient
- UNOS
United Network for Organ Sharing
- US
United States
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Khush KK, Zaroff JG, Nguyen J et al. National decline in donor heart utilization with regional variability: 1995-2010. Am J Transplant 2015;15(3):642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colvin M, Smith JM, Hadley N et al. OPTN/SRTR 2017 Annual Data Report: Heart. Am J Transplant 2019;19 Suppl 2:323–403. [DOI] [PubMed] [Google Scholar]
- 3.Kobashigawa J, Khush K, Colvin M et al. Report From the American Society of Transplantation Conference on Donor Heart Selection in Adult Cardiac Transplantation in the United States. Am J Transplant 2017;17(10):2559–2566. [DOI] [PubMed] [Google Scholar]
- 4.Aliabadi-Zuckermann AZ, Gokler J, Kaider A et al. Donor heart selection and outcomes: An analysis of over 2,000 cases. J Heart Lung Transplant 2018. [DOI] [PubMed] [Google Scholar]
- 5.Chen C, Atluri P. Expanded donor selection criteria can increase organ utilization. J Heart Lung Transplant 2018;37(3):427. [DOI] [PubMed] [Google Scholar]
- 6.Khush KK, Menza R, Nguyen J et al. Donor predictors of allograft use and recipient outcomes after heart transplantation. Circ Heart Fail 2013;6(2):300–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trivedi JR, Cheng A, Gallo M et al. Predictors of Donor Heart Utilization for Transplantation in United States. Ann Thorac Surg 2017;103(6):1900–1906. [DOI] [PubMed] [Google Scholar]
- 8.Boyle A, Colvin-Adams M. Recipient selection and management. Semin Thorac Cardiovasc Surg 2004;16(4):358–363. [DOI] [PubMed] [Google Scholar]
- 9.Costanzo MR, Dipchand A, Starling R et al. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J Heart Lung Transplant 2010;29(8):914–956. [DOI] [PubMed] [Google Scholar]
- 10.Quader M, Wolfe L, Katlaps G et al. Donor Heart Utilization following Cardiopulmonary Arrest and Resuscitation: Influence of Donor Characteristics and Wait Times in Transplant Regions. J Transplant 2014;2014:519401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solomon NA, McGiven JR, Alison PM et al. Changing donor and recipient demographics in a heart transplantation program: influence on early outcome. Ann Thorac Surg 2004;77(6):2096–2102. [DOI] [PubMed] [Google Scholar]
- 12.Shuhaiber JH, Moore J, Dyke DB. The effect of transplant center volume on survival after heart transplantation: a multicenter study. J Thorac Cardiovasc Surg 2010;139(4):1064–1069. [DOI] [PubMed] [Google Scholar]
- 13.Zou H, Hastie T. Regularization and variable selection via the elastic net. Journal of the Royal Statistical Society: Series B 2005;67(2):301–320. [Google Scholar]
- 14.Tibshirani R Regression shrinkage and selection via the lasso. Journal of the Royal Statistical Society: Series B 1996:267–288. [Google Scholar]
- 15.Team RC. R: A language and environment for statistical computing. Vienna, Austria, 2017. [Google Scholar]
- 16.Friedman J, Hastie T, Tibshirani R. Regularization Paths for Generalized Linear Models via Coordinate Descent. Journal of Statistical Software 2010;33(1):1–22. [PMC free article] [PubMed] [Google Scholar]
- 17.Hothorn T, Hornik K, Zeileis A. Unbiased Recursive Partitioning: A Conditional Inference Framework. Journal of Computational and Graphical Statistics 2006;15(3):651–674. [Google Scholar]
- 18.Hothorn T, Zeileis A. partykit: A Modular Toolkit for Recursive Partitioning in R. Journal of Machine Learning Research 2015;16:3905–3909. [Google Scholar]
- 19.Hoerl A, Kennard R. Ridge regression In. Encyclopedia of Statistical Sciences. New York: Wiley, 1998: 129–136. [Google Scholar]
- 20.Kobashigawa J, Teuteberg J, Colvin M et al. Proceedings of the AST heart allocation meeting at the American Transplant Congress, Philadelphia, Pennsylvania, May 4, 2015. Clin Transplant 2016;30(5):641–648. [DOI] [PubMed] [Google Scholar]
- 21.Meyer DM, Rogers JG, Edwards LB et al. The future direction of the adult heart allocation system in the United States. Am J Transplant 2015;15(1):44–54. [DOI] [PubMed] [Google Scholar]
- 22.Wey A, Valapour M, Skeans MA et al. Heart and lung organ offer acceptance practices of transplant programs are associated with waitlist mortality and organ yield. Am J Transplant 2018;18(8):2061–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith JW, O’Brien KD, Dardas T et al. Systematic donor selection review process improves cardiac transplant volumes and outcomes. J Thorac Cardiovasc Surg 2016;151(1):238–243. [DOI] [PubMed] [Google Scholar]
- 24.Smedira NG, Hoercher KJ, Yoon DY et al. Bridge to transplant experience: factors influencing survival to and after cardiac transplant. J Thorac Cardiovasc Surg 2010;139(5):1295–1305, 1305, e1291–1294. [DOI] [PubMed] [Google Scholar]
- 25.Steffen RJ, Blackstone EH, Smedira NG et al. Optimal Timing of Heart Transplant After HeartMate II Left Ventricular Assist Device Implantation. Ann Thorac Surg 2017;104(5):1569–1576. [DOI] [PubMed] [Google Scholar]
- 26.Madan S, Saeed O, Vlismas P et al. Outcomes After Transplantation of Donor Hearts With Improving Left Ventricular Systolic Dysfunction. J Am Coll Cardiol 2017;70(10):1248–1258. [DOI] [PubMed] [Google Scholar]
- 27.Zaroff JG, Babcock WD, Shiboski SC et al. Temporal changes in left ventricular systolic function in heart donors: results of serial echocardiography. J Heart Lung Transplant 2003;22(4):383–388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


