Abstract
Introduction
Sexual activity is important for marital quality, especially in cervical cancer survivors. Vagina extension following laparoscopic radical hysterectomy with bilateral ovarian preservation (VEOP), vagina extension following laparoscopic radical hysterectomy with bilateral oophorectomy (VEBO), radical hysterectomy with bilateral ovarian preservation (RHOP), and radical hysterectomy with bilateral oophorectomy (RHBO) are the common surgeries for young cervical cancer patients.
Aim
To investigate the effect of the 4 surgical methods on female/male sexual activity and marital quality in early-stage cervical cancer survivors.
Methods
Multiple linear regression analysis was conducted in 205 patients with stage Ia1–IIa2 cervical cancer to evaluate the factors that affected male/female sexual function and marital quality.
Main Outcome Measure
Female Sexual Function Index (FSFI), modified Kupperman Index (KI), modified Sexual Life Quality Questionnaire (mSLQQ-QoL), and ENRICH marital inventory were used to reflect changes in female/male sexual function and marital quality in the 4 groups.
Results
Female/male sexual function and marital quality were both highest in the VEOP group and lowest in the RHBO group. The regression results showed that ovarian preservation and vaginal extension were associated with female/male sexual function and marital quality. Furthermore, when vaginal extension and ovarian preservation were replaced by vaginal length, sexual psychological change, and hormone level index (KI), respectively; male sexual function was associated with vaginal length and sexual psychological change, whereas female sexual function and marital quality were only associated with hormone level and sexual psychological change. Clinical statistics found that four-fifths of the recurrent patients had vaginal extension (P = .042), and 3-quarters of these patients had large tumors.
Conclusion
Ovarian preservation and vaginal extension are both important for male/female sexual activity and marital quality. Vaginal extension may play a positive role in female sexual life via psychology and in male sexual life via vaginal length. Vaginal extension may not be suitable for patients with large masses.
Zhang Y, Sun S, Ding J, et al. The Effect of Different Surgical Methods on Female and Male Sexual Activity and Marital Quality in Patients With Early-Stage Cervical Cancer. Sex Med 2020;8:307–314.
Key Words: Cervical Cancer, Vaginal Extension, Female Sexual function, Marital Quality
Introduction
Although cervical cancer is the 4th most common female cancer in the world, the number of cases has continuously declined in regions where screening programs have been implemented. However, in resource-poor areas, where screening programs are not well established, cervical cancer continues to affect and kill hundreds of thousands of women. In China, about 130,000 cases of cervical cancer are identified each year.1 The mean age at diagnosis is 44.7 years, which is 5–10 years younger than mean ages reported before 2000 in China. Early-stage diagnoses are most common in these patients: 57.3% are stage I, 33.9% are stage II, and 4.3% are stage III or IV.2 For early-stage (stage I and II) cervical cancer patients, the 5-year survival rate is as high as 90–92%.1 Thus, these younger patients have significant additional life expectancy after treatment completion and therefore face years of potential treatment-related side effects.
Radical surgery and radiotherapy are the most common therapies for cervical cancer patients.3 These treatments may cause problems such as loss of fertility, earlier menopause, and sexual problems.4, 5, 6 In their systematic review, Ye et al conclude that vaginal dryness, dyspareunia, short vagina, and sexual dissatisfaction are prominent types of sexual dysfunction and vaginal change in cervical cancer survivors (CCSs).7 In couples, the quality of sexual function in one partner predicts the other partner's sexual quality. Female sexual arousal and overall sexual satisfaction are important factors that contribute to good sexual functioning in men.8
Marital quality is defined in terms of several dimensions, including positive and negative aspects of marriage, attitudes, and behaviors and interaction patterns.9 One of the most important factors that affect marital quality is a safe and pleasurable sexual relationship.10 A meta-analysis by Robles et al, which included 72,000 participants in studies across 126 published papers spanning half a century, indicated that greater marital quality is related to better physical health (particularly cardiovascular reactivity during marital conflict discussions), regardless of study design, marital quality measure, and publication year.9 The effects of marital functioning on physiology may be stronger for women than for men.11 Moreover, sexual activity mediates the association between self and partner's physical health and positive marital quality.12
These previous findings indicate that it would make sense for there to be an effect on marriage from cervical cancer via sexual function. Thus, it is imperative to improve sexual function in CCSs, particularly young patients. Jiang et al reported that the vaginal extension surgical process can improve sexual function.13 The most common surgical treatment methods for cervical cancer patients in our hospital are vagina extension following laparoscopic radical hysterectomy with bilateral ovarian preservation (VEOP), vagina extension following laparoscopic radical hysterectomy with bilateral oophorectomy (VEBO), radical hysterectomy with bilateral ovarian preservation (RHOP), and radical hysterectomy with bilateral oophorectomy (RHBO). In this study, we compared the effect of these 4 surgical methods on marital quality and male and female sexual function to identify possible ways to improve the sexual function of CCSs.
