Abstract
Atrial fibrillation (AF) is a common perioperative arrhythmia. However, its occurrence and implications remain poorly defined in setting of non-cardiac procedures. We sought to define the incidence, prevalence, and prognostic implications of AF among patients with atherosclerotic cardiovascular disease (ASCVD) undergoing non-cardiac surgery. Using a previously validated approach that employed unique patient-linked variables in the New York State Inpatient Database from January 1, 2012, to December 31, 2014, the frequency of new-onset and pre-existing AF was determined in adults with ASCVD aged ≥18 years undergoing non-cardiac surgery. The secondary outcomes were stroke within one month and all-cause mortality. Using multivariable logistic models the factors and outcomes associated with new-onset AF after non-cardiac surgery were assessed. Nine surgical sub-groups of major non-cardiac surgery served as exposure. A total of 184,775 patients were identified during the study period. Age ≥65, anemia, history of heart failure, valvular heart disease, and thoracic surgery were predictors of new-onset AF after non-cardiac surgery. Among 3,806 patients (2.5%) developed new-onset AFand 31,603 (17.5%) patient had pre-existing AF. After multivariable-adjusted modeling, new-onset AF was associated with increased odds of stroke within one month (OR: 1.31, 95% CI: 1.12–1.53; p<0.001)], mortality (OR: 3.74; 95% CI: 3.30–4.24; p<0.001) and longer length of stay in the hospital (10 days; interquartile range: 6–16 days; p<0.001). New-onset AF portends a poor prognosis in patients with ASCVD undergoing non-cardiac surgeries. The risk profile of patients that develop new-onset AF differs across patient phenotypes and by surgical procedure.
Keywords: Atrial Fibrillation, Mortality, Non-Cardiac Surgery, Postoperative stroke, Stroke
Introduction
Atrial fibrillation is a commonly encountered post-operative arrhythmia1 and has been associated with adverse clinical outcomes such as increased length of stay, stroke, and perioperative mortality.2–4 Prior data exploring AF among patients undergoing non-cardiac surgery has been limited in size or only examined new-onset AF in the context of specific procedures or surgical specialties. There is also a dearth of data on the impact of new-onset AF on outcomes among patients with pre-existing atherosclerotic cardiovascular disease (ASCVD). With >40 million non-cardiac surgeries performed yearly within the United States, the implications of new-onset AF in ASCVD patients warrant further investigation.5 Additionally, given the heterogeneity of non-cardiac surgical procedures, there is value in investigating the risk factors stratified by surgical procedures and comorbidity. We hypothesized that new-onset AF would be a common condition among patients with clinical ASCVD undergoing non-cardiac surgery and that AF would also adversely impact in-hospital mortality and stroke outcomes after non-cardiac surgery. We also sought to describe the phenotypic characteristics that were associated with the development of postoperative new-onset AF in patients with ASCVD. We present an investigation evaluating the incidence, prevalence, and prognostic implications of AF among patients with clinical ASCVD who are undergoing non-cardiac surgery using a previously validated approach from the New York State Inpatient Database.
Methods
The New York State Inpatient Database was used to identify the study population. This database contains inpatient data from acute care hospitals, residential health care facilities, and diagnostic centers, with each entry linked to a unique patient identifier. Patient-level data can be discerned to identify the mode of admission, length of stay, and a maximum of 26 diagnoses and 15 procedural fields. Each diagnosis and procedural field is also linked with a present on arrival indicator. This helps to differentiate between the conditions that were present on admission and those that developed during hospitalization.6
Adult patients aged ≥18 years who underwent a major therapeutic operating room procedure (i.e., principle Clinical Classifications Software Procedure Class 4) and had established clinical ASCVD were identified using the New York State Inpatient Database from 2012 through 2014. Patients with clinical ASCVD were identified using a validated methodology as described in previous studies7–10 by having one of the following International Classification of Diseases 9th Revision, Clinical Modification (ICD-9-CM) diagnoses codes: coronary artery disease (defined as a history of coronary artery disease, angina, myocardial infarction, percutaneous coronary intervention, or history of coronary artery bypass grafting), cerebrovascular disease (history of transient ischemic attacks or history of stroke), peripheral arterial disease and history of cardiac arrest (Supplementary Table 1). Major surgery sub-groups were identified using aggregated primary ICD-9-CM procedure codes generated from Clinical Classification Software.4 Patients who underwent cardiac surgery (n=179,581), procedures outside of the operating room (n=527), bone marrow transplantation (n=10,834), ophthalmic surgery (n=2,319), dental surgery (n=155), and ‘other’ procedures (n=1,390) were excluded.4 All readmissions and admissions for non-cardiac surgery where acute coronary syndrome, stroke, and/or unstable angina were present on admission were also excluded (Figure 1). The surgical procedures were divided into 9 major sub-groups using Clinical Classification Software: general (including gastrointestinal, hepatobiliary surgery, and hernia repair surgery), vascular, thoracic, neurosurgery, skin/burn, genitourinary, orthopedic, obstetrics, and others (otorhinolaryngology, endocrine, breast, gynecology).
Figure 1.
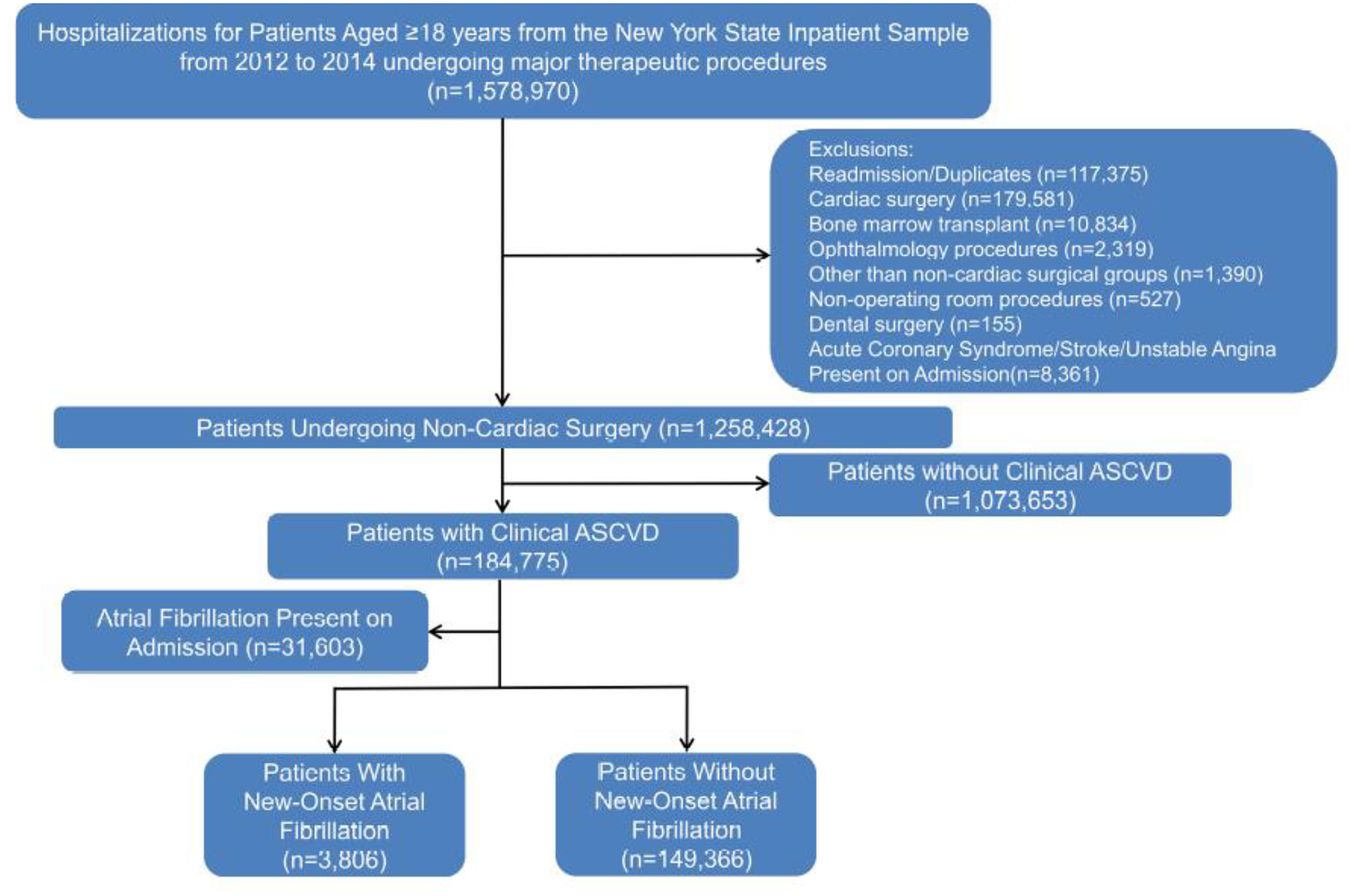
Flowchart for Cohort Selection
Baseline demographics were identified via the New York State Inpatient Database variables. The previously published ICD-9-CM codes and the Elixhauser comorbidity index were used to identify baseline comorbidities.11
The primary outcome was the incidence of AF among patients with clinical ASCVD undergoing non-cardiac surgery. The secondary outcomes were new-onset stroke within one month of surgery, mortality, and length of stay.
