Abstract
Introduction
Peyronie's disease (PD) is a connective tissue disorder of the penis characterized by an abnormality in collagen structure of penile tunica albuginea.
Aim
We sought to investigate the prevalence, risk factors, and the relationship between erectile dysfunction (ED) and PD in male patients aged 30–80 years seeking urological care.
Methods
This is a cross-sectional study using data collected from October 2016 to October 2017 in an outpatient clinic associated with the Brazilian Public Health System. All men aged 30 to 80 years were invited to participate. Data collected were related to the clinical history and sexual habits of patients using the International Index of Erectile Function, in addition to the physical examination of the penis and laboratory parameters.
Main Outcome Measure
Descriptive statistics and multivariate logistic regression models tested the prevalence, risk factors, and the relationship between ED and PD in male patients.
Results
The study included 656 individuals, who were distributed as per age, marital status, race, educational level, and income. Of these participants, 86 (13.11%) presented with fibrous plaques compatible with PD at the physical examination. Among the risk factors evaluated, PD was associated with diabetes, smoking, and obesity in 43.02, 64.17, and 26.74% of patients, respectively. The presence of penile plaques compatible with PD was more prevalent in men with ED, history of penile trauma, and complaint of penile deformity. There was a higher prevalence of plaques in the distal penis.
Conclusion
The PD among the studied population was associated with risk factors such as diabetes, smoking, and obesity. Other clinical characteristics, such as history of penile trauma, penile deformity, and ED, were reported in patients with PD. There was a higher prevalence of plaques in the distal penis, specifically in the corona of the glans penis. The prevalence of PD was different from that in the published literature, our results show that numbers thus more studies are needed.
Segundo A, Glina S. Prevalence, Risk Factors, and Erectile Dysfunction Associated With Peyronie's Disease Among Men Seeking Urological Care. Sex Med 2020;8:230–236.
Key Words: Peyronie's Disease, Risk Factors, Erectile Dysfunction, Fibrosis, Penis
Introduction
Peyronie's disease (PD) is a connective tissue disorder of the penis characterized by an abnormality in the collagen structure of the penile tunica albuginea. This disorder may promote the formation of a fibrous plaque containing a considerable amount of collagen, then modifying elastin framework and fibroblastic proliferation. It can result in alterations in penile anatomy such as penile deformity, a palpable lump in the penis, penile pain during erection, and erectile dysfunction (ED).1
According to several authors, PD diagnosis does not necessarily require a complaint as the reason for seeking medical care. A classic study conducted by Smith2 established a prevalence of PD of 22% when analyzing penile plaques in autopsies, regardless of symptoms. Most patients seek medical care because of penile deformity and/or pain during erection. However, the presence of penile plaques is enough to characterize PD.3 The minimum requirements for diagnosis of PD include an assessment of clinical history and a comprehensive physical examination of the genitalia.4
Several risk factors have been associated with PD, including hypertension, smoking, diabetes, and hyperlipidemia, and some of them may aggravate the symptoms in patients. Studies have demonstrated an important association between a more rigid plaque and poor metabolic control of diabetes in PD.5,6
Repetitive penile trauma during sexual intercourse would cause lesions in the tunica albuginea with extravasation of blood, which would lead to fibrosis by modifying the mechanism of scar formation. In a recent study, Cohen et al7 were able to trigger alterations in the extracellular matrix similar to that found in PD after the instillation of blood in the tunica albuginea tissue of rats, which showed increased expression of transforming growth factor-β; matrix metallopeptidase 9, heparanase and biglycan associated with decreased expression of syndecan-1 and aggrecan, suggesting modifications in extracellular matrix remodeling in relation to the physiopathology of PD.7
Despite several studies, the incidence and prevalence rates of PD are still unclear. Such rates vary depending on each study, methodology, and population studied. Prevalence rates ranging from 0.39 to 22.5% have been reported in previous studies, which depend on the sampling methodology used. Table 1 shows epidemiological studies on the prevalence of PD based on the methodology and the population studied.8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21
Table 1.
