Abstract
Heparanase is known to enhance the progression of many cancer types and is associated with poor patient prognosis. We recently reported that after patients with multiple myeloma were treated with high dose chemotherapy, the tumor cells that emerged upon relapse expressed a much higher level of heparanase than was present prior to therapy. Because tumor cells having stemness properties are thought to seed tumor relapse, we investigated whether heparanase had a role in promoting myeloma stemness. When plated at low density and grown in serum-free conditions that support survival and expansion of stem-like cells, myeloma cells expressing a low level of heparanase formed tumor spheroids poorly. In contrast, cells expressing a high level of heparanase formed significantly more and larger spheroids than did the heparanase low cells. Importantly, heparanase-low expressing cells exhibited plasticity and were induced to exhibit stemness properties when exposed to recombinant heparanase or to exosomes that contained a high level of heparanase cargo. The spheroid-forming heparanase-high cells had elevated expression of GLI1, SOX2 and ALDH1A1, three genes known to be associated with myeloma stemness. Inhibitors that block the heparan sulfate degrading activity of heparanase significantly diminished spheroid formation and expression of stemness genes implying a direct role of the enzyme in regulating stemness. Blocking the NF-κB pathway inhibited spheroid formation and expression of stemness genes demonstrating a role for NF-κB in heparanase-mediated stemness. Myeloma cells made deficient in heparanase exhibited decreased stemness properties in vitro and when injected into mice they formed tumors poorly compared to the robust tumorigenic capacity of cells expressing higher levels of heparanase. These studies reveal for the first time a role for heparanase in promoting cancer stemness and provide new insight into its function in driving tumor progression and its association with poor prognosis in cancer patients.
Keywords: heparanase, cancer, myeloma, stem cells, exosomes, chemoresistance
Introduction
Heparanase is upregulated in many cancer types where it promotes aggressive tumor progression and chemoresistance and is associated with poor patient prognosis [1, 2]. In multiple myeloma, despite the development of new therapies in recent years, tumor relapse occurs in most patients. This relapse is likely fueled by a persistent cancer stem cell subpopulation [3]. In a previous study we reported that heparanase expression is relatively low in tumor cells harvested from myeloma patients at initial diagnosis. In contrast, after those same patients were treated with high dose chemotherapy, upon relapse their tumor cells exhibited a much higher level of heparanase expression [4]. Tumor cells from these patients at relapse also expressed elevated levels of ALDH1A1, a gene associated with the myeloma stem cell phenotype [5, 6]. In other studies we demonstrated that heparanase promoted resistance of myeloma cells to anti-myeloma drugs and that by inhibiting heparanase enzyme activity the tumor cells were rendered susceptible to therapy in vitro and in vivo [4, 7, 8]. Together these discoveries indicate that heparanase may be associated with the emergence of myeloma stemness resulting in increased chemoresistance and aggressive tumor behavior.
To test this, in the present study we utilized a cell culture model based on the concept that growth of cells plated at low density in medium devoid of serum and supplemented with basic fibroblast growth factor and epidermal growth factor (“stem cell medium”) will enrich for cells having stem-like properties [9]. Cultures are plated at low cell density to encourage formation of multicellular clusters that arise from a single cell and proliferate to form spheroids (or tumorospheres) whereas cells lacking stemness will not thrive [9]. This spheroid-forming model has been widely employed predominantly to study solid tumors (e.g., breast, prostate, ovarian) [9]. Although not often recognized, myeloma shares characteristics with solid tumors including focal lesions consisting of a high density of tumor cells and a microenvironment rich in blood vessels, immune cells and stromal cells [10]. Using the spheroid-forming model we found that myeloma cells having high heparanase expression readily formed numerous and large spheroids compared to cells having low heparanase expression. Spheroids formed by heparanase-high expressing cells had elevated levels of expression of GLI1, SOX2 and ALDH1A1, three genes associated with the myeloma stemness phenotype [6, 11, 12]. When injected into SCID mice these cells readily formed tumors whereas cells deficient in heparanase expression formed tumors poorly. Together these results implicate heparanase as an important component of myeloma stemness and provide additional rationale for targeting heparanase therapeutically.
Results
Heparanase enhances stemness characteristics of myeloma cells
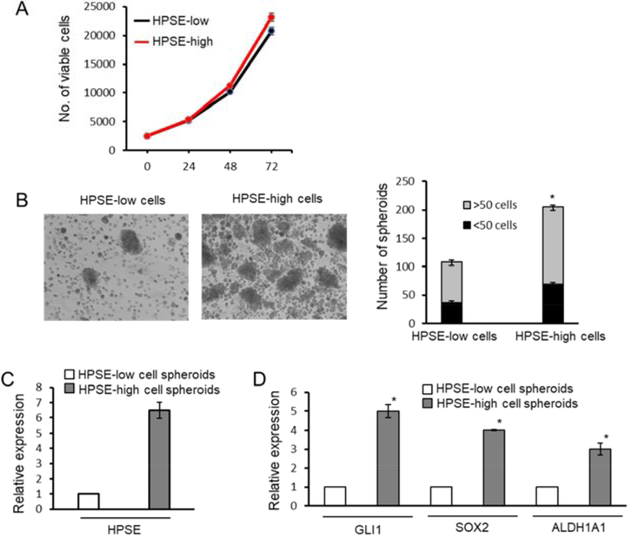
To determine whether heparanase impacts myeloma stemness, we first utilized the human myeloma cell line CAG expressing either a low or high level of heparanase [13]. These HPSE-low cells (transfected with empty vector) and HPSE-high cells (transfected with a cDNA for human heparanase) exhibit a 4-fold difference in their level of heparanase enzyme activity and thus reflect a range of heparanase levels similar to those found in the bone marrow of myeloma patients [13]. When grown in normal cell culture conditions, high cell density and in serum containing medium, the HPSE-low and HPSE-high expressing cells proliferated at almost identical rates (Fig. 1A). When HPSE-low cells and HPSE-high cells were grown at low density in stem cell medium, the number and size of the spheroids formed was significantly higher in cultures containing the CAG HPSE-high cells (Fig. 1B). The level of heparanase expression was assessed by RT-qPCR to confirm that heparanase expression remained high following spheroid formation (Fig. 1C).
Figure 1. Heparanase promotes spheroid formation and expression of genes associated with the cancer stem cell phenotype.
CAG human myeloma cells that endogenously express heparanase at a low level were transfected with empty vector or with a vector containing the cDNA for human heparanase to generate HPSE-low and HPSE-high cells, respectively. A, Cells were grown under normal culture conditions and their proliferation monitored over 72 h. B, HPSE-low and HPSE-high cells were grown in stem cell medium and the size and number of spheroids was determined. Micrographs are representative examples. Original magnification, x100. *P<0.01 for spheroids >50 cells; P<0.05 for spheroids <50 cells. C, Spheroids were assessed for heparanase expression by RT-qPCR. D, GLI1, SOX2 and ALDH1A1 expression was assessed by RT-qPCR in spheroids formed by HPSE-low and HPSE-high cells. *P<0.05. Quantitative data in all panels are the mean of the three independent experiments.
