Abstract
Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN) are potentially life-threatening, immune-mediated adverse reactions characterized by widespread erythema, epidermal necrosis, and detachment of skin and mucosa. Efforts to grow and develop functional international collaborations and a multidisciplinary interactive network focusing on SJS/TEN as an uncommon but high burden disease will be necessary to improve efforts in prevention, early diagnosis and improved acute and long-term management. SJS/TEN 2019: From Science to Translation was a 1.5-day scientific program held April 26–27, 2019, in Vancouver, Canada. The meeting successfully engaged clinicians, researchers, and patients and conducted many productive discussions on research and patient care needs.
Keywords: Stevens-Johnson syndrome (SJS), Toxic epidermal necrolysis (TEN), Human leukocyte antigen (HLA), Pharmacogenomics, Pharmacovigilance, Severe cutaneous adverse reaction (SCAR)
INTRODUCTION
Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN) are severe, life-threatening, and mainly drug-induced cutaneous adverse reactions, causing blistering, mucosal sloughing and epidermal necrosis. The global clinical and financial burden of SJS/TEN is considerable, resulting in prolonged hospital stays, mortality of up to 50% in the elderly and considerable long-term multi-system physical and mental health morbidity that is still poorly understood qualitatively and quantitatively [1]. The last 15 years have seen significant advancements in our understanding of the immunopathogenesis and genetic risk factors for SJS/TEN that have fueled preventive efforts leading to successful pre-prescription screening programs in some countries [2–5]. Research progress requires a collective effort to advance and translate science into prediction, prevention, earlier diagnosis and more targeted and effective treatments that will lead to improved short and long-term patient outcomes.
The SJS/TEN 2019 meeting built upon the outcomes and success of a 2015 workshop [6] and the inaugural SJS/TEN 2017 meeting [1] by further expanding the multidisciplinary engagement and communication between Networks and participants. Cutting-edge research and treatment presentations, interactive discussions, and breakout sessions were featured to present the recent advances and provide a global context of SJS/TEN. This article is a summary of the proceedings of the conference that brought together healthcare providers, researchers, regulators, government agencies and funders, as well as patients and families in a 1.5-day networking meeting to define strategies for multidisciplinary collaboration to address critical research gaps and improve SJS/TEN outcomes.
Pharmacogenomic Network and Panel Discussion
An opening plenary from Neil Shear provided a thoughtful perspective on the past, present, and future of the SJS/TEN research. Dr. Shear emphasized the “just do” aspect of implementation science necessary to move research and translation forward and the critical nature of teamwork in building global research networks. A pharmacogenomics panel was made up of diverse stakeholders from six different countries. The panel covered a wide range of topics but highlighted that regulations aimed at facilitating the routine clinical use of pharmacogenomics should follow evidence-based science and that diverse groups should be involved in these decisions. It was also mentioned that health economic and social science studies are increasingly important, as well as improving pharmacogenomic decision support systems and turnaround times. Finally, these systems should be dynamic, to allow for the inclusion of new biomarkers as they are discovered and replicated, and that the community should explore the repurposing of disease-related genomic data for pharmacogenomic applications.
Unmet need: To build a global research network and develop consensus on the implementation of pharmacogenomic testing in order to improve the prevention and treatment of SJS/TEN.
Regional Networks and Registries
Nine representatives from regional or international networks and registries focused on studies associated with severe immune-mediated adverse reactions and shared recent progress in the field of SJS/TEN (Figure 1). These leading groups have committed time and energy to establishing strong networks to facilitate prospective studies of genetic and mechanistic basis and provide an evidence base for treatment approaches. This has included the implementation of post-marketing safety surveillance systems and patient health information paired with the development of biological banks to store DNA, RNA, PBMCs and tissue samples. The risk of SJS/TEN has significant racial/ethnic disparities across drugs used and risk. Currently, Asians have been reported as a significantly affected population where much progress has been made on the discovery of etiologic genetic markers; however, the burden of TB-HIV co-infection in the African continent is high and the incidence of SJS/TEN and associated genetic risk factors have not been adequately studied.
Figure 1. SJS/TEN 2019: From Science to Translation Conference Participants.
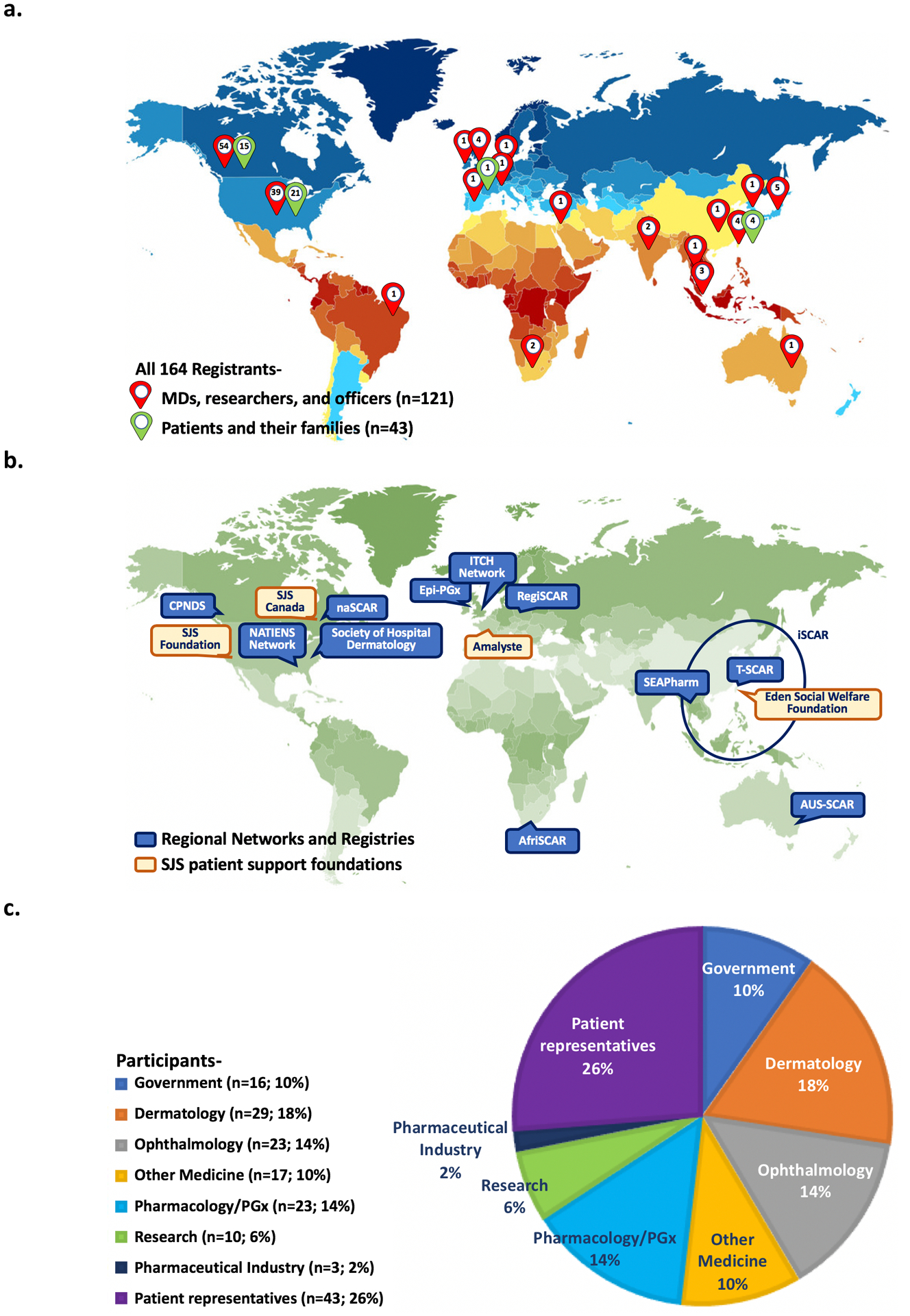
The SJS/TEN 2019: From Science to Translation conference was organized by the three co-chairs of the congress: Drs. Elizabeth J. Phillips (Vanderbilt University Medical Center), Bruce C. Carleton (University of British Columbia), and Wen-Hung Chung (Chang Gung University). a. Global distribution of participants. A total of 164 participants, representing 19 countries across six continents, engaged in this meeting, which took place at the British Columbia Children’s Hospital Research Institute in Vancouver, Canada. b. Regional Networks and Registries and SJS patient support foundations. This was of special significance because it was the largest SJS/TEN event that gathered together 16 government representatives, as well as 12 regional SCAR networks and registries from countries in North America, Europe, Asia, Africa, and Australia. Forty-three local and international SJS/TEN survivors, their families, and local community advocacy groups also attended. Six representatives from government drug regulatory and research funding agencies in the United States and Canada provided updates on regulatory science and funding opportunities related to SCAR and drug safety. c. Each sector shows the percentage of each group of participants. Participants comprised 43 (27%) patient participants, 29 (18%) dermatologists, 23 (14%) ophthalmologists, 23 (14%) experts in pharmacogenomics or clinical pharmacology, 17 (10%) other medical disciplines, 16 (10%) government officers, 10 (6%) basic science researchers, and 3 (2%) from the pharmaceutical industry.