The presence of AF was identified using ICD-9-CM diagnostic code 427.3. To identify new-onset and prevalent atrial fibrillation, a previously validated approach employing a present on admission indicator was used.6 This present on admission indicator was also used to identify patients with clinical ASCVD. This approach to identifying new-onset and prevalent AF has been previously described to have 95% sensitivity and 99% specificity.12 Additionally, previous studies have validated this methodology of discriminating new-onset and prevalent AF with blinded chart review and had an overall agreement of 90% and a kappa statistic of 0.74.13,14 To define stroke, the absence of ICD-9-CM codes 433, 434, or 436 on admission was used. This approach to identifying in-hospital ischemic stroke has been previously validated and showed 86% sensitivity and 95% specificity.15 All strokes occurring during the in-hospital period after surgery or identified during readmission for any cause within the same month or subsequent month following index hospitalization were defined to be the outcome of interest as stroke within one-month.
All statistical analyses were done in SAS 9.4 (SAS Institute, Cary, NC, U.S.A.). All analyses were performed to adhere to the Agency for Healthcare Research and Quality recommended practices. Continuous variables were presented as means and standard deviations. Categorical variables were presented as counts and percentages. The incidence of new-onset AF in both the overall population and the surgical subgroups were calculated and presented as events per 100 patients.
A logistic regression model utilizing an automated step-wise selection of variables (p<0.1) was used to examine the factors associated with new-onset AF. We included demographics (age categories, sex, race), obesity, tobacco use, alcohol abuse, each of the Elixhauser derived comorbidities, and type of surgery as covariates. Furthermore, a heat map was constructed to depict the relative importance of factors associated with new-onset of AF utilizing the proportion of Wald chi-square statistic of that factor to overall global Wald chi-square. Darker shades within the heat map signify a greater contribution to the overall global Wald chi-square score in the regression model. This implies a stronger relationship with new-onset AF after non-cardiac surgery. Lighter shades imply a lack of relationship or lower odds of new-onset AF after non-cardiac surgery.
Multivariable logistic regression models were used to estimate the odds of stroke within 1 month and mortality among patients with new-onset AF. Age categories, sex, race, obesity, tobacco use, alcohol abuse, and Elixhauser derived comorbidities were used as covariates. The estimated risks of mortality and stroke within 1 month were assessed using odds ratios (OR) with 95% confidence intervals (95% CI). Linear regression models adjusting for the abovementioned covariates were used to assess the association of new-onset AF with the length of stay in the hospital.
The association of prevalent AF with stroke and mortality in patients undergoing non-cardiac surgery was also evaluated. This was done by first stratifying patients with and without prevalent AF who had undergone major non-cardiac surgical procedures. Patients with new-onset AF after non-cardiac surgery for this analysis were excluded. This analysis was conducted by incrementally adjusting the logistic regression model. Model one was an unadjusted logistic regression model. Model two was adjusted for age, sex, and race. Model three was adjusted for age categories, sex, race, obesity, tobacco use, alcohol abuse, and Elixhauser derived comorbidities. Using the abovementioned covariates linear regression models were used to assess the association of pre-existing AF with the length of stay in the hospital.
We performed a sensitivity analysis by assessing patients who developed AF after non-cardiac surgery and were free of 427.3 ICD-9-CM code prior to the current hospitalization. It should be noted that in this sensitivity analysis, we did not find any patients with 427.3 ICD-9-CM code prior to the current hospitalization for non-cardiac surgery which indicated the robustness of our methodology. We further examined the factors associated with new-onset AF after non-cardiac surgery.
Results
Among 1,258,428 patients from 2012 through 2014, a total of 184,775 patients with clinical ASCVD, who underwent major non-cardiac surgery were identified (Figure 1). From these patients undergoing non-cardiac surgery, 31,603 patients had prevalent AF. The baseline characteristics of the study population stratified by their AF occurrence are outlined in Table 1. In the cohort of patients undergoing non-cardiac surgery, the mean age of subjects was 71.2 years, with more males (43.3%) and with a higher prevalence of whites (70.1%). The most frequent types of surgery were orthopedic, general, and vascular (Table 1).
Table 1.