Epidemiological studies on the prevalence of Peyronie's disease
| Authors and year | Country | Evaluation | Age range | Prevalence (%) |
|---|---|---|---|---|
| Lindsay et al, 19918 | USA | General population | Not specified | 0.38 |
| Schwarzer et al, 20019 | Germany | General population | 30–80 years | 3.2 |
| La Pera et al, 200110 | Italy | Smokers | 50–60 years | 7.2 |
| Rhoden et al, 200111 | Brazil | Prostate cancer screening | 52–77 years | 3.67 |
| Mulhall et al, 200412 | USA | Prostate cancer screening | 40–80 years | 8.9 |
| Kadioglu A. et al, 200413 | Turkey | Erectile dysfunction | Not specified | 16 |
| El-Sakka 200614 | Egypt | Erectile dysfunction | Not specified | 7.9 |
| Arafa et al, 200615 | Egypt | Diabetic patients and ED | Not specified | 20.3 |
| Tefekli et al, 200616 | Turkey | Diabetic patients | Not specified | 22.5 |
| Akbal et al, 200817 | Turkey | Retroperitoneal fibrosis | Not specified | 20 |
| Dibenedetti et al, 201118 | USA | General population | >18 years | 0.5 to 13 |
| Shiraishi 201219 | Japan | General population and men undergoing hemodialysis | Not specified | 0.6 to 9.2 |
| Romero et al, 201320 | Brazil | Prostate cancer screening | >40 years | 0.9 |
| Stuntz et al, 201621 | USA | General population | >18 years | 10.8 |
ED = erectile dysfunction.
There are few studies in the Brazilian literature to characterize the epidemiology of PD. Among these studies, Rhoden et al11 reported a prevalence of PD of 3.67% in men older than 50 years living in the city of Porto Alegre, Southern Brazil. In another survey conducted by Romero et al20, the presence of fibrosis suggestive of PD was reported in 0.9% of the studied population, especially in men older than 60 years and diabetic patients living in Curitiba. The objective of this study was to identify the prevalence of PD and presence of penile plaque in male patients aged 30–80 years attending a general urology outpatient clinic in the Northeast region of Brazil, independent of the complaint.
Methods
The study was carried out following the regulations governing human subject research and was evaluated and approved by the Research Ethics Committee of ABC Medical School. This is a prospective cross-sectional study carried out from October 2016 to October 2017 in an outpatient clinic associated with the Brazilian Public Health System in the city of Patos, in the state of Paraiba, Brazil. In this clinic, patients seeking urological care in several areas of urology are attended regardless of gender and age, characterizing it as a general urology outpatient clinic.
Population
All men aged 30 to 80 years attending the urology outpatient clinic, independent of the complaint, were invited to participate in the study. Exclusion criteria were patients who did not agree or did not undergo some procedure of the study, were not able to answer the questionnaire, or did not accept to be examined.
A total of 2,108 urological consultations were performed during the data collection period, including consultations and return visits of patients, regardless of age. Inclusion criteria were all men aged 30 to 80 years who attended the urology outpatient clinic during the given period of data collection. Exclusion criteria were subjects who did not agree or did not perform any stage of the investigative processes, did not have the ability to answer the questionnaire, or did not agree to be examined. The studied population was selected, with 666 men aged 30 to 80 years by using inclusion criteria, and 10 men did not agree to be examined. A total of 656 men were included to participate.
Initially, patients were assessed for age, marital status, race, education level, family income, and main complaint (grouped in asymptomatic, penile pain, penile curvature, sexual dysfunction, and other complaints). The antecedents (diabetes, obesity, hypertension, dyslipidemia, and Dupuytren's disease), lifestyle (alcohol use, smoking, and drug use), and the regular use of medication were evaluated.