To determine if spheroids formed by HPSE-high cells expressed genes known to be associated with stemness, the levels of GLI1, SOX2 and ALDH1A1 were assessed by RT-qPCR using RNA isolated from spheroids. All three of these stem cell associated genes were significantly higher in spheroids formed by HPSE-high cells compared to spheroids formed by HPSE-low cells (Fig 1D).
Next, we tested inhibitors that block heparanase enzyme activity to determine if these would diminish spheroid formation by the HPSE-high cells. Inhibitors included Roneparstat, a chemically modified heparin having low anti-coagulant activity [14, 15] and OGT 2115, a small molecule chemical inhibitor of heparanase [16]. Each of these heparanase inhibitors diminished spheroid formation by ~50% or greater (Fig. 2), indicating that robust spheroid formation is dependent on heparanase enzyme activity. To determine if this diminished spheroid formation resulting from heparanase inhibition was associated with reduced expression of stemness genes, the expression of GLI1, SOX2 and ALDH1A1 were quantified following exposure of HPSE-high cells to OGT2115. Results reveal that expression of all three genes is dramatically reduced by heparanase inhibition (Fig. 2C). Together these results demonstrate that heparanase enzyme activity is associated with elevated expression of genes that promote stemness as manifested by formation of tumor cell spheroids in culture. Thus, the upregulation of heparanase expression that is often seen in cancer patients may help drive the emergence of a subpopulation of cells having characteristics of tumor stem cells.
Figure 2. Inhibition of heparanase enzyme activity diminishes stemness.
HPSE-high CAG cells were grown in stem cell medium in the absence (control) or presence of heparanase inhibitors (A) Roneparstat (5 nM added every other day beginning on day 2) or (B) small molecule heparanase inhibitor OGT 2115 (10 μM added every other day beginning on day 2). Spheroids composed of >25 cells were counted. Original magnification, x100. *P<0.05. C, GLI1, SOX2 and ALDH1A1 expression was assessed by RT-qPCR in cells grown in stem cell medium in the absence or presence of heparanase inhibitor OGT2115. *P<0.05. Micrographs shown in panels A and B are representative examples and quantitative data in all panels are the mean of the three independent experiments.
Previous studies demonstrated that heparanase promotes translocation of the NF-κB complex to the nucleus with resulting transcription of genes driven by NF-κB [17, 18]. Thus we speculated that activation of the NF-κB pathway was a possible mechanism by which heparanase was enhancing stemness. To test this, HPSE-high CAG cells were exposed to IT-901, a small molecule chemical inhibitor that potently blocks binding of the NF-κB subunit protein c-Rel to DNA [19]. c-Rel is a unique member of the NF-κB family of proteins well known for its role in B and T cell differentiation and function [20]. In the presence of IT-901, a dramatic decrease in the number of spheroids composed of more than 50 cells was observed indicating that NF-κB activity was driving spheroid formation and likely also activating genes that promote stemness (Fig. 3A). This was confirmed by RT-qPCR that revealed decreased stemness was associated with decreased transcription of GLI1, SOX2 and ALDH1 in spheroid cultures exposed to IT-901 (Fig. 3B). ChIP assays demonstrated a direct interaction of NF-κB with the promoter regions of each of these genes (Fig. 3C). Together these results indicate that activation of the NF-κB pathway in HPSE-high cells leads to transcription of genes that support tumor cell stemness.
Figure 3. Heparanase promotes expression of stemness via activation of the NF-kb pathway.
A, Heparanase-high CAG cells were grown in spheroid forming conditions for 48 h followed by addition of DMSO (control) or 3 μM of the NF-κB small molecule chemical inhibitor IT-901. Media were replaced every 48 h and fresh DMSO or IT-901 were added and spheroids were quantified. *P<0.05 for spheroids >50 cells and for spheroids <50 cells. B, The level of expression of GLI1, SOX2 and ALDH1A1 in the absence or presence of IT-901 was assessed by RT-qPCR. *P<0.05. C, NF-kB binding to promoter regions of GLI1, SOX2 and ALDH1A1 was determined by ChIP assay utilizing monoclonal antibody to the P65 protein subunit of NF-κB. IgG was used as a control for immunoprecipitation. NF-κB consensus binding sequences within promoter regions of the three genes are shown in the boxes to the right of the gels. Micrographs shown in panel A are representative examples and quantitative data in A and B are the mean of the three independent experiments.
Development of drug resistance in myeloma cells is associated with enhanced heparanase expression and stemness properties
Substantial evidence indicates that cancer stem cells contribute to the development of cancer chemoresistance [3]. Our previous work demonstrated that as myeloma patients relapse and become refractory to chemotherapy, their tumor cells exhibit a dramatic increase in heparanase expression [4]. We also found that elevation of heparanase expression contributes to myeloma cell chemoresistance [4]. To further explore the role of heparanase in driving myeloma stemness and resulting chemoresistance, we utilized the RPMI-8226/DOX40 cell line. These cells were derived from the RPMI-8226 human myeloma cell line and became drug resistant following exposure to increasing concentrations of doxorubicin [21]. When grown in stem cell medium, the DOX-resistant cells exhibited more and larger spheroids than their wild-type RPMI-8226 DOX-sensitive counterparts (Fig. 4A). Because this was similar to the pattern of spheroid formation by CAG HPSE-high and HPSE-low myeloma cells (Fig. 1), we speculated that heparanase expression was elevated in spheroids formed by DOX-resistant cells. This was confirmed by RT-qPCR (Fig. 4B). Blocking heparanase activity with Roneparstat diminished spheroid formation confirming a role for heparanase in stemness of these DOX-resistant cells (Fig. 4C). Furthermore, spheroids formed by the DOX-resistant cells had elevated levels of cancer stem cell associated genes GLI1, SOX2, and ALDH1A1 (Fig. 4D). Together these data imply a strong relationship between elevation of heparanase expression, development of drug resistance and enhanced tumor cell stemness.
Figure 4. Myeloma cells resistant to doxorubicin exhibit enhanced heparanase expression and stemness properties.
A, RPMI-8226 wild-type (DOX-sensitive) and 8226/DOX40 (DOX-resistant) cells growing in stem cell medium were examined for their capacity to form spheroids. Original magnification, x100. *P<0.01 for spheroids >50 cells; P<0.05 for spheroids <50 cells. B, Spheroids formed by Dox-sensitive or Dox-resistant cells were assessed for heparanase expression by RT-qPCR. C, Spheroid formation was assessed in the absence or presence of Roneparstat (5 nM added every other day beginning on day 2). *P<0.05. D, Spheroids formed by DOX-resistant and DOX-sensitive cells were assessed by RT-qPCR for expression of GLI1, SOX2 and ALDH1A1. *P<0.05 Micrographs shown in panel A are representative examples and quantitative data in all panels are the mean of the three independent experiments.
