Abstract
Background
An increasing body of evidence suggests that inflammation plays a key role in stroke, in particular stroke of atherosclerotic origin. Anti‐inflammatory medications are a widely heterogeneous group of drugs that are used to suppress the innate inflammatory pathway and thus prevent persistent or recurrent inflammation. Anti‐inflammatory agents have the potential to stabilise atherosclerotic plaques by impeding the inflammatory pathway. By targeting specific cytokines, the inflammatory pathway may be interrupted at various stages.
Objectives
To assess the benefits and harms of anti‐inflammatory medications plus standard care versus standard care with or without placebo for prevention of vascular events (stroke, myocardial infarction (MI), non‐fatal cardiac arrest, unstable angina requiring revascularisation, vascular death) and all‐cause mortality in people with a prior history of ischaemic stroke or transient ischaemic attack (TIA).
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; last searched 29 May 2019); MEDLINE (1948 to 29 May 2019); Embase (1980 to 29 May 2019); the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to 29 May 2019); and Scopus (1995 to 29 May 2019). In an effort to identify additional published, unpublished, and ongoing trials, we searched several grey literature sources (last searched 30 May 2019). We incorporated all identified studies into the results section. We applied no restrictions with respect to language, date of publication, or study setting.
Selection criteria
We considered randomised controlled trials (RCTs) and cluster‐randomised controlled trials that evaluated anti‐inflammatory medications for prevention of major cardiovascular events following ischaemic stroke or TIA.
Data collection and analysis
Two review authors independently assessed for inclusion titles and abstracts of studies identified by the search. Two review authors independently reviewed full‐text articles for inclusion in this review. We planned to assess risk of bias and to apply the GRADE method.
Main results
We identified no studies that met the inclusion criteria.
Authors' conclusions
There is currently a paucity of evidence on the use of anti‐inflammatory medications for prevention of major cardiovascular events following ischaemic stroke or TIA. RCTs are needed to assess whether use of anti‐inflammatory medications in this setting is beneficial.
Plain language summary
Anti‐inflammatory medications for preventing major heart events and strokes following ischaemic stroke (stroke due to a clot) or mini‐stroke
Review question Do anti‐inflammatory medications have a role in preventing future serious heart conditions or stroke in people who have previously had a stroke or a transient ischaemic attack (TIA, or 'mini‐stroke') as the result of a blood clot?
Background Anti‐inflammatory medications are used to reduce inflammation in several inflammatory conditions. Inflammation may be involved in the occurrence of stroke, mainly through the development of fatty deposits in the vessel (artery). Currently, a variety of medications are available to reduce the risk of heart attacks and strokes or mini‐strokes, but 1 in 20 people will have a further stroke or heart attack.
Search date Searching was complete on 29 May 2019.
Study characteristics Eligible studies could involve any medication whose main use was anti‐inflammatory in any setting in adult patients who had a previous stroke or TIA caused by a clot.
Key results Although we conducted a thorough search of the medical literature for randomised controlled trials assessing anti‐inflammatory medications versus no anti‐inflammatory medications in people with previous stroke or TIA due to a blood clot, we were unable to identify any studies that had been conducted to explore this topic. Therefore, we cannot say whether anti‐inflammatory medications alter heart and stroke outcomes following stroke due to a clot, or what the harms and benefits of this treatment might be. Trials are needed to compare the use of anti‐inflammatory medications in combination with usual treatment versus usual treatment alone.
Quality of the evidence No evidence and no studies were available.
Summary of findings
Summary of findings 1. Summary of findings.
| Anti‐inflammatory therapy with usual care compared with usual care for preventing stroke and other vascular events after ischaemic stroke or transient ischaemic attack | |||||||
|
Patient or population: patients with TIA or ischaemic stroke Settings: worldwide, post‐hyperacute care Intervention: anti‐inflammatory therapy plus usual care Comparison: usual care with or without placebo | |||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | ||
| Assumed risk | Corresponding risk | ||||||
| Usual care | Anti‐inflammatory therapy plus usual care | ||||||
| Number of patients with all vascular events (stroke, MI, non‐fatal cardiac arrest, unstable angina requiring revascularisation, vascular death) at 90 days and at 1 year | 90 days | − | − | − | − | No data available | |
| 1 year | − | − | − | − | No data available | ||
| Number of patients with safety events (total AEs, infections, SAEs) | − | − | − | − | No data available | ||
| Number of patients with recurrent stroke (fatal and non‐fatal) at 90 days and at 1 year | 90 days | − | − | − | − | No data available | |
| 1 year | − | − | − | − | No data available | ||
| Number of patients with all‐cause death | − | − | − | − | No data available | ||
| Number of patients with cardiovascular death | − | − | − | − | No data available | ||
| Number of patients with infection | − | − | − | − | No data available | ||
| Number of patients with non‐scheduled hospitalisation | − | − | − | − | No data available | ||
AE: adverse event. MI: myocardial infarction. SAE: serious adverse event. TIA: transient ischaemic attack.
Background
Description of the condition
The importance of stroke
The American Stroke Association describes ischaemic stroke as "an episode of neurological dysfunction caused by a focal cerebral, spinal, or retinal infarction" (Sacco 2013), and further describes an infarction as "pathological, imaging, or other objective evidence of cerebral, spinal cord, or retinal focal ischaemic injury in a defined vascular distribution; or clinical evidence of cerebral, spinal cord, or retinal focal ischaemic injury based on symptoms persisting ≥ 24 hours or until death, and other etiologies excluded" (Sacco 2013). Although definitions of transient ischaemic attack (TIA) vary, the American Stroke Association has defined a TIA as a focal neurological deficit caused by focal ischaemia of the brain, spinal cord, or retina that does not result in acute infarction (Easton 2009).
The World Health Organization (WHO) estimates that cardiovascular diseases are the leading cause of death globally, accounting for 17.5 million deaths in 2012, of which 6.7 million were directly attributable to stroke (WHO 2016). As a result, stroke is one of the leading causes of non‐communicable death worldwide. It is also a major contributor to healthcare costs and is the second leading cause of disability after dementia (WHO 2016).
Stroke recurrence
Several observational studies undertaken in the late 1990s and the early 2000s describe a 90‐day recurrent stroke/vascular risk in the order of 12% to 20%. However, a more recent study reports a much lower 90‐day risk of recurrent stroke of 3.7% and a one‐year risk of 5.1% after a TIA or a minor ischaemic stroke (Amarenco 2016). Amarenco 2016 also found that individuals with symptomatic large artery atherosclerosis had a greater than twofold risk of recurrent stroke compared with all other stroke aetiologies despite the early introduction of best medical therapy. Additionally, it has been demonstrated that, despite optimal surgical and medical therapy, risk of a recurrent stroke is proportional to the degree of ipsilateral carotid stenosis, with risk of 5.4% in those with less than 50% stenosis to 17.2% in those with severe stenosis/occlusion (Sheehan 2010). In a meta‐analysis of data from the Oxford Vascular Study and the Oxfordshire Community Stroke Project, large artery atherosclerosis conveyed an increased risk of stroke recurrence at seven days (odds ratio (OR) 3.3, 95% confidence interval (CI) 1.5 to 7.0), 30 days (OR 2.9, 95% CI 1.7 to 4.9), and three months (OR 2.9, 95% CI 1.9 to 4.5) compared with other stroke subtypes (Lovett 2004). The reason for this increased risk is unclear; however, accumulating evidence suggests that inflammation‐related plaque instability may be a major contributor.
More recently, randomised controlled trials (RCTs) of secondary prevention strategies following TIA and minor stroke have reported similar rates of stroke recurrence to those reported in recent observational studies. In SOCRATES, the rate of ischaemic stroke recurrence in 90 days was between 5.8% and 6.7% (Johnston 2016). A slightly higher recurrence rate was found in the CHANCE study, in which the 90‐day recurrence rate for ischaemic stroke was between 8.4% and 11.9% (Wang 2013).
The risk of myocardial infarction (MI) and of vascular death is substantial among long‐term stroke/TIA survivors. Touzé 2005 (a systematic review including 39 studies and 65,996 participants) reported a 2.1% per year rate of non‐stroke vascular death and a 0.9% per year rate of non‐fatal MI in stroke survivors.
The importance of inflammation
An increasing body of evidence suggests that inflammation plays a key role in stroke, in particular in stroke of atherosclerotic origin. Five key lines of evidence currently support this view.
First, experimental data indicate that chronic inflammation plays a key role in atherogenesis across all stages. As a result of numerous stressors (such as shear flow, smoking toxins, angiotensin 2), the surface of the endothelial layer overlying the vascular wall expresses selective adhesion molecules, such as vascular cell adhesion protein‐1 (VCAM‐1), which in turn bind to leucocytes. These monocytes penetrate the intima, where they participate in and perpetuate a local inflammatory response. Monocytes undergo differentiation into macrophages, which express receptors for oxidised low‐density lipoprotein (LDL) molecules, absorbing them to form foam cells. These foam cells undergo cell death, and inefficient efferocytosis of these cells contributes to establishment of the necrotic core of an atherosclerotic lesion (Libby 2002). Simultaneously, macrophages, as a result of the action of monocyte chemotactic protein‐1, differentiate into M1 macrophages, which are proinflammatory, leading to the production of proinflammatory cytokines, such as interleukin‐1beta (IL‐1β) and tumour necrosis factor‐alpha (TNF‐α). Other cytokines, namely, platelet‐derived growth factors, stimulate the migration of vascular smooth muscle cells from the adventitia and media to the intima. Here, they form the fibrous caps of atheromatous plaques. Erosion and rupture of these fibrous caps result from the expression of cytokines and metalloproteinases on macrophages and other cells. The presence of tissue factor, produced by endothelial cells and macrophages, triggers the clotting cascade, thus leading to thrombus formation. Embolisation of this thrombus may then lead to ischaemic stroke and TIA.
The second line of evidence relates to key epidemiological studies that have examined the correlation between risk of stroke recurrence and levels of inflammatory markers. Many epidemiological studies have described correlations between the central inflammatory pathway (involving the key cytokines IL‐1, IL‐6, and TNF‐α, and in particular 'downstream' markers such as C‐reactive protein (CRP)) and vascular disease, including stroke. The Emerging Risk Factors Collaboration examined the correlation between CRP and incident stroke in over 160,000 participants without known vascular disease in prospective studies. They found a 27% increase in the risk ratio (RR) of stroke for every threefold increase in CRP: RR 1.27, 95% CI 1.15 to 1.40 (ERFC 2010). This relationship held true after adjustments for age, sex, study, and vascular risk factors. Similarly, in the Framingham Study, during 12 to 14 years of follow‐up, it was found that men and women with CRP in the highest quartile were at a 2‐ and 2.7‐fold higher risk of ischaemic stroke/TIA, respectively, independent of age, when compared with those with CRP in the lowest quartile (Rost 2001). In addition to CRP, other proatherogenic cytokines, such as IL‐6 and TNF‐α, have been associated with increased stroke risk. IL‐6 and TNF‐α are secreted by macrophages, lymphocytes, natural killer cells, and vascular smooth muscle cells, all of which play a key role in development and progression of atherosclerosis. The Health ABC Study, in which 2225 community‐dwelling individuals were prospectively followed‐up for seven years, found a 1.6‐fold (95% CI 1.23 to 2.09) increase in the risk ratio of stroke per log increase in IL‐6 levels (pg/mL) after adjustment for multiple risk factors, including age, gender, smoking, diabetes, hypertension, total cholesterol, and LDL cholesterol, among others (Cesari 2003). A similar correlation was found in the Emerging Risk Factors Collaboration Study (ERFC 2010).
Third, it has been demonstrated that genes affecting the upstream inflammatory markers IL‐1 and IL‐6 can influence an individual's risk of stroke. The Ischaemic Stroke Genetics Study demonstrated that in two North American cohorts, alterations in the allele encoding the IL‐1 receptor antagonist gene were found in nearly 25% of people with stroke ‐ 2.80 (95% CI 1.29 to 6.11; P = 0.03) times more frequently than in healthy controls (Worrall 2007). Two mendelian studies of IL‐6 receptor mutations also showed a correlation between gene polymorphisms causing impaired binding of IL‐6 to its receptor, decreased signalling of the IL‐6 receptor, and thus decreased levels of CRP and fibrinogen, along with a decrease in cardiovascular risk (ILR6 Genetics Consortium 2012; IL6R MR 2012). Stroke was not reported as an outcome in these studies.
Fourth, plaque imaging also supports the inflammation hypothesis with regards to ischaemic stroke. Positron emission tomography‐computed tomography (PET‐CT) with 18‐fluorodeoxyglucose (18‐FDG) is a validated method used to assess the degree of metabolic activity in a plaque (18‐FDG uptake is proportional to the macrophage content within an atherosclerotic plaque). In areas of high macrophage activity, there is high 18‐FDG uptake, which relates to the level of proinflammatory activation. 18‐FDG uptake has been shown to correlate closely with the degree of macrophage infiltration on histological sampling. With regards to stroke prediction, in a prospective observational study of 60 participants with recent stroke due to large vessel disease, there was an independent correlation between the degree of FDG uptake in the ipsilateral carotid and stroke recurrence (adjusted hazard ratio (HR) 6.1, 95% CI 1.3 to 28.8; P = 0.02) after data were adjusted for degree of stenosis (Marnane 2012). Furthermore, among over 900 people with cancer who underwent PET‐CT as part of staging work‐up and who were asymptomatic from a vascular viewpoint, increased 18‐FDG uptake in the major arteries was demonstrated to be the strongest predictor of a subsequent vascular event, including stroke, MI, or revascularisation (Rominger 2009). A recent study published in the journal Stroke concluded that 18‐FDG uptake (measured as target‐to‐background ratio standardised uptake value (SUV)) was higher in individuals with symptomatic carotid disease than in those with asymptomatic disease (median 2.07 versus 1.78; P < 0.0018) and in participants who had a positive microembolic signal on transcranial Doppler than in those who had negative microembolic signals (median 2.14 versus 1.86; P < 0.008) (Muller 2014).