Patients were asked about several aspects of sexual habits, such as numbers of sexual activities, condom use, lubricant use, masturbation frequency, sexual position preferences, most frequent penetration type (vaginal or anal), if the patient have ever had or is having sexual intercourse with animals, age at the beginning of sexual life and sexual preferences, history of penile trauma and complaint of some penile deformity, and, if present, for how many years. The validated International Index of Erectile Function questionnaire was applied for quantifying ED in each age-group and men with and without PD.22
For the study, the diagnosis of diabetes was based on self-report and medical diagnosis, use of hypoglycemic drugs, or fasting blood glucose level ≥ 126 mg/dL. Patients with dyslipidemia were diagnosed by self-report and medical diagnosis, use of medication, or when blood cholesterol values were ≥240 mg/dL or blood triglycerides values were ≥200 mg/dL. Body weight and height were evaluated for classification of obesity, and the body mass index (BMI) was calculated by dividing the patient's body weight by the square of the height. Patients with a BMI between 18.5 and < 25 were classified as eutrophic, whereas those with BMI between 25 and 30 were classified as overweight, and men with BMI ≥30 were classified as obese.
Smoking behavior was classified as nonsmokers, ex-smokers, or current smokers. The number of packs of cigarettes smoked per year was calculated for both current smokers and ex-smokers.
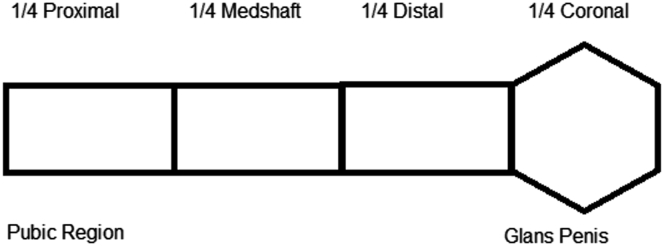
All patients underwent a physical examination of the genitalia to identify fibrous plaques, independent of the complaint. After exposure of the genitalia, with the patient in the dorsal decubitus position, the penile shaft was visually inspected and bimanually palpated, extending from the pubic region to the ventral and dorsal glans penis for assessment of hardened areas and sensitivity. Individuals were diagnosed with PD (if fibrous plaques were present) or without PD. In addition, the plaque was classified based on the location, number, and pain in the affected area in patients classified as having PD. The presence and location of penile plaques were evaluated based on their distribution in 4 regions: one-fourth proximal, one-fourth midshaft, one-fourth distal, and one-fourth distal (coronal), in addition to the sensitivity and perception by the patient with plaque (Figure 1).
Figure 1.
Schematic drawing of the location of the penile plaque.
Statistical Analyses
The data were analyzed descriptively using absolute and relative frequencies. A chi-square test was used for inferential analysis. The dependent variable (group 0, without PD and group 1, with PD) and independent variables (diabetes, obesity, smoking, and age range) were included in the regression model. The prevalence ratio was calculated using multiple Poisson regression (PR) analysis with robust variance and stepwise forward strategy for variable entry.23,24 The significance level was set at P < .05. The program used for analysis was Stata 12.
Results
The study included 656 individuals, who were distributed based on age range, marital status, race, educational level, and income. Because it is a general urology outpatient clinic, the reasons for urologic consultation in most patients (96.07%) were not sexually related (Table 2). Of these participants, 86 (13.11%) had PD, whereas 570 patients (86.89%) did not present any penile fibrous plaque. Of the 86 patients in whom penile plaques were identified, only 2 presented with complaint of penile curvature as the reason for seeking urologic care. The highest prevalence of PD was observed in men older than 50 years; however, the group with plaque had fewer individuals in the 30–40, 41–50, and 71–80 age-groups than the group without fibrous plaques (Table 2). In this study, the prevalence of PD was 13.11% among the 656 patients interviewed. When stratified by age, it was possible to observe a higher prevalence of individuals in the age-group 41–50 (17.89%) years, followed by men in the age-groups of 61–70 and 51–60 years (Figure 2).
Table 2.