Unmet need: Coordination of research networks to coordinate mechanistic, genetic and treatment studies across ethnically diverse populations.
Clinical Approaches and Management
The clinical approach to the management of SJS/TEN is multidisciplinary, including dermatologists, burn surgeons, ophthalmologists, gynecologists, pharmacologists, immunologists, psychiatrists, pharmacists, and other healthcare providers, involved in rehabilitation as indicated by the clinical case.
Diagnosis of SJS/TEN is critical to optimal management and subsequent outcomes analysis. Recent work has highlighted that up to 1/3 cases may be misdiagnosed, which emphasizes the importance of gaining histological confirmation from a skin biopsy at the outset of the rash [7]. The management of SJS/TEN should be undertaken in specialized centers with capabilities for complex skin care and appropriate intensive care for more severe cases, such as dermatology departments or burn units, which has been shown to improve outcomes [8]. Although stopping the culprit drug is associated with a better prognosis, every day of delay also worsens the outcomes [9]. However, identification of the causal drug can be challenging particularly acutely and currently relies mainly on expert judgment and clinical causality assessment. Further research is critical to develop better ways of “immunophenotyping” patients such as with novel validated biomarkers, immunoassays and genetic studies for acute identification of the causal drug.
Acute active management is controversial, and there is little consensus on medical interventions because of the lack of high-level evidence that any treatment (such as steroids and IVIg) is superior to supportive care alone. Newer treatments such as etanercept (TNF-α receptor antagonist) and cyclosporine (calcineurin inhibitor, immunosuppressant) have shown promise in a recent non-blinded randomized controlled study (etanercept) and several observational studies (cyclosporine and etanercept) [10,11]. Information on the management of children versus adults is also lacking given that a much higher proportion of cases in children are mediated by infectious and non-drug triggers. However, recent guidelines are useful for clinicians if such cases arise [12].
In management of the skin, there is consensus about the important need for non-adherent dressings and generous and frequent application of paraffin emollient. While some centers undertake debridement of blistered areas, others do not recommend this approach, and the issue remains a source of disagreement [13]. This would be a high priority area to address with future research, so that the field can develop a unified approach to skin care. Urogynecologic manifestations of SJS/TEN warrant further attention as evidenced by the fact that scarring and stenosis arise in 18–28% of cases [14,15]. All female patients of SJS/TEN should be seen by a gynecologist early where interventions including topical corticosteroid therapy, catheterization, and vaginal dilation may be considered. These patients also need to schedule follow-up appointments following discharge to ensure any vaginal adhesions and other complications that could lead to long-term reproductive morbidity are adequately managed.
Unmet need: To improve evidence-based approaches to the acute and chronic management of SJS/TEN (Table 1).
Table 1.
Summarized key points from breakout sessions
| Patients’ perspective- |
|---|
Diagnosis-
|
Acute Care Management-
|
Discharge-
|
| Clinicians’ perspective- |
|
| Pharmacogenomics experts’ and basic scientists’ perspective- |
|
Ocular Science
SJS/TEN is commonly accompanied by acute ocular disease, leading to chronic complications. Ophthalmology should be involved as early as possible and at the least there should be a bedside eye exam within 24–48 hours of disease onset and/or diagnosis. Long-term eye morbidity is prevalent even in the absence of defined acute disease and continued regular follow-up after discharge is recommended.
Acute ocular involvement presents with eyelid margin inflammation and hyperkeratosis, conjunctivitis with membranes/pseudomembranes, as well as corneal/conjunctival epithelial defects (which can progress to corneal melt, perforation, or infectious keratitis). Mild disease can be managed with topical corticosteroids, antibiotics, frequent administration of lubricants, and careful periodic removal of membrane/pseudomembranes. For more severe cases, urgent placement of amniotic membrane over the ocular surface within the first week can potentially avoid severe debilitating chronic complications [16].
Chronic manifestations include dry eye, eyelid margin keratinization, symblepharon and eyelid malposition, chronic conjunctivitis, limbal stem cell deficiency (LSCD), corneal thinning/melt, and infectious keratitis, resulting in discomfort, pain and potential vision loss. Treatment options include topical corticosteroids, lubricants, and antibiotics, specialized contact lenses for ocular surface protection and visual rehabilitation, eyelid malposition corrective surgery, and surgical procedures for LSCD. These include cultivated limbal epithelial cell transplantation (CLET), cultivated oral mucosal epithelial transplantation (COMET), and simple oral mucosal epithelial transplantation (SOMET). Boston type 1 and 2 keratoprosthesis may be helpful in the more severe cases [17]. Though sometimes necessary, outcomes following ocular surface surgeries are generally poor in SJS/TEN. As such, recent work has emphasized the importance of early intervention with amniotic membrane grafting [18]. This approach may be accomplished in a sutureless manner, thus avoiding the necessity to transfer patients to an ophthalmic theatre [19].
Finally, genetic risk factors may be associated with SJS/TEN with “severe ocular complications (SOC)” and this warrants further study in particular to prioritize patients for prognostication and follow-up. SOC has been linked to “cold-medicines” in some populations with HLA-B*44:03 (Japanese, Thai, Brazilian, and Indian) and HLA-A*02:06 (Japanese and Korean) [20]. However, it has been suggested that these HLA-alleles may rather reflect an infectious trigger than a heterogenic group of drugs that were initiated to treat the prodromal symptoms of SJS/TEN [21]. A network of susceptibility genes for SJS/TEN (TLR3, EP3, and IKZF1) may trigger the inflammation associated with SJS/TEN with SOC (Supplemental Figure S1)[22].
Unmet need: To further evidence-based approaches to understanding short- and long-term mechanisms of morbidity and the prevention and treatment of ocular disease associated with SJS/TEN (Table 1).
Updates in Global Regulatory Science, Pharmacovigilance, and Data Mining
Cases of SJS/TEN and SCAR can be identified from many sources, including post-marketing adverse event reporting systems, disease registries, electronic health records (EHRs), literature, observational studies, and clinical trials. Cross-sectional studies used EHR allergy lists to identify SCAR cases. Causative drugs, including some rarely implicated in SJS/TEN in the literature, and differences in patient demographics, were reported [23,24]. An English-language PubMed literature search from 1980–2017 yielded 851 cases categorized as “probable” or “definite” SJS/TEN cases, 80.6% of which were drug-induced (unpublished data).
In FDA regulatory actions involving labeling for SJS/TEN from 2016–2018, products with SJS/TEN labeled in Warnings/Precautions at initial approval or added post-market included 17 hematology/oncology products, 8 antimicrobials, 6 radiocontrast agents, deflazacort, and febuxostat. Post-market reports were the primary source of information for the labeling actions.
The EMA Pharmacovigilance Risk Assessment Committee (PRAC) monitors the Eudravigilance database (>50 million records) and uses disproportionality tools (electronic Reaction Monitoring Report, eRMR) to identify emerging signals. Since July 2012, PRAC has evaluated 21 drugs for SCAR risk [25]. From the Eudravigilance data, the fatality of SJS, SJS/TEN overlap and TEN is 7.4%, 12.1%, and 22.4%, respectively.