The final, fifth line of evidence comes from clinicopathological specimens, which also support a correlation between inflammation and stroke. The Oxford Plaque Study, which examined resected plaques from 526 symptomatic individuals, found that in two‐thirds of participants, there was evidence of marked inflammation in the symptomatic carotid artery (Redgrave 2006). Furthermore, extensive macrophage infiltration was independently associated with plaque rupture (adjusted OR 3.39, 95% CI 2.31 to 4.98; P < 0.001) (Redgrave 2006). Further evidence for this line of thinking can be found in a systematic review by Golledge 2000, which histologically examined studies comparing symptomatic and asymptomatic plaques of similar stenosis severity. Golledge 2000 found that symptomatic plaques had a thinner fibrous cap and a higher degree of inflammation, with increased numbers of macrophages and T cells, than asymptomatic plaques. Furthermore, in a pooled cohort of over 1500 participants who consecutively underwent carotid endarterectomy within six months of presenting with symptomatic stenosis (in the Oxford Plaque Study and in the Athero‐Express Study), it was found that individuals with cerebral events rather than isolated ocular symptoms had a significantly higher degree of inflammation in the carotid plaque (OR 1.32, 95% CI 1.02 to 1.72; P = 0.04) (Howard 2013).
There is also accumulating evidence for the use of anti‐inflammatory medications in cardiovascular disease. The LoDoCo trial, over a median follow‐up of 36 months, showed a 67% reduction in the risk of major cardiovascular events among participants with stable coronary artery disease treated with low‐dose colchicine (HR 0.33, 95% CI 0.18 to 0.59; P < 0.001) (Nidorf 2013). Several RCTs investigating anti‐inflammatory medications for the treatment of cardiovascular disease are currently under way. Canakinumab is currently under investigation, in the CANTOS trial, for individuals with stable coronary artery disease and CRP ≥ 2 mg/L. Other anti‐inflammatory agents under evaluation in this non‐stroke population include methotrexate (CIRT), colchicine (LoDoCo2), and tocilizumab (ENTRACTE).
Description of the intervention
Anti‐inflammatory medications are a widely heterogeneous group of drugs that are used to suppress the innate inflammatory pathway and thus prevent persistent or recurrent inflammation. Broadly, they consist of steroids (e.g. glucocorticoids), non‐steroidal anti‐inflammatory drugs (NSAIDs), cyclo‐oxygenase‐2 (COX‐2) inhibitors, colchicine, antimetabolite drugs such as methotrexate and azathioprine, and anticytokine agents including TNF‐α‐binding proteins (e.g. infliximab). Mycophenolate is another immune‐modulating drug. All of these types of drugs have different pharmacokinetics and pharmacodynamics.
Glucocorticoids
Glucocorticoids are potent anti‐inflammatory agents targeting both early and late phases of inflammation. Glucocorticoids target inflammation through multiple pathways including decreased activation of neutrophils, macrophages, and T‐helper cells. They also downregulate the production of multiple cytokines, including IL‐1, IL‐2, IL‐6, and TNF‐α, through inhibition of gene transcription. In addition, glucocorticoids inhibit the production of prostanoids through decreased expression of COX‐2 and are responsible in part for the upregulation of anti‐inflammatory factors, including IL‐10 and annexin 1.
Non‐steroidal anti‐inflammatory drugs
NSAIDs are very commonly used anti‐inflammatory medications. They are weak organic acids that are highly metabolised to form an active agent. Their anti‐inflammatory activity primarily results from the biosynthesis of prostaglandins; however, NSAIDs also inhibit chemotaxis, IL‐1 production, and free‐radical formation. NSAIDs decrease the sensitivity of blood vessels to bradykinin and histamine, and they decrease the production of lymphokines from T lymphocytes.
Cyclo‐oxygenase‐2 inhibitors
The COX‐2 isoenzyme is induced by proinflammatory cytokines (e.g. IL‐1β, TNF‐α) and endotoxins in response to inflammation, and is responsible for an increased level of prostaglandins during inflammation. By inhibiting this isoenzyme, the inflammatory process is downregulated as the production of regulatory cytokines is not inhibited, and B‐ and T‐cell proliferation is reduced.
Colchicine
The anti‐inflammatory effect of colchicine is mediated primarily through inhibition of the microtubule assembly. This inhibits inflammasome activation, cell chemotaxis, and production of leukotrienes and cytokines, thus inhibiting inflammation. By inhibiting the inflammasome, colchicine inhibits IL‐1β activation from its precursor, proIL‐1β, thus limiting the inflammatory response. Colchicine is also responsible for inhibiting the expression of IL‐1β, IL‐6, and TNF‐α through reduced activation of macrophages.
Antimetabolites (e.g. methotrexate, azathioprine)
Different antimetabolite drugs have different mechanisms of action. Methotrexate, an antifolate drug that interferes with DNA replication, exerts its anti‐inflammatory action through inhibition of cytokines. The exact mechanism by which this inhibition occurs remains unknown. Methotrexate is also believed to inhibit lymphocyte proliferation through inhibition of purine synthesis. Azathioprine, a purine analogue that also inhibits DNA synthesis, inhibits T‐cell proliferation through complex genetic interactions, leading to a depressed immune response.
Anticytokine drugs
This is a class of recombinant engineered antibodies that interact with the immune system. Within this broad class of immunomodulating agents, different agents target different cytokines involved in the inflammatory pathway. The main targets are TNF‐α (infliximab, adalimumab), IL‐1 antagonism (canakinumab, anakinra), and IL‐2 antagonism (daclizumab).
How the intervention might work
Anti‐inflammatory agents have the potential to stabilise atherosclerotic plaques by impeding the inflammatory pathway. By targeting specific cytokines, the inflammatory pathway may be interrupted at various stages.
Non‐specific agents, including NSAIDs, COX‐2 inhibitors, and colchicine, inhibit inflammation through downregulation of prostaglandin synthesis and chemokine modulation. Inflammatory cells, such as neutrophils and mast cells, as well as endothelial cell adhesion molecules, are inhibited, thus impeding the initiation and intensification of inflammation.
With medications such as infliximab, etanercept, and adalimumab, which target TNF‐α (a multi‐functional proinflammatory cytokine that may be involved in the development of atherosclerosis), the production of more downstream inflammatory cytokines such as IL‐6, as well as the proliferation of leucocytes, may be impeded. TNF‐α antagonism may also inhibit the earliest stages of atherosclerosis development, as this may downregulate smooth muscle proliferation and inhibit the expression of VCAM‐1, thus decreasing adherence of leucocytes to endothelial cells.
Targeting IL‐6 with agents such as tocilizumab may cause both membrane‐bound and circulating IL‐6 receptors to be blocked. This may help minimise endothelial dysfunction and reduce arterial stiffness, in addition to minimising the proinflammatory effect of IL‐6.
IL‐1β is also a promising target. IL‐1β receptor inhibition may reduce leucocyte adhesion in vascular endothelial cells, which leads to procoagulant activity and serves as a trigger for human vascular smooth muscle cell proliferation. Such agents include canakinumab and anakinra.
However, certain harms may be associated with the use of anti‐inflammatory agents. By impeding anti‐inflammatory pathways, many anti‐inflammatory medications also affect the overall immune system, leaving recipients of these drugs vulnerable to infection, in particular, opportunistic infection, such as systemic fungal infections and those caused by mycobacteria and other atypical organisms. These infections can be potentially life threatening. Some anti‐inflammatory drugs, such as methotrexate and cyclophosphamide, can lead to myelosuppression, causing a decrease in the white cell count and suppression of the immune system. NSAIDs, COX‐2 inhibitors, and steroids have also been shown to increase the risk of gastrointestinal mucosal injury through inhibition of prostaglandins.
Why it is important to do this review
Currently, the incidence of stroke is rising, particularly in low‐ and middle‐income countries, and absolute numbers are rising in high‐income countries due to greater longevity. Although secondary prevention is effective in reducing the burden of recurrent stroke, recent clinical trials have reported the rate of risk of recurrent stroke to range from 2.5% to 4% per year, even in individuals receiving established secondary prevention medications such as high‐dose statins and antiplatelet agents (SPS3 2013). Statistics from the American Stroke Association for 2017 reveal that 795,000 individuals suffer a new or recurrent stroke each year, with about 185,000 of these being recurrent events (Benjamin 2017). It is therefore imperative that novel, effective treatments for prevention of recurrent stroke and vascular events are identified.
Objectives
To assess the benefits and harms of anti‐inflammatory medications plus standard care versus standard care with or without placebo for prevention of vascular events (stroke, myocardial infarction (MI), non‐fatal cardiac arrest, unstable angina requiring revascularisation, vascular death) and all‐cause mortality in people with a prior history of ischaemic stroke or transient ischaemic attack (TIA).
Methods
Criteria for considering studies for this review
Types of studies
We considered randomised controlled trials (RCTs) and cluster‐RCTs, including studies published as full text or in abstract form and with unpublished data. From randomised cross‐over trials, we regarded only data from the first phase as eligible for inclusion. We excluded 'N of 1' trials and non‐randomised (observational) research.
Types of participants
We included adult men and women (aged 18 years and over) with a history of ischaemic stroke or TIA. We noted the definition of TIA in use at the time of publication of the article.
Types of interventions
We planned to include trials comparing treatment with anti‐inflammatory medications for any cardiovascular condition with no or any other treatment (including usual care or placebo) not containing anti‐inflammatory medications for participants following a first ever stroke or TIA. We would have accepted any co‐interventions provided they were identical in the compared study groups and were not part of the randomised treatment. We intended to include any medication, the primary effect of which is anti‐inflammatory, including (but not limited to) NSAIDs, COX‐2 inhibitors, colchicine, and antimetabolite and anticytokine medications. For the purposes of this review, we did not consider any medication with pleiotropic actions, the primary mechanism of action of which is not inhibition of inflammation (e.g. statins, allopurinol).
Types of outcome measures
Primary outcomes
The primary outcomes for this review were the numbers of patients with:
all vascular events (stroke, MI, non‐fatal cardiac arrest, unstable angina requiring revascularisation, vascular death) at 90 days and at one year; however when follow‐up is completed at different time points and all other criteria are met, the studies will be included for analysis; and
safety of interventions (total adverse events (AEs), infections, serious AEs).
In this review, we defined recurrent stroke as any stroke that is reported during the study period after the study intervention has been administered. We defined a serious AE using the US Food and Drug Administration (FDA) definition: "it results in any of the following outcomes: death, a life‐threatening adverse event, inpatient hospitalisation or prolongation of existing hospitalisation, a persistent or significant incapacity or substantial disruption of the ability to conduct normal life functions, or a congenital anomaly/birth defect."
Secondary outcomes
Secondary outcomes for this review were the numbers of patients with:
recurrent ischaemic stroke (fatal and non‐fatal) at 90 days and at one year; however when follow‐up is completed at different time points and all other criteria are met, the studies will be included for analysis;
all‐cause death;
all cardiovascular deaths;
non‐scheduled hospitalisations (all‐cause and for cardiovascular reasons);
infection; and
disability (measured via a validated scoring system (e.g. Barthel Index, modified Rankin score)).
Search methods for identification of studies
See the methods for the Cochrane Stroke Group Specialised register. We searched for trials published in any language and would have arranged for translation of relevant papers when required. We initially performed the searches on 4 May 2018 and updated them on 29 May 2019. We assessed and included all results in the review.
Electronic searches
We searched the Cochrane Stroke Group trial register and the following electronic databases.
Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 5), in the Cochrane Library (last searched 29 May 2019) (Appendix 1).
MEDLINE (1948 to 29 May 2019) (Appendix 2) using Ovid.
Embase (1980 to 29 May 2019) (Appendix 3) using Ovid.
Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to 29 May 2019) (Appendix 4) using EBSCO.
Scopus (1995 to 29 May 2019) (Appendix 5).
The subject strategies for databases will be modelled on the search strategy designed for MEDLINE by the Cochrane Stroke Group’s Information Specialist (Appendix 2). All search strategies deployed were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying RCTs and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions).
We searched the following ongoing trial registers (Appendix 6).
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 29 May 2019).
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched 29 May 2019).
Internet Stroke Centre Trials Registry (strokecenter.org/trials/; searched 29 May 2019).