Exosomes containing a high level of heparanase cargo enhance the stemness of their target cells
Data in Figs. 1 and 3 demonstrate that upregulation of heparanase either by transfection or during development of chemoresistance endows myeloma cells with stemness properties. We recently discovered that another mechanism for increasing the level of heparanase in cells is via transfer of heparanase by exosomes into target cells. When CAG HPSE-high myeloma cells were treated with anti-myeloma drugs (either bortezomib, carfilzomib or melphalan), exosome biogenesis and secretion was dramatically enhanced and those exosomes, referred to as chemoexosomes, contained a high level of heparanase as cargo [22]. The heparanase was present on the exosome surface and was active. When incubated with tumor or host cells, chemoexosomes transferred heparanase to those cells and impacted their biological behavior [22]. When wild-type CAG myeloma cells (that express a low level of heparanase) growing in stem cell medium were exposed to chemoexosomes secreted by bortezomib-treated myeloma cells, the cells formed larger and more spheroids than cells exposed to exosomes harvested from cells not treated with bortezomib (control exosomes) (Fig. 5A). These control exosomes contain a low level of heparanase cargo [22]. Spheroids formed in the presence of chemoexosomes express elevated levels of GLI1, SOX2 and ALDH1A1 compared to spheroids formed in the presence of control exosomes (Fig. 5C). Because exosomes carry cargo composed of an array of proteins, nucleic acids and other components, to confirm heparanase as a driver of stemness we added purified recombinant heparanase (rHPSE) directly to CAG wild-type cells. We and others have demonstrated that rHPSE when added to cells can be taken up by those cells and impact their behavior [23–26]. Results in Fig. 5B confirm that rHPSE alone can enhance spheroid formation by myeloma cells. This is important because we have demonstrated that soluble active heparanase is present in the plasma harvested from bone marrow of myeloma patients [13] and thus could be available to impact stemness of tumor cells within the marrow.
Figure 5. Exosomes secreted following chemotherapy enhance stemness.
A, CAG wild-type cells (that express a low level of heparanase) growing in spheroid forming conditions were treated with control exosomes or chemoexosomes isolated from the medium of HPSE-high CAG cells exposed to bortezomib. B, CAG wild-type cells growing in spheroid forming conditions without or with addition every other day of 10 ng/ml of enzymatically active recombinant human heparanase. C, CAG wild-type cells growing in spheroid forming conditions were treated with control exosomes or chemoexosomes and assessed by RT-qPCR for the level of expression of GLI1, SOX2 and ALDH1A1. *P<0.05. D, Exosomes were isolated from the serum of a 68 year old male patient treated with revlimid and having active myeloma disease. The control was from an age and sex-matched healthy subject. Equal numbers of exosomes from each sample were extracted, loaded on an SDS-PAGE gel and probed by western blotting for expression of HPSE and CD63. E, Exosomes isolated from a healthy control or myeloma patient were incubated with CAG wild-type cells growing in spheroid forming conditions. F, CAG cells in which heparanase was knocked down (HPSE KD) were treated with control exosomes or myeloma patient-derived exosomes and spheroid formation was examined. Original magnification, x100. Micrographs shown in panels A, B, E and F are representative examples and quantitative data in panel C are the mean of the three independent experiments.
To examine the potential clinical relevance of these findings, exosomes were isolated from the serum of a patient that had previously undergone anti-myeloma drug therapy and from a healthy control. Western blotting of extracts of equal numbers of purified exosomes revealed that heparanase was readily detectable in myeloma patient exosomes but very low in exosomes from the healthy control (Fig. 5D). Western blotting for CD63, a marker present on most exosomes, confirmed equivalent loading of the gel. Incubation of these purified, myeloma patient-derived exosomes with wild-type CAG cells enhanced spheroid formation compared to CAG cells incubated with exosomes isolated from healthy control (Fig. 5E). Because wild-type CAG cells express a low level of heparanase that might contribute to spheroid formation, we also tested the impact of exosomes from a myeloma patient and control on CAG cells in which heparanase had been knocked down [27]. Exosomes from the healthy control had little effect on cells lacking heparanase and only very few small spheroids formed. In contrast, exosomes from the myeloma patient promoted abundant spheroid formation by the heparanase deficient cells (Fig. 5F). Together these data indicate that the exosomes secreted following exposure of cells to chemotherapy have the potential to enhance stemness properties of cancer cells.
Myeloma cells deficient in heparanase lack stemness characteristics and grow poorly at metastatic sites in vivo
We previously reported that the heparanase inhibitor Roneparstat significantly inhibits human myeloma tumor cell growth in SCID mice [15]. This is consistent with a role for heparanase in enhancing stemness as demonstrated in Fig. 2 where Roneparstat blocked spheroid formation by myeloma cells. To further confirm the role of heparanase in stemness and tumor growth, we hypothesized that knockdown of heparanase would diminish the stemness properties of myeloma cells resulting in poor growth of the cells in vivo. We tested this in two different cell lines using multiple shRNA sequences. Knockdown of heparanase expression did not diminish viability of CAG cells growing in normal serum-containing culture medium (Fig. 6A). However, when grown in stem cell medium, spheroids were dramatically reduced in number and size compared to control knockdown cells, indicating that depletion of heparanase diminishes the number of stem-like cells (Fig. 6B, left panel). Expression of genes associated with the stem cell phenotype is substantially reduced in the cells following knockdown of heparanase expression (Fig. 6B, right panel). To determine if loss of stemness in the heparanase knockdown cells is associated with their ability to form tumors in vivo, control and heparanase knockdown cells were injected into the tail vein of SCID mice. Heparanase knockdown cells grew poorly in mice compared to control knockdown cells as demonstrated by measuring the level of human kappa light chain in mouse plasma and by bioluminescent imaging of the animals five weeks after tumor cell injection (Fig. 6C). To confirm these findings we also utilized MM1.R cells, a human myeloma cell line resistant to dexamethasone that expresses a high level of heparanase (Fig. 7A, left panel). Heparanase was dramatically reduced in knockdown cells and this reduction of heparanase level did not impact cell viability when cells were grown under normal culture conditions (Fig. 7A, right panel). Similar to what was seen with CAG cells, the MM1.R heparanase knockdown cells had a diminished ability to form spheroids (Fig. 7B) and grew poorly in SCID mice (Fig. 7C) compared to control knockdown cells.
Figure 6. Knockdown of heparanase diminishes spheroid formation and tumor growth in vivo.
A, CAG control knockdown (Ctrl KD) and CAG HPSE KD cells were tested for their viability in normal culture conditions using an MTT assay (left panel). B, Control and heparanase knockdown cells were analyzed for their ability to form spheroids (left panel; original magnification X100) and expression of stem cell genes assessed by RT-qPCR (right panel). C, To examine the effect of loss of stemness on tumor formation in vivo, Ctrl KD or HPSE KD cells were injected via the tail vein of SCID mice. Sera were collected 5 weeks after tumor cell injection and tumor burden determined by measuring the amount of human kappa light chain in the sera of the mice (left panel; each symbol represents the kappa light chain level in an individual animal, bars denote the mean kappa level for each group, *p<0.05 vs Ctrl KD). Bioluminescent imaging confirms low tumor burden in animals injected with HPSE KD cells (right panel).
Figure 7. Knockdown of heparanase diminishes spheroid formation and tumor growth in vivo.
A, Western blot demonstrating HPSE level following transduction of MM1.R cells with either control shRNA (Ctrl KD) or with one of two non-overlapping shRNAs that target HPSE expression (KD1, KD2) (left panel). MTT assay demonstrates control and knockdown cells grow at the same rate in normal culture conditions (right panel). B, MM1.R Ctrl KD and HPSE KD2 cells were examined in spheroid formation assays. C, MM1.R Ctrl KD and HPSE KD1 or HPSE KD2 cells were injected intravenously and tumor burden was determined at two week intervals by quantifying human lambda light chain present in sera of the mice. # p<0.05 versus MM1.R HPSE KD1 and * p<0.05 versus MM1.R HPSE KD2.
