Abstract
Introduction
Deep dyspareunia is a cardinal symptom of endometriosis, and as many as 40% of people with this condition experience comorbid superficial dyspareunia.
Aim
To evaluate the relationship between sexual pain and infertility concerns among women with endometriosis.
Methods
This is a cross-sectional study conducted at a university-based tertiary center for endometriosis. 300 reproductive-aged participants in the prospective Endometriosis Pelvic Pain Interdisciplinary Cohort (ClinicalTrials.gov Identifier: NCT02911090) with histologically confirmed endometriosis were included (2013–2017).
Main Outcome Measure
The total score on the infertility concerns module of the Endometriosis Health Profile-30 categorized into 5 groups (0, 1–4, 5–8, 9–12, 13–16).
Results
The odds of infertility concerns did not increase with severity of deep dyspareunia (odds ratio = 1.02, 95% CI: 0.95–1.09, P = .58). However, the odds of infertility concerns increased with severity of superficial dyspareunia (odds ratio = 1.09, 95% CI: 1.02–1.16, P = .011); this relationship persisted after adjusting for endometriosis-specific factors, infertility risk factors, reproductive history, and demographic characteristics (adjusted odds ratio [AOR] = 1.14, 95% CI: 1.06–1.24, P < .001). Other factors in the model independently associated with increased infertility concerns were previous difficulty conceiving (AOR = 2.09, 95% CI 1.04–4.19, P = .038), currently trying to conceive (AOR = 5.23, 95% CI 2.77–9.98, P < .001), nulliparity (AOR = 3.21, 95% CI 1.63–6.41, P < .001), and younger age (AOR = 0.94, 95% CI: 0.89–0.98, P = .005).
Conclusion
Severity of superficial dyspareunia, but not deep dyspareunia, was associated with increased odds of infertility concerns among women with endometriosis. Strengths of the study included the use of a validated measure of infertility concerns and disaggregation of sexual pain into deep and superficial dyspareunia. Limitations included the setting of a tertiary center for pelvic pain, which affects generalizability to fertility clinic and primary care settings. Women experiencing introital dyspareunia, who can have difficulties with achieving penetrative intercourse, may be concerned about their future fertility and should be counselled appropriately.
Wahl KJ, Orr NL, Lisonek M, et al. Deep Dyspareunia, Superficial Dyspareunia, and Infertility Concerns Among Women With Endometriosis: A Cross-Sectional Study. Sex Med 2020;8:274–281.
Key Words: Endometriosis, Dyspareunia, Infertility, Fertility, Psychological Distress
Introduction
Endometriosis is a common gynecological condition that counts sexual pain among its cardinal symptoms, along with painful menstruation, chronic pelvic pain, and infertility. Endometriosis is known to cause deep dyspareunia, which is defined as pelvic pain occurring with deep penetration during sexual activity.1 Women with endometriosis may also experience superficial dyspareunia, or pain at the vaginal introitus, as a consequence of comorbidities such as provoked vestibulodynia or pelvic floor dysfunction.2,3 Deep and superficial dyspareunia seem to co-occur in populations seeking tertiary care; in 1 study approximately 40% of women with dyspareunia reported both types of pain.3
Because endometriosis is associated with difficulties conceiving,4, 5, 6 future fertility is a cause for concern among women who are diagnosed with the condition and hope to start or grow a family.7,8 A limited literature suggests that the anticipated impact of endometriosis on fertility causes feelings of inadequacy, depression, and worry among women diagnosed with the condition.9 One qualitative study showed that individuals who were very negatively affected by their potential infertility experienced panic attacks and moderate to severe depression,10 and follow-up research suggested that greater psychological distress was related to individual beliefs about the stigma of infertility.11
In some cases, the presence of dyspareunia may be associated with the occurrence of infertility. For example, some existing research suggests that infertility may be associated with an increase in pain related to the stress of infertility and its treatment.12 Pain may be a factor in infertility when superficial dyspareunia makes intercourse difficult to achieve13 or deep dyspareunia makes intercourse difficult to sustain,14 sometimes causing affected individuals to avoid sexual stimuli.15 In the latter cases, it may be the interruption or avoidance of intercourse, rather than infertility related to abnormalities in ovulation, fertilization, or implantation, that reduces the likelihood of pregnancy.
A separate question is whether dyspareunia is associated with patients' concerns about infertility. Patients with superficial dyspareunia may worry about achieving the vaginal penetration required for natural conception. Patients with deep dyspareunia, who experience pain internally in the pelvic region, may worry about the health of their reproductive organs. Infertility concerns among women with endometriosis may also be affected by factors that are unrelated to the condition. For example, younger age, nulliparity, and nonwhite race have been shown to predict concerns about ability to conceive among reproductive-age women faced with the prospect of impaired fertility.7,16,17 Previous difficulties in achieving pregnancy and the presence of known risk factors for infertility may also affect perceptions of reproductive potential. However, it is important to note that psychological concerns about infertility may not directly correlate to the degree of infertility. It is possible for a patient with a briefer duration of infertility related to reversible cause to have significant concerns about their future fertility, more so than patient with a longer duration of infertility due to multifactorial causes, depending on the individual psychosocial and medical circumstances of the patient.