Demographics and Baseline Characteristics for Patients without Prevalent Atrial Fibrillation Who Underwent Non-Cardiac Surgery: Stratified by Presence or Absence of New-Onset Atrial Fibrillation
| New-Onset Atrial Fibrillation | ||||
|---|---|---|---|---|
| Characteristics | Overall Populations Undergoing Non-Cardiac Surgery (N=184,775) | Yes (N=3,806) | No (N=149,366) | Pre-Existing Atrial Fibrillation (N=31, 603) |
| Age, median (IQR), years | 53.5 (19.3%) | 75.6 (10.6%) | 69.8 (12.5%) | 77.5 (10.2%) |
| Female | 80,022 (43.3%) | 1,637 (43.0%) | 655,506 (43.9%) | 12,879 (40.8%) |
| White | 129,445 (70.06%) | 2,802 (73.6%) | 101,794 (68.2%) | 24,849 (78.6%) |
| Black | 19,359 (10.5%) | 283 (7.4%) | 17,036 (11.4%) | 2,040 (6.5%) |
| Hispanic | 14,002 (7.6%) | 228 (6.0) | 12,073 (8.1%) | 1,701 (5.4%) |
| Other | 21,907 (11.9%) | 491 (12.9) | 18,414 (12.3%) | 3,002 (9.5%) |
| Alcohol Abuse | 4,013 (2.2%) | 96 (2.5%) | 3,338 (2.2%) | 579 (1.8%) |
| Anemia | 32,222 (17.4%) | 817 (21.5%) | 24,619 (16.5%) | 6,786 (21.5%) |
| Chronic Kidney Disease | 34,890 (18.9%) | 752 (19.8%) | 25,927 (17.4%) | 8,211 (26.0%) |
| Chronic Pulmonary Disease | 42,892 (23.2%) | 1,130 (29.7%) | 33,622 (22.5%) | 8,140 (25.8%) |
| Coronary Artery Disease | 126,376 (10.0%) | 2,663 (70.0%) | 100,170 (67.1%) | 23,543 (74.5%) |
| Diabetes Mellitus | 51,042 (27.6%) | 888 (23.3%) | 41,684 (27.9%) | 8,470 (26.8%) |
| End-Stage Renal Disease | 11,165 (6.0%) | 217 (5.7%) | 8,641 (5.8%) | 2,307 (7.3%) |
| Prior Heart Failure | 26,054 (14.1%) | 830 (21.8%) | 15,842 (10.6%) | 9,382 (29.7%) |
| Prior Venous Thromboembolism | 6,867 (3.7%) | 120 (3.2%) | 5,286 (3.5%) | 1,461 (4.6%) |
| Dyslipidemia | 104,040 (56.3%) | 2,038 (53.6%) | 85,340 (57.1%) | 16,662 (52.7%) |
| Hypertension | 145,827 (78.9%) | 2,994 (78.7%) | 117,491 (78.7%) | 25,342 (80.2%) |
| Malignancy | 4,035 (2.2%) | 104 (2.7%) | 3,122 (2.1%) | 809 (2.6%) |
| Obesity | 25,479 (13.8%) | 463 (12.2%) | 21,083 (14.1%) | 3,933 (12.5%) |
| Peripheral Arterial Disease | 52,801 (28.6%) | 1,178 (31.0%) | 43,221 (28.9%) | 8,402 (26.6%) |
| Prior Coronary Artery Bypass Graft | 32,585 (17.6%) | 614 (16.1%) | 25,023 (16.8%) | 6,948 (22.0%) |
| Prior Percutaneous Coronary Intervention | 38,230 (20.7%) | 671 (17.6%) | 32,316 (21.6%) | 5,243 (16.6%) |
| Prior Stroke/TIA | 32,270 (17.5%) | 582 (15.3%) | 25,757 (17.2%) | 5,931 (18.8%) |
| Smoker | 59,827 (32.4%) | 1,204 (31.6%) | 50,210 (33.6%) | 8,413 (16.9%) |
| Valvular Heart Disease | 40,706 (3.2%) | 512 (13.5%) | 10,335 (6.9%) | 5,344 (16.9%) |
| General | 32,613 (17.7%) | 1,103 (29.0%) | 25,782 (17.3%) | 5,728 (18.1%) |
| Genitourinary | 12,578 (6.8%) | 189 (5.0%) | 10,290 (6.9%) | 2,099 (6.6%) |
| Neurosurgery | 7,970 (4.3%) | 123 (3.2%) | 6,684 (4.5%) | 1,163 (3.7%) |
| Orthopedic | 70,349 (38.1%) | 962 (25.3%) | 57,074 (38.2%) | 12,313 (39.0%) |
| Other Surgeries | 6,617 (3.6%) | 79 (2.1%) | 5,829 (3.9%) | 709 (2.2%) |
| Skin/Burn | 5,706 (3.1%) | 58 (1.5%) | 4,409 (3.0%) | 1,239 (3.9%) |
| Thoracic | 5,863 (3.2%) | 409 (10.8%) | 4,375 (2.9%) | 1,079 (3.4%) |
| Vascular | 43,079 (23.3%) | 883 (23.2%) | 34,923 (23.4%) | 7,273 (23.0%) |
| Length of Stay, median (IQR) | 5 (3–9) | 10 (6–16) | 4 (2–8) | 6 (3–11) |
IQR: Inter-Quartile Range, Other Surgeries include breast, endocrine, gynecological, obstetric and otorhinolaryngological surgery.
Results are presented as counts and percentages.
New-onset AF occurred in 3,806 patients, and prevalent AF was present in 31,603 major non-cardiac surgery patients. This corresponds to a prevalence of 2.5% and 17.5%, respectively. Patients that developed new-onset AF were older (mean age of 75.6 years) and more often whites (73.6%) (Table 1). Coronary artery disease, chronic kidney disease, chronic pulmonary disease, heart failure, hypertension, and valvular heart disease were more prevalent in patients who developed new-onset AF compared with those that did not.
The frequency of new-onset AF by the type of surgery is depicted in Figure 2. Patients who underwent thoracic surgery were more likely to develop new-onset AF (8.6 per 100 patients) followed by individuals who underwent general (4.1 per 100 patients) and vascular (2.5 per 100 patients) surgeries (Figure 2).
Figure 2. Incidence of New-Onset Atrial Fibrillation After Non-Cardiac Surgery.
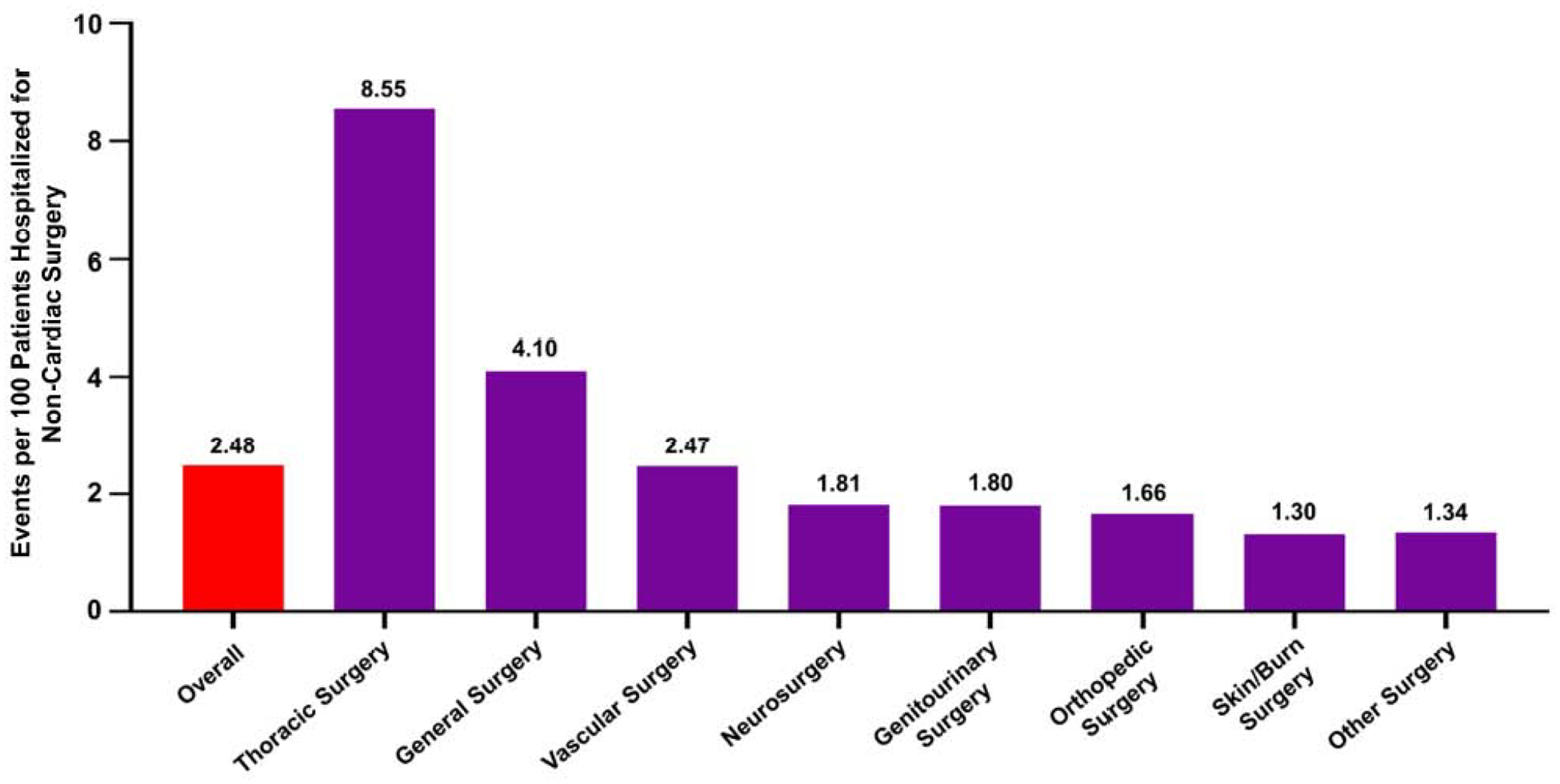
Data are presented as events per 100 patients with clinical ASCVD, admitted for non-cardiac surgery. The red bar represents the incidence of new-onset atrial fibrillation in the overall population. The purple bars represent the incidence of new-onset atrial fibrillation by sub-groups of non-cardiac surgery.
