Abstract
Purpose
To evaluate safety and clinical response of Low-intensity Shockwave Therapy (Li-SWT) for the treatment of erectile dysfunction.
Materials & Methods
A single-institution, 2 arm, phase II randomized clinical trial was conducted between February 2017 and April 2019. Patients were randomized into 2 groups, with Li-SWT delivering a total of 3,600 shocks over 5 days (720 once a day, Group A) or over 2 weeks (600 once a day, 3 times a week, Group B). Patients were evaluated for the safety of therapy and completed the International Index of Erectile Function-Erectile Function domain and the Erectile Hardness Scale assessment at baseline, and at 1, 3, and 6 months visits.
Results
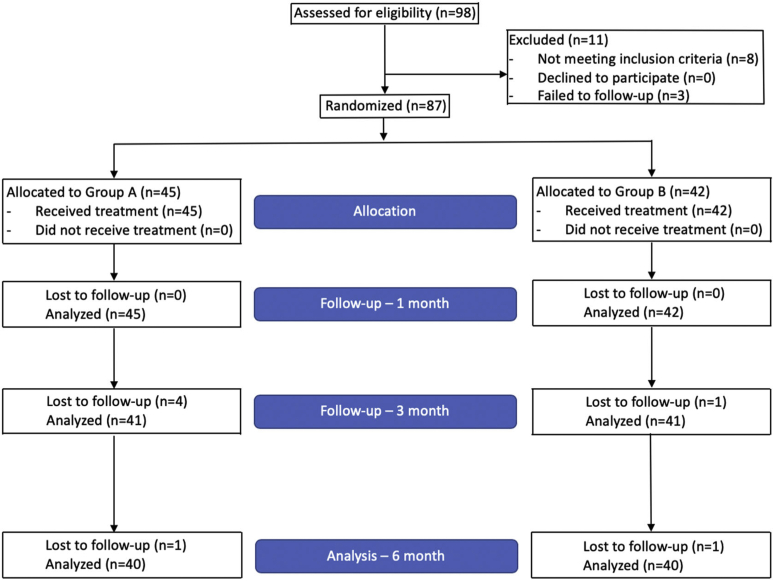
Among 87 evaluable patients, 45 and 42 were allocated to Groups A and B treatment schedules, respectively, and 80 patients (40 per group) completed the 6-month evaluation. No adverse events were reported during treatment or during follow-up. There were statistically significant (P < .05) improvements in International Index of Erectile Function-Erectile Function score (mean increase of 2.7 [95% CI = 1.2, 4.2] and 2.7 points [95% CI = 1.4, 4.1] for Groups A and B, respectively) and in Erectile Hardness Scale (mean increase of 0.6 points (95% CI = 0.3, 0.8) and 0.5 (95% CI = 0.2, 0.8) for Groups A and B, respectively) at 6 months, with no differences between groups.
Conclusion
No difference in outcomes was found when Li-SWT 3,600 shocks were delivered over 1 or 2 weeks at 6 months follow-up and both schedules were safe with no adverse events during or after treatment. Further trials with longer follow-up and sham arm will provide valuable information regarding treatment efficacy and durability.
Patel P, Katz J, Lokeshwar SD, et al. Phase II Randomized, Clinical Trial Evaluating 2 Schedules of Low-Intensity Shockwave Therapy for the Treatment of Erectile Dysfunction. Sex Med 2020;8:214–222.
Key Words: Clinical Trial, Erectile Dysfunction, Low-Intensity Shockwave Therapy, Restorative Therapy
Introduction
Erectile dysfunction (ED) is defined as the inability to attain and/or maintain penile erection sufficient for satisfactory sexual performance and affects 150 million men worldwide1,2 The current armamentarium for the treatment of ED includes oral therapies, intraurethral suppositories, intracavernosal injections, and prosthetic devices.2 These treatments do not reverse the underlying conditions that lead to the development of ED. Therefore, the utility of Low-Intensity Shockwave Therapy (Li-SWT) has gained interest for the treatment of ED. Li-SWT is theorized to potentially reverse some of the underlying pathophysiology of ED and may allow patients to minimize chronic ED treatment.3
With the recent rise in ED clinics offering restorative therapies such as Li-SWT,4 it is imperative to further investigate Li-SWT. Li-SWT is gaining traction in mainstream media with celebrity endorsements,5 regardless of American Urological Association guidelines defining Li-SWT as investigational (Conditional Recommendation; Evidence Level: Grade C). Although Li-SWT is being applied to patients, a gold-standard treatment protocol has not yet been investigated. Therefore, the objective of this study is to better define a Li-SWT protocol suitable for maximal benefit without compromising patient safety in the treatment of ED, since this treatment is widely being offered to patients as differing and unstandardized treatment schedules. We performed a phase II clinical trial to evaluate the safety and clinical response of the MoreNova Linear Shockwave Device for the treatment of ED using 2 different treatment schedules. We hypothesized that the 2 treatment schedules with Li-SWT would be safe and lead to similar significant improvement in validated ED questionnaire scores during follow-up assessments.
Methods
This single institution, prospective, randomized, 2 arm, phase II clinical trial was conducted between February 2017 and April 2019 at the University of Miami, Miami, Florida, U.S.A (ClinicalTrials.gov NCT03067987). The protocol was approved by an institutional review board. Participants provided written informed consent prior to enrollment. The study was conducted in accordance with the World Health Organization Declaration of Helsinki and all amendments, and the International Conference on Harmonization and Good Clinical Practice Guideline.