Performance and quality of pharmacogenetic tests assessing SJS/TEN drug-associated risks are also regulated by some health authorities; risks to patients with life-threatening diseases of treatment decisions based on erroneous testing are also considered. Health Canada’s evaluations are becoming more context-aware, placing more emphasis on patients’ needs, real-world evidence issues and collaborative health system models, which will inform evolutions in regulatory science and decisions about pharmacogenetic testing and patient safety.
An example of beneficial impact of regulatory action on reducing SJS/TEN is a “Dear Health Care Professional Letter” issued by the Singapore Health Sciences Authority and Ministry of Health in 2013, advising that genotyping for HLA-B*15:02 would be standard of care in Singapore before initiating carbamazepine (CBZ) in new patients of Southeast Asian ancestry. SCAR guides highlighted the importance of prompt withdrawal of drugs in suspected SCAR cases. Post-market reports of CBZ-induced SCAR cases subsequently decreased by >95% [26]. Usage of CBZ decreased modestly overall, though new CBZ users declined by 40%. Meanwhile, new users of levetiracetam increased 2.7-fold highlighting other factors that have contributed to the reduction in SJS/TEN in Singapore overall.
Unmet need: Studies are needed to leverage large-scale EHR data and advanced informatics technology to improve local and international SCAR case-finding methods to advance the science of SCAR research (Table 1).
Prediction, Prevention, Earlier Diagnosis, and Treatment
A foundation of research to identify predictors of SJS/TEN is careful ascertainment and specialist clinical phenotyping, to facilitate accurate diagnosis. In this context, RegiSCAR has developed an algorithm for assessment of drug causality for epidermal necrolysis (ALDEN), which is being used by a number of collaborative networks studying SJS/TEN across genetically diverse populations [27]. The Canadian Pharmacogenomics Network for Drug Safety (CPNDS) has also developed data collection materials to ensure proper SJS/TEN case ascertainment is occurring at each of its centers across Canada. In addition, an algorithm for causality assessment has been developed, from which, CPNDS recommendations have been made regarding the use of HLA-B*15:02 and HLA-A*31:01 genetic testing to reduce the risk of carbamazepine-induced hypersensitivity reactions [28]. Genetic studies continue to refine the existing scientific knowledge and identify novel predictors of drug-induced hypersensitivity reactions (Table 2) [2–5,29–31]. A recent meta-analysis of two genome-wide association studies (GWAS) identified HLA-A*31:01 as a strong predictor of both CBZ-induced SCAR and drug-induced liver injury (DILI) [32]. The same study reported a new association between variation in the anaplastic lymphoma kinase (ALK) gene and CBZ-induced SCAR [32].
Table 2.
Evidence-based pharmacogenomics and clinical implementation.
| Associated drug | Genetic variant | Hypersensitivity | Ethnicity and Carriage rate (%) | Level of evidence | Stage of implementation |
|---|---|---|---|---|---|
| Abacavir | HLA-B*57:01 | Hypersensitivity syndrome (NOT SJS/TEN) [2] |
|
||
| Allopurinol | HLA-B*58:01 | SJS/TEN and DRESS [5] |
|
|
|
| Carbamazepine | HLA-B*15:02 | SJS/TEN [3,27,31] |
|
|
|
| HLA-A*31:01 | SJS/TEN and DRESS and MPE [27,31] |
|
|
|
|
| Dapsone | HLA-B*13:01 | DRESS and SJS/TEN [4] |
|
|
Not available |
| Oxcarbazepine | HLA-B*15:02 | SJS/TEN [29] |
|
|
|
| Phenytoin | CYP2C9*3 | SJS/TEN and DRESS and MPE [28] |
|
|
|
| HLA-B*15:02 | SJS/TEN and DRESS [28] |
|
|
|
|
| Vancomycin | HLA-A*32:01 | DRESS [30] |
|
|
|
CPIC, Clinical Pharmacogenetics Implementation Consortium (https://cpicpgx.org); CYP, Cytochromes P450; DRESS, drug reaction with eosinophilia and systemic symptoms; EMA, European Medicines Agency; FDA, Food and Drug Administration; HLA, human leukocyte antigen; MPE, maculopapular eruption; PharmGKB, a pharmacogenomics knowledge resource (https://www.pharmgkb.org); PMDA, Pharmaceuticals and Medical Devices Agency in Japan; PGx, pharmacogenomics; SJS/TEN, Stevens-Johnson syndrome and toxic epidermal necrolysis; TFDA, Taiwan Food and Drug Administration.
The levels of evidence graded by the Clinical Pharmacogenetics Implementation Consortium as defined at https://cpicpgx.org/levels-of-evidence
PharmGKB Clinical Annotation Levels of Evidence as defined at https://www.pharmgkb.org/page/clinAnnLevels
PharmGKB Drug Label Annotations- https://www.pharmgkb.org/labelAnnotations
U.S FDA Table of Pharmacogenomic Biomarkers in Drug Labeling- https://www.fda.gov/drugs/science-and-research-drugs/table-pharmacogenomic-biomarkers-drug-labeling; https://www.fda.gov/media/124784/download.
NPV of HLA-B*58:01 for SJS/TEN and DRESS and Africans and Europeans is lower than Southeast Asians (explains approximately 60% of disease).
Actionable PGx- Product labeling includes specific actions to be taken based on the biomarker information.
Another novel report is the association between variation in the complement factor H (CFH) gene and phenytoin-induced maculopapular exanthema in individuals of European-ancestry [33]. With all the hypersensitivity-related genetic data that is being generated globally, an opportunity exists for a large meta-analysis to identify additional predictors and inform accurate prevention models. Strong associations have been reported between IL-15 and granulysin levels and severity and mortality of SJS/TEN suggesting that these could be utilized in earlier identification and prognostication [34,35]. Current suggested interventions and therapeutic strategies are highlighted in Supplemental Figure S2. Further prospective, randomized controlled studies are needed to provide more definitive conclusions and determine optimal treatment strategies in patients with SJS/TEN.
Unmet need: To fuel discovery and implementation of additional genetic predictors and biomarkers for earlier diagnosis and treatment across diverse populations (Table 1).
Special Populations
The etiology, pharmacogenomic risks, epidemiology, clinical features and outcomes of SJS/TEN vary considerably in special patient populations. SJS/TEN in children is more frequently non-drug related; with SJS/TEN very uncommon in the very young (< 2 years old) [36]. SJS/TEN is considerably more common (up to 150-fold) in certain immunocompromized patient populations, such as patients with cancer and HIV/AIDS [37]. Newer immunomodulatory treatments such as the immune checkpoint inhibitors used for treatment of previously untreated cancers such as melanoma have been transformative but have been associated with severe and unpredictable adverse events. In these populations, there is still much to be learned about the clinical presentation and treatment, pre-disposing factors including host genetics, the tumor type and the type and combination of immunomodulatory therapy used. Since SJS/TEN has been reported several months after the administration of these agents a high index of suspicion needs to be maintained. SJS/TEN mortality is higher in the elderly with malignant co-morbidity (>50%) and pre-existing hepatic disease [37–39].
Although the smaller number of patients in certain subgroups and unclear pathogenesis increase difficulties in SJS/TEN research in special populations, pharmacogenomics and associated mechanistic studies show promise for predicting SJS/TEN to population relevant drugs as was illustrated in the now routine pre-prescription screening of HLA-B*57:01 to prevent abacavir hypersensitivity (Table 2) [2]. Large scale therapeutic intervention and long-term outcome studies should also endeavor to include populations equally balanced across age, sex, pregnancy, ethnicity and co-morbidities.
Unmet need: To better understand and personalize approaches to SJS/TEN across the heterogeneous populations affected (Table 1).