The predictors of new-onset AF after non-cardiac surgery are outlined in Table 2. Compared with younger individuals (ages 18–35), the odds of developing AF were higher among older patients (age 50–65 years: OR: 2.49, 95% CI: 1.17–5.27; p=0.02 and age ≥65 years: OR: 6.21, 95% CI: 2.94–13.11; p <0.001). Male sex was also associated with higher odds of developing AF (OR: 1.11, 95% CI: 1.04–1.19; p = 0.002). Patients with a history of baseline anemia, chronic pulmonary disease, coronary artery disease, heart failure, peripheral arterial disease, and valvular heart disease were more likely to develop new-onset AF. Among surgical sub-specialties, patients who underwent thoracic surgery (OR: 2.08, 95% CI: 1.84–2.35; p <0.001) were more likely to have new-onset AF compared to those who underwent general surgery. Patients who underwent other surgeries had lower odds of developing new-onset AF compared with patients who underwent general surgery (Table 2).
Table 2:
Predictors of New-Onset Atrial Fibrillation After Non-Cardiac Surgery
| Variable | Odds Ratio (95% Confidence Interval) | p-value |
|---|---|---|
| Demographics | ||
| Age 35–50 vs. Age 18–35 | 1.62 (0.73–3.58) | 0.23 |
| Age 50–65 vs. Age 18–35 | 2.49 (1.17–5.27) | 0.02 |
| Age ≥65 vs. Age 18–35 | 6.21 (2.94–13.11) | <0.001 |
| Male vs. Female | 1.11 (1.04–1.19) | 0.002 |
| Black vs. White | 0.65 (0.57–0.73) | <0.001 |
| Hispanic vs. White | 0.73 (0.63–0.84) | <0.001 |
| Other vs. White | 1.01 (0.92–1.12) | 0.82 |
| Income Group (centile) | ||
| <25th vs. >75th | 0.97 (0.87–1.08) | 0.58 |
| 25th-50th vs. >75th | 0.95 (0.87–1.04) | 0.28 |
| 50th-75th vs. >75th | 0.97 (0.89–1.06) | 0.50 |
| Comorbidities | ||
| Alcohol Abuse | 1.25 (1.01–1.54) | 0.04 |
| Anemia | 1.29 (1.18–1.40) | <0.001 |
| Chronic Kidney Disease | 0.98 (0.89–1.08) | 0.69 |
| Chronic Pulmonary Disease | 1.22 (1.13–1.31) | <0.001 |
| Coronary Artery Disease | 1.12 (1.03–1.22) | 0.006 |
| Prior Percutaneous Coronary | 0.79 (0.72–0.86) | <0.001 |
| Intervention | ||
| Prior Coronary Artery Bypass Grafting | 0.78 (0.71–0.86) | <0.001 |
| Diabetes Mellitus | 0.76 (0.71–0.83) | <0.001 |
| Dyslipidemia | 0.87 (0.82–0.93) | <0.001 |
| End-Stage Renal Disease | 0.97 (0.83–1.14) | 0.71 |
| History of Heart Failure | 2.02 (1.86–2.20) | <0.001 |
| History of Venous Thromboembolism | 0.89 (0.74–1.08) | 0.24 |
| Hypertension | 1.0 (0.92–1.08) | 0.97 |
| Malignancy | 0.96 (0.78–1.17) | 0.68 |
| Obesity | 1.08 (0.97–1.19) | 0.15 |
| Peripheral Arterial Disease | 1.15 (1.06–1.24) | <0.001 |
| Tobacco Use | 0.87 (0.81–0.94) | <0.001 |
| Valvular Heart Disease | 1.77 (1.52–1.85) | <0.001 |
| Surgery Type | ||
| General Surgery | Reference | |
| Genitourinary | 0.42 (0.36–0.49) | <0.001 |
| Skin/Burn | 0.29 (0.22–0.38) | <0.001 |
| Neurosurgery | 0.45 (0.38–0.55) | <0.001 |
| Obstetric | 0.16 (0.12–0.22) | <0.001 |
| Orthopedic | 0.37 (0.34 –0.40) | <0.001 |
| Other Surgeries | 0.40 (0.32–0.51) | <0.001 |
| Thoracic | 2.08 (1.84–2.35) | <0.001 |
| Vascular | 0.63 (0.58–0.70) | <0.001 |
The C-statistic was 0.85 for this model.
The heat map to illustrate the Wald score grading the relative strength of the predictors of AF is depicted in Figure 3. Increasing age, history of heart failure, and valvular heart disease were the most strongly associated with new-onset AF.
Figure 3. Heat Map for Factors Associated with New-Onset Atrial Fibrillation After Non-Cardiac Surgery.