Inclusion/Exclusion Criteria
Participants were recruited from a cohort of men referred to the Principal Investigator (R.R.) for ED. Inclusion/exclusion criteria were based on previous studies investigating the efficacy of Li-SWT to ensure comparability.6 Stringent criteria were designed to eliminate confounding variability. Eligible patients included male patients who were >30 and < 80 years of age, regardless of phosphodiesterase type 5 inhibitor (PDE-5i) responsiveness (PDE-5i patients discontinued medication for 4 weeks before initial International Index of Erectile Function-Erectile Function domain [IIEF-EF] assessment and during the remainder of the study); were able to stop taking PDE-5i's until completion of the trial; were in a stable sexual relationship for over 3 months with a minimum of 2 sexual attempts per month for at least 1 month prior to enrollment; had ED lasting for over 6 months and <5 years; had a baseline IIEF-EF score between 11 and 25, testosterone level 300–1,000 ng/dL; and if diabetic, hemoglobin A1C level ≤7.5% within 1 month prior to enrollment. Exclusion criteria included current or previous patient participation in another study within the past 3 months that may interfere with the proposed study; history of radical prostatectomy or extensive pelvic surgery; pelvic region radiation therapy within 12 months prior to enrollment; recovering from any cancer within 12 months prior to enrollment; neurological disease like Alzheimer's or Parkinson's which can affect erectile function; a psychiatric diagnosis or medications such as antidepressants which can affect erectile function; anatomical malformation of the penis, including Peyronie's disease; androgen deprivation treatment in the last year; history of spinal cord injury; and the use of systemic anticoagulants (eg, Coumadin, Clopidogrel).
Treatment Device
Patients were treated with MoreNova, a shockwave device developed by Hikkonu Ltd of DirexGroup, Israel. This device uses an electromagnetic generator designed to deliver “linear” shockwaves, that is, wave fronts that are geometrically-matched to the shape of the corpora and crura. The underlying mechanism of shock delivery of this device is equivalent to that published in previously studies.7,8 Unlike other electromagnetic shockwave generators, this device does not apply a point-focuser, but instead uses the patented Large Area Shockwave Technology to simultaneously deliver shockwaves to whole segments of the corpora and crura. Compared to radial shockwaves, the focused shockwave has a much shorter pulse duration, deeper penetration depth, and has its effect at the cellular rather than superficial tissue level. The treatment device can target a focused area with greater pressure. The energy intensity is 0.09 mJ/mm2 and the pulse frequency is 1 Hz.
Study Design
This trial implemented 2 single-arm designs in parallel to test 2 treatment schedules. Patients were equally randomized into the 2 treatment groups to receive a total of 3,600 shocks of Li-SWT with prior studies demonstrating clinically significant outcomes with the same number of shocks delivered.7,8 Shocks were administered in 5 locations on the stretched penis, left and right cavernosa, and the parallel diverging segments of the crura. Patients in Group A received once a day treatment over 5 consecutive days (Monday to Friday), in which 720 shocks of Li-SWT were applied in every session, half to each treated region (left and right corpora cavernosa and crura). Group B consisted of once a day 3 sessions per week (Monday, Wednesday, and Friday) for 2 consecutive weeks, in which 600 shocks of Li-SWT were applied in every session to the aforementioned treatment areas.
The randomization list was generated, with treatment options printed on paper and then sealed in individual envelopes. The envelope was opened after a patient signed the informed consent and was ready to receive treatment. The Principal Investigator (R.R.) was responsible for patient initial evaluation and recruitment, whereas the study coordinator (M.M.) was responsible for patient randomization, treatment administration, and follow-up. The Principal Investigator (R.R.) was blinded to which arm the patients were allocated. De-identified data were collected for each participant and entered into a designated Research Electronic Data Capture Database. No protocol deviations or modifications were noted.
Outcomes
In this trial, we aimed to evaluate safety and clinical response of the 2 treatment schedules of Li-SWT. During shockwave administration, patients were evaluated for penile bruising, edema, and pain. At subsequent follow-up appointments, patients were evaluated for bruising, hematuria, penile curvature development, lower urinary tract symptoms, pain, and worsening erectile function. The primary clinical response outcomes, assessed in each group, were the changes in IIEF-EF scores9 from baseline to follow-ups, 1, 3, and 6 months after treatment, and the percentage of patients that reached minimal clinically important difference (MCID) in IIEF-EF at the end of 6 months. MCID was defined as an increase in IIEF-EF of ≥2 for patients with baseline mild ED (IIEF-EF scores 17–25) and ≥5 for patients with baseline moderate ED (IIEF-EF scores 11–16).10 The Erectile Function domain of the IIEF is a validated tool and provides valuable information for distinguishing men with and without ED, as well as stratifying the severity of ED.9 Secondary outcomes were to evaluate changes in the Erectile Hardness Scale (EHS) scores. EHS is a self-reported tool which scores erectile hardness on a validated 4-point scale. EHS scores 1–2 represent inability to have penetrative intercourse, while EHS scores 3–4 identify patients that are capable of penetrative intercourse.11
Power Analysis
The number of patients studied was based on power analysis for the primary endpoint, the change in IIEF-EF scores between baseline and the 6 months follow-up. A total of 40 men per group provided 90% power to detect an effect size of 0.576, based on 1-sample t-test at a 2-sided 2.5% significance level, adjusting for 2 tests (1 per group). The observed powers for Groups A and B were 90.4% and 92.7% based on estimated effect sizes 0.580 (6-month IIEF-EF score change mean = 2.700, SD = 4.659) and 0.604 (mean = 2.675, SD = 4.428), respectively. Allowing possible dropouts, accrual of 90 patients (45 per group) was randomized.