Discussion
The work reported here demonstrates for the first time that elevation of heparanase promotes stem-like properties of human myeloma cells. The data supporting this includes: i) heparanase enhances tumor spheroid formation, ii) heparanase stimulates expression of genes associated with stemness, iii) inhibition of heparanase enzyme activity diminishes spheroid formation and expression of stemness genes, and iv) cells deficient in heparanase expression, even when injected in high numbers, form tumors poorly in mice compared to cells that express the enzyme. Additionally, mechanistic studies revealed that the impact of heparanase on stemness is due at least in part to activation of the NF-κB pathway that results in expression of genes associated with stemness. Together these studies reveal that heparanase can influence myeloma stemness thereby contributing to tumor drug resistance, survival and proliferation (Fig. 8).
Figure 8. Model depicts the proposed role of HPSE in promoting stemness.
(1) Heparanase levels were increased experimentally by transfection, by exposure of cells to chemotherapy, to recombinant HPSE (rHPSE) or to exosomes carrying heparanase cargo. (2) The increase in HPSE leads to activation of the NF-κB pathway, upregulating the expression of stemness genes GLI1, SOX2 and ALDH1A1. (3) Expression of stemness genes promotes drug resistance, tumor cell survival and proliferation. Treatment of cells with heparanase inhibitors or NF-κB inhibitors diminishes the cells ability to form spheroids and inhibits expression of stemness genes.
The intriguing finding that the presence of heparanase can shift cells toward a stemness phenotype implies that a population of these cells maintain a high level of plasticity. This is consistent with studies demonstrating conversion of bulk tumor cells into cancer stem cells. For example, in glioblastoma, the capacity for self-renewal and tumor initiation are not restricted solely to a homogeneous population of stem cells but can include cells expressing a range of markers [28]. Importantly, in the myeloma cells studied here, we observed plasticity in several different myeloma cell lines and this occurred when the level of heparanase in cells was enhanced by multiple methods including transfection with a heparanase cDNA, transfer of heparanase via exosomes [29] or exposure of cells to rHPSE (Figs. 1 and 5). Additionally, we saw the same stemness promoting effect of heparanase when its expression was acquired during development of chemoresistance by RPMI-8226/DOX40 or MM1.R myeloma cells (Figs. 4 and 7). This indicates that the mechanism by which heparanase is enhanced is not critical, rather it’s the presence of heparanase that enhances the stem-like phenotype.
The finding that heparanase enzyme activity is required for heparanase-mediated promotion of stemness of these cells implies a role for heparan sulfate (Fig. 2). Heparanase clips heparan sulfate chains, leaving them shortened but still attached to the proteoglycan core protein, which subsumes a critical role in matrix modeling and remodeling [30–33]. However, this activity of heparanase does release fragments of heparan sulfate that can have a multitude of bound growth factors and cytokines that potentially regulate stemness. For example, heparan sulfate modulates the notch, hedgehog and Wnt pathways, all of which are associated with the stem cell phenotype [34]. Several roles for heparan sulfate in stem cell biology have been identified. Heparan sulfates were linked to regulation of embryonic stem cells [35] where it was demonstrated that impaired heparan sulfate expression or undersulfation of heparan sulfate inhibited embryonic stem cell differentiation [36–38]. In Drosophila, glypican heparan sulfate proteoglycans were found to restrict morphogens to distinct locales and then activate stem cells in trans thereby ensuring retention of their pluripotent state [39]. Thus, it is reasonable to envision that heparanase modification of heparan sulfate could regulate stem cell behavior.
Although to date there is limited information on the role of heparanase in stem cell biology, embryonic stem cells from heparanase transgenic mice proliferated more rapidly than wild-type stem cells and overexpression of heparanase in neural stem and progenitor cells enhanced Erk and Akt phosphorylation [40]. Heparanase also modulates the clonogenicity, proliferation and migration of bone marrow-derived mesenchymal stem cells [41] and heparanase overexpressing mice exhibited increased proliferation and retention of primitive, undifferentiated Sca-1+/c-Kit+/Lin− cells in the bone marrow [42]. Of additional importance is the fact that shortening of heparan sulfate chains by heparanase enhances the shedding of proteoglycans from cell surfaces including syndecan-1 (CD138), a heparan sulfate proteoglycan present in high levels on myeloma cells [43]. This shed proteoglycan remains biologically active and can bind to factors within the extracellular milieu or bind to and activate cell surface receptors [24, 44].
As an indicator of stemness, in the present study we focused not only on spheroid formation but also on the expression of the GLI1, SOX2 and ALDH1A1 genes, all of which have been associated with myeloma stemness. The hedgehog pathway is known to be activated in stem cells from several types of cancer including myeloma [11]. GLI1 is a downstream effector of hedgehog signaling and drives the transcription of genes required for myeloma stem cell self-renewal and the maintenance of those cells in an undifferentiated state [11, 45]. Tumor cells expressing SOX2 can initiate and propagate tumor growth and are strongly associated with the cancer stem cell phenotype of multiple cancer types [46]. Moreover, SOX2 is required for clonogenic growth of myeloma cells [47] and T-cell immunity against SOX2 has been shown to correlate with reduced risk of progression of monoclonal gammopathy to clinical myeloma [48].
The finding of ALDH1A1 upregulation in HPSE-high spheroids is notable because ALDH1 activity is known to be increased in myeloma stem cells [6]. ALDH1A1 promotes tumor initiation, proliferation and drug resistance and it activates drug efflux pumps as well as survival proteins AKT and BCL2. Serial gene expression profiling of purified tumor cells from myeloma patients over the course of their therapy revealed that ALDH1A1 is the predominant form of ALDH1 expressed in myeloma [5]. The level of expression of ALDH1A1 increased substantially upon tumor relapse following high dose chemotherapy and autologous stem cell transplant in all nine patients tested. Probing of those same patient samples for their level of heparanase expression demonstrated that in eight of the nine patients, heparanase also increased substantially upon relapse following high dose therapy and transplant [4]. This remarkable correlation between ALDH1A1 and heparanase expression in patients provides a strong clinical correlation to our finding that heparanase enhances spheroid formation and ALDH1A1 gene expression in vitro.
Interestingly, ALDH1A1 also increases the mRNA and protein level of NIMA-related kinase 2 (NEK2). NEK2 in turn activates the NF-κB pathway leading to increased heparanase expression in myeloma [49]. In the present work, we found an NF-κB binding site in the ALDH1A1 gene and that inhibition of NF-κB diminished ALDH1A1 expression (Fig. 3). We speculate that when heparanase levels are enhanced there is subsequent feedback on the NF-κB pathway that stimulates nuclear translocation of NF-κB leading in turn to increased ALDH1A1 expression (see Fig. 8).