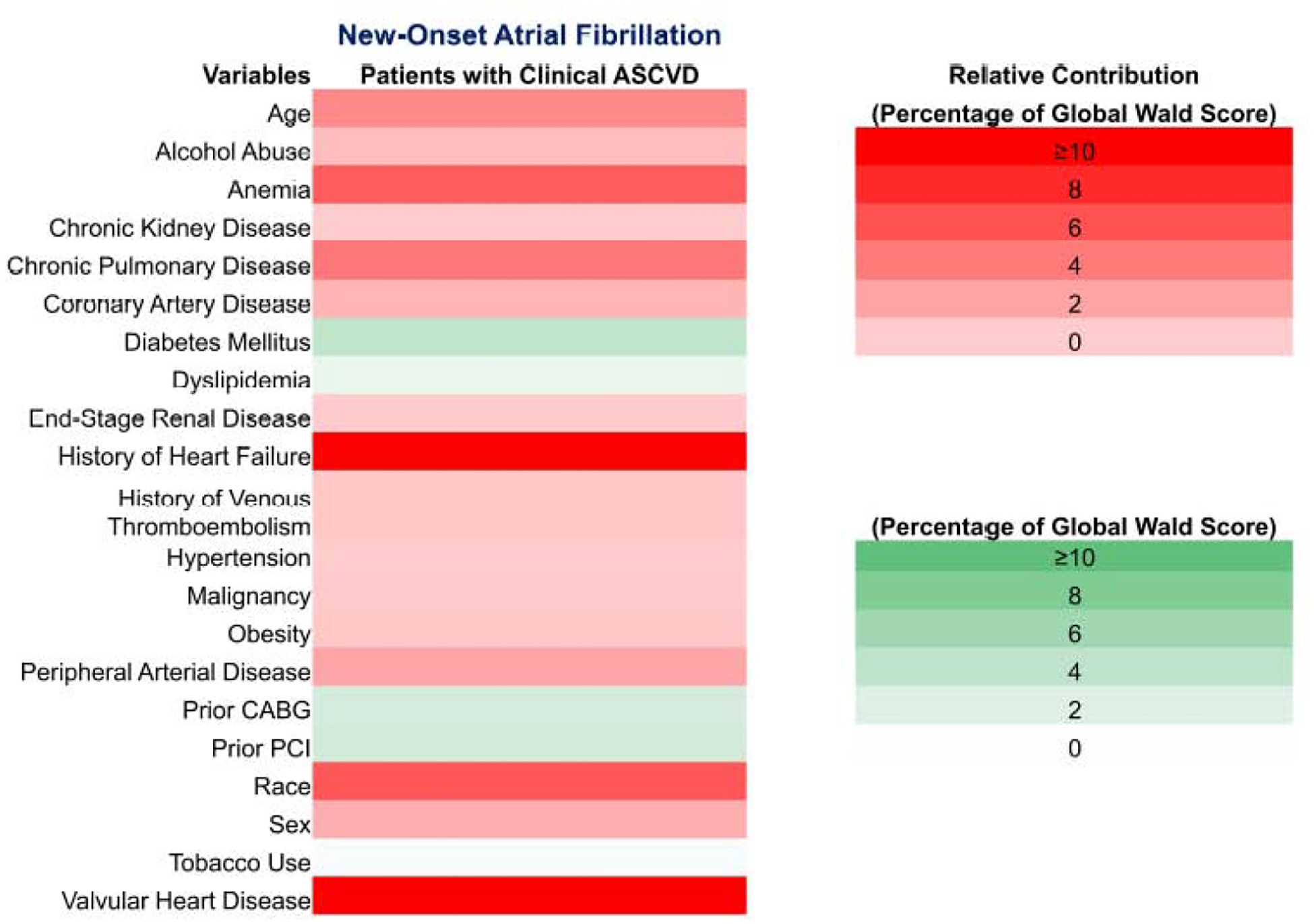
Factors are coded in shades of red or green showing the direction of effect. Red bars indicates positive association and green bars indicate negative association. Darker shades signify a greater contribution to the global Wald score in the logistic regression model. This implies a stronger relationship with new-onset AF after non-cardiac surgery. Lighter shades imply a lack of relationship or lower odds of new-onset AF after non-cardiac surgery.
Stroke occurring within 1 month of surgery was seen in 3.3% in ASCVD patients without AF. In comparison, 4.9% and 3.9% patients with new-onset and pre-existing AF, respectively, had stroke within one month of surgery. In-hospital mortality was witnessed in 0.75% patients without AF, but occurred in 7.92% and 4.34% patients with new-onset AF and pre-existing AF, respectively. In the multivariable-adjusted analyses of patients with clinical ASCVD, the odds of in-hospital mortality with new-onset AF after non-cardiac surgery were 3.74 times (95% CI: 3.30–4.24; p<0.001) those of patients who did not develop new-onset AF (Figure 4). Similarly, the odds of stroke within one month were higher among patients with new-onset AF compared to those without new-onset AF after non-cardiac surgery (OR: 1.31, 95% CI: 1.12–1.53; p <0.001) (Figure 4). In multivariable-adjusted analyses, the length of stay [10 days; Inter-Quartile Range (IQR):6–16 days] was longer in patients with new-onset AF compared to those who did not develop AF (4 days; IQR:2–8 days) (p <0.001).
Figure 4. In-Hospital Mortality and Stroke Within 1 Month Among Patients Developing New-Onset Atrial Fibrillation After Non-Cardiac Surgery.
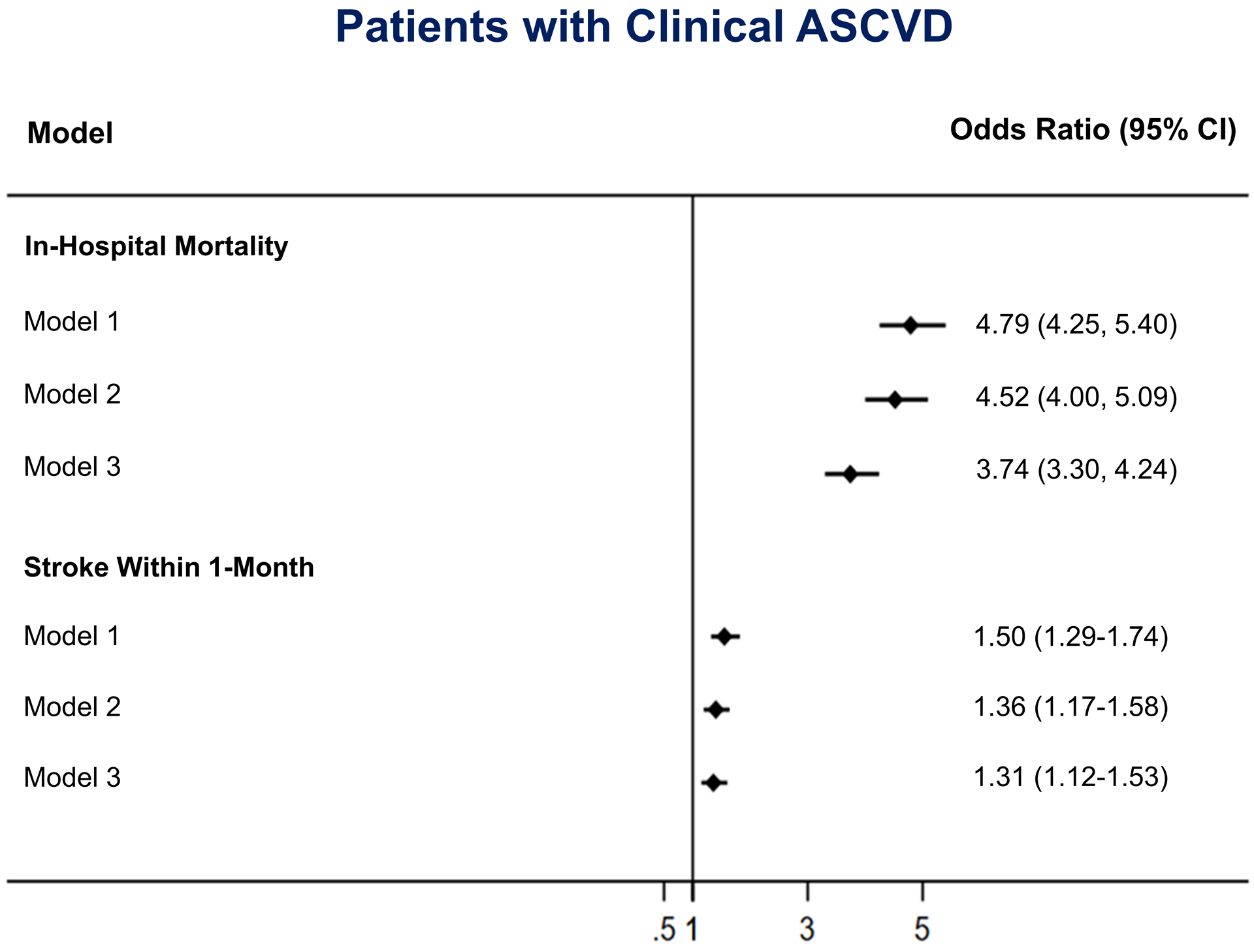
Data are presented as forest plot for odds of in-hospital mortality and stroke within 1 month among patients with clinical ASCVD. Model 1: Unadjusted odds ratio. Model 2: Adjusted for age, sex, race. Model 3: Model 2 + obesity, tobacco use, alcohol abuse, and Elixhauser derived comorbidities.