Searching other resources
In an effort to identify additional published, unpublished, and ongoing trials, we:
checked the bibliography and references of any relevant systematic reviews identified for further reference to relevant trials;
used Science Citation Index Cited Reference Search for forward tracking of relevant articles;
contacted experts in the field who may be aware of additional unpublished RCTs for inclusion; and
-
searched grey literature sources, dissertation and theses databases, and databases of conference abstracts including:
ProQuest Dissertation and Theses (search.proquest.com/index) (Appendix 7);
Epistemonikos (epistemonikos.org/) (Appendix 8);
BIOSIS Citation Index (last searched 29 May 2019) (Appendix 9); and
Web of Science Conference Proceedings Citation Index‐Science (Appendix 9) (last searched 29 May 2019).
Data collection and analysis
Selection of studies
Two review authors (SC, JM) independently screened the titles and abstracts of references obtained as a result of our searching activities and excluded obviously irrelevant reports. We retrieved full‐text articles for the remaining references, and two review authors (SC, PK) independently screened the full‐text articles to identify studies for inclusion, and to identify and record reasons for exclusion of ineligible studies. We resolved any disagreements through discussion. We collated multiple reports of the same study so that each study, not each reference, was the unit of interest in the review. We recorded the selection process and completed a PRISMA flow diagram.
Data extraction and management
For those studies deemed eligible for inclusion, three review authors (SC, SM, PK) would have independently reviewed the papers for data extraction using a data extraction template. We would have extracted and recorded the following data.
Methods: study design, method of randomisation, allocation concealment and blinding, follow‐up schedule.
Participants: number of participants assigned to each arm, details of diagnosis, demographics, attrition rates.
Interventions: drug(s) studied, doses, administration, side effects.
Outcomes: types of outcomes assessed, AEs, rates of outcomes.
Publication: year of publication, conflicts of interest.
We planned to resolve any disagreement through discussion and, when necessary, to consult a fourth review author (MO'D). When data were insufficient or missing, we intended to contact the study authors to ask for additional information.
Assessment of risk of bias in included studies
Two review authors (SC, PK) would have independently assessed the risk of bias in each included study. We planned to categorise studies as being at high, low, or unclear risk based on the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We would have resolved any disagreements by discussion or by consultation with another review author (SM). We intended to make decisions based on consensus opinion.
We planned to assess the risk of bias in each of the following domains.
Selection bias: random sequence generation, allocation concealment.
Performance bias: blinding of investigator and trial participant.
Detection bias: blinding of outcomes.
Attrition bias: missing data, loss to follow‐up rates.
Reporting bias: selective outcome reporting.
Other bias.
We would have presented in 'Risk of bias' tables the justification for our allocation of high, low, or unclear risk judgements.
Measures of treatment effect
Dichotomous data
We planned to measure the treatment effect by using risk ratios (RRs) with 95% confidence intervals (CIs). We would have extracted the actual data for outcome events and the numbers of participants randomised in each trial to calculate pooled RRs for each outcome.
Continuous data
When a standard measurement scale had been used in included studies, we would have used mean differences (MDs) with 95% CIs to measure the treatment effect; when different measurement scales had been used (e.g. disability), we would have used standardised mean differences (SMDs) with 95% CIs.
Unit of analysis issues
For cluster‐RCTs, we intended to adjust standard errors using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We would have sought advice from a statistician to ensure analysis at the level of the individual when possible. When this was not possible, we would have used a summary measurement from each cluster. For cross‐over trials, we would have included data from the first phase only.
Dealing with missing data
In the event of missing data, we intended to contact the first‐named author on the paper, or the lead investigator for ongoing trials, to obtain this information.
During data extraction, we would have recorded the quantity of missing data from outcome variables. If this had been greater than 20% and was felt to be 'not missing at random', we would have assumed that the missing data showed no benefit from the intervention. We planned to perform a sensitivity analysis to assess the effect of this assumption on the summary rate ratios. We would have liaised with an experienced statistician to ensure accuracy.
Assessment of heterogeneity
We intended to use the I² statistic to measure heterogeneity among the trials in each analysis. We planned to accept heterogeneity less than 40%.
Assessment of reporting biases
If 10 or more studies investigating a particular outcome had been included, thus ensuring the ability of tests to distinguish chance from real asymmetry, we proposed to use funnel plots to assess the possibility of publication bias.
Data synthesis
We intended to analyse data using RevMan 5.3 (RevMan 2014).
If there were sufficient similarities within subgroups (e.g. similarity of interventions) supported by an I² statistic less than 40%, we would have performed a meta‐analysis of the pooled appropriate data.
As we predict that the effects of anti‐inflammatory drugs would vary widely across the included studies, we proposed that a random‐effects model should be used (DerSimonian 1986), with a continuity correction of 0.5 to synthesise the included treatment effects.
We planned to analyse data on an intention‐to‐treat basis. We planned to use the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it relates to the studies that contribute data to the meta‐analyses for prespecified outcomes (Atkins 2004). We would have used the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), via GRADEpro GDT software (GRADEpro GDT 2015). We would have justified all decisions to downgrade the quality of studies using footnotes, and we intended to make comments to aid the reader's understanding of the review when necessary.
We would have created a 'Summary of findings' table to display our findings using the following outcomes.
All vascular events (stroke, MI, non‐fatal cardiac arrest, unstable angina requiring revascularisation, vascular death) at 90 days and at one year.
Safety of interventions (total adverse events (AEs), infections, serious AEs).
Recurrent stroke (fatal and non‐fatal) at 90 days and at one year.
All‐cause death.
Non‐scheduled hospitalisations (all‐cause and for cardiovascular reasons).
All cardiovascular death.
Infection.
Subgroup analysis and investigation of heterogeneity
When data were sufficient, we would have performed subgroup analyses for the primary outcomes, by drug class, based on:
inflammatory marker analysis: when available, the data will be studied for outcomes based on baseline and follow‐up inflammatory markers (divided into tertiles);
age: younger than 45 years old, 45 to 65 years old, and older than 65 years; and
anti‐inflammatory agent class.
We would have used a formal test for subgroup interactions in Review Manager 5.3 (RevMan 2014).
Sensitivity analysis
We intended to carry out sensitivity analyses on the primary outcomes if we found studies with variable follow‐up or with substantial missing data. To assess the robustness of the pooled RRs, we proposed analysis of data excluding studies with substantial missing data.
Results
Description of studies
No studies were available for inclusion in this review.
Results of the search
We identified 59,155 reports in total: 41,882 through searches of electronic databases, and a further 17,273 records through review of other sources, primarily grey literature searches. We identified 6600 duplicates (Figure 1).
1.
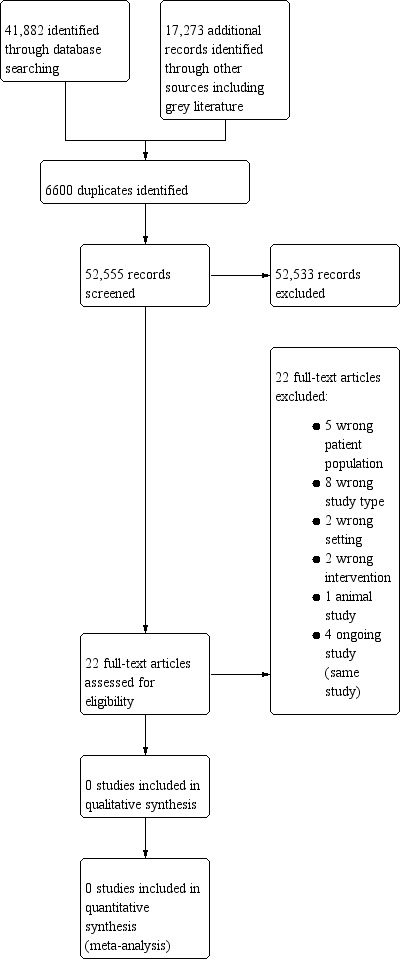
Study flow diagram.
Two review authors (SC, JM) screened titles and abstracts and identified 22 full‐text articles related to 18 completed studies (ACTRN12605000174684; ACTRN12614000093684; Deftereos 2013; Drieu 2018; Duris 2017; EudraCT2015‐003959‐22; Gonzalez‐Valcarcel 2016; IRCT201601289014N90; NCT02282098; Nidorf 2013; Raju 2012; Ridker 2017; Ridker 2018; Roubille 2015; Schink 2018; Smith 2018; Tsivgoulis 2018; Xu 1999).
Included studies
No studies met the inclusion criteria for our review, so we were unable to perform analyses to assess the efficacy of anti‐inflammatory therapy for prevention of stroke and other cardiovascular events following ischaemic stroke or transient ischaemic attack. As results become available, we will update this review.
Excluded studies
We excluded 18 studies for the following reasons: five studies included people with ischaemic heart disease alone, with no one enrolled who had suffered a stroke; two studies related to acute stroke treatment only; three were observational studies; five were review articles; two studies examined medicinal products whose primary effect was not anti‐inflammatory; and one was an ongoing study (four articles identified for this trial) (Characteristics of excluded studies). Although one study did recruit patients following acute ischaemic stroke and randomly assigned them to colchicine or usual care, the outcome measure was change in high‐sensitivity CRP at 30 days, and this study did not assess clinical outcomes (Raju 2012).
Ongoing studies
We identified one ongoing study − CONVINCE (NCT02898610).
Risk of bias in included studies
No study met inclusion criteria.
Allocation
No trials were available for analysis.
Blinding
No trials were available for analysis.
Incomplete outcome data
No trials were available for analysis.
Selective reporting
No trials were available for analysis.
Other potential sources of bias
No trials were available for analysis.
Effects of interventions
See: Table 1
No trials were available for analysis.
Discussion
In this review, we did not identify any studies or randomised controlled trials that examined the use of anti‐inflammatory therapies for prevention of stroke and other cardiovascular events following stroke or transient ischaemic attack (TIA).
Despite increasing evidence for the use of anti‐inflammatory therapies for secondary prevention of atherosclerotic cardiovascular disease, no randomised controlled trials to date have been undertaken in people with ischaemic cerebrovascular pathology. Currently one randomised trial is under way ‐ the CONVINCE trial, which is due to be completed in 2021, compares colchicine plus usual care with usual care alone for prevention of stroke and major adverse cardiovascular events in people following minor stroke or TIA (NCT02898610).
Recently published trials in the cardiovascular literature include CANTOS (Ridker 2017), CIRT (Ridker 2019), and LoDoCo (Nidorf 2013). In CANTOS, which compared canakinumab to placebo in people following myocardial infarction, there was a 15% (95% confidence interval (CI) 2% to 26%) reduction in the risk of major adverse cardiovascular events among those treated with 150 mg of canakinumab every three months versus placebo. In LoDoCo, which compared low‐dose colchicine plus usual care to usual care alone, there was a significant reduction in risk of the composite end point of acute coronary syndrome, out‐of‐hospital cardiac arrest, or non‐cardioembolic ischaemic stroke with a hazard ratio of 0.33 (95% CI 0.18 to 0.59). In contrast, in CIRT, there was no difference in the incidence of major adverse cardiovascular events among people receiving methotrexate versus placebo, among people with myocardial disease or multi‐vessel coronary disease, among people who also had a diagnosis of diabetes mellitus or metabolic syndrome.
Atherosclerosis is a major contributor to ischaemic stroke and TIA, and as such it could be hypothesised that anti‐inflammatory therapies could be beneficial for secondary prevention following ischaemic stroke and TIA, given results of trials described above related to atherosclerotic heart disease.
Although this is an empty review, it adds valuable information and should not be discounted. Given the important role that inflammation plays in ischaemic stroke, increased focus is required on the development of effective therapies in this area. This review should help to guide researchers regarding the currently available literature. Given increasing focus in this area, it is envisaged that future reviews will add significantly to the literature on this topic.
Summary of main results
In the absence of suitable trials in this area, we were unable to perform any analysis.
Overall completeness and applicability of evidence
Currently, no studies or randomised trials support the use of anti‐inflammatory therapy for prevention of future stroke or major adverse cardiovascular events following ischaemic stroke or TIA. Further studies are needed to address this topic.
Quality of the evidence
Currently, no evidence is available for inclusion in this review.
Potential biases in the review process
This was a rigorously conducted review for which review authors reviewed in excess of 50,000 articles. We minimised risk of bias by strictly adhering to Cochrane methods. Searches of the electronic databases were conducted by an Information Specialist, and searches of the grey literature and other sources were conducted under his guidance. We did not limit our search by date, language, document type, or publication status. More than one review author independently selected studies. As no studies were identified, there was no risk of bias in data extraction or interpretation.
Agreements and disagreements with other studies or reviews
It is not possible to comment on this because no studies were identified.
Authors' conclusions
Implications for practice.
As this is an empty review, we can conclude that high‐quality evidence on the use of anti‐inflammatory therapy for prevention of future strokes and cardiovascular events following ischaemic stroke and transient ischaemic attack is lacking, and therefore there is no evidence to support the routine use of anti‐inflammatory medications in this setting.
Implications for research.
Given the current lack of high‐quality evidence, randomised controlled trials on this topic are needed.
History
Protocol first published: Issue 11, 2017 Review first published: Issue 5, 2020
Acknowledgements
We thank Joshua Cheyne (Information Specialist) from the Cochrane Stroke Group for his help and guidance in performing this review. We also thank the Cochrane Stroke Editorial Board for their constructive comments during development of this review, and Hazel Fraser (Managing Editor, Cochrane Stroke Group) for her invaluable support and advice.