Sociodemographic data of patients
| Variable | Without PD |
With PD |
All patients | P value |
|---|---|---|---|---|
| (n = 570) n (%) | (n = 86) n (%) | |||
| Patients | ||||
| 30–80 years | 570 (86.89) | 86 (13.11) | 656 (100) | |
| Age range | ||||
| 30–40 years | 62 (10.88) | 3 (3.49) | 65 (9.91) | |
| 41–50 years | 101 (17.72) | 22 (25.58) | 123 (18.75) | |
| 51–60 years | 159 (27.89) | 26 (30.23) | 185 (28.20) | |
| 61–70 years | 158 (27.72) | 28 (32.56) | 186 (28.35) | |
| 71–80 years | 90 (15.79) | 7 (8.14) | 97 (14.79) | |
| Race | ||||
| White | 278 (48.77) | 37 (43.02) | 315 (48.02) | |
| Black | 92 (16.14) | 11 (12.79) | 103 (15.70) | .252 |
| Brown | 200 (35.09) | 38 (44.19) | 238 (36.28) | |
| Reason to seek urologic care | ||||
| Rectal examination | 303 (53.16) | 51 (59.30) | 354 (53.96) | |
| Penile curvature | 0 (0) | 2 (2.33) | 2 (0.30) | |
| Erectile dysfunction | 18 (3.16) | 6 (6.98) | 24 (3.66) | |
| Hardening of the penis | 0 (0) | 0 (0) | 0 (0) | |
| Others | 249 (43.68) | 27 (31.40) | 276 (42.07) | |
| Diabetes | ||||
| No | 450 (78.95) | 47 (54.65) | 497 (75.76) | |
| Yes | 95 (16.67) | 37 (43.02) | 132 (20.12) | <.001∗ |
| Do not know | 25 (4.39) | 2 (2.33) | 27 (4.12) | |
| Hypertension | ||||
| No | 188 (64.37) | 53 (61.63) | 428 (65.24) | |
| Yes | 375 (35.63) | 32 (37.21) | 220 (33.54) | |
| Do not know | 7 (1.23) | 1 (1.16) | 8 (1.22) | |
| Dyslipidemia | ||||
| No | 393 (68.95) | 57 (66.28) | 450 (68.60) | |
| Yes | 177 (31.05) | 29 (33.72) | 206 (31.40) | |
| Obesity | ||||
| No | 485 (85.09) | 63 (73.26) | 548 (83.54) | .006∗ |
| Yes | 85 (14.91) | 23 (26.74) | 108 (16.46) | |
| Smoking behavior | ||||
| Smoker | 66 (11.58) | 16 (18.60) | 82 (12.50) | |
| Nonsmoker | 294 (51.58) | 30 (34.88) | 324 (49.39) | |
| Exsmoker | 210 (36.84) | 40 (46.51) | 250 (38.11) | |
| Alcohol use | ||||
| No | 406 (71.23) | 69 (80.23) | 476 (72.31) | |
| Yes | 164 (28.77) | 17 (19.77) | 181 (27.59) | |
| Triglycerides | ||||
| <199 mg/dL | 466 (81.75) | 66 (76.74) | 532 (81.10) | |
| ≥200 mg/dL | 104 (18.25) | 20 (23.26) | 124 (18.90) | |
| Total cholesterol | ||||
| <239 mg/dL | 480 (84.21) | 74 (86.05) | 554 (84.45) | |
| ≥240 mg/dL | 90 (15.79) | 12 (13.95) | 102 (15.55) |
PD = Peyronie's disease.
Chi-squared analysis.
Figure 2.

Prevalence of Peyronie's disease by age range.
The prevalence of diabetes, obesity, and smoking in individuals with PD was 37 (43.02%), 23 (26.74%), and 56 (65.11%), respectively. A significant statistical difference was observed for patients with diabetes and obesity, as well as for lifestyle habits, including smoking (Table 2). Multiple PR analysis is shown in Table 3. The most common risk factors among patients with PD were diabetes (PR = 2.99), smoking for patients who were ex-smokers or smokers (PR = 1.93 and PR = 1.81), and being in the age-group of 41–50 years (PR = 3.53).
Table 3.