The finding that NF-κB regulates the heparanase driven emergence of stemness sheds new light on the relationship between this important transcription factor and heparanase function. Previous studies revealed that HSV-1 infection of cells upregulates heparanase through NF-κB activation [50]. Moreover, when human corneal epithelial cells that overexpressed heparanase were infected with HSV-1, NF-κB translocation from the cytoplasm to nucleus was significantly enhanced [17]. A similar feedback loop was proposed as a mechanism for heparanase promotion of the tumor inflammatory microenvironment. In this scenario, tumor associated macrophages induce heparanase expression in tumor cells; heparanase then feeds back on the macrophages to stimulate NF-κB activation and gene expression [18]. We previously demonstrated that exposure of myeloma cells to anti-myeloma drugs activates the NF-κB pathway and this upregulates heparanase expression [8]. In the present work we demonstrate that enhanced heparanase expression activates NF-κB to drive expression of stemness genes (Fig. 3). The documentation of this relationship between NF-κB, heparanase expression and subsequent activation of the NF-κB pathway in three different biological settings underscores the importance of this mechanism in heparanase related disease pathogenesis.
Our findings related to heparanase and NF-κB provide important mechanistic insight into how anti-tumor drugs cultivate the emergence of stem-like clones that are resistant to those drugs, thereby leading to tumor relapse. We previously reported that several drugs used in myeloma therapy stimulate the expression and release of heparanase that can be taken up by other tumor cells potentially enhancing their stemness and chemoresistance [8]. This implies that inhibition of heparanase activity would diminish tumor relapse, and in previous work we found this to be the case [4]. This implies that other heparanase inhibitors such as PG545 might have the same effect on diminishing stem cells and eventual tumor relapse [51]. Following injection of heparanase-high cells in mice, they were treated with melphalan for two weeks followed by treatment for two weeks with the heparanase inhibitor Roneparstat or followed by treatment with vehicle only. Tumor relapse was detected in 3 of 10 mice receiving Roneparstat but in 7 of 11 mice receiving vehicle [4]. Based on the new data we report here on the relationship between heparanase and stemness, we speculate that the reduced relapse in the Roneparstat treated animals was due to a diminished population of stem-like cells resulting from heparanase inhibition. This speculation is further supported by knockdown studies reported here. Knockdown of heparanase expression had no effect on growth of tumor cells in serum containing medium, but when grown in conditions favorable for stem cell proliferation the heparanase knockout cells formed spheroids poorly. Additionally, knockdown of heparanase dramatically diminished the ability of these cells to form tumors in vivo compared to controls (Figs. 6 and 7). The inability of these heparanase deficient cells to form spheroids in vitro or colonize and grow in vivo strongly indicates that the number of stem-like tumor initiating cells diminished substantially in the absence of heparanase. Together, these results demonstrate a role for heparanase in promoting a stem-like phenotype in myeloma and provide further rationale for therapeutic targeting of heparanase in patients to interfere with tumor relapse.
Experimental Procedures
Cell lines and reagents
Human myeloma cell lines utilized were: CAG (obtained from Dr. Joshua Epstein), MM1.R (obtained from Dr. Steven Rosen) and RPMI-8226/DOX40 cells (obtained from Dr. William Dalton). All cell lines were expanded and frozen in multiple vials upon receipt. All experiments were carried out within six weeks of thawing cells and not passaged more than five times. Cells were routinely tested for mycoplasma and retested prior to in vivo experiments. Generation of CAG cells expressing a high level of heparanase (HPSE-high) has been described [13]. The level of heparanase activity in HPSE-high cells is comparable to levels detected in bone marrow of many myeloma patients [13] and thus represent a physiologically relevant model for studying heparanase function in myeloma. These cells also express luciferase, which enabled monitoring of their location and growth in vivo. Myeloma cell lines were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum.
Antibodies to the various proteins used in this study included the following: anti-human heparanase polyclonal antibody 1453, a kind gift from Dr. Israel Vlodavsky [52], anti-human CD63 (abcam), and anti-human GAPDH (Cell Signaling). The heparanase inhibitor Roneparstat was provided by Leadiant Biosciences S.A. and heparanase inhibitor OGT2115 and NF-κB inhibitor IT-901 were from Tocris Biosciences.
Spheroid assays
Myeloma cells growing under normal cell culture conditions in log phase were harvested, washed and counted. Cells were seeded at low density (3,000 – 10,000 cells/well) and grown in conditions that support stem-like cell growth and spheroid cluster formation as previously described [53, 54]. In brief, cells were carefully dispersed as single cells, and cultured in a six well plate containing 2 ml/well of stem cell specific serum free media composed of DMEM/F-12 (1:1 ratio) supplemented with 1% penicillin-streptomycin, B27 and N2 supplements and recombinant human epidermal growth factor (EGF) and fibroblast growth factor (FGF) (both from Invitrogen). This defined media supports and enriches for the growth of stem cells, and with time cells proliferate to form floating spheroids. Cells in each well were pipetted every other day to release cells that were loosely attached to spheroids. Cultures were grown for 10–12 days with additional EGF (20 ng/ml) and FGF (20 ng/ml) added to the media every 72 h to boost growth. Cell cultures were observed daily to ensure that spheroids were forming as a result of cell multiplication and not due to clustering of multiple cells. At termination of the experiment, cultures were examined under an inverted phase contrast microscope and the number of spheroids in each well with >50 cells (large spheroids) or with 15–50 cells (small spheroids) was determined.
RNA isolation and RT-qPCR
Spheroids were washed thrice with PBS and RNA was isolated using RNeasy columns (Qiagen). 1 μg of total RNA was reverse transcribed using Applied Biosystems High Capacity cDNA synthesis kit for RT-qPCR. (ThermoFisher Scientific). 20–100 ng of cDNA was used to perform RT-qPCR using Applied Biosystems gene expression analysis kit with Taqman assays (ThermoFisher Scientific) according to the manufacturer’s protocol. The primers used were GLI1 (Hs01110772_g1), SOX2 (Hs01053049_s1), ALDH1A1 (Hs00355908_m1), HPSE (Hs00935036_m1), GAPDH (Hs02786624_g1). The real time PCR cycle parameters and analyses were performed according to the manufacturer’s instructions.
ChIP assay
Chromatin was isolated from spheroids formed by CAG HPSE-high cells and subjected to ChIP using the Chromatin Immunoprecipitation Kit following manufacturer’s suggested protocol (Millipore). Mouse IgG supplied with the kit was utilized as a negative control and chromatin containing NF-κB was immunoprecipitated using a monoclonal antibody to NF-κB-P65. Promoter regions (defined as 1000 bp upstream of the ATG) for GLI1, SOX2 and ALDH1A1 were analyzed for putative NF-κB binding sites using MatInspector (Genomatix, Munich, Germany). The primers used for PCR were GLI1 5’-ATCTGGTTGTCGGGGCCTCT-3’ (forward) and 5’-TGGAGGATGAGGCGGGGTAG-3’ (reverse), SOX2 5’-AAGGCGTGTGGTGTGACCTG-3’ (forward) and 5’-ACGGGGGCTGTCAGGGAATA-3’ (reverse), ALDH1A1 5’-CTGGCCTTAGTGGCCAGAGC-3’ (forward) and 5’-ACACCTAGGGCAGGAAGCCT-3’ (reverse).