The objective of this study was to examine the association between deep or superficial dyspareunia and infertility concerns among women with endometriosis. We hypothesized that greater severity of deep dyspareunia and greater severity of superficial dyspareunia would each have an independent association with increased infertility concerns. To account for the effect of other factors on the relationship between sexual pain and infertility concerns, we conducted multivariable analyses that included endometriosis-specific and reproductive covariates with potentially confounding effects.
Material and methods
We examined the relationship between severity of sexual pain and infertility concerns using a cross-sectional design that is well suited to exploratory analyses that are proposed on a theoretical basis.18 Ethics approval was obtained from the University of British Columbia Children's and Women's Research Ethics Board (H16-00264).
Participants
We performed a cross-sectional study of consenting patients who were newly referred or re-referred to the BC Women's Center for Pelvic Pain and Endometriosis Center, Vancouver, between 2013 and 2017.19,20 Data on the study subjects were obtained from the Endometriosis Pelvic Pain Interdisciplinary Cohort (EPPIC, ClinicalTrials.gov Identifier: NCT02911090) prospective data registry (institutional review board approval: H16-00264). EPPIC was designed to measure patient-reported outcomes and to identify factors associated with different types of pelvic pain, particularly among women with endometriosis.19 Before the gynecologist consultation, patients completed baseline questionnaires online within EPPIC, including the Endometriosis Health Profile-30 (EHP-30) and its infertility concerns subscale (module F).21 At the time of the consultation, the gynecologist entered additional data from examination and chart review into the EPPIC. This study included patients who then prospectively underwent surgery at the center, typically performed approximately 3–6 months after baseline. Surgical data were entered by the surgeon or fellow immediately after the surgery. Once available, pathology data from surgical specimens were also entered into EPPIC.
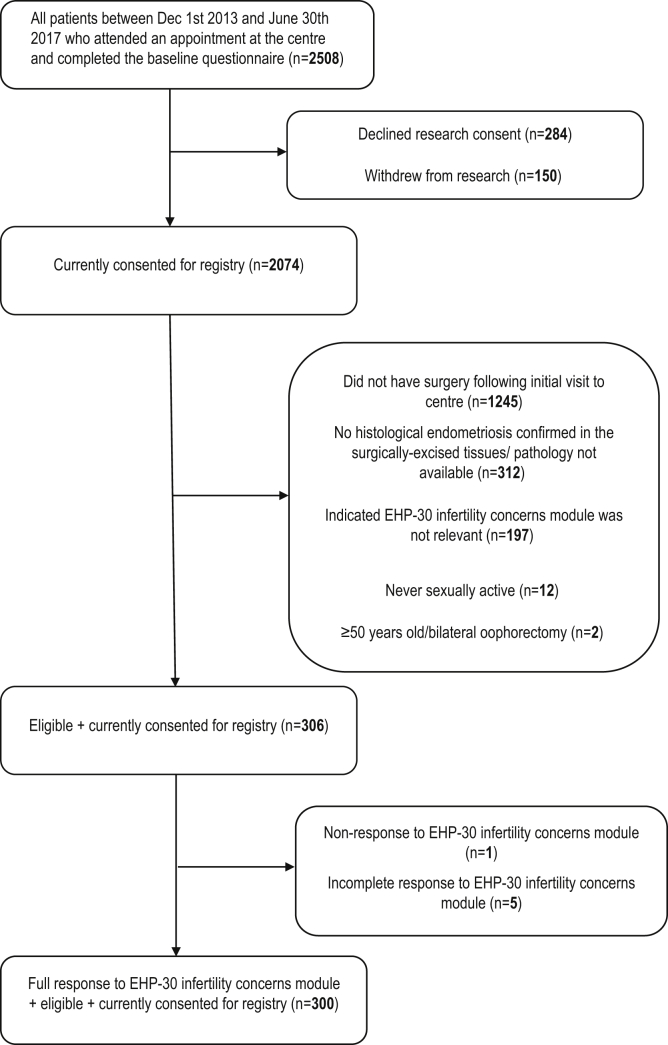
Inclusion criteria for this study were entry into the EPPIC registry between December 2013 and June 2017, with surgery prospectively performed at the center and histologically confirmed endometriosis in at least one excised tissue specimen. Exclusion criteria were self-reported status as “never sexually active,” spontaneous or surgical menopause, and patients choosing the “not relevant” option for the EHP-30 infertility concerns subscale (eg, patients who had completed childbearing). Individuals with incomplete or missing data on the infertility concerns module of the EHP-30 questionnaire were also removed from analysis. Figure 1 shows how the study population was defined, including reasons for exclusion at each stage of selection.
Figure 1.
Selection of study sample. Flow chart of included and excluded cases.
