Abstract
Introduction
Hypothyroidism and subclinical hypothyroidism (SCH) are common metabolic diseases with severe psychological and physiological effects, which may be the risk factors of sexual dysfunction.
Aim
The purpose of this study is to explore the influence of hypothyroidism and SCH on female sexual function through systematic literature review.
Methods
Until February 2020, systematic searches were conducted on Pubmed, Web of Science, EMBASE, and Clinicalkey to obtain eligible studies to report the mean and standard deviation of Female Sexual Function Index (FSFI) in various fields in women with clinical hypothyroidism, SCH, and healthy controls. In accordance with the results of heterogeneity test, a random effect model or fixed effect model was selected to aggregate the scores of each field. The scores of female patients with hypothyroidism and healthy controls were compared using forest plot. Stata (version 15.1) uses meta-analysis.
Main Outcome Measure
Evaluation values of various fields of FSFI in clinical hypothyroidism, SCH, and healthy controls.
Results
This study included 7 studies, including 88 women with clinical hypothyroidism, 337 women with SCH, and 2056 healthy controls. Compared with healthy controls, patients with hypothyroidism scored lower in all FSFI dimensions (desire, arousal, lubrication, orgasm, satisfaction, and pain), especially in lubrication. And, only arousal and orgasm decreased in patients with SCH. Hypothyroidism (odds ratio = 3.912, P = .002) rather than SCH (odds ratio = 1.036, P = .886) was a risk factor for female sexual dysfunction.
Conclusion
Hypothyroidism does impair female sexual function to varying degrees. SCH has little effect on female sexual function. It is essential to measure and evaluate the thyroid function of women with sexual dysfunction regularly, which can help clinicians improve sexual function and sexual quality of life.
Wang Y and Wang H. Effects of Hypothyroidism and Subclinical Hypothyroidism on Sexual Function: A Meta-Analysis of Studies Using the Female Sexual Function Index. Sex Med 2020;8:156–167.
Key Words: Hypothyroidism, Subclinical Hypothyroidism, Sexual Function, FSFI
Introduction
Thyroid hormone deficiency can lead to hypothyroidism, which results in poor health. The clinical manifestations of this chronic disease are generally non-specific, so the diagnosis of hypothyroidism is based on the concentration of thyroid stimulating hormone (TSH) and free thyroxine.1 Another biochemical state, subclinical hypothyroidism (SCH), is characterized by high serum TSH levels with normal free thyroxine and free triiodothyronine.2
Generally speaking, sexual dysfunctions are defined as “disturbances in sexual desire and in the psychophysiological changes that characterize the sexual response cycle and cause marked distress and inter-personal difficulty.”3 In addition, female sexual dysfunction (FSD) included disorders of desire/libido, arousal, pain/discomfort, and inhibited orgasm.4
Hypothyroidism is a slowdown of systemic activity that results in lethargy, cold tolerance, fatigue, dry skin, and poor quality of life.5,6 Although the change of thyroid function on metabolism has been recognized, the role of thyroid disease on sexual function is still controversial. In accordance with reports, hypothyroidism could damage female sexual function, and FSD may also be a signal of serious endocrine diseases.7,8 However, owing to the lack of evidence, the relationship between SCH and FSD is not clear.9 By raising awareness of hypothyroidism and sexual dysfunction, sexologists can identify those sexual symptoms and treat patients with potential thyroid diseases earlier.10 With the improvement of social and economic status, female health is no longer a negligible problem.
The evaluation of sexual function plays an important role in the measurement of chronic diseases, and the sexual function of patients with hypothyroidism is paid increasing attention. The main instrument used in sexual dysfunction evaluation in hypothyroidism is the Female Sexual Function Index (FSFI), and it is widely used to measure sexual dysfunction in female hypothyroidism. Here, we focused on FSFI, a 19-item self-report tool used to assess women's sexual function, as mentioned by Rosen et al,11 which analyzed 6 areas. FSFI includes 6 areas of sexual function: desire, arousal, lubrication, orgasm, satisfaction, and pain.
Despite reports of hypothyroidism affecting sexual function, there are different conclusions on the role of clinical or SCH on female sexual function.12 Meta-analysis, as a mature method to analyze and sum up the collected data, can help us take stock of the burden of FSD in a larger sample. Our aim was to compare sexual function between hypothyroidism, SCH, and healthy controls (HC) and to review effects of hypothyroidism and SCH on various dimensions of female sexual function.