Statistical Analysis
Descriptive statistics were used to characterized demographic and baseline characteristics, and primary and secondary outcomes. Chi-square test, the Fisher's exact test, or the 2-sample Students' t-test was performed to evaluate baseline differences between groups. 1-sample t-test was used to test the significance of change of IIEF-EF score from baseline to 6 months follow-up in each group. Longitudinal data consisting of IIEF-EF and EHS scores were also analyzed using repeated measures analysis of variance, using maximum likelihood estimation and assuming any missing data are missing at random. This analytical approach for repeated measures accommodates missing data and allows for flexible specification of covariance structure. We assumed a heterogeneous autoregressive covariance matrix to account for the correlated data structure. The model for each outcome, IIEF-EF and EHS scores, included group, time, their interaction and adjustment for age (<60, ≥60), body mass index (BMI; 18.5–24.9, 25–29.9, ≥30), number of comorbidities (0, 1, 2+), and serum testosterone value. The statistical significance was set at P < .05 and analysis were performed in SAS 9.4.
Results
90 men were randomized into Group A and Group B, respectively. 3 patients did not return for any posttreatment evaluation and were considered not evaluable for the study of efficacy outcomes. Among the 87 evaluable patients, 45 and 42 were allocated to Groups A and B, respectively. A total of 80 patients completed the 6-month evaluation (40 in each group) (Figure 1). Baseline characteristics were similar between both groups with no statistically significant differences (Table 1). Overall mean patient age was 51.9 (SD = 12.2) with a mean BMI of 29 (SD = 4.4). Age was divided into young or older than 60 to eliminate confounding variability of elder age and to facilitate the elucidation of bias regarding psychogenic ED, as this is a more common etiology of ED in younger men.12 37.9% of the total population had a BMI ≥30, which corresponds to obesity. With respect to comorbidities, 3.4% (n = 3), 33.3% (n = 29), 20.7% (n = 18), and 10.3% (n = 9) had a history of coronary artery disease, hypertension, dyslipidemia, and diabetes mellitus, respectively. All patients denied being a current smoker. Mean hemoglobin A1C and serum testosterone were 5.5 (SD = 0.6) and 513.8 (SD = 155.6), respectively. Of patients that had previously tried PDE-5i therapy, 32 (76.2%) were responders. Overall, 60 (69%) and 27 (31%) patients had mild (IIEF: 17–25) and moderate (11–16) ED, respectively.
Figure 1.
Patient flow diagram.
Table 1.
Baseline patient characteristics
| Variable | Total N (%) |
Group A N (%) |
Group B N (%) |
P |
|---|---|---|---|---|
| Total patients | 87 (100) | 45 (100) | 42 (100) | |
| Age (years) | ||||
| <60 years | 63 (72.4) | 32 (71.1) | 31 (73.8) | .778 |
| ≥60 years | 24 (27.6) | 13 (28.9) | 11 (26.2) | |
| Mean (SD) | 51.9 (12.2) | 53.2 (12) | 50.5 (12.4) | .316 |
| Median (range) | 54 (30, 78) | 54 (30, 78) | 53.5 (30, 78) | |
| BMI (kg/m2): | ||||
| 18.5–24.9 | 13 (14.9) | 6 (13.3) | 7 (16.7) | .873 |
| 25–29.9 | 41 (47.1) | 21 (46.7) | 20 (47.6) | |
| ≥30 | 33 (37.9) | 18 (40) | 15 (35.7) | |
| Mean (SD) | 29.0 (4.4) | 29.2 (4.2) | 28.8 (4.8) | .670 |
| Median (range) | 27.8 (20.7, 44.7) | 27.9 (21.9, 38.3) | 27.7 (20.7, 44.7) | |
| Not current smoker | 87 (100) | 45 (100) | 42 (100) | NA |
| CAD | 3 (3.4) | 2 (4.4) | 1 (2.4) | 1.0 |
| HTN | 29 (33.3) | 17 (37.8) | 12 (28.6) | .327 |
| Dyslipidemia | 18 (20.7) | 8 (17.8) | 10 (23.8) | .487 |
| DM | 9 (10.3) | 4 (8.9) | 5 (11.9) | .733 |
| No. of comorbidities | ||||
| 0 | 48 (55.2) | 22 (48.9) | 26 (61.9) | .082 |
| 1 | 24 (27.6) | 17 (37.8) | 7 (16.7) | |
| 2+ | 15 (17.2) | 6 (13.3) | 9 (21.4) | |
| Hemoglobin A1C at baseline (%) | ||||
| 4–5.6% (normal) | 55 (63.2) | 28 (62.2) | 27 (64.3) | .841 |
| 5.7–6.4% (pre-diabetes) | 25 (28.7) | 14 (31.1) | 11 (26.2) | |
| 6.5% or higher (diabetes) | 7 (8) | 3 (6.7) | 4 (9.5) | |
| Mean (SD) | 5.5 (0.6) | 5.6 (0.6) | 5.5 (0.6) | .609 |
| Median (range) | 5.5 (4.2, 7.4) | 5.5 (4.2, 7.2) | 5.4 (4.5, 7.4) | |
| T-level (ng/dL) | ||||
| Mean (SD) | 513.8 (155.6) | 524.1 (158.3) | 502.8 (153.9) | .527 |
| Median (range) | 488 (307, 998) | 486 (307, 998) | 491.5 (314, 929) | |
| PDE-5i response | (n = 42) | (n = 20) | (n = 22) | |
| No | 10 (23.8) | 5 (25.0) | 5 (22.7) | 1.00 |
| Yes | 32 (76.2) | 15 (75.0) | 17 (77.3) | |
| ED | ||||
| Mild (IIEF-EF scores 17–25) | 60 (69) | 32 (71.1) | 28 (66.7) | .654 |
| Moderate (IIEF-EF scores 11–16) | 27 (31) | 13 (28.9) | 14 (33.3) |
BMI = body mass index; CAD = coronary artery disease; DM = diabetes mellitus; ED = erectile dysfunction; HTN = hypertension; IIEF-EF = International Index of Erectile Function-Erectile Function domain; NA = not applicable; PDE-5i = phosphodiesterase type 5 inhibitor.