Multiple Poisson regression model for factors associated with Peyronie's disease
| Variables | PR | CI95% | P value |
|---|---|---|---|
| Diabetes | |||
| No | 1 | - | |
| Yes | 2.99 | 2.01; 4.44 | <.001∗ |
| Obesity | |||
| No | 1 | - | |
| Yes | 1.41 | 0.90; 2.21 | .129 |
| Smoking behavior | |||
| Nonsmoker | 1 | - | |
| Exsmoker | 1.93 | 1.09.; 3.42 | .022∗ |
| Smoker | 1.81 | 1.12; 2.93 | .014∗ |
| Age range | |||
| 30–40 years | 1 | - | |
| 41–50 years | 3.53 | 1.09; 11.42 | .035∗ |
| 51–60 years | 2.42 | 0.74; 7.88 | .140 |
| 61–70 years | 1.80 | 0.54; 6.00 | .333 |
| >71 years | 0.89 | 0.23; 3.36 | .868 |
PR = Poisson regression.
Chi-squared analysis.
More patients with penile plaque reported a history of penile trauma (9; 10.47%) and a complaint of penile deformity (16; 18.60%) than patients without plaque (Table 4). The prevalence of ED by age, as shown in Table 5, higher prevalence and severity of ED were observed in older men. Table 6 shows the prevalence of ED and score in patients with or without PD. Patients with PD had a higher prevalence of moderate (9 patients, 10.47%), mild to moderate (18 patients - 20.93%), and mild (12 patients - 13.95%) ED.
Table 4.
Clinical characteristics of the patients with or without Peyronie's disease
| Variable | Without PD |
With PD |
P value |
|---|---|---|---|
| (n = 570) n (%) | (n = 86) n (%) | ||
| History of penile trauma | |||
| No | 553 (97.01) | 77 (89.53) | .002∗ |
| Yes | 17 (2.99) | 9 (10.47) | |
| Penile deformity | |||
| No | 548 (96.14) | 70 (81.40) | |
| Yes | 22 (3.86) | 16 (18.60) | |
| Palpable penile plaque | |||
| No | 569 (100) | 71 (82.56) | |
| Yes | 0 (0.00) | 15 (17.44) |
PD = Peyronie's disease.
Chi-squared analysis.
Table 5.
Classification of erectile dysfunction by age range
| Category (score) | 30–40 years |
41–50 years |
51–60 years |
61–70 years |
71–80 years |
P value |
|---|---|---|---|---|---|---|
| (n = 656) n (%) | ||||||
| Severe (6–10) | 1 (1.59) | 7 (4.88) | 10 (5.41) | 50 (26.34) | 42 (43.30) | |
| Moderate (11–16) | 1 (1.59) | 1 (0.81) | 9 (4.86) | 21 (11.29) | 11 (11.34) | |
| Mild to moderate (17–21) | 2 (3.18) | 16 (13.01) | 20 (10.81) | 28 (15.05) | 21 (21.65) | <.001∗ |
| Mild (22–25) | 5 (7.94) | 14 (11.38) | 28 (15.14) | 11 (5.91) | 14 (14.43) | |
| No erectile dysfunction (26–30) | 54 (85.7) | 86 (69.92) | 118 (63.78) | 77 (41.40) | 9 (9.28) | |
Chi-squared analysis.
Table 6.
Prevalence of erectile dysfunction and score in patients with or without Peyronie's disease
| Category (score) | Without PD |
With PD |
P value |
|---|---|---|---|
| (n = 570) n (%) | (n = 86) n (%) | ||
| Severe (6–10) | 97 (17.02) | 11 (12.79) | |
| Moderate (11–16) | 34 (5.96) | 9 (10.47) | |
| Mild to moderate (17–21) | 70 (12.28) | 18 (20.93) | <.01∗ |
| Mild (22–25) | 61 (10.70) | 12 (13.95) | |
| No erectile dysfunction (26–30) | 308 (54.04) | 36 (41.86) |
PD = Peyronie's disease.
Chi-squared analysis.
The different locations of fibrous plaque in the penis were evaluated based on their distribution in 4 regions: one-fourth proximal, one-fourth midshaft, one-fourth distal, and one-fourth distal (coronal) (Figure 1). There was a higher prevalence of plaques in the distal penis, specifically in the corona of the glans corresponding to the distal cavernous body (69.7%), followed by the midshaft area (24.7%). The prevalence of plaques was lowest in the proximal region (8.1%), as shown in Table 7.