Appendices
Appendix 1. CENTRAL search strategy
ID Search Hits #1 MeSH descriptor: [Cerebrovascular Disorders] this term only #2 MeSH descriptor: [Basal Ganglia Cerebrovascular Disease] this term only #3 MeSH descriptor: [Brain Ischemia] explode all trees #4 MeSH descriptor: [Carotid Artery Diseases] this term only #5 MeSH descriptor: [Carotid Artery Thrombosis] this term only #6 MeSH descriptor: [Intracranial Arterial Diseases] this term only #7 MeSH descriptor: [Cerebral Arterial Diseases] this term only #8 MeSH descriptor: [Intracranial Embolism and Thrombosis] explode all trees #9 MeSH descriptor: [Stroke] explode all trees #10 (isch?emi* near/6 (stroke* or apoplex* or cerebral vasc* or cerebrovasc* or cva or attack*)):ti,ab,kw (Word variations have been searched) #11 ((brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA* or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) near/5 (isch?emi* or infarct* or thrombo* or emboli* or occlus* or hypoxi*)):ti,ab,kw (Word variations have been searched) #12 tia:ti,ab,kw (Word variations have been searched) #13 {or #1‐#12} #14 MeSH descriptor: [Anti‐Inflammatory Agents] explode all trees #15 (acetaminophen* or acetophenide or alclometasone or adalimumab or beclomethasone* or benzydamine or betamethasone or budesonide or clobetasol or clofazimine or corticosterone or cortisone or desonide or desoximetasone or dexamethason* or fludrocortisone or flufenamic* or flumethasone or fluocinolon* or fluocinonide or fluorometholone or fluprednisolone or flurandrenolone or fluticasone or glycyrrhetinic acid or glycyrrhizic* or halcinonide or hydrocortisone or methylprednisolone or mometasone* or nedocromil or paramethasone or prednisolone or prednisone or tilorone or triamcinolone):ti,ab,kw (Word variations have been searched) #16 NSAID*:ti,ab,kw (Word variations have been searched) #17 MeSH descriptor: [Prostaglandin‐Endoperoxide Synthases] explode all trees #18 ((coxib* or cyclooxygenase or COX) near/3 inhibit*):ti,ab,kw (Word variations have been searched) #19 ((Prostaglandin* or PTGS2) near/3 inhibit*):ti,ab,kw (Word variations have been searched) #20 MeSH descriptor: [Glucocorticoids] explode all trees #21 (glucocorticoids or beclomethasone or betamethasone or budesonide or clobetasol or desoximetasone or dexamethasone or diflucortolone or flumethasone or fluocinolone acetonide or fluocinonide or fluocortolone or fluorometholone or fluprednisolone or flurandrenolone or fluticasone‐salmeterol or melengestrol acetate or methylprednisolone or paramethasone or prednisolone or prednisone or triamcinolone):ti,ab,kw (Word variations have been searched) #22 corticosteroid*:ti,ab,kw (Word variations have been searched) #23 (adalimumab or adapalene or ampyrone or anakinraantipyrine or apazone or acetylsalicyl* or bufexamac or celecoxib or clonixin or curcumin or daclizumab or dexibuprofen or dexketoprofen or diclofenac or diflunisal or dipyrone or etodolac or epirizole or etanercept or fenoprofen or feprazone or flurbiprofen or ibuprofen or indomethacin or indomethacin or indoprofen or ketoprofen or ketorolac or masoprocol or meclofenamic acid or mefenamic acid or meloxic or mesalamine or naproxen or niflumic acid or olopatadine hydrochloride or oxyphenbutazone or phenylbutazone or piroxicam or salicylate* or sulfasalazine or sulindac or suprofen or tenoxicam or tiaprofenic* or tolmetin):ti,ab,kw (Word variations have been searched) #24 (A 771726 or aceclofenac or acemetacin or acetosyringone or acetovanillone or alclofenac or alminoprofen or amiprilose or andrographolide or anisodam* or apremilast or arteparon or arthrotec or atrinositol or azulene or baicalin or balsalazide or bendazac* or benorilate or benoxaprofen or benzobarbital or berbamine or betulinic* or bevonium or biphenylylacetic* or boldine or boswellic* or bredinin or bromfenac or bucillamine or bumadizone or butibufen or canakinumab or carbaspirin* or carprofen or caryophyllene or castanospermine or CDP 571 or cepharanthine or chloroquine* or choline magnesium trisalicylate or chrysarobin or CP 96345 or colchicine or dauricine or difenpiramide or dimephosphon or diucifon or droxicam or DuP 697 or ebselen or efamol or eltenac or enfenamic* or ethenzamide or ethonium or etofenamate or etoricoxib or fenamic acid or fenbufen or fenclofenac or fenflumizole or fentiazac or fepradinol or ferulic* or floctafenine or flosulide or flunixin* or flunoxaprofen or fluproquazone or FR 167653 or FR 173657 or glucametacin or guacetisal or helenalin or heliodermin or hemodes or higenamine or ibuproxam or icatibant or IH 764‐3 or imidazole* or indobufen or infliximab):ti,ab,kw (Word variations have been searched) #25 (iodoantipyrine or isoxicam or kebuzone or L 745337 or L 778736 or lipoxin* or lisofylline or lobenzarit or lonazolac or lornoxicam or loxoprofen or lumiracoxib or magnolol or manoalide or meloxicam or methotrexate or mofebutazone or mofezolac or nabumetone or nafamostat or nebacetin or nepafenac or nifenazone or nimesulide or nitroaspirin or olsalazine or olvanil or orgotein or oxaprozin or palmidrol or parecoxib or parthenolide or peoniflorin or phenidone or pimecrolimus or pirfenidone or pirprofen or proglumetacin or propacetamol or propionylcarnitine or propyphenazone or proquazone or pyranoprofen or pyrazolone or pyrogenal or resveratrol or rofecoxib or rosmarinic* or rumalon or salicin or salicylamide or salicylsalicylic* or SB 203580 or SC 299 or SC 41930 or SC 560 or semapimod or seratrodast or serratiopeptidase or sinapaldehyde or ST 679 or suxibuzone or tanshinone or taxifolin or tenidap or tenoxicam or tepoxalin or tiaprofenic* or tiaramide or tinoridine or tolfenamic* or tranilast or tribenoside or ursolic* or valdecoxib or zileuton or zomepirac):ti,ab,kw (Word variations have been searched) #26 MeSH descriptor: [Macrophage Inflammatory Proteins] explode all trees #27 ((inflam* near/5 protein*) or chemokin*):ti,ab,kw (Word variations have been searched) #28 (CCL3 or CCL4 or CCL19 or CCL20 or CXCL2):ti,ab,kw (Word variations have been searched) #29 MeSH descriptor: [Colchicine] explode all trees #30 {or #14‐#29} #31 MeSH descriptor: [Antimetabolites] this term only #32 MeSH descriptor: [Antimetabolites, Antineoplastic] this term only #33 MeSH descriptor: [Nucleic Acid Synthesis Inhibitors] this term only #34 MeSH descriptor: [Cytokines] this term only and with qualifier(s): [Antagonists & inhibitors ‐ AI] #35 (antimetabolit* or anti‐metabolit*):ti,ab,kw (Word variations have been searched) #36 (anticytokin* or (cytokine near/3 inhibit*)):ti,ab,kw (Word variations have been searched) #37 (ancitabine or azacitidine or azacosterol or azaguanine or azaserine or azathioprine or azauridine or betaine or bezafibrate or bromodeoxyuridine or bromouracil or buthionine sulfoximine or butoxamine or capecitabine or carbon monoxide or cerulenin or chitosan or cholestyramine resin or choline or clofenapate or clofibrate or clofibric acid or colesevelam hydrochloride or colestipol or cycloserine or cytarabine or deoxyglucose or deoxyuridine or diazooxonorleucine or didanosine or dideoxyadenosine or dideoxynucleosides or ethionamide or ethionine or floxuridine or flucytosine or fluorouracil or halofenate or isoniazid or meglutol or methotrexate or metyrapone or nafenopin or niacin or niceritrol or oxythiamine or peptichemio or probucol or proguanil or propylthiouracil or puromycin or pyridinolcarbamate or pyrithiamine or ribavirin or stavudine or tegafur or tetrahydrouridine or thioguanine or thioinosine or thiouridine or toyocamycin or dihydrochloride or triclosan or trifluridine or trimetrexate or triparanol or tubercidin or vidarabine or vidarabine phosphate or zalcitabine or zidovudine):ti,ab,kw (Word variations have been searched) #38 {or #31‐#37} #39 #30 or #38 #40 #13 and #39
Appendix 2. MEDLINE search strategy
1. cerebrovascular disorders/ or basal ganglia cerebrovascular disease/ or exp brain ischemia/ or carotid artery diseases/ or carotid artery thrombosis/ or intracranial arterial diseases/ or cerebral arterial diseases/ or exp "intracranial embolism and thrombosis"/ or exp stroke/ 2. (isch?emi$ adj6 (stroke$ or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva or attack$)).tw. 3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA$ or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw. 4. tia.tw. 5. 1 or 2 or 3 or 4 6. exp anti‐inflammatory agents/ 7. (antiinflam$ or anti‐inflam$ or DMARD$).tw. 8. (acetaminophen$ or acetophenide or alclometasone or adalimumab or beclomethasone$ or benzydamine or betamethasone or budesonide or clobetasol or clofazimine or corticosterone or cortisone or desonide or desoximetasone or dexamethason$ or fludrocortisone or flufenamic$ or flumethasone or fluocinolon$ or fluocinonide or fluorometholone or fluprednisolone or flurandrenolone or fluticasone or glycyrrhetinic acid or glycyrrhizic$ or halcinonide or heparinoid$ or hydrocortisone or methylprednisolone or mometasone$ or nedocromil or paramethasone or prednisolone or prednisone or tilorone or triamcinolone).tw. 9. exp anti‐inflammatory agents, non‐steroidal/ or exp cyclooxygenase inhibitors/ 10. NSAID$.tw. 11. exp Prostaglandin‐Endoperoxide Synthases/ 12. ((coxib$ or cyclooxygenase or COX) adj3 inhibit$).tw. 13. ((Prostaglandin$ or PTGS2) adj3 inhibit$).tw. 14. corticosteroid$.tw. 15. (adapalene or ampyrone or antipyrine or apazone or aspirin or acetylsalicyl$ or bufexamac or celecoxib or clonixin or curcumin or dexibuprofen or dexketoprofen or diclofenac or diflunisal or dipyrone or etodolac or epirizole or etanercept or fenoprofen or feprazone or flurbiprofen or ibuprofen or indomethacin or indomethacin or indoprofen or ketoprofen or ketorolac or masoprocol or meclofenamic acid or mefenamic acid or meloxic or mesalamine or naproxen or niflumic acid or olopatadine hydrochloride or oxyphenbutazone or phenylbutazone or piroxicam or salicylate$ or sulfasalazine or sulindac or suprofen or tenoxicam or tolfenamic acid or tiaprofenic acid or tolmetin).tw. 16. (A 771726 or aceclofenac or acemetacin or acetosyringone or acetovanillone or alclofenac or alminoprofen or amiprilose or andrographolide or anisodam$ or apremilast or arteparon or arthrotec or atrinositol or azulene or baicalin or balsalazide or bendazac$ or benorilate or benoxaprofen or benzobarbital or berbamine or betulinic$ or bevonium or biphenylylacetic$ or boldine or boswellic$ or bredinin or bromfenac or bucillamine or bumadizone or butibufen or canakinumab or carbaspirin$ or carprofen or caryophyllene or castanospermine or CDP 571 or cepharanthine or chloroquine$ or choline magnesium trisalicylate or chrysarobin or CP 96345 or colchicine or dauricine or dexketoprofen$ or difenpiramide or dimephosphon or diucifon or droxicam or DuP 697 or ebselen or efamol or eltenac or enfenamic$ or ethenzamide or ethonium or etofenamate or etoricoxib or fenamic acid or fenbufen or fenclofenac or fenflumizole or fentiazac or fepradinol or ferulic$ or floctafenine or flosulide or flunixin$ or flunoxaprofen or fluproquazone or FR 167653 or FR 173657 or glucametacin or guacetisal or helenalin or heliodermin or hemodes or higenamine or ibuproxam or icatibant or IH 764‐3 or imidazole$ or indobufen or infliximab or iodoantipyrine or isoxicam or kebuzone or L 745337 or L 778736 or lipoxin$ or lisofylline or lobenzarit or lonazolac or lornoxicam or loxoprofen or lumiracoxib or magnolol or manoalide or meloxicam or methotrexate or mofebutazone or mofezolac or nabumetone or nafamostat or nebacetin or nepafenac or nifenazone or nimesulide or nitroaspirin or olsalazine or olvanil or orgotein or oxaprozin or palmidrol or parecoxib or parthenolide or peoniflorin or phenidone or pimecrolimus or pirfenidone or pirprofen or proglumetacin or propacetamol or propionylcarnitine or propyphenazone or proquazone or pyranoprofen or pyrazolone or pyrogenal or resveratrol or rofecoxib or rosmarinic$ or rumalon or salicin or salicylamide or salicylsalicylic$ or SB 203580 or SC 299 or SC 