Measures and Covariates
All variables were collected when patients presented at the center and were recruited into the EPPIC registry, except for endometriosis stage which was obtained at the time of surgery.
The primary outcome was the infertility concerns subscale (Module F) of the EHP-30, a validated instrument that measures health-related quality of life in women with endometriosis.21 Module F has a 4-week recall period and asks whether the possibility of infertility has caused respondents to feel (i) worry, (ii) inadequacy, (iii) depression, or (iv) relationship strain. For every item, respondents indicated whether they never, rarely, sometimes, often, or always experienced the feeling in the last 4 weeks. Each response option was assigned a value from 0 to 4, for a total maximum possible score of 16 (a higher score indicates higher concern). The score was also converted to a percentage, after dividing by 16.
The main variables of interest were sexual pain scores at baseline. Respondents reported severity of deep dyspareunia and severity of superficial dyspareunia on previously published 11-point numeric rating sales, anchored with “no pain” at 0 and “worst pain imaginable” at 10.19 The items reflect the Initiative on Methods, Measurement and Pain Assessment in Clinical Trials recommendations that 11-point numeric rating scales are appropriate for pain scoring in endometriosis.22
Potential confounders were obtained from the data registry and included endometriosis-specific factors, demographic characteristics, and reproductive variables. Endometriosis-specific factors were endometriosis stage at the time of surgery (American Society for Reproductive Medicine stage I/II vs stage III/IV23), as well as baseline dysmenorrhea and chronic pelvic pain reported on the same 11-point numeric scale as sexual pain. Demographic data included age; nulliparity (yes/no); marital status (partnered; yes/no); ethnicity (Caucasian, other); education (some high school, graduated high school or earned General Educational Development diploma, some college, graduated 2 year college, graduated 4 year college, postgraduate degree, other); and household income (<$20,000, $20,000–$39,999, $40,000–$59,999, $60,000–$79,999, $80,000–$99,999, ≥$100,000). We also collected data for other variables that could affect reproductive potential such as previous difficulties conceiving (“Have you ever had problems getting pregnant [infertility] when you wanted to?” never tried/yes/no); currently trying to conceive (yes/no); previous miscarriage (yes/no); use of hormonal contraception (oral contraceptives, intrauterine devices, injectable medications, transdermal patches, and/or transvaginal rings: never/ever); oligomenorrhea (>35 days between menstrual periods; yes/no)24; history of chlamydia, gonorrhea, and/or pelvic inflammatory disease (yes/no)25; smoking (never, ever)26; weekly alcohol use (≤7 drinks, >7 drinks)21, 22, 23, 24; and body mass index (<18.5, 18.5–30, >30 kg/m2).27
Statistical Analyses
Statistical analyses were performed using R,28 with AER29 and MASS30 packages. When the EHP-30 infertility concerns score was left as a continuous variable (0–16), the assumptions of linearity and homogeneity of variance for linear regression were not met. Therefore, we grouped the EHP-30 infertility score into 5 categories for ordinal regression: those without infertility concerns (ie, 0 category) formed one category and those with infertility concerns were divided into equal quartiles (ie, 1–4, 5–8, 9–12, 13–16 categories). Listwise deletion of missing data was used given the small proportion of partial respondents. Nonresponse and partial response rates are reported in Figure 1 and Table 1.
Table 1.
Characteristics of the study population (n = 300)
| Characteristic | Mean ± SD or n (%) |
|---|---|
| Infertility concerns score (0–16) | 7.94 (5.47) |
| 0 | 53 (18) |
| 1–4 | 38 (13) |
| 5–8 | 67 (22) |
| 9–12 | 75 (25) |
| 13–16 | 67 (22) |
| Superficial dyspareunia (0–10) | 3.63 ± 3.16 |
| Deep dyspareunia (0–10) | 6.24 ± 3.08 |
| Age | 32.7 ± 6.3 |
| Marital status | |
| Partnered | 247 (82) |
| Unpartnered | 53 (18) |
| Race | |
| White | 210 (70) |
| Other | 90 (30) |
| Education | |
| Some high school | 8 (3) |
| High school or GED diploma | 26 (9) |
| Some college | 66 (22) |
| 2 year college degree | 46 (15) |
| 4 year college degree | 81 (27) |
| Postgraduate degree | 64 (21) |
| Other | 9 (3) |
| Income | |
| <$20,000 | 34 (11) |
| $20,000–$39,000 | 55 (18) |
| $40,000–$59,999 | 39 (13) |
| $60,000–$79,999 | 56 (19) |
| $80,000–$99,999 | 43 (14) |
| ≥100,000 | 73 (25) |
| Nulliparous | 221 (74) |
| Amenorrhea due to hormonal therapy | 32 (11) |
| Previous miscarriage | 48 (16) |
| Previous difficulty conceiving | |
| Never tried | 130 (43) |
| Yes | 122 (41) |
| No | 48 (16) |
| Currently trying to conceive | 89 (30) |
| Endometriosis stage | |
| I/II | 192 (64) |
| III/IV | 98 (33) |
| Missing | 10 (3) |
GED = General Educational Development.