Materials and methods
Search Strategy
To identify all relevant studies published, Pubmed, Web of Science, EMBASE and ClinicalKey databases were searched until February 2020. We used the following terms to search in PubMed, Web of Science and ClinicalKey: ((female sexual function index) OR (FSFI) OR (sexual function) OR (sexual dysfunction)) AND (hypothyroidism OR hypothyroid) AND (women OR female). The terms used to search in EMBASE were the following: ('female sexual function index’ OR ‘fsfi’ OR ‘sexual function’ OR ‘sexual dysfunction’) AND (hypothyroidism OR hypothyroid) AND (women OR female). The search was restricted to literature in English. Articles, reviews, editorials, and reference lists in the proceedings of international conferences have also been manually searched for useful studies.
Eligibility of Relevant Studies
Studies that reported female sexual function in patients with hypothyroidism and/or SCH and HC were considered suitable for this review. Studies were included if they met the following criteria: (1) case-control, cohort, and cross-sectional design; (2) female sexual function assessed using the FSFI; (3) female sexual function must have all 6 FSFI domains (Desire, Arousal, Lubrication, Orgasm, Satisfaction, and Pain) recorded as means with standard deviation (SD); and (4) HC were derived from a population within the same geographic area and ethnic background as hypothyroidism. Studies were excluded if they (1) were reviews, letters to the editor, case reports, expert opinion, or consensus statements; (2) reported duplicated or useless data.
Data Extraction and Quality Assessment
The following information was extracted independently by one reviewer from each included study: first author, year of publication, country, study design, case, sampling method, study quality, characteristics of the hypothyroidism (scores of the domains of FSFI), mean age of patients and HC, and so on. The data set was double-checked and completed through communication with another author.
2 reviewers assessed the quality of included studies using the Newcastle-Ottawa scale (NOS) for assessing the quality of non-randomized studies in meta-analysis. The parts of the NOS scale include selection, comparability, exposure, and outcome. A study can be awarded a maximum of one star for each numbered item within the selection and exposure categories. A maximum of 2 stars can be given for comparability. Quality of articles was divided into the following 3 levels: low (0–3), medium (4–6), and high (7–9) quality.
Data Synthesis and Statistical Analysis
The scores on questionnaires used to evaluate sexual function in patients with hypothyroidism, SCH, and HC in each study were extracted as mean differences (MD) ± SD. MD and 95% confidence intervals (CIs) were calculated for scores in each study eligible for the meta-analysis and combined by using fixed or random effects model according to Dersimonian and Nan.13 I2 was used to evaluate the heterogeneity, with the value less than 50% denoting small level of between-study variation, and then a fixed effect model (the inverse variance method) was used. Otherwise, a random effect model (I–V heterogeneity method) was used.14 Statistical heterogeneity in the results of different studies was examined by X2 tests for significance. Sensitivity analysis is used to analyze the impact of each study on the results. Publication bias was assessed with a visual inspection of a funnel plot. All statistical tests were with a significance level of 0.05.
Sensitivity analysis is used to analyze which article has a greater impact on the overall results. Considering the small number of studies included, no subgroup analysis was carried out.
The meta-analysis was conducted using Stata (version 15.1). This meta-analysis had been registered on the systematic review database of PROSPERO, with the registration number CRD42018104509 (https://www.crd.york.ac.uk/PROSPERO). We used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 checklist15 to generate the current report.
Results
Search Results and Quality Assessment
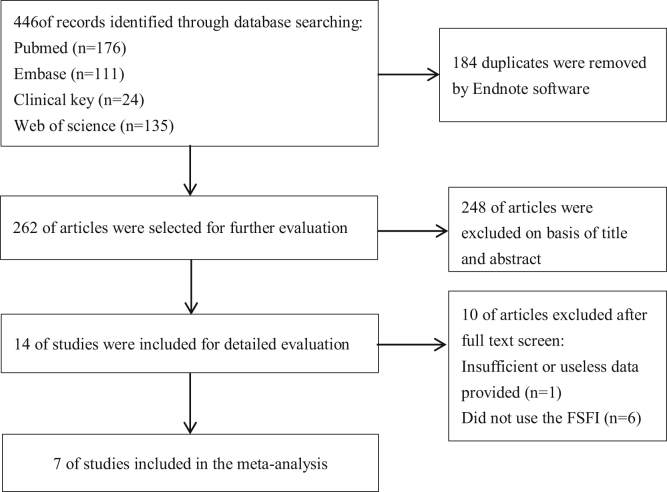
We identified 446 studies following search strategy; 184 duplicates were removed by Endnote software. A total of 248 of articles were excluded on basis of title and abstract. Of the remaining 14 studies, one study was excluded because of insufficient or useless data and 6 studies that did not use the FSFI questionnaire were also excluded. Finally, a total of 7 studies7,9,16, 17, 18, 19, 20 were included in the meta-analysis, of which 3 articles provided FSFI scores of hypothyroidism, 3 articles provided FSFI scores of SCH, and one article provided both. Among them, 3 were conducted in Italy, one in Turkey, one in Korea, one in Poland, and one in China. Detailed inclusion procedure is shown in Figure 1.