41930 or SC 560 or semapimod or seratrodast or serratiopeptidase or sinapaldehyde or ST 679 or suxibuzone or tanshinone or taxifolin or tenidap or tenoxicam or tepoxalin or tiaprofenic$ or tiaramide or tinoridine or tolfenamic$ or tranilast or tribenoside or ursolic$ or valdecoxib or zileuton or zomepirac).tw. 17. exp Macrophage Inflammatory Proteins/ 18. ((inflam$ adj5 protein$) or chemokin$).tw. 19. (CCL3 or CCL4 or CCL19 or CCL20 or CXCL2).tw. 20. or/6‐19 21. Antimetabolites/ or Antimetabolites, Antineoplastic/ 22. Nucleic Acid Synthesis Inhibitors/ 23. Cytokines/ai [Antagonists & Inhibitors] 24. (Antimetabolit* or anti‐metabolit*).tw. 25. (anticytokin$ or (cytokine adj3 inhibit$)).tw. 26. (ancitabine or azacitidine or azacosterol or azaguanine or azaserine or azathioprine or azauridine or betaine or bezafibrate or bromodeoxyuridine or bromouracil or buthionine sulfoximine or butoxamine or capecitabine or carbon monoxide or cerulenin or chitosan or cholestyramine resin or choline or clofenapate or clofibrate or clofibric acid or colesevelam hydrochloride or colestipol or cycloserine or cytarabine or deoxyglucose or deoxyuridine or diazooxonorleucine or didanosine or dideoxyadenosine or dideoxynucleosides or ethionamide or ethionine or ezetimibe or fenofibrate or floxuridine or flucytosine or fluorouracil or gemfibrozil or halofenate or isoniazid or lovastatin or meglutol or methotrexate or metyrapone or nafenopin or niacin or niceritrol or oxythiamine or peptichemio or probucol or proguanil or propylthiouracil or puromycin or pyridinolcarbamate or pyrithiamine or ribavirin or stavudine or tegafur or tetrahydrouridine or thioguanine or thioinosine or thiouridine or toyocamycin or dihydrochloride or triclosan or trifluridine or trimetrexate or triparanol or tubercidin or vidarabine or vidarabine phosphate or zalcitabine or zidovudine).tw. 27. or/21‐26 28. 20 or 27 29. Randomized Controlled Trials as Topic/ 30. Random Allocation/ 31. Controlled Clinical Trials as Topic/ 32. control groups/ 33. clinical trials as topic/ or clinical trials, phase i as topic/ or clinical trials, phase ii as topic/ or clinical trials, phase iii as topic/ or clinical trials, phase iv as topic/ 34. double‐blind method/ 35. single‐blind method/ 36. Placebos/ 37. placebo effect/ 38. cross‐over studies/ 39. randomized controlled trial.pt. 40. controlled clinical trial.pt. 41. (clinical trial or clinical trial phase i or clinical trial phase ii or clinical trial phase iii or clinical trial phase iv).pt. 42. (random$ or RCT or RCTs).tw. 43. (controlled adj5 (trial$ or stud$)).tw. 44. (clinical$ adj5 trial$).tw. 45. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 46. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 47. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 48. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 49. (cross‐over or cross over or crossover).tw. 50. (placebo$ or sham).tw. 51. trial.ti. 52. (assign$ or allocat$).tw. 53. controls.tw. 54. or/29‐53 55. exp animals/ not humans.sh. 56. 5 and 28 and 54 57. 56 not 55
Appendix 3. Embase search strategy
1. cerebrovascular disease/ or cerebral artery disease/ or cerebrovascular accident/ or stroke/ or vertebrobasilar insufficiency/ or carotid artery disease/ or exp carotid artery obstruction/ or exp brain infarction/ or exp brain ischemia/ or exp occlusive cerebrovascular disease/ 2. (isch?emi$ adj6 (stroke$ or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva or attack$)).tw. 3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw. 4. tia.tw. 5. or/1‐4 6. exp antiinflammatory agent/ 7. (antiinflam$ or anti‐inflam$ or DMARD$).tw. 8. (acetaminophen$ or acetophenide or alclometasone or adalimumab or beclomethasone$ or benzydamine or betamethasone or budesonide or clobetasol or clofazimine or corticosterone or cortisone or desonide or desoximetasone or dexamethason$ or fludrocortisone or flufenamic$ or flumethasone or fluocinolon$ or fluocinonide or fluorometholone or fluprednisolone or flurandrenolone or fluticasone or glycyrrhetinic acid or glycyrrhizic$ or halcinonide or hydrocortisone or methylprednisolone or mometasone$ or nedocromil or paramethasone or prednisolone or prednisone or tilorone or triamcinolone).tw. 9. exp prostaglandin synthase inhibitor/ 10. NSAID$.tw. 11. prostaglandin synthase/ 12. ((coxib$ or cyclooxygenase or COX) adj3 inhibit$).tw. 13. ((Prostaglandin$ or PTGS2) adj3 inhibit$).tw. 14. corticosteroid/ or glucocorticoids/ 15. (glucocorticoids or beclomethasone or betamethasone or budesonide or clobetasol or desoximetasone or dexamethasone or diflucortolone or flumethasone or fluocinolone acetonide or fluocinonide or fluocortolone or fluorometholone or fluprednisolone or flurandrenolone or fluticasone‐salmeterol or melengestrol acetate or methylprednisolone or paramethasone or prednisolone or prednisone or triamcinolone).tw. 16. (adalimumab or adapalene or ampyrone or anakinraantipyrine or apazone or acetylsalicyl$ or bufexamac or celecoxib or clonixin or curcumin or daclizumab or dexibuprofen or dexketoprofen or diclofenac or diflunisal or dipyrone or etodolac or epirizole or etanercept or fenoprofen or feprazone or flurbiprofen or ibuprofen or indomethacin or indomethacin or indoprofen or ketoprofen or ketorolac or masoprocol or meclofenamic acid or mefenamic acid or meloxic or mesalamine or naproxen or niflumic acid or olopatadine hydrochloride or oxyphenbutazone or phenylbutazone or piroxicam or salicylate$ or sulfasalazine or sulindac or suprofen or tenoxicam or tolfenamic$ or tolmetin).tw. 17. (A 771726 or aceclofenac or acemetacin or acetosyringone or acetovanillone or alclofenac or alminoprofen or amiprilose or andrographolide or anisodam$ or apremilast or arteparon or arthrotec or atrinositol or azulene or baicalin or balsalazide or bendazac$ or benorilate or benoxaprofen or benzobarbital or berbamine or betulinic$ or bevonium or biphenylylacetic$ or boldine or boswellic$ or bredinin or bromfenac or bucillamine or bumadizone or butibufen or canakinumab or carbaspirin$ or carprofen or caryophyllene or castanospermine or CDP 571 or cepharanthine or chloroquine$ or choline magnesium trisalicylate or chrysarobin or CP 96345 or colchicine or dauricine or difenpiramide or dimephosphon or diucifon or droxicam or DuP 697 or ebselen or efamol or eltenac or enfenamic$ or ethenzamide or ethonium or etofenamate or etoricoxib or fenamic acid or fenbufen or fenclofenac or fenflumizole or fentiazac or fepradinol or ferulic$ or floctafenine or flosulide or flunixin$ or flunoxaprofen or fluproquazone or FR 167653 or FR 173657 or glucametacin or guacetisal or helenalin or heliodermin or hemodes or higenamine or ibuproxam or icatibant or IH 764‐3 or imidazole$ or indobufen or infliximab or iodoantipyrine or isoxicam or kebuzone or L 745337 or L 778736 or lipoxin$ or lisofylline or lobenzarit or lonazolac or lornoxicam or loxoprofen or lumiracoxib or magnolol or manoalide or meloxicam or methotrexate or mofebutazone or mofezolac or nabumetone or nafamostat or nebacetin or nepafenac or nifenazone or nimesulide or nitroaspirin or olsalazine or olvanil or orgotein or oxaprozin or palmidrol or parecoxib or parthenolide or peoniflorin or phenidone or pimecrolimus or pirfenidone or pirprofen or proglumetacin or propacetamol or propionylcarnitine or propyphenazone or proquazone or pyranoprofen or pyrazolone or pyrogenal or resveratrol or rofecoxib or rosmarinic$ or rumalon or salicin or salicylamide or salicylsalicylic$ or SB 203580 or SC 299 or SC 41930 or SC 560 or semapimod or seratrodast or serratiopeptidase or sinapaldehyde or ST 679 or suxibuzone or tanshinone or taxifolin or tenidap or tenoxicam or tepoxalin or tiaprofenic$ or tiaramide or tinoridine or tolfenamic$ or tranilast or tribenoside or ursolic$ or valdecoxib or zileuton or zomepirac).tw. 18. macrophage derived chemokine/ or macrophage inflammatory protein/ or macrophage inflammatory protein 1/ or macrophage inflammatory protein 1alpha/ or macrophage inflammatory protein 1beta/ or macrophage inflammatory protein 2/ or macrophage inflammatory protein 3alpha/ or macrophage inflammatory protein 3beta/ 19. chemokine/ 20. ((inflam$ adj5 protein$) or chemokin$).tw. 21. (CCL3 or CCL4 or CCL19 or CCL20 or CXCL2).tw. 22. colchicine/ 23. or/6‐22 24. exp antimetabolite/ 25. antineoplastic antimetabolite/ 26. nucleic acid synthesis inhibitor/ 27. cytokine/ 28. (antimetabolit$ or anti‐metabolit$).tw. 29. (anticytokin$ or (cytokine adj3 inhibit$)).tw. 30. (ancitabine or azacitidine or azacosterol or azaguanine or azaserine or azathioprine or azauridine or betaine or bezafibrate or bromodeoxyuridine or bromouracil or buthionine sulfoximine or butoxamine or capecitabine or carbon monoxide or cerulenin or chitosan or cholestyramine resin or choline or clofenapate or clofibrate or clofibric acid or colesevelam hydrochloride or colestipol or cycloserine or cytarabine or deoxyglucose or deoxyuridine or diazooxonorleucine or didanosine or dideoxyadenosine or dideoxynucleosides or ethionamide or ethionine or floxuridine or flucytosine or fluorouracil or halofenate or isoniazid or meglutol or methotrexate or metyrapone or nafenopin or niacin or niceritrol or oxythiamine or peptichemio or probucol or proguanil or propylthiouracil or puromycin or pyridinolcarbamate or pyrithiamine or ribavirin or stavudine or tegafur or tetrahydrouridine or thioguanine or thioinosine or thiouridine or toyocamycin or dihydrochloride or triclosan or trifluridine or trimetrexate or triparanol or tubercidin or vidarabine or vidarabine phosphate or zalcitabine or zidovudine).tw. 31. or/24‐30 32. 23 or 31 33. Randomized Controlled Trial/ or "randomized controlled trial (topic)"/ 34. Randomization/ 35. Controlled clinical trial/ or "controlled clinical trial (topic)"/ 36. control group/ or controlled study/ 37. clinical trial/ or "clinical trial (topic)"/ or phase 1 clinical trial/ or phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/ 38. Crossover Procedure/ 39. Double Blind Procedure/ 40. Single Blind Procedure/ or triple blind procedure/ 41. placebo/ or placebo effect/ 42. (random$ or RCT or RCTs).tw. 43. (controlled adj5 (trial$ or stud$)).tw. 44. (clinical$ adj5 trial$).tw. 45. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 46. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 47. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 48. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 49. (cross‐over or cross over or crossover).tw. 50. (placebo$ or sham).tw. 51. trial.ti. 52. (assign$ or allocat$).tw. 53. controls.tw. 54. or/33‐53 55. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 56. 5 and 32 and 54 57. 56 not 55
Appendix 4. CINAHL search strategy