Descriptive data were summarized as n (%) for categorical variables and mean ± SD for continuous variables. Simple ordinal regression was used to test the bivariate relationships between the categorized infertility concerns subscale and deep dyspareunia, superficial dyspareunia, endometriosis-specific variables, factors from reproductive history, and demographic variables. Multivariable ordinal regression was then performed for the categorized EHP-30 infertility concerns subscale score, including significant dyspareunia variables and all potential confounders. Because it is recommended that each of the 5 categories of the infertility concerns score should have a sample size greater than 10 multiplied by the number of variables in the model,31 we built additional models with smaller subsets of potential confounders to confirm the robustness of the results.
Results
Sample Description
The study flow chart is shown in Figure 1, and Table 1 shows the characteristics of the 300 women in our study. On average, participants were 32.7 ± 6.3 years old. 82% (247/300) of participants were partnered, 70% (210/300) were Caucasian, and 74% (221/300) were nulliparous. 66% of participants (200/300) had at least a 2-year college degree, and 57% (172/300) had a household income of $60,000 or greater. 30% of participants (89/300) were trying to conceive at the time of the study, and 41% (122/300) reported previous difficulty conceiving. At the time of surgery, 64% (192/300) were found to have stage I/II endometriosis, 33% (98/300) were found to have stage III/IV endometriosis, and 3% (10/300) were missing staging information. The mean infertility concerns score was 7.94 ± 5.47, the mean superficial dyspareunia score was 3.63 ± 3.16, and the mean deep dyspareunia score was 6.24 ± 3.08.
Relationship Between Infertility Concerns and Dyspareunia
Bivariate analyses indicated that increased severity of superficial dyspareunia was associated with infertility concerns subscale score (odds ratio = 1.09, 95% CI 1.02–1.16, P = .011) but increased severity of deep dyspareunia was not (odds ratio = 1.02, 95% CI 0.95–1.09, P = .58). Ancillary analyses showed that participants who self-reported previous difficulties conceiving had significantly higher superficial dyspareunia than those who reported no difficulties, and the group who had never tried to conceive had the highest average superficial dyspareunia (data not shown). As shown in Table 2, younger age, race, nulliparity, previous difficulties conceiving, currently trying to conceive, greater severity of dysmenorrhea, and stage III/IV endometriosis were also associated with greater infertility concerns score.
Table 2.
Bivariate associations with the EHP-30 infertility concerns score
| Characteristic | OR (95% CI)∗ | P∗ |
|---|---|---|
| Deep dyspareunia | 1.02 (0.95–1.09) | .58 |
| Superficial dyspareunia | 1.09 (1.02–1.16) | .011 |
| Dysmenorrhea | 1.13 (1.04–1.13) | .005 |
| Chronic pelvic pain | 1.02 (0.95–1.09) | .53 |
| Endometriosis stage, III/IV | 1.65 (1.07–2.55) | .024 |
| Age, years | 0.94 (0.91–0.98) | <.001 |
| Marital status, partnered | 1.09 (0.65–1.84) | .74 |
| Race, other | 1.62 (1.04–2.55) | .035 |
| Education (reference group: some high school) | ||
| High school or GED diploma | 0.26 (0.04–1.61) | .14 |
| Some college | 0.34 (0.06–1.94) | .22 |
| 2-year college degree | 0.30 (0.05–1.73) | .17 |
| 4-year college degree | 0.39 (0.07–2.17) | .27 |
| Postgraduate degree | 0.38 (0.06–2.15) | .27 |
| Other | 0.48 (0.06–3.49) | .46 |
| Income (reference group: <20,000) | ||
| $20,000–$39,000 | 1.58 (0.73–3.45) | .25 |
| $40,000–$59,999 | 1.56 (0.67–3.62) | .30 |
| $60,000–$79,999 | 1.62 (0.75–3.51) | .25 |
| $80,000–$99,999 | 1.17 (0.52–2.61) | .70 |
| ≥100,000 | 1.19 (0.56–2.52) | .65 |
| Nulliparous | 6.68 (3.98–11.39) | <.001 |
| Previous miscarriage | 0.66 (0.38–1.16) | .15 |
| Previous difficulty conceiving (reference group: never tried) | ||
| Yes | 2.11 (1.35–3.31) | .001 |
| No | 0.13 (0.07–0.25) | <.001 |
| Currently trying to conceive | 5.69 (3.57–9.21) | <.001 |
| Hormonal contraceptive use, ever | 0.64 (0.35–1.15) | .14 |
| Oligomenorrhea | 1.63 (0.74–3.60) | .22 |
| STI | 1.22 (0.69–2.18) | .50 |
| Smoking status, yes | 1.14 (0.63–2.06) | .67 |
| >7 alcoholic beverages/week | 1.07 (0.43–2.67) | .89 |
| BMI (reference group: 18.5–30 kg/m2) | ||
| <18.5 | 2.24 (0.78–6.68) | .14 |
| >30 | 0.73 (0.42–1.28) | .28 |
EHP-30 = Endometriosis Health Profile-30.