Beyond Acute Care and Long-term Considerations
Awareness of SJS/TEN-associated long-term sequelae is increasing. The RegiSCAR study was the first to systematically quantify higher mortality, morbidity and lower quality of life (QOL) beyond the acute stage by following up a cohort of SJS/TEN survivors at 8±2 weeks, one year and five years. At eight-weeks, 88% and 70% of survivors reported skin and eye symptoms respectively. These persisted in 77% and 61% at one year, and 73% and 67% respectively at five years. Ocular symptoms, reported by patients as the most bothersome, developed despite optimal acute care and sometimes only months later. Oral and genital sequelae manifested features reflective of localized scarring and functional dryness such as dental caries and genital pain, bleeding, dyspareunia, and hypogeusia. Surprisingly, severity of mucosal sequelae did not correlate with disease severity in the acute stage (unpublished data) [38].
A pattern of psychological sequelae amongst survivors is also emerging. Clinical criteria for anxiety, posttraumatic stress disorder and depression were fulfilled in approximately half, one-third and one-third respectively in several studies [40,41]. Five years post-discharge, >50% of survivors still avoid medication. These long-term sequelae do not only reduce patient’s QOL, but also their ability to work. Five years after the acute stage of SJS/TEN, <50% had returned to their normal premorbid activities. Approximately 10% of survivors were not back to gainful employment after five years compared to 25% at one-year follow-up. (unpublished data) [38].
Cutaneous scarring and dyspigmentation are common features of SJS/TEN and occur in 46% and 77% of cases respectively [42]. The amount of scarring present may be extensive and associated with hypertrophic and keloid variants resulting in chronic pain and pruritus [13,43–45]. The etiology of the scarring is unknown; however, it is possibly impacted by the following: delayed re-epithelialization, non-standardized/optimized wound care, differences in systemic treatments and comorbid conditions, and genetic predisposition to develop hypertrophic scars.
Conventional treatment options have been adapted from lessons learned by caring for burn patients. Common modalities include scar massage, silicone sheeting and the use of pressure garments. While there is evidence that these modalities have been and continue to be helpful, there have been many recent advancements in the non-operative management of scarring with an emphasis on the use of medical laser devices [46,47].
More specifically, medical laser devices have been shown to improve scar tissue pliability and flexibility leading to improvements in range of motion and symptomatic improvements that can result in decreased pain, burning and pruritus. Additionally, restoration of pilosebaceous unit functionality with return of sweating and hair growth has been observed. These benefits have been achieved predominantly through the use of devices that target hemoglobin and water, including the 595nm pulsed dye laser and fractional ablative carbon dioxide lasers. SJS/TEN patients with symptomatic and/or disfiguring scarring should be considered for such treatments [46,47].
With increasing awareness, multidisciplinary and system-specific strategies and protocols are needed to prevent, diagnose and treat these sequelae. Routine ophthalmic and psychiatric follow-up assessments of survivors are recommended [40].
Unmet need: To understand the nature of, prevalence of and risk factors for long-term complications and to develop holistic and novel approaches to their management (Table 1).
Models and Mechanism
The immunopathogenesis of SJS/TEN remains to be fully elucidated, thus hampering prevention and treatment efforts. A major breakthrough arose from the discovery that specific HLA alleles predispose and, in most cases, appear necessary but not sufficient for the development of SJS/TEN and other SCAR upon exposure to particular drugs, which directly implicated T cells as key mediators of disease. Drugs, considered as foreign antigens, likely interact with particular HLA/peptide/T-cell receptor (TCR) complexes on keratinocytes to trigger the adaptive immune response and adverse reactions. CD8+ cytotoxic T lymphocytes (CTLs), that recognize HLA-drug epitopes along with natural killer (NK) and NK T cells infiltrate skin lesions and secrete cytolytic proteins/chemokine mediators (e.g. granulysin), causing disseminated keratinocyte death in SJS/TEN [1]. Multiple predictive genomic markers (Table 2) are subsequently determined to prevent drug-specific SJS/TEN and serum biomarkers such as IL-15 and granulysin [34,35] may have roles in predicting the prognosis of acute stage SJS/TEN. Single-cell (sc) T-cell receptor (TCR) sequencing and repertoire analysis are novel approaches to investigate drug-specific T cell populations and can be paired with sc-RNAseq and Cite-seq to examine expression of the related transcriptome and proteome of total cell populations on interest [48–49]. Dominant TCRαβ clonotypes have been identified in single cells sorted from blister samples of patients with HLA-B*58:01 restricted allopurinol-SJS/TEN and HLA-B*15:02 restricted carbamazepine-SJS/TEN which in the case of the latter represent a public TCRαβ clonotype that is shared amongst unrelated HLA-B*15:02 positive patients with carbamazepine SJS/TEN [48–49]. These new technologies, combined with traditional analysis of prospectively collected blister fluid, skin, and blood, allow the identification of new biomarkers of disease and an avenue to define novel and more targeted treatment approaches. The insights generated from these combined efforts have led to the development of much-needed mouse models of SJS/TEN and other SCAR. A mouse model of abacavir hypersensitivity provides a potential mechanism to explain tolerance in the presence of the HLA-B*57:01 risk allele [50]. For SJS/TEN, mouse models have allowed for further delineation of disease pathogenesis and provide a system to test potential therapeutic interventions [1,50]. Collectively, though much research remains to be done in SJS/TEN, a solid framework is now in place upon which further progress can be built.
Unmet need: To utilize new technologies and scalable approaches to defining the specific immunopathogenesis of SJS/TEN that will lead to biomarkers for prevention, earlier diagnosis and treatment (Table 1).
The Patient and Family Perspective
Most notably, this conference offered an opportunity to identify critical unmet needs within the SJS/TEN patient community. Patients described SJS/TEN as a disease that burned their body from the inside out, that ravaged and charred their bodies, altered their appearance, and wrecked their lives.
From the symptomatic phase through hospitalization and discharge, survivors identified multiple gaps in the continuum of care that they felt contributed to the sequelae of SJS/TEN. The three most notable areas were diagnosis, acute care management, and discharge care plans, discussed in Table 1.
Overall, due to the atypical and rare features of SJS/TEN, many patients and their families felt an overwhelming disconnect with the medical community and could only hope that the healthcare providers and scientists working together as represented at SJS/TEN 2019 to move science and clinical care forward would be the ones to bridge the gap.
Unmet need: To develop universal patient-centered approaches to diagnosis, acute management and follow-up with significant involvement of patients and survivor groups and families in this process (Table 1).
Conclusions
As the global landscape of treatment for high burden diseases, such as tuberculosis, HIV and cancer, evolve, and as an even larger number of new drugs are administered globally, increasing concerns arise about the severe adverse drug reactions such as SJS/TEN that threaten public health and drug safety. The SJS/TEN 2019: From Science to Translation conference highlighted how clinical implementation of predictive screening for HLA risk alleles before initiating some well-known culprit drugs has made important progress in lowering the incidence of SJS/TEN and improving the safety of medication use. In vitro tests, animal models, and novel experimental approaches for SJS/TEN research have facilitated a better understanding of the causative drugs, the drug-gene interactions, the immune response, and the pathogenic mechanisms. Further research is still needed to address the clinical burden, epidemiology, drug and population-specific genetic basis and immunopathogenesis of SJS/TEN globally. Leveraging existing resources and integrating research networks, registries and clinical experts will help facilitate this cause. The ultimate goal is the development of evidence-based and personalized approaches to patients with SJS/TEN that will fuel prediction, prevention, and improved short- and long-term clinical outcomes at the population and individual levels.
Supplementary Material
ACKNOWLEDGEMENTS
We gratefully acknowledge all the SJS/TEN patients and their families- Cheryl Barlow, Jim Barlow, Jacob Bonebreak, Allison Brimhall, Carolyn Burr, Arthur Burr, Janice Casebeer, Hsing-Chun Chang, Carl Chapman, Joseph Croasdaile, Tricia Eads, Jane Fleiss-Brogger, Amy Ford, Trevor Ford, Pawan Gaikwad, Katie Grant, Jamie Kidwell, Coleen Lambert, Trent Lowe, Grace Lu, Alysia MacGrotty, Mark Mills, Jeff Niemeyer, Anastasia Patrignani, Deborah Patrignani, Christopher Patrignani, Amani Saini, Keith Stryker, and Brock Whale, for their participation in the SJS/TEN 2019: From Science to Translation conference. We appreciate all participants not listed as co-authors who attended and provided important insights regarding SJS/TEN at the meeting. We also would like to thank University of British Columbia and British Columbia Children’s Hospital Research Institute for their continue support, as well as Canadian Pharmacogenomics Network for Drug Safety (CPNDS) members for their assistance in organizing the meeting. We thank Ms. Linda Coyne for assistance in the preparation of this manuscript.