Materials and methods
Patient Recruitment and Data Collection
The patient recruitment procedure was almost identical to that described by Jiang et al.13 Briefly, 205 (50 received VEOP, 9 received VEBO, 84 received RHOP, and 62 received RHBO) patients with International Federation of Gynecology and Obstetrics (FIGO 2009) stage Ia1 (with lymphovascular space invasion or positive margins for carcinoma, determined by specimens from conization of the cervix) to IIa2 cervical cancer who had received laparoscopic surgery at the authors' university hospital, China, between January 2013 and December 2015 were recruited after providing informed consent.
The criteria for recruitment and inclusion were 1) preoperative pathological diagnosis of cervical cancer; 2) regular follow-up; 3) heterosexually active and intending to be so after recovery from surgery; 4) negative history of previous gynecologic operations and the absence of pelvic adhesion as confirmed during the operation; and 5) negative vaginal margins as confirmed by intraoperative frozen pathological evaluation; 6) nonfertility sparing. All eligible patients were given a detailed explanation of the surgical procedure and the possible risks and benefits of the 4 surgical methods. If the patient was younger than 45 years and the preoperative imaging examination showed no ovarian involvement, OP and transposition was recommended to her before surgery; this was performed if consent was given and if a gross ovarian biopsy was found to be negative during surgery. If the patient was menopausal, ovarian removal was recommended to her before the operation or recommended during the operation if a gross ovarian biopsy was positive. If the patient was older than 45 years and was menstruating regularly, the patient made the final decision about ovarian removal. All patients made the final decision about whether to have a vagina extension. Modified LRH (type B, following Ref.14) was performed on 8 patients with FIGO stage Ia1 cervical cancer coupled with positive margin, and LRH (type C, following Ref.14) was performed on the other patients at FIGO stages Ia2 to IIa2 (FIGO 2009) cervical cancer. LRH and pelvic lymphadenectomy were performed as described in Ref.15 and the uterus was removed through the vaginal canal after RH. The vagina cuff was closed with a running locking suture for patients who had not chosen vagina extension. For patients who required vagina extension, the vagina extension procedure was a slightly modified peritoneal vaginoplasty, as described by Rangaswamy et al.16 Briefly, the rectouterine peritoneum was sutured to the posterior vaginal wall when the uterovesical peritoneum was sutured to the anterior vaginal wall. Then a purse-string stitch was performed with the peritoneum of the bladder and on the surface of the rectum 4–5 cm above the vaginal cuff after suturing the peritoneum on both sides. After this procedure, a 10-cm long and 3-cm wide soft vagina were made.
None of the participants received any neoadjuvant chemotherapy or radiotherapy before the surgery. Informed consent was obtained before the operation. Postoperative adjuvant chemotherapy, radiotherapy, or both were recommended to patients according to NCCN guidelines.13 All patients were followed up to at least 1 year after surgery. Vagina length measurement was performed after vault dehiscence was excluded after a vaginal examination with a speculum. A sterile cotton swab was inserted into the apex of the vagina gently without any tension, and a mark was made at the vaginal outlet. Finally, a Vernier caliper was used to measure the distance from the tip of the swab to the mark, which was the length of the vagina.
Questionnaires
The Female Sexual Function Index (FSFI), the ENRICH marital inventory, and the modified Kupperman Index (KI) were administered within 1 week before surgery and 1 year after surgery; the Modified Sexual Life Quality Questionnaire (SLQQ-QoL) was administered 1 year after surgery.
The FSFI is a well-validated, self-report multiple-choice questionnaire that assesses key dimensions or domains of sexual function and quality of life in women. A Chinese version of the scale has been validated.17 The FSFI comprises 6 domains: genital desire, arousal, lubrication, orgasm, sexual satisfaction, and pain. We added one question to investigate sexual psychological change before and after surgery: (Before surgery) How would you evaluate your level of confidence in your physical ability to have a full sexual life before vaginal sexual activity? 0 = I am suffering from cervical disease and cannot have a full sexual life; 1 = I am suffering from cervical disease and most of the time my sexual life is affected; 2 = I am suffering from cervical disease and my sexual life is occasionally affected; 3 = Although I am suffering from cervical disease, it does not affect my sexual life. (After surgery) How would you evaluate your level of confidence in your physical ability to have a full sexual life before vaginal sexual activity? 0 = I am suffering from cervical disease and cannot have a full sexual life, although I have had surgery; 1 = I am suffering from cervical disease and most of the time my sexual life is affected, although I have had surgery; 2 = I am suffering from cervical disease and since the surgery, my sexual life is occasionally affected; 3 = Although I am suffering from cervical disease, since the surgery, my sexual life has not been affected.
The Chinese version of the ENRICH marital inventory, originally created by Olson, was used to describe marital dynamics.18 The scale comprises 124 items and 12 subscales. Items are rated on a 5-point Likert scale (1 = agree, 2 = somewhat agree, 3 = neither agree nor disagree, 4 = somewhat disagree, 5 = disagree). Scores on each subscale range from 10 to 50 points; a score of 50 is the most positive outcome. To meet the research objectives, we used three 30-item subscales: Marital Satisfaction, Communication, and Sexual Relationship.