Table 7.
Distribution of fibrous plaque in the penis
| Penile plaque | Without PD |
With PD |
|---|---|---|
| (n = 570) n (%) | (n = 86) n (%) | |
| Absent | 570 (100) | 0 (0.00) |
| Proximal | 0 (0) | 3 (3.49) |
| Midshaft | 0 (0) | 7 (8.14) |
| Distal | 0 (0) | 16 (18.60) |
| Distal (coronal) | 0 (0) | 60 (69.77) |
PD = Peyronie's disease.
Discussion
In the literature, the prevalence of PD varies depending on each study and sampling methodology. Moreover, it is also believed that there may be an underreporting of the disease because of patients' reluctance to seek diagnosis or treatment.25
In this study, where patients with different urological complaints were attended, the prevalence of PD was 13.11% among the 656 patients interviewed. When stratified by age, it was possible to observe a higher prevalence of individuals in the age-group 41–50 years (17.89%), followed by men in the age-groups of 61–70 and 51–60 years (Figure 2). The fact that the studied population is composed of patients seeking urology care, especially men older than 40 years, can have caused an overestimation of PD prevalence. The prevalence of PD is higher for older men, especially those older than 40 years. Increasing age may decrease the stiffness of the penis, making it more susceptible to microtrauma and the development of fibrous plaques.11 It is important to highlight that most patients with penile plaque were asymptomatic.
Our data suggest an association between PD and diabetes, obesity, and smoking with prevalence rates of 43.02, 26.74, and 65.11%, respectively, in patients with PD (Tables 2 and 3). Several studies already described the association between PD and some risk factors. Romero et al20 reported that the prevalence of diabetes among patients with PD was 10.7% and that diabetes can lead to the development of PD, besides aggravating penile curvature. For men, being in the age-group of 30–59 and above 65 years, being white or brown, as well as being obese were directly associated with a higher prevalence of diabetes.20
Smoking is highly associated with PD. La Pera et al10 observed increased rates of PD with an increasing number of packs of cigarettes smoked per year, especially for individuals who smoked more than 10,000 packs a year. In addition to describing the association between PD and the number of cigarettes/day, El-Sakka and Tayeb14 also demonstrated an association with smoking time, observing a higher prevalence of PD in individuals who have smoked for more than 5 years.
It is believed that penile traumas during sexual activity cause delamination of the septal fibers with extravasation of blood into the intraluminal spaces, which is associated with perivascular lymphocytic infiltration and plasmocyte inflammatory-cell infiltration in the areolar connective tissue.26 Our study corroborates these previous results because individuals with PD had a history of penile trauma and penile deformity (Table 4).
This study observed ED in most patients with PD, with the highest frequency for individuals with “mild to moderate ED” (Table 6). When asked the main reason for seeking urological care, 24 (3.66%) men cited ED. In this study, 6.98% of the individuals with penile plaque reported ED as the main reason for seeking medical consultation, in contrast to 3.16% in the group without PD (Table 2). The association between PD and ED is a subject of great interest by researchers. Previous studies demonstrate that the prevalence of ED among patients with PD can reach up to 54%.11
According to several authors, the most common location of the penile plaques were more commonly found at the base of the penis, followed by the midshaft area and the distal penis.27 In contrast, our study showed a higher prevalence of plaques in the distal penis, specifically in the corona of glans corresponding to the distal cavernous body (69.7%), followed by the midshaft area (24.7%) and the proximal region (8.1%), as shown in Table 7. We believe that distal plaques would reduce the penile curvature and be less noticeable, thus being less diagnosed. As this study focused on search for hardening in the penile region, we were able to find different results from the literature. Of the 86 men with plaques, only 15 (17.44%) reported having noticed the presence of plaque in their genital organs.
The main limitation is that this study was performed in only one center, which may prevent the generalization of the results. Furthermore, the results differed from the published literature, and the penile plaques were clinically irrelevant because most men were asymptomatic. It would be helpful to follow these men to understand if they will become symptomatic.