Web resources and support services for patients with SJS/TEN: General SJS Foundation (http://www.sjsupport.org); Stevens-Johnson Syndrome Canada (http://www.sjscanada.org); Amalyste (France; http://www.amalyste.fr); Taiwan Eden Social Welfare Foundation (Mandarin/English; https://eden.international).
For more information, please visit the SJS/TEN2019 official website at https://medsites.mc.vanderbilt.edu/sjsmeeting/welcome or you may view the presentations from the meeting at https://nexuswebcast.mediasite.com/Mediasite/Catalog/catalogs/sjs-ten-2019-event.
FUNDING SOURCES
Funding for this conference was made possible (in part) by NIH (1 R13-AR74889-01) National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) and all co-funding support provided by: National Human Genome Research Institute (NHGRI), National Center for Advancing Translational Sciences (NCATS), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Eye Institute (NEI), National Institute of Allergy and Infectious Diseases (NIAID), U.S. Food and Drug Administration, Canadian Institutes of Health Research, Genome British Columbia.
Funding was also supported by the University of British Columbia Faculty of Medicine; University of British Columbia Faculty of Pharmaceutical Sciences; BC Children’s Hospital Research Institute; Dr. Hongsheng Wang (Institute of Dermatology, Chinese Academy of Medical Sciences & Peking Union Medical College) and Dr. Wen-Hung Chung (Chang Gung Memorial Hospital); British Columbia Provincial Health Services Authority; Stevens-Johnson Syndrome Consulting Group, Inc; Eli Lilly Canada, Inc; Murdoch Global University, Australia, Dubai, Singapore; Dynacare; Illumina; Sandoz Canada, Biopharmaceuticals; Tissue Tech/Ocular Surface; Pharmigene, Inc. Dr. Stuart MacLeod.
This publication has emanated in part from research supported in part by a research grant from Science Foundation Ireland (SFI) under Grant Number 16/RC/3948 and co-funded under the European Regional Development Fund and by FutureNeuro industry partners. T. Bellón’s research was supported in part by grant PI18/00718 (cofounded by FEDER) from ISCII, Spain. S. J. Divito has received funding from National Institutes of Health (NIH DP5OD023091). M. E. Lacouture is funded in part through the NIH/NCI Cancer Center Support Grant P30 CA 008748. M. Pirmohamed wishes to thank the MRC Centre for Drug Safety Science, and the International Serious Adverse Event Consortium (ISAEC) for research support. S.I. Hung’s and W.H. Chung’s research were supported in part by a grant (MOST 108-2320-B-182A-023 -MY3) from the Ministry of Science and Technology, Taiwan, and research grants (CIRPG3I0041, CIRPG3I0021) from Chang Gung Memorial Hospital, Linkou Branch, Taoyuan, Taiwan. The German Registry of Severe Skin Reactions (dZh), representing the German part of the multinational RegiSCAR-study since 2003, was mainly funded by a research grant from the European Commission (QLRT-2002-01738) and by a grant from the Federal Ministry for Education and Research (Bundesministerium für Bildung und Forschung (BMBF); grant no. 01KG1018). The dZh also received a grant / donation by Erika- and Werner Messmer-Foundation for clinical research (grant no. 1020.0355.01a), a private donation (C.H.R., Nailsea, UK) for SCAR-research (grant no. 1020.0355.01b) and a grant / donation by the German Dermatology Foundation (Deutsche Stiftung zur Förderung wissenschaftlicher Arbeit auf dem Gebiet der Dermatologie; grant no. 1020.0355.01c). Additional financial support was provided by several pharmaceutical companies (Bayer vital, Boehringer-Ingelheim, Cephalon, GlaxoSmithKline, Grünenthal, MSD Sharp and Dome, Merck, Novartis, Pfizer, Sanofi-Aventis, Servier, Tibotec-Janssen). M. Mockenhaupt received the Else Kröner Memorial Stipendium for support of clinical research through Else Kröner-Fresenius-Foundation. Methodological considerations were partly supported by German Research Foundation (Deutsche Forschungsgemeinschaft; FOR 534).
ABBREVIATIONS USED-
- ADR
Adverse drug reaction
- AIDS
Acquired immunodeficiency syndrome
- ALDEN
Algorithm of drug causality for epidermal necrolysis
- ALK
Anaplastic lymphoma kinase
- AUS-SCAR
Australian registry of severe cutaneous adverse reactions
- CBZ
Carbamazepine
- CFH
Complement factor H
- CLET
Cultivated limbal epithelial sell transplantation
- COMET
Cultivated oral mucosal epithelial transplantation
- CPNDS
Canadian Pharmacogenomics Network for Drug Safety
- DILI
Drug-induced liver injury
- DRESS
Drug reaction with eosinophilia and systemic symptoms
- EHR
Electronic health records
- EMA
European Medicines Agency
- EpiPGx
Epilepsy Pharmacogenomics
- eRMR
Electronic Reaction Monitoring Report
- FDA
Food and Drug Administration
- GWAS
Genome-wide association study
- HIV
Human immunodeficiency virus
- HLA
Human leukocyte antigen
- IVIg
Intravenous immune globulin
- iSCAR
International Congress on Cutaneous Adverse Drug Reaction
- ITCH
International Consortium on Drug Hypersensitivity
- LSCD
Limbal stem cell deficiency
- MPE
Maculopapular eruption
- NATIENS
North American Therapeutics in Epidermal Necrolysis Syndrome Network
- PGx
Pharmacogenomics
- PMDA
Pharmaceuticals and Medical Devices Agency (in Japan)
- PRAC
Pharmacovigilance Risk Assessment Committee
- QOL
Quality of life
- RegiSCAR
Multinational registry of Severe Cutaneous Adverse Reactions to drugs and collection of biological samples
- SCAR
Severe cutaneous adverse reaction
- SDH
Society of Dermatology Hospitalists
- SEAPharm
Southeast Asian Pharmacogenomic Network
- SOMET
Simple oral mucosal epithelial transplantation
- SJS
Stevens-Johnson syndrome
- TB
Tuberculosis
- TCR
T cell receptor
- TEN
Toxic epidermal necrolysis
- TFDA
Taiwan Food and Drug Administration
- T-SCAR
Taiwan Severe Cutaneous Adverse Reaction Consortium
Footnotes
CONFLICTS OF INTEREST