Exosome isolation from cell culture and from sera of myeloma patients
Exosomes were isolated from media conditioned by human myeloma cell lines. Cells were washed thrice with PBS and grown in serum-free medium for 16 h. Exosomes secreted into the medium by myeloma cells were isolated by differential centrifugation and characterized as described previously [22]. For exosomes isolated from myeloma patient sera, samples were obtained from a multiple myeloma patient or healthy control enrolled in the Molecular And Genetic Epidemiology (iMAGE) study of myeloma who met the revised and updated International Multiple Myeloma Working Group classification criteria for myeloma [55, 56]. Approval from the Institutional Review Board in accordance with the Declaration of Helsinki was obtained prior to study initiation, and informed consent was obtained from all individual participants included in the study. Exosomes were isolated from myeloma patient sera using an ExoQuick isolation kit (System Biosciences). Briefly, 30 μl of ExoQuick solution was added to 100 μl of serum and incubated at 4°C for 1 h followed by centrifugation at 1500 × g for 30 min. Particle size and number were assessed using a NanoSight 300 according to manufacturer’s protocol.
Stable knockdown of heparanase expression
Heparanase knockdown in CAG cells was previously described [27]. Heparanase expression was knocked down in MM1.R cells using MISSION® lentiviral transduction particles from Sigma. Briefly, 50 μl of lentiviral particles bearing non-overlapping shRNAs (TRCN0000142009 or TRCN0000142328) targeting human heparanase were mixed with cells in serum free media and incubated for 24 h. Cells were washed with complete growth media and stable transfectants were selected using puromycin (5 μg/mL). Heparanase knockdown was verified by western blotting. The control was prepared by transducing MM1.R cells with MISSION® non-target shRNA lentiviral transduction particles (Sigma, SHC002V).
Western blotting
For preparing whole cell lysates, cells were washed and incubated in lysis buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 0.5% Triton X-100) containing 1× HALT protease and phosphatase inhibitor mixture (Pierce) for 30 min. Lysates were centrifuged at 12,000 × g at 4 °C for 15 min and supernatants were removed from the pellets. Equal amounts of total protein were loaded onto 4–20% gradient SDS-polyacrylamide gels (Bio-Rad, Richmond, CA), transferred to a nitrocellulose membrane (Schleicher & Schuell) and probed with specific primary antibodies followed by horseradish peroxidase-conjugated secondary anti-mouse antibody (GE Healthcare). Immunoreactive bands were detected using enhanced chemiluminescence (GE Healthcare).
For exosomes, following quantification of exosome number using a NanoSight 300, samples containing equal numbers of exosomes were lysed using ice-cold RIPA buffer supplemented with protease and phosphatase inhibitors (Roche) and prepared in 5× Laemmli’s buffer and heated to 100°C for 15 min. Samples were denatured and resolved by SDS-PAGE. Gels were electroblotted onto a nitrocellulose membrane and blocked 1 h in 5% BLOTTO milk powder in Tris-buffered saline containing 0.1% Tween-20 (TBST). Membranes were incubated with primary antibody for 2 h, followed by peroxidase-conjugated secondary antibody in TBST containing 0.1% Tween-20 for 1 h. Immunoreactive bands were detected using enhanced chemiluminescence (Pierce).
Animal studies
CB.17/ICR SCID female mice (weight 20 g, age eight weeks) were obtained from Charles River Breeding Laboratories and were housed and monitored in the animal facility at the University of Alabama at Birmingham (UAB). All the animals were handled as per protocols and procedures approved by the UAB Institutional Animal Care and Use Committee (Protocol:100408732). To establish disseminated myeloma tumors, 8X106 MM.1R cells or 3X106 CAG cells were injected intravenously into the lateral tail vein of SCID mice. In each experiment, ten animals were included in each experimental group. Sera from animals were collected at specified intervals, stored at −80 °C and the level of human immunoglobulin kappa (CAG) or lambda (MM1.R) light chain was determined by ELISA (Bethyl Laboratories) as a measure of whole animal tumor burden. The animals were monitored regularly, weighed, and animals bearing CAG tumors were imaged weekly for bioluminescence using an IVIS-100 system (Xenogen Corporation).
Statistical analyses
All experiments were analyzed by Student t-test using GraphPad Prism software or Excel. P values less than 0.05 was considered statistically significant. All data are presented as mean plus or minus standard error of the mean (SEM).
Highlights.
Elevation of heparanase expression in myeloma cells leads to enhanced tumor cell stemness.
Blocking heparanase enzyme activity causes loss of stemness.
Mechanistically, NF-κB acts downstream of heparanase to promote expression of genes associated with stemness.
Tumor cells made deficient in heparanase exhibit decreased stemness properties in vitro and poor growth in vivo.
Acknowledgments
Funding: This work was supported by National Institutes of Health grants CA138340 (RDS), CA211752 (RDS), CA186646 (EEB) and the United States – Israel Binational Science Foundation grant 2015240 (RDS).
Abbreviations
- ChIP
chromatin immunoprecipitation
- EGF
epidermal growth factor
- FGF
fibroblast growth factor
- HPSE
heparinase
- KD
knockdown
- NF-κB
nuclear factor kappa light chain enhancer of activated B cells
- Nek-2
NIMA-related kinase 2
- rHPSE
recombinant heparanase
Footnotes
Conflict of interest disclosure: Roneparstat is a proprietary drug of Leadiant Biosciences SA. RDS previously received grant funding from Leadiant Biosciences SA and was a member of their Scientific Advisory Board. No potential conflicts of interest were disclosed by the other authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Vlodavsky I, Singh P, Boyango I, Gutter-Kapon L, Elkin M, Sanderson RD, Ilan N, Heparanase: From basic research to therapeutic applications in cancer and inflammation, Drug Resist Updat 29 (2016) 54–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Boyango I, Barash U, Fux L, Naroditsky I, Ilan N, Vlodavsky I, Targeting heparanase to the mammary epithelium enhances mammary gland development and promotes tumor growth and metastasis, Matrix Biol 65 (2018) 91–103. [DOI] [PubMed] [Google Scholar]