Group A: 5 daily treatments (720 shocks/d). Group B: 3 times a week for 2 weeks (600 shocks/d).
P: P-value from the chi-square test, the Fisher's exact test, or the 2-sample Student's t test.
Regarding safety of therapy, all patients underwent their respective treatment schedule without development of any adverse events. Adverse events were reported at 6 months follow-up with no patients reporting pain, edema, bruising, hematuria, penile curvature development, or lower urinary tract symptoms. However, 2 patients (5%) in Group A did develop worsening erectile function.
Table 2 provides a univariable logistic regression and identifies potential predictors of MCID in IIEF-EF at 6 months. The 6-month follow-up included 40 patients in each group due to patient attrition. 1 patient was not included in Group B for EHS changes from baseline due to lack of questionnaire response. Mean improvement in IIEF-EF score at 6 months was 2.7 (95% CI = 1.2–4.2; P < .001) and 2.7 (95% CI = 1.3–4.1; P < .001) for Groups A and B, respectively. Overall, 52.5% of patients reached MCID in IIEF-EF at 6 months, 57.5% and 47.5% in Group A and Group B, respectively (P = .502). ED was defined as none, mild, moderate, and severe. For Group A, 71.1% and 28.9% patients had mild and moderate ED at baseline, respectively. After 6 months, 62.5% and 12.5% of patients had mild and moderate ED, respectively, with 20% reporting no ED and the remaining 5% reporting severe ED. Similarly, for Group B, 66.7% and 33.3% patients had mild and moderate ED at baseline, respectively. After 6 months, 60% and 20% of patients had mild and moderate ED, respectively, with the remaining 20% reporting no ED. For patients with a baseline EHS score of 1–2 (inability to have penetrative intercourse), 76.9% (10 out of 13) and 90.9% (10 out of 11) reached an EHS score of 3–4 (ability to have penetrative intercourse) at 6 months follow-up for Group A and Group B, respectively (P = .596).
Table 2.
IIEF-EF and EHS scores and related outcomes
| Baseline |
1 month |
3 months |
6 months |
|||||
|---|---|---|---|---|---|---|---|---|
| Group A | Group B | Group A | Group B | Group A | Group B | Group A | Group B | |
| IIEF-EF | ||||||||
| N | 45 | 42 | 45 | 42 | 41 | 41 | 40 | 40 |
| Mean | 18.1 | 18.0 | 20.6 | 19.3 | 19.9 | 20.6 | 21.0 | 20.8 |
| 95% CI | (17.1, 19.1) | (16.9, 19.2) | (18.9, 22.3) | (17.9, 20.6) | (18.1, 21.6) | (19.2, 22) | (19.4, 22.6) | (19.3, 22.2) |
| Range | (11, 25) | (11, 25) | (11, 30) | (9, 28) | (3, 30) | (10, 30) | (7, 30) | (12, 30) |
| IIEF-EF change from baseline | ||||||||
| N | NA | NA | 45 | 42 | 41 | 41 | 40 | 40 |
| Mean | NA | NA | 2.5 | 1.2 | 1.7 | 2.4 | 2.7 | 2.7 |
| 95% CI | NA | NA | (1.1, 4) | (−0.1, 2.6) | (0.3, 3.1) | (1.2, 3.7) | (1.2, 4.2) | (1.3, 4.1) |
| Range | NA | NA | (−8, 14) | (−8, 9) | (−12, 12) | (−4, 12) | (−10, 10) | (−8, 14) |
| MCID in IIEF-EF, n (%) | ||||||||
| Yes | NA | NA | 25 (55.6) | 14 (33.3) | 20 (48.8) | 21 (51.2) | 23 (57.5) | 19 (47.5) |
| No | NA | NA | 20 (44.4) | 28 (66.7) | 21 (51.2) | 20 (48.8) | 17 (42.5) | 21 (52.5) |
| ED, n (%) | ||||||||
| No | - | - | 12 (26.7) | 4 (9.5) | 7 (17.1) | 5 (12.2) | 8 (20.0) | 8 (20.0) |
| Mild | 32 (71.1) | 28 (66.7) | 21 (46.7) | 27 (64.3) | 23 (56.1) | 28 (68.3) | 25 (62.5) | 24 (60.0) |
| Moderate | 13 (28.9) | 14 (33.3) | 12 (26.7) | 10 (23.8) | 9 (22.0) | 7 (17.1) | 5 (12.5) | 8 (20.0) |
| Severe | - | - | - | 1 (2.4) | 2 (4.9) | 1 (2.4) | 2 (5.0) | - |
| EHS | ||||||||
| N | 45 | 42 | 45 | 42 | 41 | 41 | 40 | 39 |
| Mean | 2.6 | 2.7 | 3.2 | 3 | 3.1 | 3 | 3.2 | 3.2 |
| 95% CI | (2.4, 2.8) | (2.5, 2.9) | (2.9, 3.4) | (2.8, 3.2) | (2.9, 3.3) | (2.8, 3.2) | (2.9, 3.4) | (2.9, 3.4) |
| Range | (1, 4) | (1, 3) | (1, 4) | (1, 4) | (1, 4) | (2, 4) | (0, 4) | (1, 4) |
| EHS change from baseline | 45 | 42 | 41 | 41 | 40 | 39 | ||
| N | NA | NA | 0.6 | 0.3 | 0.5 | 0.3 | 0.6 | 0.5 |
| Mean | NA | NA | (0.3, 0.8) | (0.1, 0.5) | (0.2, 0.8) | (0, 0.5) | (0.3, 0.8) | (0.2, 0.8) |
| 95% CI | NA | NA | (−1, 2) | (−1, 2) | (−1, 3) | (−1, 2) | (−1, 2) | (−2, 3) |
| Range | NA | NA | 45 | 42 | 41 | 41 | 40 | 39 |
| MCID in EHS, n (%) | ||||||||
| N | NA | NA | 15 | 12 | 13 | 11 | 13 | 11 |
| Yes | NA | NA | 12 (80.0) | 8 (66.7) | 8 (61.5) | 9 (81.8) | 10 (76.9) | 10 (90.9) |
| No | NA | NA | 3 (20.0) | 4 (33.3) | 5 (38.5) | 2 (18.2) | 3 (23.1) | 1 (9.1) |
ED = erectile dysfunction; EHS = Erectile Hardness Scale; IIEF-EF = International Index of Erectile Function-Erectile Function domain; MCID = minimal clinically important difference; NA = not applicable.