G. L. Cavalleri has received collaborative research support from Congenica. R. Lim is a Senior Science Advisor for Health Products and Food Branch. D. M. Koelle is a member of advisory boards of Curevo, Gilead, and MaxHealth. M. E. Lacouture serves as a consultant/speaker for Legacy Healthcare Services, Adgero Bio Pharmaceuticals, Amryt Pharmaceuticals, Celldex Therapeutics, Debiopharm, Galderma Research and Development, Johnson & Johnson, Novocure Inc., Lindi, Merck Sharp and Dohme Corporation, Helsinn Healthcare SA, Janssen Research & Development LLC, Menlo Therapeutics, Novartis Pharmaceuticals Corporation, F. Hoffmann-La Roche AG, Abbvie Inc., Boehringer Ingelheim Pharma Gmbh & Co. KG, Allergan Inc., Amgen Inc., E.R. Squibb & Sons LLC, EMD Serono Inc., AstraZeneca Pharmaceuticals LP, Genentech Inc., Leo Pharma Inc., Seattle Genetics, Bayer, Manner SAS, Lutris, Pierre Fabre, Paxman Coolers, Adjucare, Dignitana, Biotechspert, Teva Mexico, Parexel, OnQuality Pharmaceuticals Ltd., Novartis, Our Brain Bank, and Takeda Millenium. M. E. Lacouture also receives research funding from Veloce, US Biotest, Berg, Bristol-Myers Squibb, Lutris, Paxman, and Novocure. M. Mockenhaupt is a member of advisory boards or expert panels (pharmaceutical companies- Merck and Pfizer) and has served as an expert in litigation related to severe cutaneous adverse reactions. M. Pirmohamed has received research support from International Serious Adverse Event Consortium (ISAEC). The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES-
- [1].White KD, Abe R, Ardern-Jones M, Beachkofsky T, Bouchard C, Carleton B, Chodosh J, Cibotti R, Davis R, Denny JC, Dodiuk-Gad RP, Ergen EN, Goldman JL, Holmes JH, Hung SI, Lacouture ME, Lehloenya RJ, Mallal S, Manolio TA, Micheletti RG, Mitchell CM, Mockenhaupt M, Ostrov DA, Pavlos R, Pirmohamed M, Pope E, Redwood A, Rosenbach M, Rosenblum MD, Roujeau JC, Saavedra AP, Saeed HN, Struewing JP, Sueki H, Sukasem C, Sung C, Trubiano JA, Weintraub J, Wheatley LM, Williams KB, Worley B, Chung WH, Shear NH, Phillips EJ, SJS/TEN 2017: Building Multidisciplinary Networks to Drive Science and Translation., J. Allergy Clin. Immunol. Pract 6 (2018) 38–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mallal S, Phillips E, Carosi G, Molina JM, Workman C, Tomazic J, Jägel-Guedes E, Rugina S, Kozyrev O, Cid JF, Hay P, Nolan D, Hughes S, Hughes A, Ryan S, Fitch N, Thorborn D, Benbow A, HLA-B*5701 screening for hypersensitivity to abacavir. And Warfarin Genetic Dosage Algorithm., N. Engl. J. Med 358 (2008) 568–579. [DOI] [PubMed] [Google Scholar]
- [3].Chen P, Lin JJ, Lu CS, Ong CT, Hsieh PF, Yang CC, Tai CT, Wu SL, Lu CH, Hsu YC, Yu HY, Ro LS, Lu CT, Chu CC, Tsai JJ, Su YH, Lan SH, Sung SF, Lin SY, Chuang HP, Huang LC, Chen YJ, Tsai PJ, Liao HT, Lin YH, Chen CH, Chung WH, Hung SI, Wu JY, Chang CF, Chen L, Chen YT, Shen CY, Taiwan SJS Consortium, Carbamazepine-Induced Toxic Effects and HLA-B*1502 Screening in Taiwan., N. Engl. J. Med 364 (2011) 1126–1133. [DOI] [PubMed] [Google Scholar]
- [4].Zhang FR, Liu H, Irwanto A, Fu XA, Li Y, Yu GQ, Yu YX, Chen MF, Low HQ, Li JH, Bao FF, Foo JN, Bei JX, Jia XM, Liu J, Liany H, Wang N, Niu GY, Wang ZZ, Shi BQ, Tian HQ, Liu HX, Ma SS, Zhou Y, You JB, Yang Q, Wang C, Chu TS, Liu DC, Yu XL, Sun YH, Ning Y, Wei ZH, Chen SL, Chen XC, Zhang ZX, Liu YX, Pulit SL, Wu WB, Zheng ZY, Yang RD, Long H, Liu ZS, Wang JQ, Li M, Zhang LH, Wang H, Wang LM, Xiao P, Li JL, Huang ZM, Huang JX, Li Z, Liu J, Xiong L, Yang J, Wang XD, Yu DB, Lu XM, Zhou GZ, Yan LB, Shen JP, Zhang GC, Zeng YX, de Bakker PIW, Chen SM, Liu JJ, HLA-B*13:01 and the Dapsone Hypersensitivity Syndrome., N. Engl. J. Med 369 (2013) 1620–1628. [DOI] [PubMed] [Google Scholar]
- [5].Ko TM, Tsai CY, Chen SY, Chen KS, Yu KH, Chu CS, Huang CM, Wang CR, Weng CT, Yu CL, Hsieh SC, Tsai JC, Lai WT, Tsai WC, Yin GD, Ou TT, Cheng KH, Yen JH, Liou TL, Lin TH, Chen DY, Hsiao PJ, Weng MY, Chen YM, Chen CH, Liu MF, Yen HW, Lee JJ, Kuo MC, Wu CC, Hung SY, Luo SF, Yang YH, Chuang HP, Chou YC, Liao HT, Wang CW, Huang CL, Chang CS, Lee MTM, Chen P, Wong CS, Chen CH, Wu JY, Chen YT, Shen CY, Taiwan Allopurinol-SCAR Consortium, Use of HLA-B*58:01 genotyping to prevent allopurinol induced severe cutaneous adverse reactions in Taiwan: national prospective cohort study., BMJ. 351 (2015) h4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Manolio TA, Hutter CM, Avigan M, Cibotti R, Davis RL, Denny JC, La Grenade L, Wheatley LM, Carrington MN, Chantratita W, Chung WH, Dalton AD, Hung SI, Lee MTM, Leeder JS, Lertora JJL, Mahasirimongkol S, McLeod HL, Mockenhaupt M, Pacanowski M, Phillips EJ, Pinheiro S, Pirmohamed M, Sung C, Suwankesawong W, Trepanier L, Tumminia SJ, Veenstra D, Yuliwulandari R, Shear NH, Research Directions in Genetic Predispositions to Stevens-Johnson Syndrome / Toxic Epidermal Necrolysis., Clin. Pharmacol. Ther 103 (2018) 390–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Le HG, Saeed H, Mantagos IS, Mitchell CM, Goverman J, Chodosh J, Burn unit care of Stevens Johnson syndrome/toxic epidermal necrolysis: A survey., Burns. 42 (2016) 830–835. [DOI] [PubMed] [Google Scholar]
- [8].Lim VM, Do A, Berger TG, Nguyen AH, DeWeese J, Malone JD, Jordan K, Hom F, Tuffanelli L, Fillari P, Siu S, Grossman R, A decade of burn unit experience with Stevens-Johnson Syndrome / Toxic Epidermal Necrolysis: Clinical pathological diagnosis and risk factor awareness., Burns. 42 (2016) 836–843. [DOI] [PubMed] [Google Scholar]
- [9].Garcia-Doval I, LeCleach L, Bocquet H, Otero XL, Roujeau JC, Toxic epidermal necrolysis and Stevens-Johnson syndrome: does early withdrawal of causative drugs decrease the risk of death?, Arch. Dermatol 136 (2000) 323–327. [DOI] [PubMed] [Google Scholar]
- [10].González-Herrada C, Rodríguez-Martín S, Cachafeiro L, Lerma V, González O, Lorente JA, Rodríguez-Miguel A, González-Ramos J, Roustan G, Ramírez E, Bellón T, de Abajo FJ, Bellón T, Cabañas R, Cachafeiro L, García de Lorenzo A, González-Ramos J, Hernández O, Herranz P, Ramírez E, Bravo ER, Alonso Y, Aramburu JA, Cámara N, González O, González-Herrada C, Laosa O, Lorente JA, Moscoso A, Payares C, Roustan G, de Abajo FJ, Quesada A, Lerma V, Rodríguez-Martín S, Cyclosporine Use in Epidermal Necrolysis Is Associated with an Important Mortality Reduction: Evidence from Three Different Approaches., J. Invest. Dermatol 137 (2017) 2092–2100. [DOI] [PubMed] [Google Scholar]
- [11].Wang CW, Yang LY, Chen CB, Ho HC, Hung SI, Yang CHCY, Chang CJ, Su SC, Hui RCY, Chin SW, Huang LF, Lin YYW, Chang WY, Fan WL, Yang CHCY, Ho JC, Chang YC, Lu CW, Chung WH, the Taiwan Severe Cutaneous Adverse Reaction (TSCAR) Consortium, Randomized, controlled trial of TNF-α antagonist in CTL-mediated severe cutaneous adverse reactions., J. Clin. Invest 128 (2018) 985–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].McPherson T, Exton LS, Biswas S, Creamer D, Dziewulski P, Newell L, Tabor KL, Wali GN, Walker G, Walker R, Walker S, Young AE, Mohd Mustapa MF, Murphy R, British Association of Dermatologists’ guidelines for the management of Stevens–Johnson syndrome/toxic epidermal necrolysis in children and young people, 2018., Br. J. Dermatol 181 (2019) 37–54. [DOI] [PubMed] [Google Scholar]