The modified KI was used to assess ovarian function. It consists of 13 items19: urinary infections, sexual complaints, sweating/hot flushes, palpitations, vertigo, headaches, paresthesia, formication, arthralgia, myalgia (categorized as somatic symptoms), fatigue, nervousness, and melancholia (categorized as psychological symptoms). Complaint severity is rated on a scale ranging from 0 to 3. The weighting factors were the same as those used in the original KI.20 The total score ranges from 0 to 63 and is calculated as the sum of all items by the weighting factor. Score ranges of 0–6, 7–15, 16–30, and >30 represented the degree of severity as none, mild, moderate, and severe, respectively.
The Sexual Life Quality Questionnaire (SLQQ) is a validated, multidimensional instrument that consists of 2 domains: Sexual quality of life (QoL) (10 questions) and Treatment satisfaction (6 questions).21 The SLQQ-QoL domain compares the patient's current sexual experience with their experience before the onset of the patient's ED. We modified this domain to compare male sexual partners' current sexual experience with their experience before their female sexual partner's surgery. We named this measure as mSLQQ-QoL. The score range is 10–90 points: score from 10 to 90 represents the worst to the best outcome and 50 indicates no change in sexual experience before and after surgery.
Statistical Analysis
All statistical analyses were carried out using the Statistical Package for Social Sciences (SPSS) 22.0. P values of 0.05 were considered statistically significant. The count data are presented in terms of numbers and percentages, and the differences between the 4 surgical groups were tested using the chi-square test. The measurement data are presented in terms of means and standard deviations. The difference between the 4 surgical groups was tested using analysis of variance and independent t-tests for 2 groups (the vaginal extension following radical hysterectomy [VERH] younger than 45 vs radical hysterectomy groups [RH] younger than 45); the nonconformity distribution is presented with the median and the range values, and the difference between the 4 surgical groups was tested using nonparametric statistical methods. Male/female sexual life and marriage quality scores before and after operation were the final evaluation index. A linear regression analysis was carried out to evaluate which factors affected male and female sexual function and marital quality.
Results
Clinicopathological and Surgical Data in the 4 Groups
As Table 1 shows, the average age of participants in the ovary removal groups was higher than that of those in the ovary preservation groups. Patients who chose vagina extension were relatively young. There were fewer patients in the vagina extension with ovary resection group (VEBO group) than in the other 3 groups, and their average age was 44.73 years. Menstruation status also differed across the 4 groups. The proportion of stage II cases was higher in the RHBO group (Table 1). The other clinicopathological parameters, such as length of resected vaginal wall, and postoperative adjuvant therapy, were comparable across the 4 groups.
Table 1.
Clinical and pathological characteristics of patients in 4 surgery groups
| Variable | VEOP N = 50 | RHOP N = 84 | VEBO N = 9 | RHBO N = 62 | P value |
|---|---|---|---|---|---|
| Age (years) | 36.36 ± 4.34 | 38.92 ± 4.87 | 44.73 ± 4.62 | 51.69 ± 7.29 | .000 |
| Menstruation | .000 | ||||
| Regular | 49 (98%) | 83 (97.6%) | 6 (75.0%) | 19 (30.6%) | |
| Irregular | 1 (2.0%) | 2 (2.4%) | 0 (0.0%) | 7 (11.3%) | |
| Menopause | 0 (0%) | 0 (0%) | 2 (25.0%) | 36 (58.1%) | |
| FIGO stage | .002 | ||||
| Ia1 | 0 (0.0%) | 9 (10.6%) | 0 (0.0%) | 0 (0.0%) | |
| Ia2 | 1 (2.0%) | 5 (5.9%) | 0 (0.0%) | 0 (0.0%) | |