Group A: 5 daily treatments (720 shocks/d). Group B: 3 times a week for 2 weeks (600 shocks/d).
MCID in IIEF-EF was defined as increase of ≥2 for patients with baseline mild ED (baseline IIEF-EF score of 17–25), and ≥5 for patients with baseline moderate ED (score of 11–16).
MCID in EHS was defined as a change to score 3 or 4, among patients with baseline scores of 1 or 2.
ED categories based on the IIEF-EF score (range 0–30): scores 26–30, no; 17–25, mild; 11–16, moderate; 0–10, severe ED.
EHS range 0–4.
MCID in IIEF-EF within 6 months were 71% (32/45) and 59.5% (25/42) in Groups A and B, respectively, and the difference between groups was not statistically significant (P = .270).
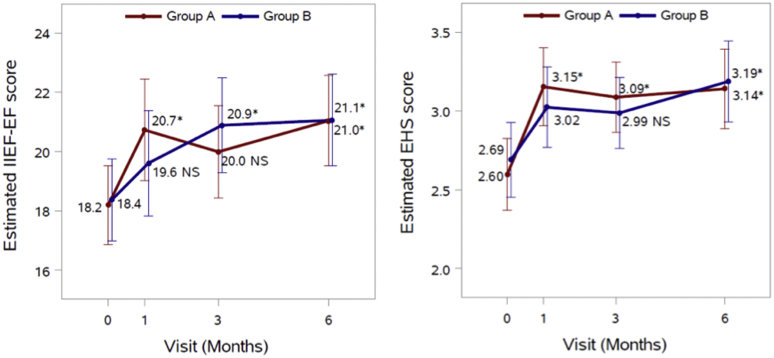
In Figure 2, for each outcome we fit a repeated measures analysis of variance model including group, visit time, and their interaction, with adjustment for age (<60, ≥60), BMI (18.5–24.9, 25–29.9, ≥30), number of comorbidities (0, 1, 2+), and T-level. The estimated mean scores from the fitted repeated measures models presented in Figure 2 were very similar to the observed mean scores in Table 2. Overall, for both type of scores, there was no statistically significant difference between treatment groups at any visit time (P > .05), there was a significant effect of time (P < .0001), and the group × time interaction was not statistically significant (P = .076 for IIEF-EF and P = .437 for EHS). Without multiple comparison adjustment, the mean scores at the 3-month follow-up visit were all statistically significantly higher than the mean score at baseline (P < .0001). After applying Bonferroni's correction for multiple comparisons, 2 comparisons to baseline became statistically non-significant (P > .05) as indicated in Figure 2. As per Table 3, both Group A and B were combined to identify predictors of reaching MCID in IIEF-EF at 6 months. Patient age, comorbidities, testosterone level, treatment group, or baseline IIEF-EF predicted MCID at 6 months. This may be due to the limited number of patients included in the study.
Figure 2.
Estimated means with corresponding 95% CI for IIEF-EF and EHS scores. Asterisks (∗) represent statistically significant difference between baseline and the specific follow-up visit (P < .05). NS = not significant (P > .05) difference.
Table 3.