Using patients with clinical ASCVD and those who did not develop new-onset AF as a reference, odds of in-hospital mortality and stroke within 1 month among various groups were generated and are depicted in Figure 5.
Figure 5. In-Hospital Mortality and Stroke Within 1 Month Among Patients After Non-Cardiac Surgery.
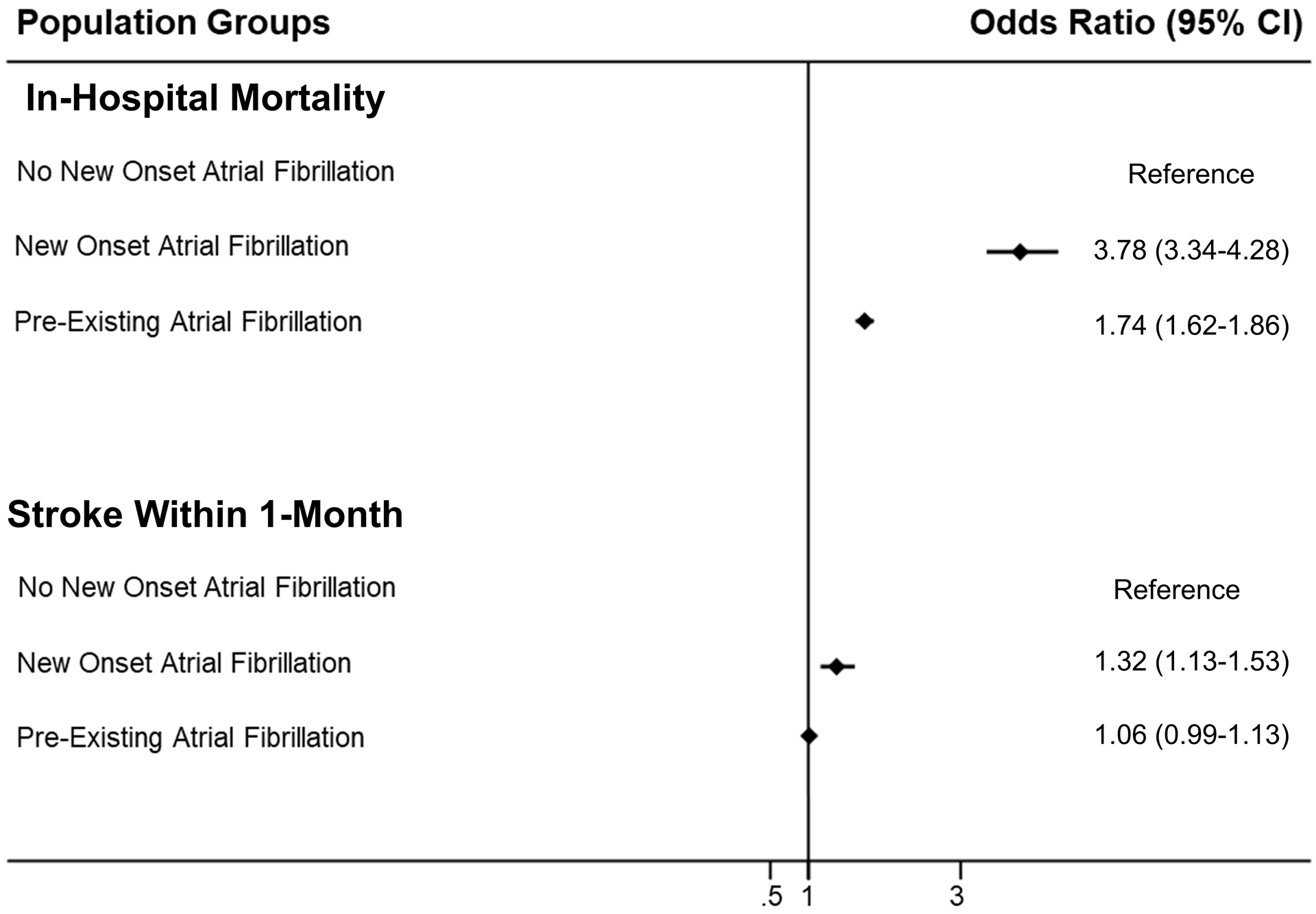
Data are presented as forest plot for the odds of in-hospital mortality and stroke within 1 month among patients admitted for non-cardiac surgery, stratified by the presence of new-onset and pre-existing atrial fibrillation.
After adjustment, patients with prevalent AF and clinical ASCVD had significantly higher odds of in-hospital mortality (OR: 1.73, 95% CI: 1.62–1.86; p <0.001) compared to ASCVD patients without prevalent AF (Table 3). In multivariable-adjusted analyses, pre-existing AF in patients undergoing non-cardiac surgery was associated with a longer length of stay in hospital (6 days; IQR: 3–11 days; p <0.001).
Table 3:
In-Hospital Mortality and Stroke Within 1-Month Among Patients With Clinical ASCVD and Prevalent Atrial Fibrillation Undergoing Non-Cardiac Surgery
| Model | Odds Ratio (95% Confidence Interval) | p-value |
|---|---|---|
| In-Hospital Mortality | ||
| Model 1 | 2.50 (2.35–2.67) | <0.001 |
| Model 2 | 2.34 (2.19–2.49) | <0.001 |
| Model 3 | 1.73 (1.62–1.86) | <0.001 |
| Stroke Within 1 Month | ||
| Model 1 | 1.20 (1.13–1.28) | <0.001 |
| Model 2 | 1.07 (1.01–1.14) | 0.03 |
| Model 3 | 1.03 (0.96–1.10) | 0.40 |
Model 1: Unadjusted odds ratio.
Model 2: Adjusted for age, sex, race.
Model 3: Model 2 + obesity, tobacco use, alcohol abuse and Elixhauser derived comorbidities.
Discussion
In summary, we found that the incidence and prevalence of AF in patients with clinical ASCVD undergoing non-cardiac surgery is 2.5% and 17.5%, respectively. New-onset AF was associated with increasing age, greater comorbidity burden, and the type of non-cardiac surgical procedure. In multivariable-adjusted analyses, we found that both new-onset and prevalent AF increased the odds of in-hospital mortality after major non-cardiac surgery. Additionally, new-onset AF increased the odds of stroke within 1 month of the index hospitalization. Taken together, the occurrence of new-onset AF in ASCVD patients appears to be a surrogate for having a high risk of in-hospital mortality, stroke within 1 month (Figure 5), and a longer length of stay.