GED = General Educational Development.
Bivariate ordinal logistic regression.
Multivariable ordinal regression models are shown in Table 3. For model A, a unit increase in the superficial dyspareunia score (ie, an increase of 1/10 in severity rating) was associated with a 14% higher odds of infertility concerns (adjusted odds ratio [AOR] = 1.14, 95% CI: 1.06–1.24, P < .001), adjusting for age, race, nulliparity, previous difficulties conceiving, currently trying to conceive, severity of dysmenorrhea, and endometriosis stage. A unit decrease in age (ie, 1 year younger) was associated with a 6% higher odds of infertility concerns (AOR = 0.94, 95% CI: 0.89–0.98, P = .005). For the binary variables in model A, currently trying to conceive was associated with a fivefold higher odds of infertility concerns (AOR = 5.23, 95% CI: 2.77–9.98, P < .001), nulliparity was associated with a threefold higher odds of infertility concerns (AOR = 3.21, 95% CI 1.63–6.41, P < .001), and previous difficulty conceiving was associated with a twofold higher odds of infertility concerns (AOR = 2.09, 95% CI 1.04–4.19, P = .038). Race, endometriosis stage, and severity of dysmenorrhea were not significantly associated with infertility concerns in model A.
Table 3.
Multivariable ordinal regression models
| Variables | Unadjusted |
Model A |
Model B |
Model C |
Model D |
|---|---|---|---|---|---|
| n = 300 |
n = 259∗ |
n = 259∗ |
n = 300 |
n = 300 |
|
| OR (95% CI, P) | AOR (95% CI, P) | AOR (95%CI, P) | AOR (95% CI, P) | AOR (95% CI, P) | |
| Superficial dyspareunia | 1.09 (1.02–1.16, .011) | 1.14 (1.06–1.24, <.001) | 1.10 (1.03–1.19, .007) | 1.07 (1.00–1.15, .042) | 1.13 (1.06–1.22, <.001) |
| Previous difficulty conceiving, yes | 2.09 (1.04–4.19, .038) | 0.87 (0.50–1.50, .61) | |||
| Previous difficulty conceiving, no | 0.40 (0.16–0.94, .038) | 0.12 (0.06–0.24, <.001) | |||
| Currently trying to conceive | 5.23 (2.77–9.98, <.001) | 6.82 (3.81–12.38, <.001) | |||
| Age | 0.94 (0.89–0.98, .005) | 0.98 (0.94–1.01, .24) | |||
| Race | 1.11 (0.63–1.94, .72) | 1.74 (1.10–2.78, .019) | |||
| Nulliparity | 3.21 (1.63–6.41, <.001) | 5.82 (3.37–10.22, <.001) | |||
| Endometriosis, stage III/IV | 1.48 (0.86–2.56, .16) | 1.96 (1.24–3.14, .005) | |||
| Dysmenorrhea | 1.09 (1.00–1.20, .057) | 1.11 (1.02–1.21, .015) |
AOR = adjusted odds ratio; OR = odds ratio.
Excludes participants who are amenorrheic because of hormonal suppression and participants with missing endometriosis stage.
Additional regression models were built incorporating endometriosis-associated factors alone (model B; Table 3), demographic variables alone (model C), and reproductive history alone (model D). In each of these models, superficial dyspareunia remained significantly associated with infertility concerns (model B, AOR = 1.10, 95% CI: 1.03–1.19, P = .007; model C, AOR = 1.07, 95% CI: 1.00–1.15, P = .042; model D, AOR = 1.13, 95% CI: 1.06–1.22, P < .001).
Discussion
We hypothesized that both deep dyspareunia and superficial dyspareunia would be independently associated with infertility concerns among women with endometriosis; however, only superficial dyspareunia was significantly related to infertility concerns in bivariate analyses. Adjusting for other variables in a multivariable model, superficial dyspareunia, currently trying to conceive, nulliparity, previous difficulties trying to conceive, and younger age remained significantly related to infertility concerns.
There are several possible explanations for the observed difference between deep dyspareunia and superficial dyspareunia. These types of pain differ significantly with regard to whether vaginal penetration is possible—whereas women with deep dyspareunia may achieve penetration and endure pain until their partner ejaculates,32 those with superficial dyspareunia can find initial penetration very difficult or impossible and may delay trying to conceive until this symptom improves.33 Another potential explanation is that women with superficial dyspareunia may have more or different psychological comorbidities (eg, anxiety) that increase their worry about future fertility than those with deep dyspareunia; however, there has been no direct comparison of women with different types of sexual pain to address this hypothesis. Overall, the observed difference between deep dyspareunia and superficial dyspareunia should be interpreted with caution given the small absolute difference between the odds of infertility concerns associated with each of these variables.