S1(MH "Cerebrovascular Disorders") OR (MH "Basal Ganglia Cerebrovascular Disease") OR (MH "Cerebral Ischemia+") OR (MH "Intracranial Embolism and Thrombosis+") OR (MH "Intracranial Arterial Diseases") OR (MH "Carotid Artery Diseases") OR (MH "Carotid Artery Thrombosis") OR (MH "Stroke+") S2TI ( (isch?emi* N6 (stroke* or apoplex* or cerebral vasc* or cerebrovasc* or cva or attack*)). ) OR AB ( (isch?emi* N6 (stroke* or apoplex* or cerebral vasc* or cerebrovasc* or cva or attack*)). ) S3TI ( ((brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA* or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) N5 (isch?emi* or infarct* or thrombo* or emboli* or occlus* or hypoxi*)). ) OR AB ( ((brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA* or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) N5 (isch?emi* or infarct* or thrombo* or emboli* or occlus* or hypoxi*)). ) S4TI tia OR AB tia S5S1 OR S2 OR S3 OR S4 S6(MH "Antiinflammatory Agents+") S7TI ( (antiinflam* or anti‐inflam* or DMARD*) ) OR AB ( (antiinflam* or anti‐inflam* or DMARD*) ) S8TI ( (acetaminophen* or acetophenide or alclometasone or adalimumab or beclomethasone* or benzydamine or betamethasone or budesonide or clobetasol or clofazimine or corticosterone or cortisone or desonide or desoximetasone or dexamethason* or fludrocortisone or flufenamic* or flumethasone or fluocinolon* or fluocinonide or fluorometholone or fluprednisolone or flurandrenolone or fluticasone or glycyrrhetinic acid or glycyrrhizic* or halcinonide or hydrocortisone or methylprednisolone or mometasone* or nedocromil or paramethasone or prednisolone or prednisone or tilorone or triamcinolone) ) OR AB ( (acetaminophen* or acetophenide or alclometasone or adalimumab or beclomethasone* or benzydamine or betamethasone or budesonide or clobetasol or clofazimine or corticosterone or cortisone or desonide or desoximetasone or dexamethason* or fludrocortisone or flufenamic* or flumethasone or fluocinolon* or fluocinonide or fluorometholone or fluprednisolone or flurandrenolone or fluticasone or glycyrrhetinic acid or glycyrrhizic* or halcinonide or hydrocortisone or methylprednisolone or mometasone* or nedocromil or paramethasone or prednisolone or prednisone or tilorone or triamcinolone) ) S9TI NSAID* OR AB NSAID* S10(MH "Prostaglandin Antagonists") S11TI ( ((coxib* or cyclooxygenase or COX) N3 inhibit*) ) OR AB ( ((coxib* or cyclooxygenase or COX) N3 inhibit*) ) S12TI ( ((Prostaglandin* or PTGS2) N3 inhibit*) ) OR AB ( ((Prostaglandin* or PTGS2) N3 inhibit*) ) S13(MH "Adrenal Cortex Hormones+") S14TI ( (glucocorticoids or beclomethasone or betamethasone or budesonide or clobetasol or desoximetasone or dexamethasone or diflucortolone or flumethasone or fluocinolone acetonide or fluocinonide or fluocortolone or fluorometholone or fluprednisolone or flurandrenolone or fluticasone‐salmeterol or melengestrol acetate or methylprednisolone or paramethasone or prednisolone or prednisone or triamcinolone) ) OR AB ( (glucocorticoids or beclomethasone or betamethasone or budesonide or clobetasol or desoximetasone or dexamethasone or diflucortolone or flumethasone or fluocinolone acetonide or fluocinonide or fluocortolone or fluorometholone or fluprednisolone or flurandrenolone or fluticasone‐salmeterol or melengestrol acetate or methylprednisolone or paramethasone or prednisolone or prednisone or triamcinolone) ) S15TI corticosteroid* OR AB corticosteroid* S16TI ( (adalimumab or adapalene or ampyrone or anakinraantipyrine or apazone or acetylsalicyl* or bufexamac or celecoxib or clonixin or curcumin or daclizumab or dexketoprofen or diclofenac or diflunisal or dipyrone or etodolac or epirizole or etanercept or fenoprofen or feprazone or flurbiprofen or ibuprofen or indomethacin or indomethacin or indoprofen or ketoprofen or ketorolac or masoprocol or meclofenamic acid or mefenamic acid or meloxic or mesalamine or naproxen or niflumic acid or olopatadine hydrochloride or oxyphenbutazone or phenylbutazone or piroxicam or salicylate* or sulfasalazine or sulindac or suprofen or tenoxicam or tiaprofenic* or tolmetin) ) OR AB ( (adalimumab or adapalene or ampyrone or anakinraantipyrine or apazone or acetylsalicyl* or bufexamac or celecoxib or clonixin or curcumin or daclizumab or dexibuprofen or diclofenac or diflunisal or dipyrone or etodolac or epirizole or etanercept or fenoprofen or feprazone or flurbiprofen or ibuprofen or indomethacin or indomethacin or indoprofen or ketoprofen or ketorolac or masoprocol or meclofenamic acid or mefenamic acid or meloxic or mesalamine or naproxen or niflumic acid or olopatadine hydrochloride or oxyphenbutazone or phenylbutazone or piroxicam or salicylate* or sulfasalazine or sulindac or suprofen or tenoxicam or tiaprofenic* or tolmetin) ) S17TI ( (A 771726 or aceclofenac or acemetacin or acetosyringone or acetovanillone or alclofenac or alminoprofen or amiprilose or andrographolide or anisodam* or apremilast or arteparon or arthrotec or atrinositol or azulene or baicalin or balsalazide or bendazac* or benorilate or benoxaprofen or benzobarbital or berbamine or betulinic* or bevonium or biphenylylacetic* or boldine or boswellic* or bredinin or bromfenac or bucillamine or bumadizone or butibufen or canakinumab or carbaspirin* or carprofen or caryophyllene or castanospermine or CDP 571 or cepharanthine or chloroquine* or choline magnesium trisalicylate or chrysarobin or CP 96345 or colchicine or dauricine or dexketoprofen* or difenpiramide or dimephosphon or diucifon or droxicam or DuP 697 or ebselen or efamol or eltenac or enfenamic* or ethenzamide or ethonium or etofenamate or etoricoxib or fenamic acid or fenbufen or fenclofenac or fenflumizole or fentiazac or fepradinol or ferulic* or floctafenine or flosulide or flunixin* or flunoxaprofen or fluproquazone or FR 167653 or FR 173657 or glucametacin or guacetisal or helenalin or heliodermin or hemodes or higenamine or ibuproxam or icatibant or IH 764‐3 or imidazole* or indobufen or infliximab or iodoantipyrine or isoxicam or kebuzone or L 745337 or L 778736 or lipoxin* or lisofylline or lobenzarit or lonazolac or lornoxicam or loxoprofen or lumiracoxib or magnolol or manoalide or meloxicam or methotrexate or mofebutazone or mofezolac or nabumetone or nafamostat or nebacetin or nepafenac or nifenazone or nimesulide or nitroaspirin or olsalazine or olvanil or orgotein or oxaprozin or palmidrol or parecoxib or parthenolide or peoniflorin or phenidone or pimecrolimus or pirfenidone or pirprofen or proglumetacin or propacetamol or propionylcarnitine or propyphenazone or proquazone or pyranoprofen or pyrazolone or pyrogenal or resveratrol or rofecoxib or rosmarinic* or rumalon or salicin or salicylamide or salicylsalicylic* or SB 203580 or SC 299 or SC 41930 or SC 560 or semapimod or seratrodast or serratiopeptidase or sinapaldehyde or ST 679 or suxibuzone or tanshinone or taxifolin or tenidap or tenoxicam or tepoxalin or tiaprofenic* or tiaramide or tinoridine or tolfenamic* or tranilast or tribenoside or ursolic* or valdecoxib or zileuton or zomepirac) ) S18AB ( (A 771726 or aceclofenac or acemetacin or acetosyringone or acetovanillone or alclofenac or alminoprofen or amiprilose or andrographolide or anisodam* or apremilast or arteparon or arthrotec or atrinositol or azulene or baicalin or balsalazide or bendazac* or benorilate or benoxaprofen or benzobarbital or berbamine or betulinic* or bevonium or biphenylylacetic* or boldine or boswellic* or bredinin or bromfenac or bucillamine or bumadizone or butibufen or canakinumab or carbaspirin* or carprofen or caryophyllene or castanospermine or CDP 571 or cepharanthine or chloroquine* or choline magnesium trisalicylate or chrysarobin or CP 96345 or colchicine or dauricine or dexketoprofen* or difenpiramide or dimephosphon or diucifon or droxicam or DuP 697 or ebselen or efamol or eltenac or enfenamic* or ethenzamide or ethonium or etofenamate or etoricoxib or fenamic acid or fenbufen or fenclofenac or fenflumizole or fentiazac or fepradinol or ferulic* or floctafenine or flosulide or flunixin* or flunoxaprofen or fluproquazone or FR 167653 or FR 173657 or glucametacin or guacetisal or helenalin or heliodermin or hemodes or higenamine or ibuproxam or icatibant or IH 764‐3 or imidazole* or indobufen or infliximab or iodoantipyrine or isoxicam or kebuzone or L 745337 or L 778736 or lipoxin* or lisofylline or lobenzarit or lonazolac or lornoxicam or loxoprofen or lumiracoxib or magnolol or manoalide or meloxicam or methotrexate or mofebutazone or mofezolac or nabumetone or nafamostat or nebacetin or nepafenac or nifenazone or nimesulide or nitroaspirin or olsalazine or olvanil or orgotein or oxaprozin or palmidrol or parecoxib or parthenolide or peoniflorin or phenidone or pimecrolimus or pirfenidone or pirprofen or proglumetacin or propacetamol or propionylcarnitine or propyphenazone or proquazone or pyranoprofen or pyrazolone or pyrogenal or resveratrol or rofecoxib or rosmarinic* or rumalon or salicin or salicylamide or salicylsalicylic* or SB 203580 or SC 299 or SC 41930 or SC 560 or semapimod or seratrodast or serratiopeptidase or sinapaldehyde or ST 679 or suxibuzone or tanshinone or taxifolin or tenidap or tenoxicam or tepoxalin or tiaprofenic* or tiaramide or tinoridine or tolfenamic* or tranilast or tribenoside or ursolic* or valdecoxib or zileuton or zomepirac) ) S19S17 OR S18 S20TI ( ((inflam* N5 protein*) or chemokin*) ) OR AB ( ((inflam* N5 protein*) or chemokin*). ) S21TI ( (CCL3 or CCL4 or CCL19 or CCL20 or CXCL2) ) OR AB ( (CCL3 or CCL4 or CCL19 or CCL20 or CXCL2) ) S22(MH "Colchicine") S23S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S19 OR S20 OR S21 OR S22 S24(MH "Antimetabolites+") S25TI Nucleic Acid Synthesis S26(MH "Cytokines/AI") S27TI ( (antimetabolit* or anti‐metabolit*) ) OR AB ( (antimetabolit* or anti‐metabolit*) ) S28TI ( (anticytokin* or (cytokine N3 inhibit*)) ) OR AB ( (anticytokin* or (cytokine N3 inhibit*)) ) S29TI ( (ancitabine or azacitidine or azacosterol or azaguanine or azaserine or azathioprine or azauridine or betaine or bezafibrate or bromodeoxyuridine or bromouracil or buthionine sulfoximine or butoxamine or capecitabine or carbon monoxide or cerulenin or chitosan or cholestyramine resin or choline or clofenapate or clofibrate or clofibric acid or colesevelam hydrochloride or colestipol or cycloserine or cytarabine or deoxyglucose or deoxyuridine or diazooxonorleucine or didanosine or dideoxyadenosine or dideoxynucleosides or ethionamide or ethionine or floxuridine or flucytosine or fluorouracil or halofenate or isoniazid or meglutol or methotrexate or metyrapone or nafenopin or niacin or niceritrol or oxythiamine or peptichemio or probucol or proguanil or propylthiouracil or puromycin or pyridinolcarbamate or pyrithiamine or ribavirin or stavudine or tegafur or tetrahydrouridine or thioguanine or thioinosine or thiouridine or toyocamycin or dihydrochloride or triclosan or trifluridine or trimetrexate or triparanol or tubercidin or vidarabine or vidarabine phosphate or zalcitabine or zidovudine) ) S30AB ( (ancitabine or azacitidine or azacosterol or azaguanine or azaserine or azathioprine or azauridine or betaine or bezafibrate or bromodeoxyuridine or bromouracil or buthionine sulfoximine or butoxamine or capecitabine or carbon monoxide or cerulenin or chitosan or cholestyramine resin or choline or clofenapate or clofibrate or clofibric acid or colesevelam hydrochloride or colestipol or cycloserine or cytarabine or deoxyglucose or deoxyuridine or diazooxonorleucine or didanosine or dideoxyadenosine or dideoxynucleosides or ethionamide or ethionine or floxuridine or flucytosine or fluorouracil or halofenate or isoniazid or meglutol or methotrexate or metyrapone or nafenopin or niacin or niceritrol or oxythiamine or peptichemio or probucol or proguanil or propylthiouracil or puromycin or pyridinolcarbamate or pyrithiamine or ribavirin or stavudine or tegafur or tetrahydrouridine or thioguanine or thioinosine or thiouridine or toyocamycin or dihydrochloride or triclosan or trifluridine or trimetrexate or triparanol or tubercidin or vidarabine or vidarabine phosphate or zalcitabine or zidovudine) ) S31S29 OR S30 S32S24 OR S25 OR S26 OR S27 OR S28 OR S31 S33S23 OR S32 S34MH Random Assignment or MH Single‐blind Studies or MH Double‐blind Studies or MH Triple‐blind Studies or MH Crossover design or MH Factorial Design S35TI ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study") or AB ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study") or SU ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study") S36TI random* or AB random* S37AB "latin square" or TI "latin square" S38TI (crossover or cross‐over) or AB (crossover or cross‐over) or SU (crossover or cross‐over) S39MH Placebos S40AB (singl* or doubl* or trebl* or tripl*) or TI (singl* or doubl* or trebl* or tripl*) S41TI blind* or AB mask* or AB blind* or TI mask* S42S40 and S341 S43TI Placebo* or AB Placebo* or SU Placebo* S44MH Clinical Trials S45TI (Clinical AND Trial) or AB (Clinical AND Trial) or SU (Clinical AND Trial) S46S34 or S35 or S36 or S37 or S38 or S39 or S42 or S43 or S44 or S45 S47S5 AND S33 AND S46
Appendix 5. SCOPUS search strategy