Univariable logistic regression: potential predictors of MCID in IIEF-EF at 6 months
| Total patients |
MCID in IIEF-EF at 6 months |
Or (95% CI) | P | ||||
|---|---|---|---|---|---|---|---|
| N | No |
Yes |
|||||
| N | % | N | % | ||||
| All | 80 | 38 | 47.5 | 42 | 52.5 | ||
| Group | |||||||
| Group A | 40 | 17 | 42.5 | 23 | 57.5 | 1.50 (0.62, 3.61) | .371 |
| Group B | 40 | 21 | 52.5 | 19 | 47.5 | Reference | |
| Age, in years | |||||||
| <60 | 58 | 25 | 43.1 | 33 | 56.9 | 1.91 (0.70, 5.16) | .204 |
| ≥60 | 22 | 13 | 59.1 | 9 | 40.9 | Reference | |
| 1-year increase | - | - | - | - | - | 0.97 (0.93, 1.01) | .104 |
| BMI (m/kg2) | |||||||
| 18.5–24.9 | 13 | 5 | 38.5 | 8 | 61.5 | 1.49 (0.39, 5.67) | .556 |
| 25–29.9 | 38 | 19 | 50.0 | 19 | 50.0 | 0.93 (0.35, 2.45) | .889 |
| ≥30 | 29 | 14 | 48.3 | 15 | 51.7 | Reference | |
| 18.5–24.9 | 13 | 5 | 38.5 | 8 | 61.5 | 1.55 (0.46, 5.24) | .478 |
| ≥25 | 67 | 33 | 49.3 | 34 | 50.7 | Reference | |
| HTN | |||||||
| No | 55 | 25 | 45.5 | 30 | 54.5 | 1.30 (0.50, 3.35) | .587 |
| Yes | 25 | 13 | 52.0 | 12 | 48.0 | Reference | |
| Dyslipidemia | |||||||
| No | 64 | 28 | 43.8 | 36 | 56.3 | 2.14 (0.69, 6.61) | .185 |
| Yes | 16 | 10 | 62.5 | 6 | 37.5 | Reference | |
| DM | |||||||
| No | 73 | 33 | 45.2 | 40 | 54.8 | 3.03 (0.55, 16.64) | .202 |
| Yes | 7 | 5 | 71.4 | 2 | 28.6 | Reference | |
| Hemoglobin A1C at baseline | |||||||
| Normal | 51 | 20 | 39.2 | 31 | 60.8 | 2.54 (0.99, 6.48) | .052 |
| Pre-diabetes/diabetes | 29 | 18 | 62.1 | 11 | 37.9 | Reference | |
| No. of comorbidities | |||||||
| 0 | 34 | 13 | 38.2 | 21 | 61.8 | 2.49 (0.70, 8.81) | .158 |
| 1 | 34 | 17 | 50.0 | 17 | 50.0 | 1.20 (0.29, 4.93) | .800 |
| 2+ | 12 | 8 | 66.7 | 4 | 33.3 | Reference | |
| Comorbidities Y or N | |||||||
| 0 | 34 | 13 | 38.2 | 21 | 61.8 | 2.22 (0.90, 5.49) | .083 |
| 1+ | 46 | 25 | 54.3 | 21 | 45.7 | Reference | |
| ED | |||||||
| Mild | 56 | 26 | 46.4 | 30 | 53.6 | 1.15 (0.44, 3.01) | .769 |
| Moderate | 24 | 12 | 50.0 | 12 | 50.0 | Reference | |
BMI = body mass index; HTN = hypertension; DM = diabetes mellitus; ED = erectile dysfunction; IIEF-EF = International Index of Erectile Function-Erectile Function domain; MCID = minimal clinically important difference; OR = odds ratio.
Pre-diabetes: HbA1C: 5.7–6.4%.
MCID was defined as increase in IIEF-EF total score of ≥2 for patients with baseline mild ED (baseline IIEF-EF score of 17–25), and ≥5 for patients with baseline moderate ED (score of 11–16).
95% CI: 95% CI for OR. P: P-value from Wald's test.
OR estimates from univariable logistic regression modeling the probability of MCID = “Yes” at 6 months.
A multivariable analysis was conducted testing all the variables listed in this table, using stepwise model selection with entry criteria of 50% and retention criteria of 10%. Given the small sample size and data sparseness, only A1C at baseline was retained in the model.
Discussion
We demonstrate a statistically significant improvement in IIEF-EF after Li-SWT for ED treatment with an effective response at 6 months follow-up independent of treatment arm. Age, comorbidity status, baseline erectile function (mild vs moderate), and serum testosterone was not predictive of reaching MCID. With respect to Li-SWT safety, all patients completed their respective treatment protocol with no patients reporting pain, bruising, penile curvature development, hematuria, etc. However, 2 patients did develop worsening erectile function, although this may have secondary to further progression of their ED.
The detailed mechanism of how Li-SWT affects erectile function is yet to be fully understood but numerous well-conducted rodent studies have provided the framework to the mechanism of action.13,14 The best supported mechanism is via stimulation of angiogenesis and restoration of blood flow via upregulation of proangiogenic factors, such as vascular endothelial growth factor and endothelial nitric oxide synthase.1 Other mechanisms include promotion of recruitment of endogenous progenitor cells and activation of Schwann cells, which has the theoretical potential for nerve generation as demonstrated in rat models with pelvic neurovascular injuries.15 Tissue response to Li-SWT is dependent on factors which include energy flux density, number of shocks, frequency of device, treatment frequency, and interval. We selected the MoreNova device specifically because it was designed to generate shockwaves that are then focused in a geometrically matched manner to the shape of the corpora and crura. Additionally, we chose to investigate treatment protocols requiring fewer shockwaves over a shorter time duration, than prior studies, to better delineate the minimal duration of treatment with clinical response. The treatment schedules were varied by time frame due to studies demonstrating an association between patient adherence and satisfaction, economic costs, and treatment time frame.16,17
Since the initial trial by Vardi et al, several meta-analyses have been published demonstrating an improvement in IIEF-EF after Li-SWT.6,14,18, 19, 20, 21 As most randomized controlled trials have been small with short follow-up, there exists significant limitations in these studies. Although prior studies have demonstrated an increase in IIEF after Li-SWT, it is important to consider what increase in IIEF-EF is clinically meaningful. 2 double-blinded clinical trials have shown no significant difference with respect to placebo groups.22,23 Although in one study improvements did occur in the treatment group, they were not significant when compared to the sham arm.22 In a different study, investigators found higher improvements in IIEF scores within the sham arm group.23 Therefore, to find true clinical significance, we assessed MCID as defined as an increase in IIEF of ≥2 for patients with baseline mild ED (IIEF score of 17–25), and ≥5 for patients with baseline moderate ED (IIEF score of 11–16). At 6 months, 57.5% and 47.5% of patients reached MCID in Group A and Group B, respectively. Our results are consistent with prior studies which demonstrate a clinically significant improvement in IIEF-EF after Li-SWT.24,25 Our study had a modest follow-up of 6 months which demonstrated an effective response. A recent study by Kitrey et al found 53.5% of patients had a sustained response to Li-ESWT after 2 years. Non-diabetic patients with mild ED had the most pronounced response with 76% having a preserved response to therapy after 2 years.26 On univariate analysis we identified no predictors of reaching MCID when combing both Groups A and B. Further studies are required to identify ideal candidates for this treatment modality.