- [13].Schwartz RA, McDonough PH, Lee BW, Toxic epidermal necrolysis: Part II. Prognosis, sequelae, diagnosis, differential diagnosis, prevention, and treatment., J. Am. Acad. Dermatol 69 (2013) 187.e1–16. [DOI] [PubMed] [Google Scholar]
- [14].Meneux E, Wolkenstein P, Haddad B, Roujeau JC, Revuz J, Paniel BJ, Vulvovaginal involvement in toxic epidermal necrolysis: a retrospective study of 40 cases., Obstet. Gynecol 91 (1998) 283–287. [DOI] [PubMed] [Google Scholar]
- [15].Niemeijer IC, van Praag MCG, van Gemund N, Relevance and consequences of erythema multiforme, Stevens-Johnson syndrome and toxic epidermal necrolysis in gynecology., Arch. Gynecol. Obstet 280 (2009) 851–854. [DOI] [PubMed] [Google Scholar]
- [16].Gregory DG, New Grading System and Treatment Guidelines for the Acute Ocular Manifestations of Stevens-Johnson Syndrome., Ophthalmology. 123 (2016) 1653–1658. [DOI] [PubMed] [Google Scholar]
- [17].Kohanim S, Palioura S, Saeed HN, Akpek EK, Amescua G, Basu S, Blomquist PH, Bouchard CS, Dart JK, Gai X, Gomes JAP, Gregory DG, Iyer G, Jacobs DS, Johnson AJ, Kinoshita S, Mantagos IS, Mehta JS, Perez VL, Pflugfelder SC, Sangwan VS, Sippel KC, Sotozono C, Srinivasan B, Tan DTH, Tandon R, Tseng SCG, Ueta M, Chodosh J, Acute and Chronic Ophthalmic Involvement in Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis - A Comprehensive Review and Guide to Therapy. II. Ophthalmic Disease., Ocul. Surf 14 (2016) 168–188. [DOI] [PubMed] [Google Scholar]
- [18].Sharma N, Thenarasun SA, Kaur M, Pushker N, Khanna N, Agarwal T, Vajpayee RB, Adjuvant Role of Amniotic Membrane Transplantation in Acute Ocular Stevens–Johnson Syndrome., Ophthalmology. 123 (2016) 484–491. [DOI] [PubMed] [Google Scholar]
- [19].Shanbhag SS, Rashad R, Chodosh J, Saeed HN, Long-term impact of a treatment protocol for acute ocular involvement in Stevens-Johnson syndrome/toxic epidermal necrolysis., Am. J. Ophthalmol 208 (2019) 331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ueta M, Kannabiran C, Wakamatsu TH, Kim MK, Yoon KC, Seo KY, Joo CK, Sangwan V, Rathi V, Basu S, Shamaila A, Lee HS, Yoon S, Sotozono C, Gomes JÁP, Tokunaga K, Kinoshita S, Trans-ethnic study confirmed independent associations of HLA-A*02:06 and HLA-B*44:03 with cold medicine-related Stevens-Johnson syndrome with severe ocular surface complications., Sci. Rep 4 (2014) 5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Roujeau JC, Dunant A, Mockenhaupt M, Epidermal Necrolysis, Ocular Complications, and “Cold Medicines”., J. Allergy Clin. Immunol. Pract 6 (2018) 703–704. [DOI] [PubMed] [Google Scholar]
- [22].Ueta M, Results of Detailed Investigations Into Stevens-Johnson Syndrome With Severe Ocular Complications., Invest. Ophthalmol. Vis. Sci 59 (2018) DES183–DES191. [DOI] [PubMed] [Google Scholar]
- [23].Blumenthal KG, Wickner PG, Lau JJ, Zhou L, Stevens-Johnson syndrome and toxic epidermal necrolysis: A cross-sectional analysis of patients in an integrated allergy repository of a large health care system., J. Allergy Clin. Immunol. Pract 3 (2015) 277–280.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wong A, Seger DL, Lai KH, Goss FR, Blumenthal KG, Zhou L, Drug Hypersensitivity Reactions Documented in Electronic Health Records within a Large Health System., J. Allergy Clin. Immunol. Pract 7 (2019) 1253–1260.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].European Medicines Agency (EMA)/Pharmacovigilance Risk Assessment Committee (PRAC) recommendations on safety signals- List of safety signals discussed since September 2012, (2012). https://www.ema.europa.eu/en/human-regulatory/post-authorisation/pharmacovigilance/signal-management/prac-recommendations-safety-signals#list-of-safety-signals-discussed-since-september-2012-section.
- [26].Tan-Koi WC, Sung C, Chong YY, Lateef A, Pang SM, Vasudevan A, Aw D, Lui NL, Lee SX, Ren EC, Koay ES, Tay YK, Lim YL, Lee HY, Dong D, Loke C, Tan L, Limenta M, Lee EJ, Toh D, Chan CL, Tailoring of recommendations to reduce serious cutaneous adverse drug reactions: a pharmacogenomics approach., Pharmacogenomics. 18 (2017) 881–890. [DOI] [PubMed] [Google Scholar]
- [27].Sassolas B, Haddad C, Mockenhaupt M, Dunant A, Liss Y, Bork K, Haustein UF, Vieluf D, Roujeau JC, Le Louet H, ALDEN, an algorithm for assessment of drug causality in Stevens-Johnson Syndrome and toxic epidermal necrolysis: comparison with case-control analysis., Clin. Pharmacol. Ther 88 (2010) 60–68. [DOI] [PubMed] [Google Scholar]
- [28].Amstutz U, Shear NH, Rieder MJ, Hwang S, Fung V, Nakamura H, Connolly MB, Ito S, Carleton BC, CPNDS clinical recommendation group, Recommendations for HLA-B*15:02 and HLA-A*31:01 genetic testing to reduce the risk of carbamazepine-induced hypersensitivity reactions., Epilepsia. 55 (2014) 496–506. [DOI] [PubMed] [Google Scholar]
- [29].Chung WH, Chang WC, Lee YS, Wu YRYY, Yang CH, Ho HC, Chen MJ, Lin JY, Hui RCY, Ho JC, Wu WM, Chen TJ, Wu TTL, Wu YRYY, Hsih MS, Tu PH, Chang CJCN, Hsu CKCN, Wu TTL, Choon SE, Hsu CKCN, Chen DY, Liu CS, Lin CY, Kaniwa N, Saito Y, Takahashi Y, Nakamura R, Azukizawa H, Shi Y, Wang TH, Chuang SS, Tsai SF, Chang CJCN, Chang YS, Hung SI, Taiwan Severe Cutaneous Adverse Reaction Consortium, Japan Pharmacogenomics Data Science Consortium, Genetic variants associated with phenytoin-related severe cutaneous adverse reactions., JAMA. 312 (2014) 525–534. [DOI] [PubMed] [Google Scholar]
- [30].Chen CB, Hsiao YH, Wu TTL, Hsih MS, Tassaneeyakul W, Jorns TP, Sukasem C, Hsu CKCN, Su SC, Chang WC, Hui RCY, Chu CY, Chen YJ, Wu CY, Hsu CKCN, Chiu TM, Sun PL, Lee HE, Yang CYCHCS, Kao PH, Yang CYCHCS, Ho HC, Lin JY, Chang YC, Chen MJ, Lu CW, Ng CY, Kuo KL, Lin CY, Yang CYCHCS, Chen DP, Chang PY, Wu TTL, Lin YJ, Weng YC, Kuo TT, Hung SI, Chung WH, Taiwan Severe Cutaneous Adverse Reaction Consortium, Risk and association of HLA with oxcarbazepine-induced cutaneous adverse reactions in Asians., Neurology. 88 (2017) 78–86. [DOI] [PubMed] [Google Scholar]