( ( TITLE‐ABS‐KEY ( ischem* W/6 stroke* OR apoplexy OR "cerebral vasc*" OR cerebrovasc* OR cva OR attack* ) ) OR ( TITLE‐ABS‐KEY ( brain OR cerebral OR cerebellar OR intracranial OR intracerebral W/5 ischemic OR infarction OR thrombosis OR embolism OR occlusion ) ) ) AND ( TITLE‐ABS‐KEY ( inflammation OR anti‐inflammator* OR glucocorticoid* OR colchicine OR nsaid* OR cyclooxygenase OR coxib* OR cox‐2 OR antimetabolite* OR anticytokin* ) ) AND ( TITLE‐ABS‐KEY ( random* OR rct OR "controlled trial" OR placebo ) )
Appendix 6. Clinical Trials Registry search strategy
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov) ( inflammation OR anti‐inflammatory OR glucocorticoids OR colchicine OR nsaids OR cyclooxygenase OR coxibs OR cox‐2 OR antimetabolites OR anticytokines ) AND ( Infarction Cerebral OR Transient Ischemic Attack OR Ischemic Stroke OR Cerebral Arterial Diseases OR Intracranial Embolism and Thrombosis ) [DISEASE] World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch) Basic search: ischaemic AND inflammation OR ischemic AND inflammation OR ischaemic AND anti‐inflammatory OR ischemic AND anti‐inflammatory Basic search: infarction AND inflammation OR infarction AND anti‐inflammatory Basic search: cerebrovascular AND inflammation OR cerebrovascular AND anti‐inflammatory Basic search: stroke AND inflammation OR stroke AND anti‐inflammatory Basic search: cerebral AND inflammation OR cerebral AND anti‐inflammatory
Appendix 7. ProQuest search strategy
Set#: S1 Searched for: AB,TI((brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or “middle cerebral artery” or MCA* or “anterior circulation” or “posterior circulation” or “basilar artery” or “vertebral artery” or “space‐occupying”) N/5 (isch?emi* or infarct* or thrombo* or emboli* or occlus* or hypoxi*)) Set#: S2 Searched for: AB,TI(isch?emi* NEAR/5 (stroke* OR apoplex* OR cerebral OR cerebrovasc* OR cva OR attack*)) Set#: S3 Searched for: AB,TI(antiinflam* OR anti‐inflam* OR DMARD* OR cox* OR cyclooxygenase OR corticosteroid* OR prostaglandin* OR anticytokin* OR antimetabolit* OR anti‐metabolit*) Set#: S4 Searched for: S1 OR S2 Set#: S5 Searched for: S3 AND S4
Appendix 8. Epistemonikos (www.epistemonikos.org/en/) search strategy
Advanced search: (title:((isch?emi* AND (stroke* OR apoplex* OR cerebral OR cerebrovasc* OR cva OR attack*)) AND (antiinflam* OR anti‐inflam* OR DMARD* OR cox* OR cyclooxygenase OR corticosteroid* OR prostaglandin* OR anticytokin* OR antimetabolit* OR anti‐metabolit*)) OR abstract:((isch?emi* AND (stroke* OR apoplex* OR cerebral OR cerebrovasc* OR cva OR attack*)) AND (antiinflam* OR anti‐inflam* OR DMARD* OR cox* OR cyclooxygenase OR corticosteroid* OR prostaglandin* OR anticytokin* OR antimetabolit* OR anti‐metabolit*)))
Appendix 9. BIOSIS Citation Index and Web of Science Conference Proceedings Citation Index search strategies
#1TS=(isch?emi* NEAR/6 (stroke* or apoplex* or “cerebral vasc*” or cerebrovasc* or cva or attack*)) #2TS=((brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or “middle cerebral artery” or MCA* or “anterior circulation” or “posterior circulation” or “basilar artery” or “vertebral artery” or “space‐occupying”) NEAR/5 (isch?emi* or infarct* or thrombo* or emboli* or occlus* or hypoxi*)) #3TS=tia #4#1 or #2 or #3 #5TS=(antiinflam* or anti‐inflam* or DMARD*) #6TS=(acetaminophen* or acetophenide or alclometasone or adalimumab or beclomethasone* or benzydamine or betamethasone or budesonide or clobetasol or clofazimine or corticosterone or cortisone or desonide or desoximetasone or dexamethason* or fludrocortisone or flufenamic* or flumethasone or fluocinolon* or fluocinonide or fluorometholone or fluprednisolone or flurandrenolone or fluticasone or glycyrrhetinic acid or glycyrrhizic* or halcinonide or hydrocortisone or methylprednisolone or mometasone* or nedocromil or paramethasone or prednisolone or prednisone or tilorone or triamcinolone) #7TS=NSAID* #8TS=((coxib* or cyclooxygenase or COX) NEAR/3 inhibit*) #9TS=((Prostaglandin* or PTGS2) NEAR/3 inhibit*) #10TS=(glucocorticoids or beclomethasone or betamethasone or budesonide or clobetasol or desoximetasone or dexamethasone or diflucortolone or flumethasone or fluocinolone acetonide or fluocinonide or fluocortolone or fluorometholone or fluprednisolone or flurandrenolone or fluticasone‐salmeterol or melengestrol acetate or methylprednisolone or paramethasone or prednisolone or prednisone or triamcinolone) #11TS=corticosteroid* #12TS=(adalimumab or adapalene or ampyrone or anakinraantipyrine or apazone or acetylsalicyl* or bufexamac or celecoxib or clonixin or curcumin or daclizumab or dexibuprofen or dexketoprofen or diclofenac or diflunisal or dipyrone or etodolac or epirizole or etanercept or fenoprofen or feprazone or flurbiprofen or ibuprofen or indomethacin or indomethacin or indoprofen or ketoprofen or ketorolac or masoprocol or meclofenamic acid or mefenamic acid or meloxic or mesalamine or naproxen or niflumic acid or olopatadine hydrochloride or oxyphenbutazone or phenylbutazone or piroxicam or salicylate* or sulfasalazine or sulindac or suprofen or tenoxicam or tolfenamic* or tolmetin) #13TS=(A 771726 or aceclofenac or acemetacin or acetosyringone or acetovanillone or alclofenac or alminoprofen or amiprilose or andrographolide or anisodam* or apremilast or arteparon or arthrotec or atrinositol or azulene or baicalin or balsalazide or bendazac* or benorilate or benoxaprofen or benzobarbital or berbamine or betulinic* or bevonium or biphenylylacetic* or boldine or boswellic* or bredinin or bromfenac or bucillamine or bumadizone or butibufen or canakinumab or carbaspirin* or carprofen or caryophyllene or castanospermine or CDP 571 or cepharanthine or chloroquine* or choline magnesium trisalicylate or chrysarobin or CP 96345 or colchicine or dauricine or difenpiramide or dimephosphon or diucifon or droxicam or DuP 697 or ebselen or efamol or eltenac or enfenamic* or ethenzamide or ethonium or etofenamate or etoricoxib or fenamic acid or fenbufen or fenclofenac or fenflumizole or fentiazac or fepradinol or ferulic* or floctafenine or flosulide or flunixin* or flunoxaprofen or fluproquazone or FR 167653 or FR 173657 or glucametacin or guacetisal or helenalin or heliodermin or hemodes or higenamine or ibuproxam or icatibant or IH 764‐3 or imidazole* or indobufen or infliximab or iodoantipyrine or isoxicam or kebuzone or L 745337 or L 778736 or lipoxin* or lisofylline or lobenzarit or lonazolac or lornoxicam or loxoprofen or lumiracoxib or magnolol or manoalide or meloxicam or methotrexate or mofebutazone or mofezolac or nabumetone or nafamostat or nebacetin or nepafenac or nifenazone or nimesulide or nitroaspirin or olsalazine or olvanil or orgotein or oxaprozin or palmidrol or parecoxib or parthenolide or peoniflorin or phenidone or pimecrolimus or pirfenidone or pirprofen or proglumetacin or propacetamol or propionylcarnitine or propyphenazone or proquazone or pyranoprofen or pyrazolone or pyrogenal or resveratrol or rofecoxib or rosmarinic* or rumalon or salicin or salicylamide or salicylsalicylic* or SB 203580 or SC 299 or SC 41930 or SC 560 or semapimod or seratrodast or serratiopeptidase or sinapaldehyde or ST 679 or suxibuzone or tanshinone or taxifolin or tenidap or tenoxicam or tepoxalin or tiaprofenic* or tiaramide or tinoridine or tranilast or tribenoside or ursolic* or valdecoxib or zileuton or zomepirac) #14TS=((inflam* NEAR/5 protein*) or chemokin*) #15TS=CCL3 or CCL4 or CCL19 or CCL20 or CXCL2 #16#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 #17TS=(antimetabolit* or anti‐metabolit*) #18TS=(anticytokin* or (cytokine NEAR/3 inhibit*)) #19TS=(ancitabine or azacitidine or azacosterol or azaguanine or azaserine or azathioprine or azauridine or betaine or bezafibrate or bromodeoxyuridine or bromouracil or buthionine sulfoximine or butoxamine or capecitabine or carbon monoxide or cerulenin or chitosan or cholestyramine resin or choline or clofenapate or clofibrate or clofibric acid or colesevelam hydrochloride or colestipol or cycloserine or cytarabine or deoxyglucose or deoxyuridine or diazooxonorleucine or didanosine or dideoxyadenosine or dideoxynucleosides or ethionamide or ethionine or floxuridine or flucytosine or fluorouracil or halofenate or isoniazid or meglutol or methotrexate or metyrapone or nafenopin or niacin or niceritrol or oxythiamine or peptichemio or probucol or proguanil or propylthiouracil or puromycin or pyridinolcarbamate or pyrithiamine or ribavirin or stavudine or tegafur or tetrahydrouridine or thioguanine or thioinosine or thiouridine or toyocamycin or dihydrochloride or triclosan or trifluridine or trimetrexate or triparanol or tubercidin or vidarabine or vidarabine phosphate or zalcitabine or zidovudine) #20#17 or #18 or #19 #21#16 or #20 #22#4 and #21
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12605000174684 | Allocation: randomised Population: diabetic patients with acute ischaemic stroke Intervention: pioglitazone ‐ primary function not anti‐inflammatory; acute intervention |
| ACTRN12614000093684 | Allocation: randomised Participants: stable coronary heart disease |
| Deftereos 2013 | Allocation: randomised Population: diabetic patients undergoing PCI in a coronary artery |
| Drieu 2018 | Article type: review |
| Duris 2017 | Article type: review |
| EudraCT2015‐003959‐22 | Allocation: randomised Population: recent stroke or TIA Intervention: BMS986141 ‐ primary action not anti‐inflammatory |
| Gonzalez‐Valcarcel 2016 | Allocation: not randomised |
| IRCT201601289014N90 | Allocation: randomised Population: acute stroke ‐ haemorrhagic or ischaemic Intervention: citicoline ‐ primary action not anti‐inflammatory; acute intervention |
| NCT02282098 | Allocation: randomised Population: people with atrial fibrillation receiving chronic anticoagulation for at least 3 months |
| Nidorf 2013 | Allocation: randomised Population: stable coronary artery disease |
| Raju 2012 | Allocation: randomised Population: adults with diagnosis of acute coronary syndrome or people with acute ischaemic stroke Intervention: colchicine Outcome: no clinical outcomes recorder; primary outcome was blood level of hs‐CRP at 30 days |
| Ridker 2017 | Allocation: randomised Population: history of myocardial infarction and hs‐CRP ≤ 2 mg/L |
| Ridker 2018 | Article type: review |
| Roubille 2015 | Allocation: not randomised |
| Schink 2018 | Allocation: not randomised |
| Smith 2018 | Allocation: randomised Population: confirmed ischaemic stroke within 5 hours of onset Intervention: recombinant human IL‐1Ra (anakinra) or placebo Outcomes: area under the curve for the log‐transformed concentration of plasma IL‐6 between day 1 and day 3 Secondary outcomes: mRS (not recurrence) at 3 months |
| Tsivgoulis 2018 | Article type: review |
| Xu 1999 | Allocation: randomised Population: confirmed ischaemic stroke Intervention: cyclophosphamide and colchicine for 10 days plus usual care vs usual care alone Outcome: BI and NIHSS at 3 months |
BI: Barthel Index. hs‐CRP: high‐sensitivity C‐reactive protein. IL: interleukin. mRS: modified Rankin Scale. NIHSS: National Institutes of Health Stroke Scale. PCI: percutaneous intervention. TIA: transient ischaemic attack.
Characteristics of ongoing studies [ordered by study ID]
NCT02898610.
| Study name | CCOlchicine for the preventioN of Vascular Inflammation in Non CardioEmbolic Stroke (CONVINCE) |
| Methods | Prospective, open‐label, blinded endpoint design |
| Participants | Adults over 40 years of age who have suffered an ischaemic stroke or TIA (72 hours to 28 days before randomisation) not caused by cardiac embolism or other defined causes |
| Interventions | Colchicine 0.5 mg od plus usual care vs usual care alone |
| Outcomes | Recurrence of non‐fatal ischaemic stroke, non‐fatal hospitalisation for unstable angina, MI, cardiac arrest, or vascular death |
| Starting date | December 2016 |
| Contact information | isctn@ucd.ie |
| Notes | − |
od: once per day. MI: myocardial infarction. TIA: transient ischaemic attack.