The study had no source of external funding. The researchers paid all the expenses and costs related to the research.
Conclusion
The PD among the studied population was association with risk factors such as diabetes, smoking, and obesity. Other clinical characteristics, such as history of penile trauma, penile deformity, and ED, were reported in patients with PD. There was a higher prevalence of plaques in the distal penis, specifically in the corona of the glans penis. The prevalence of PD was different from that in the published literature; our results thus show that more studies are needed.
Statement of authorship
Category 1
-
(a)Conception and Design
- Antonio Segundo
-
(b)Acquisition of Data
- Antonio Segundo
-
(c)Analysis and Interpretation of Data
- Sidney Glina
Category 2
-
(a)Drafting the Article
- Antonio Segundo; Sidney Glina
-
(b)Revising It for Intellectual Content
- Antonio Segundo; Sidney Glina
Category 3
-
(a)Final Approval of the Completed Article
- Sidney Glina
Footnotes
Funding: None.
Conflict of Interest: The authors report no conflicts of interest.
References
- 1.Abern M.R., Larsen S., Levine L.A. Combination of penile traction, intralesional verapamil, and oral therapies for Peyronie’s disease. J Sex Med. 2012;9:288–295. doi: 10.1111/j.1743-6109.2011.02519.x. [DOI] [PubMed] [Google Scholar]
- 2.Smith B.H. Peyronie’s disease. Amer J Clin. 1966;45:670–678. doi: 10.1093/ajcp/45.6.670. [DOI] [PubMed] [Google Scholar]
- 3.Ralph D., Gonzalez-Cadavid N., Mirone V. The management of Peyronie's disease: evidence-based 2010 guidelines. J Sex Med. 2010;7:2359–2374. doi: 10.1111/j.1743-6109.2010.01850.x. [DOI] [PubMed] [Google Scholar]
- 4.American Urological Association Peyronie’s disease. https://www.auajournals.org/doi/full/10.1016/j.juro.2015.05.098 Available at:
- 5.Usta M.F., Bivalacqua T.J., Jabren G.W. Relationship between the severity of penile curvature and the presence of comorbidities in men with Peyronie's disease. J Urol. 2004;171:775–779. doi: 10.1097/01.ju.0000097498.34847.7c. [DOI] [PubMed] [Google Scholar]
- 6.El-Sakka A.I., Tayeb K.A. Peyronie's disease in diabetic patients being screened for erectile dysfunction. J Uro. 2005;174:1026–1030. doi: 10.1097/01.ju.0000170231.51306.32. [DOI] [PubMed] [Google Scholar]
- 7.Cohen D.J., Oliveira A.V., Theodoro T.R. Extracellular matrix alterations after blood instillation in tunica albuginea of rats. Int J Impot Res. 2018;30:85–92. doi: 10.1038/s41443-017-0015-1. [DOI] [PubMed] [Google Scholar]
- 8.Lindsay M.B., Schain D.M., Grambsch P. The incidence of Peyronie’s disease in Rochester, Minnesota, 1950 through 1984. J Urol. 1991;146:1007–1009. doi: 10.1016/s0022-5347(17)37988-0. [DOI] [PubMed] [Google Scholar]
- 9.Schwarzer U., Sommer F., Klotz T. The prevalence of Peyronie's disease: results of a large survey. BJU Int. 2001;88:727–730. doi: 10.1046/j.1464-4096.2001.02436.x. [DOI] [PubMed] [Google Scholar]
- 10.La Pera G., Pescatori E.S., Calabrese M. Peyronie’s disease: prevalence and association with cigarette smoking. Eur Uro. 2001;40:525–530. doi: 10.1159/000049830. [DOI] [PubMed] [Google Scholar]
- 11.Rhoden E.L., Teloken C., Ting H.Y. Prevalence of Peyronie's disease in men over 50-y-old from Southern Brazil. Int J Impot Res. 2001;13:291. doi: 10.1038/sj.ijir.3900727. [DOI] [PubMed] [Google Scholar]