- [3].Huff CA, Matsui W, Multiple myeloma cancer stem cells, J Clin Oncol 26 (2008) 2895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ramani VC, Zhan F, He J, Barbieri P, Noseda A, Tricot G, Sanderson RD, Targeting heparanase overcomes chemoresistance and diminishes relapse in myeloma, Oncotarget 7 (2016) 1598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yang Y, Zhou W, Xia J, Gu Z, Wendlandt E, Zhan X, Janz S, Tricot G, Zhan F, NEK2 mediates ALDH1A1-dependent drug resistance in multiple myeloma, Oncotarget 5 (2014) 11986–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Matsui W, Wang Q, Barber JP, Brennan S, Smith BD, Borrello I, McNiece I, Lin L, Ambinder RF, Peacock C, Watkins DN, Huff CA, Jones RJ, Clonogenic multiple myeloma progenitors, stem cell properties, and drug resistance, Cancer Res 68 (2008) 190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ramani VC, Purushothaman A, Stewart MD, Thompson CA, Vlodavsky I, Au JL, Sanderson RD, The heparanase/syndecan-1 axis in cancer: mechanisms and therapies, FEBS J 280 (2013) 2294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ramani VC, Vlodavsky I, Ng M, Zhang Y, Barbieri P, Noseda A, Sanderson RD, Chemotherapy induces expression and release of heparanase leading to changes associated with an aggressive tumor phenotype, Matrix Biol 55 (2016) 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Weiswald LB, Bellet D, Dangles-Marie V, Spherical cancer models in tumor biology, Neoplasia 17 (2015) 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kawano Y, Moschetta M, Manier S, Glavey S, Gorgun GT, Roccaro AM, Anderson KC, Ghobrial IM, Targeting the bone marrow microenvironment in multiple myeloma, Immunol Rev 263 (2015) 160–72. [DOI] [PubMed] [Google Scholar]
- [11].Peacock CD, Wang Q, Gesell GS, Corcoran-Schwartz IM, Jones E, Kim J, Devereux WL, Rhodes JT, Huff CA, Beachy PA, Watkins DN, Matsui W, Hedgehog signaling maintains a tumor stem cell compartment in multiple myeloma, Proc Natl Acad Sci U S A 104 (2007) 4048–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Spisek R, Kukreja A, Chen LC, Matthews P, Mazumder A, Vesole D, Jagannath S, Zebroski HA, Simpson AJ, Ritter G, Durie B, Crowley J, Shaughnessy JD Jr., Scanlan MJ, Gure AO, Barlogie B, Dhodapkar MV, Frequent and specific immunity to the embryonal stem cell-associated antigen SOX2 in patients with monoclonal gammopathy, J Exp Med 204 (2007) 831–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kelly T, Miao HQ, Yang Y, Navarro E, Kussie P, Huang Y, MacLeod V, Casciano J, Joseph L, Zhan F, Zangari M, Barlogie B, Shaughnessy J, Sanderson RD, High heparanase activity in multiple myeloma is associated with elevated microvessel density, Cancer Res 63 (2003) 8749–56. [PubMed] [Google Scholar]
- [14].Pala D, Rivara S, Mor M, Milazzo FM, Roscilli G, Pavoni E, Giannini G, Kinetic analysis and molecular modeling of the inhibition mechanism of roneparstat (SST0001) on human heparanase, Glycobiology 26 (2016) 640–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ritchie JP, Ramani VC, Ren Y, Naggi A, Torri G, Casu B, Penco S, Pisano C, Carminati P, Tortoreto M, Zunino F, Vlodavsky I, Sanderson RD, Yang Y, SST0001, a chemically modified heparin, inhibits myeloma growth and angiogenesis via disruption of the heparanase/syndecan-1 axis, Clin Cancer Res 17 (2011) 1382–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].McKenzie EA, Heparanase: a target for drug discovery in cancer and inflammation, Br. J. Pharmacol. 151 (2007) 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Agelidis AM, Hadigal SR, Jaishankar D, Shukla D, Viral Activation of Heparanase Drives Pathogenesis of Herpes Simplex Virus-1, Cell Rep 20 (2017) 439–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Goldberg R, Meirovitz A, Hirshoren N, Bulvik R, Binder A, Rubinstein AM, Elkin M, Versatile role of heparanase in inflammation, Matrix Biol 32 (2013) 234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Shono Y, Tuckett AZ, Liou HC, Doubrovina E, Derenzini E, Ouk S, Tsai JJ, Smith OM, Levy ER, Kreines FM, Ziegler CG, Scallion MI, Doubrovin M, Heller G, Younes A, O’Reilly RJ, van den Brink MR, Zakrzewski JL, Characterization of a c-Rel Inhibitor That Mediates Anticancer Properties in Hematologic Malignancies by Blocking NF-kappaB-Controlled Oxidative Stress Responses, Cancer Res 76 (2016) 377–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gilmore TD, Gerondakis S, The c-Rel Transcription Factor in Development and Disease, Genes Cancer 2 (2011) 695–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tong AW, Lee J, Wang RM, Dalton WS, Tsuruo T, Fay JW, Stone MJ, Elimination of chemoresistant multiple myeloma clonogenic colony-forming cells by combined treatment with a plasma cell-reactive monoclonal antibody and a P-glycoprotein-reactive monoclonal antibody, Cancer Res 49 (1989) 4829–34. [PubMed] [Google Scholar]
- [22].Bandari SK, Purushothaman A, Ramani VC, Brinkley GJ, Chandrashekar DS, Varambally S, Mobley JA, Zhang Y, Brown EE, Vlodavsky I, Sanderson RD, Chemotherapy induces secretion of exosomes loaded with heparanase that degrades extracellular matrix and impacts tumor and host cell behavior, Matrix Biol 65 (2018) 104–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Spyrou A, Kundu S, Haseeb L, Yu D, Olofsson T, Dredge K, Hammond E, Barash U, Vlodavsky I, Forsberg-Nilsson K, Inhibition of Heparanase in Pediatric Brain Tumor Cells Attenuates their Proliferation, Invasive Capacity, and In Vivo Tumor Growth, Mol Cancer Ther 16 (2017) 1705–1716. [DOI] [PubMed] [Google Scholar]
- [24].Purushothaman A, Uyama T, Kobayashi F, Yamada S, Sugahara K, Rapraeger AC, Sanderson RD, Heparanase-enhanced shedding of syndecan-1 by myeloma cells promotes endothelial invasion and angiogenesis, Blood 115 (2010) 2449–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yang Y, Macleod V, Miao HQ, Theus A, Zhan F, Shaughnessy JD Jr., Sawyer J, Li JP, Zcharia E, Vlodavsky I, Sanderson RD, Heparanase enhances syndecan-1 shedding: A novel mechanism for stimulation of tumor growth and metastasis, J Biol Chem 282 (2007) 13326–33. [DOI] [PubMed] [Google Scholar]
- [26].Yang Y, Ren Y, Ramani VC, Nan L, Suva LJ, Sanderson RD, Heparanase enhances local and systemic osteolysis in multiple myeloma by upregulating the expression and secretion of RANKL, Cancer Res 70 (2010) 8329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Purushothaman A, Chen L, Yang Y, Sanderson RD, Heparanase stimulation of protease expression implicates it as a master regulator of the aggressive tumor phenotype in myeloma, J Biol Chem 283 (2008) 32628–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chen R, Nishimura MC, Bumbaca SM, Kharbanda S, Forrest WF, Kasman IM, Greve JM, Soriano RH, Gilmour LL, Rivers CS, Modrusan Z, Nacu S, Guerrero S, Edgar KA, Wallin JJ, Lamszus K, Westphal M, Heim S, James CD, VandenBerg SR, Costello JF, Moorefield S, Cowdrey CJ, Prados M, Phillips HS, A hierarchy of self-renewing tumor-initiating cell types in glioblastoma, Cancer Cell 17 (2010) 362–75. [DOI] [PubMed] [Google Scholar]