| Ib1 | 36 (72.0%) | 49 (57.6%) | 6 (75.0%) | 34 (54.8%) | |
| Ib2 | 6 (12.0%) | 10 (11.8%) | 1 (12.5%) | 5 (8.1%) | |
| IIa1 | 4 (8.0%) | 5 (5.9%) | 0 (0.0%) | 17 (27.4%) | |
| IIa2 | 3 (6.0%) | 7 (8.2%) | 1 (12.5%) | 6 (9.7%) | |
| Operating time (min) | 255.22 ± 48.74 | 230.67 ± 44.09 | 237.33 ± 10.72 | 217.31 ± 69.80 | .003 |
| Amount of blood loss (mL) | 276.00 ± 231.95 | 291.29 ± 167.94 | 293.75 ± 56.3 | 249.65 ± 96.03 | .505 |
| Length of resected vaginal wall (cm) | |||||
| At 12 o'clock | 2.53 ± 0.67 | 2.62 ± 0.93 | 2.69 ± 0.39 | 2.60 ± 0.72 | .920 |
| At 3 o'clock | 2.65 ± 0.68 | 2.69 ± 0.90 | 2.55 ± 0.92 | 2.49 ± 0.64 | .444 |
| At 6 o'clock | 3.48 ± 0.89 | 3.22 ± 1.06 | 3.60 ± 1.12 | 3.13 ± 0.80 | .178 |
| At 9 o'clock | 2.54 ± 0.61 | 2.71 ± 0.90 | 2.79 ± 0.90 | 2.44 ± 0.67 | .127 |
| Hospital stay(days) | 18.62 ± 5.47 | 16.96 ± 4.42 | 17.50 ± 4.47 | 17.94 ± 5.12 | .285 |
| Postoperative adjuvant therapy | .160 | ||||
| None | 34 (68.0%) | 50 (58.8%) | 4 (50.0%) | 35 (56.5%) | |
| Chemotherapy only | 0 (0.0%) | 8 (9.4%) | 0 (0.0%) | 3 (4.8%) | |
| Radiotherapy only | 0 (0.0%) | 7 (8.2%) | 1 (12.5%) | 3 (4.8%) | |
| Chemoradiotherapy | 16 (32.0%) | 20 (23.5%) | 3 (37.5%) | 21 (33.9%) | |
| Length of vagina measured 1 year after surgery (cm) | 7.24 ± 0.66 | 5.12 ± 0.64 | 7.51 ± 0.33 | 4.69 ± 0.57 | .000 |
| Number of recurrence by the last follow-up | 3 (6.0%) | 1 (1.2%) | 1 (12.5%) | 0 (0.0%) | .042 |
| Number of death by the last follow-up | 0 (0.0%) | 1 (1.2%) | 0 (0.0%) | 0 (0.0%) | .701 |
| Postoperative sexual life | .000 | ||||
| Yes | 42 (84.0%) | 68 (80.0%) | 4 (50.0%) | 18 (29.0%) | |
| No | 8 (16.0%) | 17 (20.0%) | 4 (50.0%) | 44 (71.0%) | |
| Divorce after surgery | 3 (6.0%) | 2 (2.4%) | 0 (0.0%) | 1 (1.6%) | .498 |
According to the different operating times for the 4 groups (Table 1), we calculated the vagina extension time as 20–25 min (VEBO−RHBO = 20 min, VEOP−RHOP = 25 min) and the ovary preservation time as 13–18 min (RHOP−RHBO = 13 min, VEOP−VEBO = 18 min). Although ovary preservation and vagina extension take more time, they do not increase blood loss or hospital stay. Notably, a total of 5 patients recurred, 4 of whom had vaginal extension. Of the 4 recurrent patients with vaginal extension, 2 were Ib2 and one was IIa2 (FIGO 2009). These data indicate that vaginal extension may not be suitable for patients with large masses.
Factors Affecting Male and Female Sexual Function and Marital Quality
According to the previous reports, female sex function was affected by age and menstruation.22, 23, 24 As shown in Table 1, these 2 factors differed across the 4 groups. To eliminate any bias of age and menstrual status, we compared FSFI and ENRICH scores before and after surgery and used the reduction in scores as comparison indexes. A linear regression analysis was conducted using the parameters in Table 1 in patients with postoperative sexual life,adding ovary preservation vs no ovary preservation and vagina extension vs no vagina extension. The factors that differed across the 4 groups (age, menstruation, and cancer stage) were not associated with FSFI score reduction. Only ovary preservation and vagina extension were associated with FSFI score reduction (P < .001 and <0.001, respectively) and the standardized coefficients beta values were 0.796 and 0.376, respectively (Table 2). This indicates that ovary preservation may play a more important role than vagina extension in female sexual function. From Table 1, we calculated the ratio of patients who had no sexual life decreased by preserving ovary as 34–51% (VEBO-VEOP = 34%, RHBO-RHOP = 51%) and decreased by vagina extension as 4–21% (RHOP-VEOP = 4%, RHBO-VEBO = 21%). This calculation quantified the advantages of retaining ovaries in improving female sexual function than vaginal extension further.
Table 2.