- 12.Mulhall J.P., Creech S.D., Boorjian S.A. Subjective and objective analysis of the prevalence of Peyronie’s disease in a population of men presenting for prostate cancer screening. J Urol. 2004;171:2350–2353. doi: 10.1097/01.ju.0000127744.18878.f1. [DOI] [PubMed] [Google Scholar]
- 13.Kadioglu A., Oktar T., Kandirali E. Incidentally diagnosed Peyronie's disease in men presenting with erectile dysfunction. Int J Impot Res. 2004;16:540. doi: 10.1038/sj.ijir.3901247. [DOI] [PubMed] [Google Scholar]
- 14.El-Sakka A.I. Peyronie’s disease in diabetic patients being screened for erectile dysfunction. European Urol. 2006;49:564–569. doi: 10.1097/01.ju.0000170231.51306.32. [DOI] [PubMed] [Google Scholar]
- 15.Arafa M., Eid H., El-Badry The prevalence of Peyronie's disease in diabetic patients with erectile dysfunction. Int J Impot Res. 2007;19:213–217. doi: 10.1038/sj.ijir.3901518. [DOI] [PubMed] [Google Scholar]
- 16.Tefekli A., Kandirali E., Erol B. Peyronie's disease: a silent consequence of diabetes mellitus. Asia J Androl. 2006;8:75–79. doi: 10.1111/j.1745-7262.2006.00099.x. [DOI] [PubMed] [Google Scholar]
- 17.Akbal C., Tanıdır Y., Özgen M.B. Erectile dysfunction and Peyronie’s disease in patient with retroperitoenal fibrosis. Int Urol Neph. 2008;40:971–975. doi: 10.1007/s11255-008-9381-4. [DOI] [PubMed] [Google Scholar]
- 18.Dibenedetti D.B., Nguyen D., Zografos L. A population-based study of Peyronie's disease: prevalence and treatment patterns in the United States. Adv Urol. 2011:1–9. doi: 10.1155/2011/282503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shiraishi K., Shimabukuro T., Matsuyama H. The prevalence of Peyronie's disease in Japan: a study in men undergoing maintenance hemodialysis and routine health checks. J Sex Med. 2012;9:2716–2723. doi: 10.1111/j.1743-6109.2012.02868.x. [DOI] [PubMed] [Google Scholar]
- 20.Romero F.R., Romero A.W., Almeida R.M.S.D. Prevalence and risk factors for penile lesions/anomalies in a cohort of Brazilian men ⥠40 years of age. Int BJU. 2013;39:55–62. doi: 10.1590/S1677-5538.IBJU.2013.01.08. [DOI] [PubMed] [Google Scholar]
- 21.Stuntz M., Perlaky A., Vignes F. The prevalence of Peyronie's disease in the United States: a population-based study. PlosOne. 2016;11:0150157. doi: 10.1371/journal.pone.0150157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzáles A.I., Sties S.W., Wittkopf P.G. Validação do Índice Internacional de Função Erétil (IIFE) para Uso no Brasil. A B Card. 2013;101:176–182. doi: 10.5935/abc.20130141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barros A.J., Hirakata V.N. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Meth. 2003;3:21. doi: 10.1186/1471-2288-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kleinbaum D.G., Kupper L.L., Nizam A. Applied regression analysis and other multivariable methods. 5th. Ed. Nelson Education. 2013:134–143. [Google Scholar]
- 25.Shindel A.W., Sweet G., Thieu W. Prevalence of Peyronie's disease-Like symptoms in men presenting with Dupuytren Contractures. Sex Med. 2017;5:135–141. doi: 10.1016/j.esxm.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung E., Ralph D., Kagioglu A. Evidence-based management guidelines on Peyronie’s disease. J Sex Med. 2016;13:905–923. doi: 10.1016/j.jsxm.2016.04.062. [DOI] [PubMed] [Google Scholar]
- 27.Kalokairinou K., Konstantinidis C., Domazou M. US Imaging in Peyronie’s disease. J Clin Imaging Sci. 2012;2:63. doi: 10.4103/2156-7514.103053. [DOI] [PMC free article] [PubMed] [Google Scholar]