Differences between protocol and review
Following discussion with the Cochrane Information Specialist (JC), we excluded the Directory of Open Access Repositories from the search.
As we did not identify any studies that met the inclusion criteria, we were unable to perform aspects of methods related to data extraction, synthesis, and analysis.
Contributions of authors
SC and PK formulated the idea for the review, registered the review title, and developed the basis for the protocol. SC wrote the first draft of the protocol and the paper with contributions from PK and SM. MO'D reviewed the protocol before submission. SC and JM screened titles and abstracts. SC and PK were responsible for review of full texts. SM and MOD were available to advise throughout. All review authors were involved in reviewing this paper.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Health Research Board, Other
SC is in receipt of a HRB Cochrane Fellowship grant. The sponsors played no role in the review.
Declarations of interest
SC: none known. SM: none known. MO'D: none known. PK: is in receipt of a grant from the Health Research Board, Ireland, for the CONVINCE (COlchicine for preventioN of Vascular Inflammation in Non‐CardioEmbolic stroke) trial, for which he is Chief Investigator.
New
References
References to studies excluded from this review
ACTRN12605000174684 {published data only}
- ACTRN12605000174684. Anti-inflammatory effects of pioglitazone in acute stroke - a double-blind placebo controlled trial. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=203 (date first received 15 August 2005).
ACTRN12614000093684 {published data only (unpublished sought but not used)}
- ACTRN12614000093684. "The LoDoCo2 Trial": low dose colchicine for secondary prevention of cardiovascular disease [The LoDoCo2 Trial: a randomised controlled trial on the effect of low-dose colchicine for secondary prevention of cardiovascular disease in patients with established, stable coronary artery disease]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=363771 (date first received 20 January 2014).
Deftereos 2013 {published data only}
- Deftereos S, Giannopoulos G, Raisakis K, Kossyvakis C, Kaoukis A, Panagopoulou V, et al. Colchicine treatment for the prevention of bare-metal stent restenosis in diabetic patients. Journal of the American College of Cardiology 2013;61:1679-85. [DOI] [PubMed] [Google Scholar]
Drieu 2018 {published data only}
- Drieu A, Levard D, Vivien D, Rubio M. Anti-inflammatory treatments for stroke: from bench to bedside. Therapeutic Advances in Neurological Disorders 2018;11:1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Duris 2017 {published data only}
- Duris K, Lipkova, J, Jurajda M. Cholinergic anti-inflammatory pathway and stroke. Current Drug Delivery 2017;12(4):449-57. [DOI] [PubMed] [Google Scholar]
EudraCT2015‐003959‐22 {published data only}
- EudraCT2015-003959-22. An international study at different study sites testing the safety and effectiveness of a drug called BMS-986141 to prevent recurrence of brain infarction in patients receiving acetylsalicylic acid (ASA) following acute ischemic stroke or transient ischemic attack [A phase 2, placebo controlled, randomized, double-blind, parallel-arm study to evaluate efficacy and safety of BMS-986141 for the prevention of recurrent brain infarction in subjects receiving acetylsalicyl acid (ASA) following acute ischemic stroke or transient ischemic attack]. www.clinicaltrialsregister.eu/ctr-search/trial/2015-003959-22/ES (date first received 20 April 2016).
Gonzalez‐Valcarcel 2016 {published data only}
- Gonzalez-Valcarcel J, Sissani L, Labreuche J, Bousser MG, Chamorro A, Fisher M, et al. Paracetamol, ibuprofen, and recurrent major cardiovascular and major bleeding events in 19,120 patients with recent ischaemic stroke. European Stroke Journal 2016;1(1):732-3. [DOI] [PubMed] [Google Scholar]
IRCT201601289014N90 {published data only}
- IRCT201601289014N90. Effect of citicoline versus placebo on clinical signs in patients with acute ischemic and hemorrhagic stroke. en.irct.ir/trial/9528 (date first received 24 February 2016).
NCT02282098 {published data only}
- NCT02282098. Colchicine in atrial fibrillation to prevent stroke. clinicaltrials.gov/ct2/show/NCT02282098 (date first received 4 November 2014).
Nidorf 2013 {published data only}
- Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL. Low-dose colchicine for secondary prevention of cardiovascular disease. Journal of the American College of Cardiology 2013;61(4):404-10. [DOI] [PubMed] [Google Scholar]
Raju 2012 {published data only}
- Raju NC, Yi Q, Nidorf M, Fagel ND, Hiralal R, Eikelboom JW. Effect of colchicine compared with placebo on high sensitivity C-reactive protein in patients with acute coronary syndrome or acute stroke: a pilot randomized controlled trial. Journal of Thrombosis and Thrombolysis 2012;33:88-94. [DOI] [PubMed] [Google Scholar]
Ridker 2017 {published data only}
- Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Cantos Trial Group. Anti-inflammatory therapy with canakinumab for atherosclerotic disease. New England Journal of Medicine 2017;377(12):1119-31. [DOI] [PubMed] [Google Scholar]
Ridker 2018 {published data only}
- Ridker PM. Interleukin-1 inhibition and ischaemic stroke: has the time for a major outcomes trial arrived? European Heart Journal 2018;39(38):3518-20. [DOI] [PubMed] [Google Scholar]
Roubille 2015 {published data only}
- Roubille C, Richer V, Starnino T, McCourt C, McFarlane A, Fleming P, et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Annals of the Rheumatic Diseases 2015;74(3):480-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schink 2018 {published data only}
- Schink T, Kollhorst B, Varas Lorenzo C, Arfe A, Herings R, Lucchi S, et al. Risk of ischemic stroke and the use of individual non-steroidal anti-inflammatory drugs: a multi-country European database study within the SOS Project. PloS One 2018;13(9):e0203362. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2018 {published data only}
- Smith CJ, Hulme S, Vail A, Heal C, Parry-Jones AR, Scarth S, et al. SCIL-STROKE (Subcutaneous Interleukin-1 Receptor Antagonist in Ischemic Stroke): a randomized controlled phase 2 trial. Stroke 2018;49(5):1210-6. [DOI] [PubMed] [Google Scholar]
Tsivgoulis 2018 {published data only}
- Tsivgoulis G, Katsanos AH, Giannopoulos G, Panagopoulou V, Jatuzis D, Lemmens R, et al. The role of colchicine in the prevention of cerebrovascular ischemia. Current Pharmaceutical Design 2018;24(6):668-74. [DOI] [PubMed] [Google Scholar]
Xu 1999 {published data only}
- Xu Y, Luo Z, He L, Peng R, Zhou D. Clinical randomized controlled trial of patients with acute cerebral infarction treated with cyclophosphamide and colchicine. West China Medical Journal 1999;14(1):23-6. [Google Scholar]
References to ongoing studies
NCT02898610 {published data only}
- NCT02898610. Colchicine for prevention of vascular inflammation in noncardioembolic stroke (CONVINCE) - a randomised clinical trial [CONVINCE - (COlchicine for preventioN of Vascular Inflammation in Non-CardioEmbolic Stroke) - a randomised clinical trial of low-dose colchicine for secondary prevention after stroke]. clinicaltrials.gov/ct2/show/NCT02898610 (first received 13 September 2016).
Additional references
Amarenco 2016
- Amarenco P, Lavallee PC, Labreuche J, Albers GW, Bornstein NM, Canhão P, et al. One-year risk of stroke after transient ischemic attack or minor stroke. New England Journal of Medicine 2016;374(16):1533-42. [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al, GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Benjamin 2017
- Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart disease and stroke statistics - 2017 update: a report from the American Heart Association. Circulation 2017;135(10):e146-e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cesari 2003
- Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrel K, et al. Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation 2003;108(19):2317-22. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177-88. [DOI] [PubMed] [Google Scholar]
Easton 2009
- Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, Feldmann E, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. Stroke 2009;40:2276-93. [DOI] [PubMed] [Google Scholar]
ERFC 2010
- Emerging Risk Factors Collaboration. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet 2010;375(9709):132-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Golledge 2000
- Golledge J, Greenhalgh RM, Davies AH. The symptomatic carotid plaque. Stroke 2000;31(3):774-81. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- GRADE Working Group, McMaster University GRADEpro GDT. Hamilton (ON): GRADE Working Group, McMaster University, 2015 (accessed 10 January 2017).
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). Cochrane, 2011. Available from handbook.cochrane.org.
Howard 2013
- Howard DP, Lammeren GW, Redgrave JN, Moll FL, Vries JP, Kleijn DP, et al. Histological features of carotid plaque in patients with ocular ischemia versus cerebral events. Stroke 2013;44(3):734-9. [DOI] [PubMed] [Google Scholar]
IL6R MR 2012
- Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium. The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet 2012;379(9822):1214-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
ILR6 Genetics Consortium 2012
- IL6R Genetics Consortium and Emerging Risk Factors Collaboration. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet 2012;379(9822):1205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnston 2016
- Johnston SC, Amarenco P, Albers GW, Denison H, Easton JD, Evans SR, et al. Ticagrelor versus aspirin in acute stroke or transient ischemic attack. New England Journal of Medicine 2016;375(1):35-43. [DOI] [PubMed] [Google Scholar]
Libby 2002
- Libby P. Inflammation in atherosclerosis. Nature 2002;420(6917):868-74. [DOI] [PubMed] [Google Scholar]
Lovett 2004
- Lovett JK, Coull AJ, Rothwell PM. Early risk of recurrence by subtype of ischemic stroke in population-based incidence studies. Neurology 2004;62(4):569-73. [DOI] [PubMed] [Google Scholar]
Marnane 2012
- Marnane M, Merwick A, Sheehan OC, Hannon N, Foran P, Grant T, et al. Carotid plaque inflammation on 18F-fluorodeoxyglucose positron emission tomography predicts early stroke recurrence. Annals of Neurology 2012;71(5):709–18. [DOI] [PubMed] [Google Scholar]
Muller 2014
- Muller HFG, Viaccoz A, Fisch L, Bonvin C, Lovblad KO, Ratib O, et al. 18FDG-PET-CT: an imaging biomarker of high-risk carotid plaques. Correlation to symptoms and microembolic signals. Stroke 2014;45(12):3561-6. [DOI] [PubMed] [Google Scholar]
Redgrave 2006
- Redgrave JN, Lovett JK, Gallagher PJ, Rothwell PM. Histological assessment of 526 symptomatic carotid plaques in relation to the nature and timing of ischemic symptoms: the Oxford Plaque Study. Circulation 2006;113(19):2320-8. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, Cochrane Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, Cochrane, 2014.
Ridker 2019
- Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, for the CIRT Investigators. Low-dose methotrexate for the prevention of atherosclerotic events. New England Journal of Medicine 2019;380:752-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rominger 2009
- Rominger A, Saam T, Cyran CC, Schmidt M, Foerster S, Nikolaou K, et al. 18F-FDG PET/CT identifies patients at risk for future vascular events in an otherwise asymptomatic cohort with neoplastic disease. Journal of Nuclear Medicine 2009;50(10):1611-20. [DOI] [PubMed] [Google Scholar]
Rost 2001
- Rost NS, Wolf PA, Kelly-Hayes M, Silbershatz H, Massaro JM, D'Agostino RB, et al. Plasma concentration of C-reactive protein and risk of ischemic stroke and transient ischemic attack: the Framingham study. Stroke 2001;32(11):2575-9. [DOI] [PubMed] [Google Scholar]
Sacco 2013
- Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44(7):2064-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sheehan 2010
- Sheehan OC, Kyne L, Kelly LA, Hannon N, Marnane M, Merwick A, et al. A population-based study of ABCD2 score, carotid stenosis, and atrial fibrillation for early stroke prediction after TIA: the North Dublin TIA Study. Stroke 2010;41(5):844-50. [DOI] [PubMed] [Google Scholar]
SPS3 2013
- The SPS3 Study Group. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet 2013;382:507–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Touzé 2005
- Touzé E, Varenne O, Chatellier G, Peyrard S, Rothwell PM, Mas JL. Risk of myocardial infarction and vascular death after transient ischaemic attack and ischaemic stroke: a systematic review and meta-analysis. Stroke 2005;36(12):2748-55. [DOI] [PubMed] [Google Scholar]
Wang 2013
- Wang Y, Wang Y, Zhao X, Liu L, Wang D, Wang C, Wang C, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. New England Journal of Medicine 2013;369(1):11-9. [DOI] [PubMed] [Google Scholar]
WHO 2016
- World Health Organization. Cardiovascular Diseases Fact Sheet. www.who.int/mediacentre/factsheets/fs317/en/ (accessed 14 December 2016).
Worrall 2007
- Worrall BB, Brott TG, Brown RD Jnr, Brown WM, Rich SS, Arepalli S, et al. IL1RN VNTR polymorphism in ischemic stroke. Stroke 2007;38(4):1189-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Coveney 2017
- Coveney S, Murphy S, O'Donnell M, Kelly PJ. Anti-inflammatory therapy for preventing stroke and other vascular events after ischaemic stroke or transient ischaemic attack. Cochrane Database of Systematic Reviews 2017, Issue 11. [DOI: 10.1002/14651858.CD012825] [DOI] [PMC free article] [PubMed] [Google Scholar]