Results of linear regression analysis of FSFI score reduction, ENRICH score reduction, and mSLQQ-QoL score
| Dependent variable Model |
FSFI score reduction |
ENRICH score reduction |
mSLQQ-QoL score |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Standardized coefficients beta | t | P | Standardized coefficients beta | t | P | Standardized coefficients beta | t | P | |
| Constant | −6.779 | .000 | −9.450 | .000 | 31.605 | .000 | |||
| Extend vagina or not | .376 | 11.925 | .000 | .452 | 12.319 | .000 | −.539 | −13.504 | .000 |
| Preserve ovary or not | .796 | 19.731 | .000 | .691 | 14.705 | .000 | −.607 | −11.868 | .000 |
| Age | .020 | .469 | .640 | .025 | .492 | .624 | −.042 | −.759 | .450 |
| FIGO stage | −.059 | −1.094 | .276 | −.007 | −.113 | .910 | −.101 | −1.472 | .144 |
| Menstruation | .027 | .608 | .544 | .041 | .798 | .426 | .043 | .767 | .444 |
| Length of resected vaginal wall | |||||||||
| At 12 o'clock | .005 | .154 | .878 | .044 | 1.172 | .244 | .057 | 1.392 | .167 |
| At 3 o'clock | −.008 | −.181 | .857 | .052 | 1.078 | .283 | −.001 | −.024 | .981 |
| At 6 o'clock | .003 | .059 | .953 | −.069 | −1.380 | .170 | .092 | 1.695 | .093 |
| At 9 o'clock | −.060 | −1.466 | .145 | .042 | .891 | .375 | .013 | .256 | .798 |
| Adjuvant therapy | .006 | .173 | .863 | .045 | 1.123 | .264 | −.016 | −.366 | .715 |
FSFI = Female Sexual Function Index; mSLQQ-QoL = modified Sexual Life Quality Questionnaire.
Vagina extension leads to changes in vaginal length, and ovary preservation leads to changes in ovarian function. Moreover, this gynecological surgery may cause sexual psychological change. Thus, we changed ovary preservation and vagina extension into KI score reduction, vaginal length and sexual psychological change in the linear regression. Only KI score reduction and sexual psychological change were associated with FSFI score reduction which means vaginal length may not be important for female sexual function (Table 3). All these aforementioned findings indicate that ovary preservation plays a more important role in female sexual function and that vagina extension may affect FSFI scores via sexual psychological change.
Table 3.
Results of linear regression analysis of FSFI score reduction, ENRICH score reduction, and mSLQQ-QoL score when ovary preservation and vaginal extension were replaced with KI score reduction, vaginal length, and sexual psychological change
| Dependent variable Model |
FSFI score reduction |
ENRICH score reduction |
mSLQQ-QoL score |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Standardized coefficients beta | t | P | Standardized coefficients beta | t | P | Standardized coefficients beta | t | P | |
| Constant | −.240 | .811 | −1.595 | .114 | 17.361 | .000 | |||
| Age | .109 | 1.640 | .104 | .114 | 1.610 | .110 | −.087 | −1.364 | .175 |
| FIGO stage | −.006 | −.068 | .946 | .029 | .323 | .747 | −.126 | −1.566 | .120 |
| Menstruation | .112 | 1.681 | .096 | .126 | 1.761 | .081 | −.004 | −.058 | .953 |
| Length of resected vaginal wall | |||||||||
| At 12 o'clock | .041 | .831 | .408 | .078 | 1.456 | .148 | .028 | .588 | .557 |
| At 3 o'clock | −.059 | −.939 | .350 | .016 | .233 | .816 | .006 | .097 | .923 |
| At 6 o'clock | .072 | 1.136 | .258 | −.038 | −.556 | .579 | .082 | 1.339 | .183 |
| At 9 o'clock | −.084 | −1.345 | .181 | .040 | .602 | .549 | .006 | .095 | .925 |
| Adjuvant therapy | .000 | −.009 | .993 | .043 | .753 | .453 | −.012 | −.231 | .818 |
| Length of vagina | −.002 | −.037 | .971 | −.089 | −1.409 | .162 | .140 | 2.454 | .016 |
| Psychological changes | .214 | 3.039 | .003 | .239 | 3.165 | .002 | −.285 | −4.182 | .000 |
| KI score reduction | .611 | 8.513 | .000 | .478 | 6.204 | .000 | −.493 | −7.095 | .000 |
FSFI = Female Sexual Function Index; KI = Kupperman Index; mSLQQ-QoL = modified Sexual Life Quality Questionnaire.
In the same analysis methods as FSFI reduction, it indicated that ovary preservation and vagina extension were associated with ENRICH reduction (P < .001 and <0.001, respectively) and mSLQQ-QoL score (P < .001 and <0.001, respectively) (Table 2). When ovary preservation and vagina extension were replaced with KI score reduction, vaginal length, and sexual psychological change, all these 3 factors were associated with mSLQQ-QoL score, but only KI score reduction and sexual psychological change were associated with ENRICH reduction (Table 3). Taken together, ovary preservation and vagina extension both improve marital quality and male sexual life. Vaginal length is a useful correlate of mSLQQ-QoL score index, although ovarian function may be more important.