Other factors that were associated with more infertility concerns were previous difficulty conceiving, currently trying to conceive, nulliparity, and age. These relationships were expected: both women who have experienced dificulty conceiving and those who are trying to conceive are likely to be more concerned about their ability to do so. Similarly, younger age and nulliparity may reflect uncertainty about reproductive potential.34
Strengths of this study included the use of a validated measure for infertility concerns in the endometriosis population (EHP-30) and the robustness of the findings across ordinal regression models. The differentiation of sexual pain by type was also a strength, given that deep and superficial dyspareunia are often measured in aggregate.1 In addition, sampling and nonresponse error were unlikely to have biased the results, given the low rates of partial questionnaire completion (Figure 1, Table 1).
A primary limitation of the study was that the different relationships of superficial dyspareunia and deep dyspareunia with infertility concerns may have been driven by comorbid psychological factors.3,10,35 This possibility could not be explored because of collinearity between the infertility concerns module (ie, assessing depression and worry) and questionnaires used to measure pain catastrophizing,36 anxiety,37 and depression38 in our registry and reflects the complexities of studying the psychological burden of anticipated infertility.11 Another limitation of the study was that endometriosis was not staged at the same time that other data were collected because of the wait for surgery after the initial consultation; however, while some degree of spontaneous growth or regression of endometriosis lesions is known to occur,39 it would be clinically rare for stage I/II endometriosis to evolve into stage III/IV endometriosis within 6 months. Although the average infertility concern score in this study was consistent with the scores reported in the development of the EHP-30,21 our use of a pelvic pain data registry may limit generalizability to women with a primary complaint of infertility (eg, in a fertility clinic setting) as these individuals may have less severe dyspareunia.40
The clinical significance of these findings can only be inferred, given the cross-sectional nature of the data. A unit increase of superficial dyspareunia (ie, an increase of 1/10) was associated with a 14% higher odds of infertility concerns. This means that a woman with a superficial dyspareunia severity score of 10 out of 10 would have 70% higher odds of infertility concerns than a woman with a severity score of 5 out of 10 and 140% higher odds than a woman with severity score 0 out of 10. Thus, among patients with endometriosis and severe superficial dyspareunia resulting from comorbidities such as provoked vestibulodynia or, in rare cases, endometriosis itself, it may be helpful to explore conversations about potential infertility concerns. In particular, these patients could be advised about treatment for introital pain (eg, pelvic floor physiotherapy) to facilitate vaginal intercourse and be reassured that the presence of superficial dyspareunia does not indicate dysfunction of the reproductive organs.
Conclusion
We showed that superficial dyspareunia was associated with infertility concerns among women with endometriosis irrespective of age, reproductive history, and disease-specific factors. Given the cross-sectional design of this study, the relationship of superficial dyspareunia with infertility concerns should be further explored. Studies that account for relevant psychological variables, for example, through qualitative exploration or by controlling for a history of psychological conditions before the index endometriosis diagnosis, may help clarify the biopsychosocial complexities of infertility concerns and their relationship with dyspareunia. Considering the etiology of superficial dyspareunia through the use of vulvoscopy and a systematic assessment of Q-tip tenderness of the vulvar vestibule may also illuminate the relationship of superficial dyspareunia subtypes and infertility concerns. Clinically, the results serve as a reminder that potential infertility may produce worry among some women with endometriosis, even for those who have not yet tried to become pregnant and perhaps particularly for those experiencing superficial dyspareunia.
Statement of authorship
Category 1
-
(a)Conception and Design
- Kate J. Wahl; Paul J. Yong
-
(b)Acquisition of Data
- Kate J. Wahl; Heather Noga; Paul J. Yong
-
(c)Analysis and Interpretation of Data
- Kate J. Wahl; Natasha L. Orr; Michelle Lisonek; Mohamed A. Bedaiwy; Christina Williams; Catherine Allaire; Arianne Y. Albert; Kelly B. Smith; Susan Cox; Paul J. Yong
Category 2
-
(a)Drafting the Article
- Kate J. Wahl; Michelle Lisonek
-
(b)Revising It for Intellectual Content
- Kate J. Wahl; Natasha L. Orr; Michelle Lisonek; Heather Noga; Mohamed A. Bedaiwy; Christina Williams; Catherine Allaire; Arianne Y. Albert; Kelly B. Smith; Susan Cox; Paul J. Yong
Category 3
-
(a)Final Approval of the Completed Article
- Kate J. Wahl; Natasha L. Orr; Michelle Lisonek; Heather Noga; Mohamed A. Bedaiwy; Christina Williams; Catherine Allaire; Arianne Y. Albert; Kelly B. Smith; Susan Cox; Paul J. Yong
Footnotes
Funding: This work was supported by a Canadian Institutes of Health Research (CIHR) Operating Grant (MOP142273) and Project Grant (PGT156084), the Women’s Health Research Institute, and the BC Women’s Hospital and Health Center Foundation. Paul Yong was supported by an Investigator Award from the VGH and UBC Hospital Foundation (Mentored Clinician Scientist Award of the Vancouver Coastal Health Research Institute) and the Health Professional Investigator Award for the Michael Smith Foundation for Health Research. Funding sources had no involvement in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Conflict of Interest: Mohamed A. Bedaiwy and Catherine Allaire have financial affiliations with AbbVie and Allergan.