- [31].Konvinse KC, Trubiano JA, Pavlos R, James I, Shaffer CM, Bejan CA, Schutte RJ, Ostrov DA, Pilkinton MA, Rosenbach M, Zwerner JP, Williams KB, Bourke J, Martinez P, Rwandamuriye F, Chopra A, Watson M, Redwood AJ, White KD, Mallal SA, Phillips EJ, HLA-A*32:01 is strongly associated with vancomycin-induced drug reaction with eosinophilia and systemic symptoms., J. Allergy Clin. Immunol 144 (2019) 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nicoletti P, Barrett S, McEvoy L, Daly AK, Aithal G, Lucena MI, Andrade RJ, Wadelius M, Hallberg P, Stephens C, Bjornsson ES, Friedmann P, Kainu K, Laitinen T, Marson A, Molokhia M, Phillips E, Pichler W, Romano A, Shear N, Sills G, Tanno LK, Swale A, Floratos A, Shen Y, Nelson MR, Watkins PB, Daly MJ, Morris AP, Alfirevic A, Pirmohamed M, Shared Genetic Risk Factors Across Carbamazepine-Induced Hypersensitivity Reactions., Clin. Pharmacol. Ther 106 (2019) 1028–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].McCormack M, Gui H, Ingason A, Speed D, Wright GEB, Zhang EJ, Secolin R, Yasuda C, Kwok M, Wolking S, Becker F, Rau S, Avbersek A, Heggeli K, Leu C, Depondt C, Sills GJ, Marson AG, Auce P, Brodie MJ, Francis B, Johnson MR, Koeleman BPC, Striano P, Coppola A, Zara F, Kunz WS, Sander JW, Lerche H, Klein KM, Weckhuysen S, Krenn M, Gudmundsson LJ, Stefánsson K, Krause R, Shear N, Ross CJD, Delanty N, Pirmohamed M, Carleton BC, Cendes F, Lopes-Cendes I, Liao W, O’Brien TJ, Sisodiya SM, Cherny S, Kwan P, Baum L, Cavalleri GL, Kwan P, Baum L, International League Against Epilepsy Consortium on Complex Epilepsies, G.L. Cavalleri, Genetic variation in CFH predicts phenytoin-induced maculopapular exanthema in European-descent patients., Neurology. 90 (2018) e332–e341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chung WH, Hung SI, Yang JY, Su SC, Huang SP, Wei CY, Chin SW, Chiou CC, Chu SC, Ho HC, Yang CH, Lu CF, Wu JY, Liao YD, Chen YT, Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis., Nat. Med 14 (2008) 1343–1350. [DOI] [PubMed] [Google Scholar]
- [35].Su SC, Mockenhaupt M, Wolkenstein P, Dunant A, Le Gouvello S, Chen CB, Chosidow O, Valeyrie-Allanore L, Bellon T, Sekula P, Wang CW, Schumacher M, Kardaun SH, Hung SI, Roujeau JC, Chung WH, Interleukin-15 Is Associated with Severity and Mortality in Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis., J. Invest. Dermatol 137 (2017) 1065–1073. [DOI] [PubMed] [Google Scholar]
- [36].Paulmann M, Mockenhaupt M, Fever in Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in Pediatric Cases: Laboratory Work-up and Antibiotic Therapy., Pediatr. Infect. Dis. J 36 (2017) 513–515. [DOI] [PubMed] [Google Scholar]
- [37].Peter J, Choshi P, Lehloenya RJ, Drug hypersensitivity in HIV infection., Curr. Opin. Allergy Clin. Immunol 19 (2019) 272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sekula P, Dunant A, Mockenhaupt M, Naldi L, Bouwes Bavinck JN, Halevy S, Kardaun S, Sidoroff A, Liss Y, Schumacher M, Roujeau JC, RegiSCAR study group, Comprehensive Survival Analysis of a Cohort of Patients with Stevens–Johnson Syndrome and Toxic Epidermal Necrolysis., J. Invest. Dermatol 133 (2013) 1197–1204. [DOI] [PubMed] [Google Scholar]
- [39].Lehloenya RJ, Haitembu N, Basera W, Peter J, Lower-than-predicted mortality in a predominantly HIV-infected population with epidermal necrolysis regardless of HIV status: implications and challenges for interventional studies., J. Allergy Clin. Immunol. Pract 7 (2019) 1653–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Dodiuk-Gad RP, Olteanu C, Feinstein A, Hashimoto R, Alhusayen R, Whyte-Croasdaile S, Finkelstein Y, Burnett M, Sade S, Cartotto R, Jeschke M, Shear NH, Major psychological complications and decreased health-related quality of life among survivors of Stevens-Johnson syndrome and toxic epidermal necrolysis., Br. J. Dermatol 175 (2016) 422–424. [DOI] [PubMed] [Google Scholar]
- [41].Hefez L, Zaghbib K, Sbidian E, Valeyrie-Allanore L, Allain M, Duong TA, Colin A, Bellivier F, Romano H, de Prost N, Chazelas K, Chosidow O, Wolkenstein P, Ingen-Housz-Oro S, Post-traumatic stress disorder in Stevens-Johnson syndrome and toxic epidermal necrolysis: prevalence and risk factors. A prospective study of 31 patients., Br. J. Dermatol 180 (2019) 1206–1213. [DOI] [PubMed] [Google Scholar]
- [42].Olteanu C, Shear NH, Chew HF, Hashimoto R, Alhusayen R, Whyte-Croasdaile S, Finkelstein Y, Burnett M, Ziv M, Sade S, Jeschke MG, Dodiuk-Gad RP, Severe Physical Complications among Survivors of Stevens–Johnson Syndrome and Toxic Epidermal Necrolysis., Drug Saf. 41 (2018) 277–284. [DOI] [PubMed] [Google Scholar]
- [43].Sheridan RL, Schulz JT, Ryan CM, Schnitzer JJ, Lawlor D, Driscoll DN, Donelan MB, Tompkins RG, Long-term consequences of toxic epidermal necrolysis in children., Pediatrics. 109 (2002) 74–78. [DOI] [PubMed] [Google Scholar]
- [44].Paquet P, Jacob E, Quatresooz P, Jacquemin D, Piérard GE, Delayed reepithelialization and scarring deregulation following drug-induced toxic epidermal necrolysis., Burns. 33 (2007) 100–104. [DOI] [PubMed] [Google Scholar]
- [45].Kreft B, Lieser U, Haase R, Marsch WC, Wohlrab J, Extensive hypertrophic scarring after toxic epidermal necrolysis in a child., Pediatr. Dermatol 31 (2014) 527–528. [DOI] [PubMed] [Google Scholar]
- [46].Anderson RR, Donelan MB, Hivnor C, Greeson E, Ross EV, Shumaker PR, Uebelhoer NS, Waibel JS, Laser treatment of traumatic scars with an emphasis on ablative fractional laser resurfacing: consensus report., JAMA Dermatol. 150 (2014) 187–193. [DOI] [PubMed] [Google Scholar]
- [47].Miletta NR, Donelan MB, Hivnor CM, Management of trauma and burn scars: the dermatologist’s role in expanding patient access to care., Cutis. 100 (2017) 18–20. [PubMed] [Google Scholar]
- [48].Chung WH, Pan RY, Chu MT, Chin SW, Huang YL, Wang WC, Chang JY, Hung SI, Oxypurinol-Specific T Cells Possess Preferential TCR Clonotypes and Express Granulysin in Allopurinol-Induced Severe Cutaneous Adverse Reactions., J. Invest. Dermatol 135 (2015) 2237–2248. [DOI] [PubMed] [Google Scholar]
- [49].Pan RY, Chu MT, Wang CW, Lee YS, Lemonnier F, Michels AW, Schutte R, Ostrov DA, Chen CB, Phillips EJ, Mallal SA, Mockenhaupt M, Bellón T, Tassaneeyakul W, White KD, Roujeau JC, Chung WH, Hung SI, Identification of drug-specific public TCR driving severe cutaneous adverse reactions., Nat. Commun 10 (2019) 3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Cardone M, Garcia K, Tilahun ME, Boyd LF, Gebreyohannes S, Yano M, Roderiquez G, Akue AD, Juengst L, Mattson E, Ananthula S, Natarajan K, Puig M, Margulies DH, Norcross MA, A transgenic mouse model for HLA-B*57:01-linked abacavir drug tolerance and reactivity., J. Clin. Invest 128 (2018) 2819–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