Marital Quality Changes Across the 4 Groups
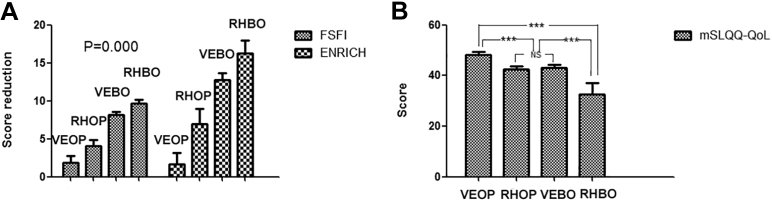
As described in Tables 1 and 2, some important clinicopathological and surgical parameters were inconsistent across the 4 groups, but they did not affect male/female sexual function or marital quality. Thus, we compared these 4 groups to identify the best surgical method in terms of marital quality. Both the FSFI score reduction and ENRICH score reduction were lowest in the VEOP group, followed by the RHOP, VEBO, and RHBO groups (FSFI: 1.890 < 4.10 < 8.200 < 9.690, respectively, P = .000; ENRICH: 1.690 < 7.000 < 12.750 < 16.310, P = .000) (Figure 1A). The mSLQQ-QoL score was highest in the VEOP group (48.210 ± 1.3170), and lowest in RHBO groups (32.670 ± 4.4980) (Figure 1B). There was no difference in divorce rate among the 4 groups (P = .498) (Table 1). The FSFI score reduction was mainly focused in the pain, lubrication, and desire domain, whereas the ENRICH score reduction was mainly in the sexual life factor (see Supplementary Tables 1–3). Taken together, these findings suggest that ovary preservation with vagina extension is the best way to improve postoperative male and female sexual function and marital quality.
Figure 1.
The FSFI and ENRICH score reductions and mSLQQ-QoL score in 4 groups. (A) FSFI and ENRICH score reductions were both significantly different in each groups. P values calculated between any 2 groups were <0.001. (B) mSLQQ-QoL score was significantly different in 4 groups. ∗∗∗Statistically highly significant, that is, P < .001; NS, statistically not significant, that is, P > .05. FSFI = Female Sexual Function Index; mSLQQ-QoL = modified Sexual Life Quality Questionnaire.
Discussion
Our study suggests that ovary preservation with vagina extension has the greatest effect on sexual function improvement in men and women, as well as marital quality and vaginal extension may play a positive role in female sexual life via psychology and in male sexual life via vaginal length. The vaginal sensory nerves are mainly concentrated in the lower two-thirds of the vagina; the upper third of the vagina, which is the area that is surgically removed, contains very few sensory nerves.25 Thus, the controversial G-spot structure,26 located in the distal third of the anterior vaginal wall, is preserved; this area is highly sensitive and involved in climax. This indicates that the upper third of the vagina that is removed during surgery has little effect on female orgasm. Therefore, the artificial vaginal extension of this part may not directly affect female sexual function. In fact, female sexual function is a combination of multiple factors, including physical, psychological, environmental, and genetic factors. Psychological factors have a large influence on sexual function.24
A couple's sexual life is mutually influential.8 Women's rejection of sexual life often conveys a negative message to men, leading to a decline in male sexual function. Male orgasm is usually accompanied by ejaculation, and men prefer to insert deeply during ejaculation. This may be because the vaginal opening is narrower than the upper part of the vagina. During ejaculation, the vaginal opening can place more pressure on the root of the penis, enhancing sexual pleasure.27 Thus, a short vagina will reduce the male orgasm experience, and consistent with our conclusions, the length of the vagina plays an important role in male sexual life.
Therefore, for patients who wish to engage in sexual behavior after surgery, we should perform vagina extension; however, this procedure should be avoided in patients with large masses (>4 cm) as shown in our data. Tumor cells in large masses are more likely to remain. These tumor cells may arise from preoperative or laparoscopic procedures, including the process of clamping the cervix to remove the uterus from the vagina. The peritoneal cavity has an important anti-tumor effect.28 If vagina extension is performed, some residual tumor cells that should be located in the abdominal cavity will be enclosed in the artificial vagina. As a result, this part of the residual tumor cells will not get the anti-tumor effect of peritoneal cavity, which may increase recurrence. Vagina extension is suitable for patients whose tumor diameter is less than 4 cm.
Another mechanism that affects sexual function is ovary removal. Oophorectomy also leads to surgical menopause. Greater impairment of sexual function may be associated with surgical versus natural menopause. In natural menopause, estrogen levels are low, but ovarian androgen output is maintained at premenopausal levels.29 In the PRESIDE study, surgical, but not natural, menopause was associated with orgasm problems and decrease in arousal was greater in women after surgical menopause.30 Our results support this conclusion and indicate that its effect is greater than vaginal elongation. Therefore, if possible, ovaries should be retained in patients with functional ovaries and no ovarian removal indications. If the ovaries cannot be retained, hormone replacement therapy (HRT) should be recommended.
Improving the sexual life of CCSs requires comprehensive management. In addition to the choice of surgical methods, postoperative management is very important. Many patients are afraid to resume their sexual life after surgery, mainly owing to treatment side effects and anxiety that sex would damage their surgical sites. Such anxiety is a result of an insufficient understanding of the disease. Patients should be guided to resume their sexual life during the follow-up process. In fact, medical workers have not done enough to address the issue of postsexual life concerns. A survey conducted by Sarah Bedell of members of the Society of Gynecologic Oncology revealed that only approximately 23% received training about sexual dysfunction.31
Unfortunately, the study limitations include (1) limited sample size, (2) nonrandomized design, (3) inability to compare the sex hormone level to the presurgery and postsurgery, and this study is an exploratory analysis. Larger controlled and long-term studies with a robust study design are required to confirm the results.