Both the American Urological Association and Sexual Medicine Society of North America do not recommend the use of Li-SWT for the treatment of ED outside an investigational setting.2,27 As such, further studies are required to determine the long-term efficacy and safety of Li-SWT for the treatment of ED. The strengths of our study include the investigator-blind randomized design, 2 varying treatment protocols, use of clinically meaningful IIEF-EF differences, evaluation of therapeutic safety, and a month of PDE-5i wash-out. There are currently only 2 other randomized control trials investigating various LiSWT protocols, and both have shown comparable findings to this study.24,25 The limitations of this phase II clinical trial study include the modest sample size, short follow-up, lack of a sham arm, stringent exclusion and inclusion criteria, and the inability to exclude psychogenic ED. Further studies investigating the treatment yield of Li-SWT on moderate ED patients vs mild ED patients in regard to MCID would elucidate whether the small increases in the IIEF-EF score are clinically relevant. Confounders such as patient lifestyle, exercise, and alcohol may have also introduced bias. Additionally, some selection bias may exist, as some of the patients did not present to the 6-month follow-up. Lastly, there was a lack of a standardized measurement for penile curvature and pain assessment score within the trial.
The lack of a placebo control arm may limit the conclusions on efficacy of treatment; however, the study was designed to test treatment schedules for safety and efficacy as they are being utilized on patients in clinics currently. Although prior PDE5-i usage and efficacy status were recorded, further studies should also evaluate the concurrent utilization of Li-SWT and continuing medication use. A phase III study is warranted to test these treatment schedules, which have been shown to be efficacious, against a placebo control.
Conclusion
Our study found no difference in outcomes when Li-SWT 3,600 shocks were delivered over 1 or 2 weeks at 6 months follow-up and both schedules were safe with no adverse events during or after treatment. Further trials with longer follow-up and a sham arm will provide valuable information regarding treatment efficacy and durability.
Statement of authorship
Category 1
-
(a)Conception and Design
- Premal Patel; Jonathan Katz; Soum D. Lokeshwar; Raul Clavijo; Ranjith Ramasamy
-
(b)Acquisition of Data
- Premal Patel; Jonathan Katz; Soum D. Lokeshwar; Manuel Molina; Isildinha M. Reis
-
(c)Analysis and Interpretation of Data
- Premal Patel; Jonathan Katz; Soum D. Lokeshwar; Isildinha M. Reis; Raul Clavijo; Ranjith Ramasamy
Category 2
-
(a)Drafting the Article
- Premal Patel; Jonathan Katz; Soum D. Lokeshwar; Raul Clavijo; Ranjith Ramasamy
-
(b)Revising It for Intellectual Content
- Premal Patel; Jonathan Katz; Soum D. Lokeshwar; Manuel Molina; Isildinha M. Reis; Raul Clavijo; Ranjith Ramasamy
Category 3
-
(a)Final Approval of the Completed Article
- Premal Patel; Jonathan Katz; Soum D. Lokeshwar; Manuel Molina; Isildinha M. Reis; Raul Clavijo; Ranjith Ramasamy
Footnotes
Conflict of Interest: Ranjith Ramasamy has served as a consultant for Coloplast, has been an investigator and a member of the advisory board of Boston Scientific, Endo, and Aytu Biosciences, and has been an investigator for Direx. Premal Patel has served as a consultant for Boston Scientific and Nestle Health and has been a member of the advisory board of Aytu Biosciences.
Funding: Investigator initiated grant from Direx and was supported by the Barton Weiss Men's Health Initiative.