There may be multiple mechanistic explanations for our findings. The precipitation of new-onset AF is thought to be due to a combination of patient substrate and context-specific triggers.14 A surgical procedure may be described as the archetypal trigger due to the combination of physiological aberrancies that it provokes such as local and systemic inflammatory responses, catecholaminergic surges, and potentially significant metabolic and hemodynamic aberrations. These may be further exacerbated by post-operative illnesses and surgical complications. As a corollary, our investigation describes the many patient substrate factors that may be relevant in provoking new-onset AF. By identifying patients with clinical ASCVD, we attempted to identify those patients with a high-risk phenotype. Patients with new-onset AF were older and had a greater prevalence of several comorbidities. The comorbidities deemed most pertinent to the development of AF, such as coronary artery disease, obesity, heart failure, and valvular heart disease, were all found to be more common in the patients that developed new-onset AF. Age had the greatest impact on the development of new-onset AF in our investigation. This may be due to any combination of altered cardiac geometry, valvular degeneration, and/or increased epicardial fat deposition.16 We speculate that new-onset AF may reflect a greater systemic upset at the time of the index procedure and thus be more deleterious. Finally, variation in the incidence of new-onset AF amongst surgical sub-groups may also be explained by the patient’s substrate and the surgical trigger (Figure 2). For example, thoracic and vascular surgical procedures typically involve older subjects and those with significant co-existent cardiovascular disease.17 In comparison, obstetric and gynecologic procedures are typically done in younger patients. This may also reflect a significant systemic derangement from the index procedure, associated illnesses, or surgical complications that were associated with the development of AF.
Our investigation also adds to the existing literature. Prior reports have evaluated the incidence and prognostic implications in the general population. Bhave et al. previously characterized the incidence, predictors, and prognostic implications of AF from an administrative database of 375 American hospitals.2 They found that new-onset AF occurred in approximately 1.0% of surgeries and was associated with increased length of stay, hospitalization, and all-cause mortality.2 Gialdini et al. also previously characterized the incidence of new-onset AF as 1.43% in a retrospective cohort study examining patients who had undergone both cardiac and non-cardiac surgery from the California State Inpatient Database.18 They found that new-onset AF was associated with a greater incidence of stroke after both cardiac and non-cardiac surgery. Smilowitz et al. examined the New York State Inpatient Database and found a significant risk of stroke in patients but an overall declining rate of major adverse cardiovascular events across multiple surgical subspecialties.4 In contrast, our investigation selectively focuses on the implications of a common postoperative arrhythmia in patients with clinical ASCVD which is a high-risk group that is undergoing a high volume of elective outpatient surgeries.
We build upon all of the above work by demonstrating that there is a higher incidence of AF in patients with clinical ASCVD in comparison to the general population. By defining a high-risk cohort and characterizing the relevant demographics, comorbidities, and surgical subtypes, we also demonstrate that the abovementioned stroke and mortality implications may be magnified in the setting of a higher risk profile. Our findings mirror those of Bhave et al. and Smilowitz et al. by suggesting that patients undergoing vascular, thoracic, and general surgery had the highest odds of developing new-onset AF. We also demonstrate the prevalence of AF in patients undergoing non-cardiac surgery and the associated implications of prevalent AF in this cohort. Our findings also parallel prior reports of the effects of new-onset AF after cardiac surgery which state that AF occurs in older patients with more comorbidities and is associated with increased stroke and in-hospital mortality.14,19–21 However, the incidence estimates of new-onset AF after cardiac surgery appear to be markedly higher with estimates ranging between 19–50%.14,19–21
Our investigation may have important public health implications. We confirm prior reports that the occurrence of new-onset AF after non-cardiac surgery is associated with a significant morbidity and mortality burden.2 There is ample evidence to suggest that the prevalence of AF will rise in the context of an aging population and the rising prevalence of AF risk factors, such as in those with clinical ASCVD.22 Clinicians should, therefore, pre-empt this rise in morbidity burden associated with new-onset AF. As a corollary, our findings support prior efforts to develop risk prediction tools to pre-operatively stratify patients’ propensity to develop AF. Mariscalco et al. previously described the preoperative use of the POAF (postoperative AF) score amongst patients undergoing cardiac surgery to target prophylactic approaches to patients that have a high risk of developing patients new-onset perioperative AF.23 We propose that similar efforts should be explored for other high-risk patients undergoing non-cardiac surgical procedures. Given the intense interest related to wearable technology to detect arrhythmias24 and the increasing usage of implantable loop recorders, they may also be utilized in pursuing preoperative monitoring of AF in select patients at high risk of developing AF. Studies pursuing longer-term monitoring have yielded significant rates of occult AF, especially in older adults.25 Finally, there is significant debate about whether new-onset AF is an acute, self-limited response to the surgical trigger or the first manifestation of an occult chronic condition.26 Our investigation suggests that shared patient-clinician counseling as part of preoperative cardiac risk stratification and decision-making regarding the possibility and prognostic considerations of new-onset AF is critically important prior to any non-cardiac procedures. Clinicians should focus these discussions to those deemed to have high-risk phenotypes and surgical procedures, such as those demonstrated in our investigation. These patients may be better served by these efforts since chronic anticoagulation is more likely to be a potential sequela of their surgical procedure.
Our investigation uses a state inpatient database that has multiple important limitations, some of which have been previously described.27 Retrospective cohort studies are labored with selection and information bias. Patients with clinical ASCVD may have been more likely deemed to be poor operative candidates, thus leading to a selection bias in favor of lower-risk candidates for elective surgeries. This may falsely decrease the incidence of perioperative AF and the associated complications. However, this means that the ‘true’ estimates of new-onset AF and the associated stroke and in-hospital mortality were likely higher. AF may also not be diagnosed in patients who are discharged after undergoing same-day surgeries. In this context, the exact methodology of how AF was diagnosed in individual patients could not be elucidated. However, the use of a state inpatient database to elucidate new-onset AF diagnoses is an approach that has been well-validated based on prior reports.13,14,18 The present on admission indicator also helps to separate patients from new-onset AF diagnoses from prevalent diagnoses. Our study design also precludes us from making assertions regarding causality. Coding errors associated with chronic comorbid conditions have been previously recognized within inpatient databases and may contribute to the observed negative associations.13 We were unable to access information regarding pre-operative and peri-operative medical therapies (such as the use of beta-blockers, anticoagulation and anti-arrhythmics), the institution of anticoagulation, and specific trends regarding individual surgical procedures. Finally, there is an increasing focus on defining AF by burden, which we could not define in this investigation.22 Regardless, contemporary guidelines suggest the pursuit of therapeutic anticoagulation for postoperative AF that lasts for a short duration, particularly if the patient has an elevated stroke risk.28,29
In conclusion, new-onset and pre-existing AF was seen in ~20% patients with clinical ASCVD undergoing non-cardiac surgical procedures. AF occurred more frequently in older patients and those with greater comorbidity burden. Both new-onset and prevalent AF are associated with an increased incidence of all-cause mortality and a longer length of stay. These findings should be used to guide shared patient-clinician counseling in high-risk patients prior to non-cardiac procedures to discuss the prognostic implications of new-onset postoperative AF.
Supplementary Material
Sources of Funding:
Dr. Pankaj Arora is supported by the National Institutes of Health Mentored Patient-Oriented Research Award 5K23HL146887–02.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: None of the authors report any significant relationships with industry or financial disclosures related to this manuscript.