Conclusions
In summary, our results suggest that ovary preservation with vagina extension has the greatest effect on sexual function improvement in men and women, as well as marital quality, although OP may play a more important role. HRT should be given to patients who cannot retain their ovaries and are not contraindicated for HRT. If possible, vagina extension should not be used for patients with large masses. During follow-up, medical workers should pay more attention to patients' sexual lives, and partners should be included in the care of such patients.
Statement of authorship
Category 1
-
(a)Conception and design
- Jingxin Ding; Keqin Hua
-
(b)Acquisition of data
- Yunqiang Zhang; Shugen Sun; Jingxin Ding, Keqin Hua
-
(c)Analysis and interpretation of data
- Yunqiang Zhang; Shugen Sun
Category 2
-
(a)Drafting the article
- Yunqiang Zhang; Shugen Sun
-
(b)Revising it for intellectual content
- Keqin Hua
Category 3
-
(a)Final approval of the completed article
- Keqin Hua
Acknowledgments
The authors thank the women who participated in this study for sharing their experiences so generously. The authors thank Diane Williams, PhD, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Footnotes
Yunqiang Zhang and Shugen Sun are contributed equally to this work.
Conflict of Interest: The authors report no conflicts of interest.
Funding: This work was funded by a grant from Shanghai Hospital Development Center (Grant/Award Number: SHDC12015117, PI: Keqin Hua).
Supplementary data related to this article can be found at https://doi.org/10.1016/j.esxm.2020.02.001.
Supplementary data
References
- 1.Tian J. Sexual well-being of cervical cancer survivors under 50 years old and the factors affecting their libido. Gynecol Obstet Invest. 2013;76:177–181. doi: 10.1159/000355104. [DOI] [PubMed] [Google Scholar]
- 2.Li S., Hu T., Lv W. Changes in prevalence and clinical characteristics of cervical cancer in the People's Republic of China: a study of 10,012 cases from a nationwide working group. Oncologist. 2013;18:1101–1107. doi: 10.1634/theoncologist.2013-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncol. 2008;26:5802–5812. doi: 10.1200/JCO.2008.16.4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burns M., Costello J., Ryan-Woolley B. Assessing the impact of late treatment effects in cervical cancer: an exploratory study of women's sexuality. Eur J Cancer Care (Engl) 2007;16:364–372. doi: 10.1111/j.1365-2354.2006.00743.x. [DOI] [PubMed] [Google Scholar]
- 5.Kim W.Y., Chang S.J., Chang K.H. Treatment patterns and outcomes in bulky stage IB2 cervical cancer patients: a single institution's experience over 14 years. Gynecol Obstet Invest. 2011;71:19–23. doi: 10.1159/000320722. [DOI] [PubMed] [Google Scholar]
- 6.Plotti F., Terranova C., Capriglione S. Assessment of quality of life and urinary and sexual function after radical hysterectomy in long-term cervical cancer survivors. Int J Gynecol Cancer. 2018;28:818–823. doi: 10.1097/IGC.0000000000001239. [DOI] [PubMed] [Google Scholar]
- 7.Ye S., Yang J., Cao D. A systematic review of quality of life and sexual function of patients with cervical cancer after treatment. Int J Gynecol Cancer. 2014;24:1146–1157. doi: 10.1097/IGC.0000000000000207. [DOI] [PubMed] [Google Scholar]
- 8.Yeoh S.H., Razali R., Sidi H. The relationship between sexual functioning among couples undergoing infertility treatment: a pair of perfect gloves. Compr Psychiatry. 2014;55(Suppl 1):S1–S6. doi: 10.1016/j.comppsych.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Robles T.F., Slatcher R.B., Trombello J.M. Marital quality and health: a meta-analytic review. Psychol Bulletin. 2014;140:140–187. doi: 10.1037/a0031859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiecolt-Glaser J.K., Newton T.L. Marriage and health: his and hers. Psychol Bulletin. 2001;127:472–503. doi: 10.1037/0033-2909.127.4.472. [DOI] [PubMed] [Google Scholar]
- 11.Galinsky A.M., Waite L.J. Sexual activity and psychological health as mediators of the relationship between physical health and marital quality. J Gerontol B Psychol Sci Soc Sci. 2014;69:482–492. doi: 10.1093/geronb/gbt165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang H., Zhu J., Guo S.W. Vaginal extension improves sexual function in patients receiving laparoscopic radical hysterectomy. Gynecologic Oncol. 2016;141:550–558. doi: 10.1016/j.ygyno.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Querleu D., Morrow C.P. Classification of radical hysterectomy. Lancet Oncol. 2008;9:297–303. doi: 10.1016/S1470-2045(08)70074-3. [DOI] [PubMed] [Google Scholar]