References
- 1.Orr N., Wahl K., Joannou A., Hartmann D., Valle L., Yong P. Deep dyspareunia: review of pathophysiology and proposed future research priorities. Sex Med Rev. 2020;8:3–17. doi: 10.1016/j.sxmr.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Yong P.J., Mui J., Allaire C. Pelvic floor tenderness in the etiology of superficial dyspareunia. J Obstet Gynaecol Can. 2014;36:1002–1009. doi: 10.1016/S1701-2163(15)30414-X. [DOI] [PubMed] [Google Scholar]
- 3.Yong P.J., Sadownik L., Brotto L.A. Concurrent deep–superficial dyspareunia: prevalence, associations, and outcomes in a multidisciplinary vulvodynia program. J Sex Med. 2015;12:219–227. doi: 10.1111/jsm.12729. [DOI] [PubMed] [Google Scholar]
- 4.Practice Committee of the American Society for Reproductive Medicine Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril. 2012;98:1103–1111. doi: 10.1016/j.fertnstert.2012.06.048. [DOI] [PubMed] [Google Scholar]
- 5.Prescott J., Farland L.V., Tobias D.K. A prospective cohort study of endometriosis and subsequent risk of infertility. Hum Reprod. 2016;31:1475–1482. doi: 10.1093/humrep/dew085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young K., Kirkman M., Holton S. Fertility experiences in women reporting endometriosis: findings from the understanding fertility management in contemporary Australia survey. Eur J Contracept Reprod Health Care. 2018;23:434–440. doi: 10.1080/13625187.2018.1539163. [DOI] [PubMed] [Google Scholar]
- 7.Young K., Fisher J., Kirkman M. Endometriosis and fertility: women’s accounts of healthcare. Hum Reprod. 2016;31:554–562. doi: 10.1093/humrep/dev337. [DOI] [PubMed] [Google Scholar]
- 8.Culley L., Hudson N., Mitchell H. The impact of endometriosis on planning for and having children: findings from the Endopart Study. J Fertil Counsel. 2014;21:22–25. [Google Scholar]
- 9.Culley L., Law C., Hudson N. The social and psychological impact of endometriosis on women’s lives: a critical narrative review. Hum Reprod Update. 2013;19:625–639. doi: 10.1093/humupd/dmt027. [DOI] [PubMed] [Google Scholar]
- 10.Facchin F., Saita E., Barbara G. “Free butterflies will come out of these deep wounds”: a grounded theory of how endometriosis affects women’s psychological health. J Health Psychol. 2018;23:538–549. doi: 10.1177/1359105316688952. [DOI] [PubMed] [Google Scholar]
- 11.Facchin F., Buggio L., Dridi D. A woman’s worth: the psychological impact of beliefs about motherhood, female identity, and infertility on childless women with endometriosis. J Health Psychol. 2019 doi: 10.1177/1359105319863093. 1359105319863093. [DOI] [PubMed] [Google Scholar]
- 12.de Mendonca C.R., Arruda J.T., Noll M. Sexual dysfunction in infertile women: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2017;215:153–163. doi: 10.1016/j.ejogrb.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 13.Jindal U.N., Jindal S. Use by gynecologists of a modified sensate focus technique to treat vaginismus causing infertility. Fertil Steril. 2010;94:2393–2395. doi: 10.1016/j.fertnstert.2010.03.071. [DOI] [PubMed] [Google Scholar]
- 14.de Ziegler D., Borghese B., Chapron C. Endometriosis and infertility: pathophysiology and management. Lancet. 2010;376:730–738. doi: 10.1016/S0140-6736(10)60490-4. [DOI] [PubMed] [Google Scholar]
- 15.Basson R. Rethinking low sexual desire in women. BJOG. 2002;109:357–363. doi: 10.1111/j.1471-0528.2002.01002.x. [DOI] [PubMed] [Google Scholar]
- 16.Jones G., Jenkinson C., Kennedy S. The impact of endometriosis upon quality of life: a qualitative analysis. J Psychosom Obstet Gynaecol. 2004;25:123–133. doi: 10.1080/01674820400002279. [DOI] [PubMed] [Google Scholar]
- 17.Ruddy K.J., Gelber S.I., Tamimi R.M. Prospective study of fertility concerns and preservation strategies in young women with breast cancer. J Clin Oncol. 2014;32:1151–1156. doi: 10.1200/JCO.2013.52.8877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Encyclopedia of survey research methods. Sage Publications; Thousand Oaks, CA: 2008. Cross-sectional data [internet]http://methods.sagepub.com/reference/encyclopedia-of-survey-research-methods/n119.xml Available at: [Google Scholar]
- 19.Yosef A., Yosef A., Allaire C. Multifactorial contributors to the severity of chronic pelvic pain in women. Am J Obstet Gynecol. 2016;215:760.e1–760.e14. doi: 10.1016/j.ajog.2016.07.023. [DOI] [PubMed] [Google Scholar]