- [29].Sanderson RD, Bandari SK, Vlodavsky I, Proteases and glycosidases on the surface of exosomes: Newly discovered mechanisms for extracellular remodeling, Matrix Biol 75–76 (2019) 160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Silagi ES, Shapiro IM, Risbud MV, Glycosaminoglycan synthesis in the nucleus pulposus: Dysregulation and the pathogenesis of disc degeneration, Matrix Biol 71–72 (2018) 368–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wight TN, A role for proteoglycans in vascular disease, Matrix Biol 71–72 (2018) 396–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gu BH, Madison MC, Corry D, Kheradmand F, Matrix remodeling in chronic lung diseases, Matrix Biol 73 (2018) 52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Karamanos NK, Theocharis AD, Neill T, Iozzo RV, Matrix modeling and remodeling: A biological interplay regulating tissue homeostasis and diseases, Matrix Biol 75–76 (2019) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Vitale D, Kumar Katakam S, Greve B, Jang B, Oh ES, Alaniz L, Gotte M, Proteoglycans and glycosaminoglycans as regulators of cancer stem cell function and therapeutic resistance, FEBS J 286 (2019) 2870–2882. [DOI] [PubMed] [Google Scholar]
- [35].Mikami T, Kitagawa H, Sulfated glycosaminoglycans: their distinct roles in stem cell biology, Glycoconj J 34 (2017) 725–735. [DOI] [PubMed] [Google Scholar]
- [36].Kraushaar DC, Rai S, Condac E, Nairn A, Zhang S, Yamaguchi Y, Moremen K, Dalton S, Wang L, Heparan sulfate facilitates FGF and BMP signaling to drive mesoderm differentiation of mouse embryonic stem cells, J Biol Chem 287 (2012) 22691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kunath T, Saba-El-Leil MK, Almousailleakh M, Wray J, Meloche S, Smith A, FGF stimulation of the Erk1/2 signalling cascade triggers transition of pluripotent embryonic stem cells from self-renewal to lineage commitment, Development 134 (2007) 2895–902. [DOI] [PubMed] [Google Scholar]
- [38].Forsberg M, Holmborn K, Kundu S, Dagalv A, Kjellen L, Forsberg-Nilsson K, Undersulfation of heparan sulfate restricts differentiation potential of mouse embryonic stem cells, J Biol Chem 287 (2012) 10853–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hayashi Y, Kobayashi S, Nakato H, Drosophila glypicans regulate the germline stem cell niche, J Cell Biol 187 (2009) 473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Xiong A, Kundu S, Forsberg M, Xiong Y, Bergstrom T, Paavilainen T, Kjellen L, Li JP, Forsberg-Nilsson K, Heparanase confers a growth advantage to differentiating murine embryonic stem cells, and enhances oligodendrocyte formation, Matrix Biol 62 (2017) 92–104. [DOI] [PubMed] [Google Scholar]
- [41].Cheng CC, Lee YH, Lin SP, Huangfu WC, Liu IH, Cell-autonomous heparanase modulates self-renewal and migration in bone marrow-derived mesenchymal stem cells, J. Biomed. Sci. 21 (2014) 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Spiegel A, Zcharia E, Vagima Y, Itkin T, Kalinkovich A, Dar A, Kollet O, Netzer N, Golan K, Shafat I, Ilan N, Nagler A, Vlodavsky I, Lapidot T, Heparanase regulates retention and proliferation of primitive Sca-1+/c-Kit+/Lin− cells via modulation of the bone marrow microenvironment, Blood 111 (2008) 4934–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ramani VC, Pruett PS, Thompson CA, DeLucas LD, Sanderson RD, Heparan sulfate chains of syndecan-1 regulate ectodomain shedding, J Biol Chem 287 (2012) 9952–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Jung O, Trapp-Stamborski V, Purushothaman A, Jin H, Wang H, Sanderson RD, Rapraeger AC, Heparanase-induced shedding of syndecan-1/CD138 in myeloma and endothelial cells activates VEGFR2 and an invasive phenotype: prevention by novel synstatins, Oncogenesis 5 (2016) e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Agarwal JR, Matsui W, Multiple myeloma: a paradigm for translation of the cancer stem cell hypothesis, Anticancer Agents Med Chem 10 (2010) 116–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Boumahdi S, Driessens G, Lapouge G, Rorive S, Nassar D, Le Mercier M, Delatte B, Caauwe A, Lenglez S, Nkusi E, Brohee S, Salmon I, Dubois C, del Marmol V, Fuks F, Beck B, Blanpain C, SOX2 controls tumour initiation and cancer stem-cell functions in squamous-cell carcinoma, Nature 511 (2014) 246–50. [DOI] [PubMed] [Google Scholar]
- [47].Tanno T, Lim Y, Wang Q, Chesi M, Bergsagel PL, Matthews G, Johnstone RW, Ghosh N, Borrello I, Huff CA, Matsui W, Growth differentiating factor 15 enhances the tumor-initiating and self-renewal potential of multiple myeloma cells, Blood 123 (2014) 725–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Dhodapkar MV, Sexton R, Das R, Dhodapkar KM, Zhang L, Sundaram R, Soni S, Crowley JJ, Orlowski RZ, Barlogie B, Prospective analysis of antigen-specific immunity, stem-cell antigens, and immune checkpoints in monoclonal gammopathy, Blood 126 (2015) 2475–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Franqui-Machin R, Hao M, Bai H, Gu Z, Zhan X, Habelhah H, Jethava Y, Qiu L, Frech I, Tricot G, Zhan F, Destabilizing NEK2 overcomes resistance to proteasome inhibition in multiple myeloma, J Clin Invest 128 (2018) 2877–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hadigal SR, Agelidis AM, Karasneh GA, Antoine TE, Yakoub AM, Ramani VC, Djalilian AR, Sanderson RD, Shukla D, Heparanase is a host enzyme required for herpes simplex virus-1 release from cells, Nat Commun 6 (2015) 6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Weissmann M, Bhattacharya U, Feld S, Hammond E, Ilan N, Vlodavsky I, The heparanase inhibitor PG545 is a potent anti-lymphoma drug: Mode of action, Matrix Biol 77 (2019) 58–72. [DOI] [PubMed] [Google Scholar]
- [52].Nadir Y, Brenner B, Fux L, Shafat I, Attias J, Vlodavsky I, Heparanase enhances the generation of activated factor X in the presence of tissue factor and activated factor VII, Haematologica 95 (2010) 1927–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kumar S, Raina K, Agarwal C, Agarwal R, Silibinin strongly inhibits the growth kinetics of colon cancer stem cell-enriched spheroids by modulating interleukin 4/6-mediated survival signals, Oncotarget 5 (2014) 4972–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Somasagara RR, Spencer SM, Tripathi K, Clark DW, Mani C, Madeira da Silva L, Scalici J, Kothayer H, Westwell AD, Rocconi RP, Palle K, RAD6 promotes DNA repair and stem cell signaling in ovarian cancer and is a promising therapeutic target to prevent and treat acquired chemoresistance, Oncogene 36 (2017) 6680–6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, Kumar S, Hillengass J, Kastritis E, Richardson P, Landgren O, Paiva B, Dispenzieri A, Weiss B, LeLeu X, Zweegman S, Lonial S, Rosinol L, Zamagni E, Jagannath S, Sezer O, Kristinsson SY, Caers J, Usmani SZ, Lahuerta JJ, Johnsen HE, Beksac M, Cavo M, Goldschmidt H, Terpos E, Kyle RA, Anderson KC, Durie BG, Miguel JF, International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma, Lancet Oncol 15 (2014) e538–48. [DOI] [PubMed] [Google Scholar]
- [56].VanValkenburg ME, Pruitt GI, Brill IK, Costa L, Ehtsham M, Justement IT, Innis-Shelton RD, Salzman D, Reddy ES, Godby KN, Mikhail FM, Carroll AJ, Reddy VB, Sanderson RD, Justement LB, Sanders PW, Brown EE, Family history of hematologic malignancies and risk of multiple myeloma: differences by race and clinical features, Cancer Causes Control 27 (2016) 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]