- 14.Nezhat C.R., Burrell M.O., Nezhat F.R. Laparoscopic radical hysterectomy with paraaortic and pelvic node dissection. Am J Obstet and Gynecol. 1992;166:864–865. doi: 10.1016/0002-9378(92)91351-a. [DOI] [PubMed] [Google Scholar]
- 15.Rangaswamy M., Machado N.O., Kaur S. Laparoscopic vaginoplasty: using a sliding peritoneal flap for correction of complete vaginal agenesis. Eur J Obstet Gynecol Reprod Biol. 2001;98:244–248. doi: 10.1016/s0301-2115(01)00313-x. [DOI] [PubMed] [Google Scholar]
- 16.Sun X., Li C., Jin L. Development and validation of Chinese version of female sexual function index in a Chinese population-a pilot study. J Sex Med. 2011;8:1101–1111. doi: 10.1111/j.1743-6109.2010.02171.x. [DOI] [PubMed] [Google Scholar]
- 17.Wang K., Li J., Zhang J.X. Psychological characteristics and marital quality of infertile women registered for in vitro fertilization-intracytoplasmic sperm injection in China. Fertil Steril. 2007;87:792–798. doi: 10.1016/j.fertnstert.2006.07.1534. [DOI] [PubMed] [Google Scholar]
- 18.Tao M., Shao H., Li C. Correlation between the modified Kupperman index and the menopause rating scale in Chinese women. Patient Prefer Adherence. 2013;7:223–229. doi: 10.2147/PPA.S42852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kupperman H.S., Blatt M.H., Wiesbader H. Comparative clinical evaluation of estrogenic preparations by the menopausal and amenorrheal indices. J Clin Endocrinol Metab. 1953;13:688–703. doi: 10.1210/jcem-13-6-688. [DOI] [PubMed] [Google Scholar]
- 20.Woodward J.M., Hass S.L., Woodward P.J. Reliability and validity of the sexual life quality questionnaire (SLQQ) Quality Life Res. 2002;11:365–377. doi: 10.1023/a:1015513228469. [DOI] [PubMed] [Google Scholar]
- 21.Dennerstein L., Alexander J.L., Kotz K. The menopause and sexual functioning: a review of the population-based studies. Annu Rev Sex Res. 2003;14:64–82. [PubMed] [Google Scholar]
- 22.Leiblum S.R., Koochaki P.E., Rodenberg C.A. Hypoactive sexual desire disorder in postmenopausal women: US results from the Women's International Study of Health and Sexuality (WISHeS) Menopause. 2006;13:46–56. doi: 10.1097/01.gme.0000172596.76272.06. [DOI] [PubMed] [Google Scholar]
- 23.Avis N.E., Stellato R., Crawford S. Is there an association between menopause status and sexual functioning? Menopause. 2000;7:297–309. doi: 10.1097/00042192-200007050-00004. [DOI] [PubMed] [Google Scholar]
- 24.Brotto L., Atallah S., Johnson-Agbakwu C. Psychological and Interpersonal dimensions of sexual function and dysfunction. J Sex Med. 2016;13:538–571. doi: 10.1016/j.jsxm.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 25.Puppo V., Puppo G. Anatomy of sex: Revision of the new anatomical terms used for the clitoris and the female orgasm by sexologists. Clinical Anatomy. 2015;28:293–304. doi: 10.1002/ca.22471. [DOI] [PubMed] [Google Scholar]
- 26.Li T., Liao Q., Zhang H. Anatomic distribution of nerves and microvascular density in the human anterior vaginal wall: prospective study. PLoS One. 2014;9:e110239. doi: 10.1371/journal.pone.0110239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alwaal A., Breyer B.N., Lue T.F. Normal male sexual function: emphasis on orgasm and ejaculation. Fertil Steril. 2015;104:1051–1060. doi: 10.1016/j.fertnstert.2015.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sedlacek A.L., Gerber S.A., Randall T.D. Generation of a dual-functioning antitumor immune response in the peritoneal cavity. Am J Pathol. 2013;183:1318–1328. doi: 10.1016/j.ajpath.2013.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang G., Basaria S., Travison T.G. Testosterone dose-response relationships in hysterectomized women with or without oophorectomy: effects on sexual function, body composition, muscle performance and physical function in a randomized trial. Menopause. 2014;21:612–623. doi: 10.1097/GME.0000000000000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shifren J.L., Monz B.U., Russo P.A. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112:970–978. doi: 10.1097/AOG.0b013e3181898cdb. [DOI] [PubMed] [Google Scholar]
- 31.Bedell S., Manders D., Kehoe S. The opinions and practices of providers toward the sexual issues of cervical cancer patients undergoing treatment. Gynecol Oncol. 2017;144:586–591. doi: 10.1016/j.ygyno.2016.12.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.