References
- 1.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener H-C, Heidbuchel H, Hendriks J. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 2.Bhave PD, Goldman LE, Vittinghoff E, Maselli J, Auerbach A. Incidence, predictors, and outcomes associated with postoperative atrial fibrillation after major noncardiac surgery. Am Heart J 2012;164:918–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polanczyk CA, Goldman L, Marcantonio ER, Orav EJ, Lee TH. Supraventricular arrhythmia in patients having noncardiac surgery: clinical correlates and effect on length of stay. Ann Intern Med 1998;129:279–285. [DOI] [PubMed] [Google Scholar]
- 4.Smilowitz NR, Gupta N, Ramakrishna H, Guo Y, Berger JS, Bangalore S. Perioperative Major Adverse Cardiovascular and Cerebrovascular Events Associated With Noncardiac Surgery. JAMA Cardiol 2017;2:181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall MJ, DeFrances CJ, Williams SN, Golosinskiy A, Schwartzman A. National Hospital Discharge Survey: 2007 summary. Natl Health Stat Report 2010:1–20, 24. [PubMed] [Google Scholar]
- 6.Coffey R, Milenkovic M, Andrews RM. The Case for the Present-on-Admission (POA) Indicator 2006. HCUP Methods Series Report # 2006–01 Online. June 26, 2006. U.S. Agency for Healthcare Research and Quality; Available: http://www.hcup-us.ahrq.gov/reports/methods.jsp. Accessed on August 21, 2019. [Google Scholar]
- 7.Virani SS, Akeroyd JM, Ramsey DJ, Chan WJ, Frazier L, Nasir K, S SR, Ballantyne CM, Petersen LA. Comparative effectiveness of outpatient cardiovascular disease and diabetes care delivery between advanced practice providers and physician providers in primary care: Implications for care under the Affordable Care Act. Am Heart J 2016;181:74–82. [DOI] [PubMed] [Google Scholar]
- 8.Faza NN, Akeroyd JM, Ramsey DJ, Shah T, Nasir K, Deswal A, Ballantyne CM, Petersen LA, Virani SS. Effectiveness of NPs and PAs in managing diabetes and cardiovascular disease. JAAPA 2018;31:39–45. [DOI] [PubMed] [Google Scholar]
- 9.McBride CL, Akeroyd JM, Ramsey DJ, Nambi V, Nasir K, Michos ED, Bush RL, Jneid H, Morris PB, Bittner VA, Ballantyne CM, Petersen LA, Virani SS. Statin prescription rates and their facility-level variation in patients with peripheral artery disease and ischemic cerebrovascular disease: Insights from the Department of Veterans Affairs. Vasc Med 2018;23:232–240. [DOI] [PubMed] [Google Scholar]
- 10.Virani SS, Akeroyd JM, Ramsey DJ, Deswal A, Nasir K, Rajan SS, Ballantyne CM, Petersen LA. Health Care Resource Utilization for Outpatient Cardiovascular Disease and Diabetes Care Delivery Among Advanced Practice Providers and Physician Providers in Primary Care. Popul Health Manag 2018;21:209–216. [DOI] [PubMed] [Google Scholar]
- 11.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 12.Glazer NL, Dublin S, Smith NL, French B, Jackson LA, Hrachovec JB, Siscovick DS, Psaty BM, Heckbert SR. Newly detected atrial fibrillation and compliance with antithrombotic guidelines. Ann Intern Med 2007;167:246–252. [DOI] [PubMed] [Google Scholar]
- 13.Walkey AJ, Wiener RS, Ghobrial JM, Curtis LH, Benjamin EJ. Incident stroke and mortality associated with new-onset atrial fibrillation in patients hospitalized with severe sepsis. JAMA. 2011;306:2248–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalra R, Patel N, Doshi R, Arora G, Arora P. Evaluation of the Incidence of New-Onset Atrial Fibrillation After Aortic Valve Replacement. JAMA Intern Med 2019; 179(8):1122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tirschwell DL, Longstreth WT, Jr. Validating administrative data in stroke research. Stroke 2002;33:2465–2470. [DOI] [PubMed] [Google Scholar]
- 16.Kitzman DW, Edwards WD. Age-Related Changes in the Anatomy of the Normal Human Heart. J Gerontol 1990;45:M33–M39. [DOI] [PubMed] [Google Scholar]
- 17.Noorani A, Walsh SR, Tang TY, Sadat U, Cooper DG, Callaghan CJ, Varty K, Gaunt ME. Atrial fibrillation following elective open abdominal aortic aneurysm repair. Int J Surg 2009;7:24–27. [DOI] [PubMed] [Google Scholar]
- 18.Gialdini G, Nearing K, Bhave PD, Bonuccelli U, Iadecola C, Healey JS, Kamel H. Perioperative atrial fibrillation and the long-term risk of ischemic stroke. JAMA 2014;312:616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinberg BA, Zhao Y, He X, Hernandez AF, Fullerton DA, Thomas KL, Mills R, Klaskala W, Peterson ED, Piccini JP. Management of postoperative atrial fibrillation and subsequent outcomes in contemporary patients undergoing cardiac surgery: insights from the Society of Thoracic Surgeons CAPS-Care Atrial Fibrillation Registry. Clin Cardiol 2014;37:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saxena A, Dinh DT, Smith JA, Shardey GC, Reid CM, Newcomb AE. Usefulness of postoperative atrial fibrillation as an independent predictor for worse early and late outcomes after isolated coronary artery bypass grafting (multicenter Australian study of 19,497 patients). Am J Cardiol 2012;109:219–225. [DOI] [PubMed] [Google Scholar]
- 21.LaPar DJ, Speir AM, Crosby IK, Fonner E Jr., Brown M, Rich JB, Quader M, Kern JA, Kron IL, Ailawadi G. Postoperative atrial fibrillation significantly increases mortality, hospital readmission, and hospital costs. Ann Thorac Surg 2014;98:527–533. [DOI] [PubMed] [Google Scholar]
- 22.Chen LY, Chung MK, Allen LA, Ezekowitz M, Furie KL, McCabe P, Noseworthy PA, Perez MV, Turakhia MP. Atrial Fibrillation Burden: Moving Beyond Atrial Fibrillation as a Binary Entity: A Scientific Statement From the American Heart Association. Circulation 2018;137:e623–e644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mariscalco G, Biancari F, Zanobini M, Cottini M, Piffaretti G, Saccocci M, Banach M, Beghi C, Angelini GD. Bedside tool for predicting the risk of postoperative atrial fibrillation after cardiac surgery: the POAF score. J Am Heart Assoc 2014;3:e000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turakhia MP, Desai M, Hedlin H, Rajmane A, Talati N, Ferris T, Desai S, Nag D, Patel M, Kowey P, Rumsfeld JS, Russo AM, Hills MT, Granger CB, Mahaffey KW, Perez MV. Rationale and design of a large-scale, app-based study to identify cardiac arrhythmias using a smartwatch: The Apple Heart Study. Am Heart J 2019;207:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Healey JS, Alings M, Ha A, Leong-Sit P, Birnie DH, Graaf JJd, Freericks M, Verma A, Wang J, Leong D, Dokainish H, Philippon F, Barake W, McIntyre WF, Simek K, Hill MD, Mehta SR, Carlson M, Smeele F, Pandey AS, Connolly SJ. Subclinical Atrial Fibrillation in Older Patients. Circulation 2017;136:1276–1283. [DOI] [PubMed] [Google Scholar]
- 26.McIntyre WF, Connolly SJ, Healey JS. Atrial fibrillation occurring transiently with stress. Curr Opin Cardiol 2018;33:58–65. [DOI] [PubMed] [Google Scholar]
- 27.Patel N, Bajaj NS, Doshi R, Gupta A, Kalra R, Singh A, Berra L, Arora G, Prabhu SD, Arora P. Cardiovascular Events and Hospital Deaths Among Patients With Severe Sepsis. Am J Cardiol 2019;123:1406–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahlsson A, Manolis AS, Casadei B, Van Putte B, Popescu BA, Atar D, Kotecha D, Hindricks G, Diener H-C, Heidbuchel H, Hendriks J, Oldgren J, Castella M, Vardas P, Schotten U, Kirchhof P, Benussi S, Group ESD. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart Jor 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 29.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014;64:e1–e76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