References
- 1.NIH Consensus Conference. Impotence. NIH Consensus development Panel on Impotence. JAMA. 1993;270:83–90. [PubMed] [Google Scholar]
- 2.Burnett A.L., Nehra A., Breau R.H. Erectile dysfunction: AUA guideline. J Urol. 2018;200:633–641. doi: 10.1016/j.juro.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Young Academic Urologists Men's Health G. Fode M., Hatzichristodoulou G., Serefoglu E.C. Low-intensity shockwave therapy for erectile dysfunction: is the evidence strong enough? Nat Rev Urol. 2017;14:593–606. doi: 10.1038/nrurol.2017.119. [DOI] [PubMed] [Google Scholar]
- 4.Scott S., Roberts M., Chung E. Platelet-rich Plasma and treatment of erectile dysfunction: Critical review of Literature and Global Trends in Platelet-Rich Plasma clinics. Sex Med Rev. 2019;7:306–312. doi: 10.1016/j.sxmr.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Torjesen I., journalist, London The cricketer promoting shockwave therapy for erectile dysfunction. BMJ. 2016;354:i4808. doi: 10.1136/bmj.i4808. [DOI] [PubMed] [Google Scholar]
- 6.Clavijo R.I., Kohn T.P., Kohn J.R. Effects of low-intensity extracorporeal shockwave therapy on erectile dysfunction: a systematic review and meta-analysis. J Sex Med. 2017;14:27–35. doi: 10.1016/j.jsxm.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Reisman Y., Hind A., Varaneckas A. Initial experience with linear focused shockwave treatment for erectile dysfunction: a 6-month follow-up pilot study. Int J impotence Res. 2015;27:108–112. doi: 10.1038/ijir.2014.41. [DOI] [PubMed] [Google Scholar]
- 8.Ruffo A., Capece M., Prezioso D. Safety and efficacy of low intensity shockwave (LISW) treatment in patients with erectile dysfunction. Int Braz J Urol. 2015;41:967–974. doi: 10.1590/S1677-5538.IBJU.2014.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cappelleri J.C., Rosen R.C., Smith M.D. Diagnostic evaluation of the erectile function domain of the International index of erectile function. Urology. 1999;54:346–351. doi: 10.1016/s0090-4295(99)00099-0. [DOI] [PubMed] [Google Scholar]
- 10.Rosen R.C., Allen K.R., Ni X. Minimal clinically important differences in the erectile function domain of the International Index of Erectile Function scale. Eur Urol. 2011;60:1010–1016. doi: 10.1016/j.eururo.2011.07.053. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein I., Lue T.F., Padma-Nathan H. Oral sildenafil in the treatment of erectile dysfunction. Sildenafil Study Group. New Engl J Med. 1998;338:1397–1404. doi: 10.1056/NEJM199805143382001. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen H.M.T., Gabrielson A.T., Hellstrom W.J.G. Erectile dysfunction in young men-A review of the Prevalence and Risk factors. Sex Med Rev. 2017;5:508–520. doi: 10.1016/j.sxmr.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Liu T., Shindel A.W., Lin G. Cellular signaling pathways modulated by low-intensity extracorporeal shock wave therapy. Int J impotence Res. 2019;31:170–176. doi: 10.1038/s41443-019-0113-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sokolakis I., Hatzichristodoulou G. Clinical studies on low intensity extracorporeal shockwave therapy for erectile dysfunction: a systematic review and meta-analysis of randomised controlled trials. Int J impotence Res. 2019;31:177–194. doi: 10.1038/s41443-019-0117-z. [DOI] [PubMed] [Google Scholar]
- 15.Li H., Matheu M.P., Sun F. Low-energy shock wave therapy Ameliorates erectile dysfunction in a pelvic neurovascular Injuries rat model. J Sex Med. 2016;13:22–32. doi: 10.1016/j.jsxm.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Plumb J.M., Guest J.F. Annual cost of erectile dysfunction to UK Society. Pharmacoeconomics. 1999;16:699–709. doi: 10.2165/00019053-199916060-00008. [DOI] [PubMed] [Google Scholar]
- 17.Normansell R., Kew K.M., Stovold E. Interventions to improve adherence to inhaled steroids for asthma. Cochrane Database Syst Rev. 2017;4:CD012226. doi: 10.1002/14651858.CD012226.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu Z., Lin G., Reed-Maldonado A. Low-intensity extracorporeal shock wave treatment Improves erectile function: a systematic review and meta-analysis. Eur Urol. 2017;71:223–233. doi: 10.1016/j.eururo.2016.05.050. [DOI] [PubMed] [Google Scholar]
- 19.Man L., Li G. Low-intensity extracorporeal shock wave therapy for erectile dysfunction: a systematic review and meta-analysis. Urology. 2018;119:97–103. doi: 10.1016/j.urology.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 20.Vardi Y., Appel B., Jacob G. Can low-intensity extracorporeal shockwave therapy improve erectile function? A 6-month follow-up pilot study in patients with organic erectile dysfunction. Eur Urol. 2010;58:243–248. doi: 10.1016/j.eururo.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Zou Z.J., Tang L.Y., Liu Z.H. Short-term efficacy and safety of low-intensity extracorporeal shock wave therapy in erectile dysfunction: a systematic review and meta-analysis. Int Braz J Urol. 2017;43:805–821. doi: 10.1590/S1677-5538.IBJU.2016.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olsen A.B., Persiani M., Boie S. Can low-intensity extracorporeal shockwave therapy improve erectile dysfunction? A prospective, randomized, double-blind, placebo-controlled study. Scand J Urol. 2015;49:329–333. doi: 10.3109/21681805.2014.984326. [DOI] [PubMed] [Google Scholar]
- 23.Fojecki G.L., Tiessen S., Osther P.J. Effect of low-energy linear shockwave therapy on erectile dysfunction-A double-blinded, sham-controlled, randomized clinical trial. J Sex Med. 2017;14:106–112. doi: 10.1016/j.jsxm.2016.11.307. [DOI] [PubMed] [Google Scholar]
- 24.Kalyvianakis D., Memmos E., Mykoniatis I. Low-intensity shockwave therapy for erectile dysfunction: a randomized clinical trial comparing 2 treatment protocols and the impact of repeating treatment. J Sex Med. 2018;15:334–345. doi: 10.1016/j.jsxm.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Kalyvianakis D., Mykoniatis I., Memmos E. Low-intensity shockwave therapy (LiST) for erectile dysfunction: a randomized clinical trial assessing the impact of energy flux density (EFD) and frequency of sessions. Int J impotence Res. 2019;16:1478–1480. doi: 10.1038/s41443-019-0185-0. [DOI] [PubMed] [Google Scholar]
- 26.Kitrey N.D., Vardi Y., Appel B. Low intensity shock wave treatment for erectile dysfunction-how long does the effect last? J Urol. 2018;200:167–170. doi: 10.1016/j.juro.2018.02.070. [DOI] [PubMed] [Google Scholar]
- 27.Patel P., Huang C., Molina M. Clinical trial update on shockwave therapy and future of erectile function restoration. Int J impotence Res. 2019;31:206–208. doi: 10.1038/s41443-019-0115-1. [DOI] [PubMed] [Google Scholar]