- 20.Orr N.L., Noga H., Williams C. Deep dyspareunia in endometriosis: role of the bladder and pelvic floor. J Sex Med. 2018;15:1158–1166. doi: 10.1016/j.jsxm.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Jones G., Kennedy S., Barnard A. Development of an endometriosis quality-of-life instrument: the endometriosis health profile-30. Obstet Gynecol. 2001;98:258–264. doi: 10.1016/s0029-7844(01)01433-8. [DOI] [PubMed] [Google Scholar]
- 22.Vincent K., Kennedy S., Stratton P. Pain scoring in endometriosis: entry criteria and outcome measures for clinical trials. Report from the Art and Science of Endometriosis meeting. Fertil Steril. 2010;93:62–67. doi: 10.1016/j.fertnstert.2008.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunselman G.A.J., Vermeulen N., Becker C. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29:400–412. doi: 10.1093/humrep/det457. [DOI] [PubMed] [Google Scholar]
- 24.Ballard K.D., Seaman H.E., De Vries C.S. Can symptomatology help in the diagnosis of endometriosis? Findings from a national case–control study—Part 1. BJOG. 2008;115:1382–1391. doi: 10.1111/j.1471-0528.2008.01878.x. [DOI] [PubMed] [Google Scholar]
- 25.Agarwal S.K., Chapron C., Giudice L.C. Clinical diagnosis of endometriosis: a call to action. Am J Obstet Gynecol. 2019;220:354.e1–354.e12. doi: 10.1016/j.ajog.2018.12.039. [DOI] [PubMed] [Google Scholar]
- 26.Greene R., Stratton P., Cleary S.D. Diagnostic experience among 4,334 women reporting surgically diagnosed endometriosis. Fertil Steril. 2009;91:32–39. doi: 10.1016/j.fertnstert.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 27.Soliman A.M., Coyne K.S., Gries K.S. The effect of endometriosis symptoms on absenteeism and presenteeism in the workplace and at home. J Manag Care Spec Pharm. 2017;23:745–754. doi: 10.18553/jmcp.2017.23.7.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2008. R: a language and environment for statistical computing [internet]https://www.R-project.org Available at: [Google Scholar]
- 29.Kleiber C., Zeileis A. Springer-Verlag; New York, NY: 2008. Applied econometrics with R. [Google Scholar]
- 30.Venables V.N., Ripley B.D. 4th ed. Springer Science; New York, NY: 2002. Modern applied statistics. [Google Scholar]
- 31.Peduzzi P., Concato J., Kemper E. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 32.Denny E., Mann C.H. Endometriosis-associated dyspareunia: the impact on women’s lives. J Fam Plann Reprod Health Care. 2007;33:189–193. doi: 10.1783/147118907781004831. [DOI] [PubMed] [Google Scholar]
- 33.Johnson N.S., Harwood E.M., Nguyen R.H.N. “You have to go through it and have your children”: reproductive experiences among women with vulvodynia. BMC Pregnancy Childbirth. 2015;15:114. doi: 10.1186/s12884-015-0544-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mazuy M, de la Rochebrochard E. Fertility problems: a widespread concern among women. Results from the French generations and gender survey [internet]. Population Association of America (PAA) Annual Meeting. April 17-19, 2018; New Orleans, LA. Available at: https://paa2008.princeton.edu/papers/81038. Accessed October 18, 2018
- 35.Brauer M., Kuile M.M. ter, Laan E. Cognitive-affective correlates and predictors of superficial dyspareunia. J Sex Marital Ther. 2008;35:1–24. doi: 10.1080/00926230802525604. [DOI] [PubMed] [Google Scholar]
- 36.Sullivan M.J.L., Bishop S.R., Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assess. 1995;7:524–532. [Google Scholar]
- 37.Spitzer R.L., Kroenke K., Williams J.B.W. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 38.Kroenke K., Spitzer R.L., Williams J.B.W. The PHQ-9. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abbott J., Hawe J., Hunter D. Laparoscopic excision of endometriosis: a randomized, placebo-controlled trial. Fertil Steril. 2004;82:878–884. doi: 10.1016/j.fertnstert.2004.03.046. [DOI] [PubMed] [Google Scholar]
- 40.Dmowski W.P., Lesniewicz R., Rana N. Changing trends in the diagnosis of endometriosis: a comparative study of women with pelvic endometriosis presenting with chronic pelvic pain or infertility. Fertil Steril. 1997;67:238–243. doi: 10.1016/S0015-0282(97)81904-8. [DOI] [PubMed] [Google Scholar]