Figure 1.
Flow diagram of our study selection process. FSFI = Female Sexual Function Index.
Included studies reported data on 2,481 individuals (88 with hypothyroidism, 337 with SCH, and 2056 HC). The subjects of hypothyroidism and SCH were 20–53 and 14–168, respectively. The median mean age of patients with hypothyroidism and SCH was 39.65 (37.04–41.7) years and 38.77 (30–52) years, respectively. The characteristics of the 7 included studies are described in Table 1 briefly.
Table 1.
Characteristics of studies included in the meta-analysis
| Study | Annamaria Veronelli‖ | Gokhan Atis# | A. Oppo‖ | D. Pasquali‖ | Hyeri Hong¶ | Robert Krysiak¶ | Han Luo¶ |
|---|---|---|---|---|---|---|---|
| Published year | 2009 | 2010 | 2011 | 2013 | 2015 | 2016 | 2018 |
| Country | Italy | Turkey | Italy | Italy | Korea | Poland | China |
| Case/HC (n) | 24/36 | 25/14/20 | 17/30 | 22/53 | 138/948 | 17/18 | 168/951 |
| Age(case/HC) | 41.7 ± 1.58/39.3 ± 1.06 | 37.04 ± 7.08/38.33 ± 5.82/39.30 ± 5.52 | 39.6 ± 6.8/37.5 ± 7.7 | 39.7 ± 8.7/39.2 ± 12.4 | 52(9)/50(9)§ | 30 ± 4.0/29 ± 4.0 | 39.2 ± 7.6/38.5 ± 7.7 |
| FSD (case/HC) | N/A | 56%/32%/15% | N/A | 41%/20.7% | 67.4%/68.4% | 41%/17% | 21.4%/27.4% |
| TSH mU/L (case/HC) | 4.4 ± 1.06/2.9 ± 0.19 | 43.1 ± 14.44/7.55 ± 0.97/2.18 ± 0.70 | 14.1 ± 7.6/1.4 ± 0.7 | 8.1 ± 4.5/N/A | N/A | 7.3 ± 1.3/1.7 ± 0.6 | 5.4(1.8)/2.3(1.2)§ |
| FT4 pmol/L (case/HC) | 13.2 ± 1.49/14.5 ± 0.38 | 0.62 ± 0.09/1.89 ± 0.34/1.93 ± 0.28 | 7.8 ± 2.8/12.6 ± 2.5 | 11.3 ± 3.4/N/A | N/A | N/A | 16.4 ± 2.1/17.1 ± 2.2 |
| Study design | Case-control | Case-control | Case-control | Case-control | Cross-sectional | Case-control | Case-control |
| NOS score∗ | 7 | 6 | 7 | 6 | 6 | 5 | 5 |
| Sampling method† | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| Setting‡ | 1 | 1 | 1 | 1 | 2 | 3 | 2 |
| Desire (case/HC) | 2.4 ± 0.21/4.0 ± 0.19 | 4.01 ± 0.93/4.14 ± 0.8/5.16 ± 0.74 | 3.5 ± 1.0/4.5 ± 0.6 | 3.1 ± 0.9/3.8 ± 1.0 | 3.0 ± 0.89/2.4 ± 1.33 | 4.30 ± 0.48/5.10 ± 0.70 | 3.5 ± 0.84/3.4 ± 0.8 |
| Arousal | 2.8 ± 0.36/2.8 ± 0.36 | 3.28 ± 1.3/4.73 ± 0.99/5.37 ± 0.8 | 7.7 ± 1.5/9.4 ± 1.3 | 3.2 ± 1.3/4.1 ± 1.9 | 3.0 ± 1.11/3.6 ± 1.11 | 4.75 ± 0.67/5.42 ± 0.61 | 3.9 ± 1.0/3.9 ± 0.9 |
| Lubrication | 3.3 ± 0.51/5.6 ± 0.11 | 4.24 ± 0.82/5.24 ± 0.58/5.4 ± 0.52 | 7.7 ± 1.5/9.4 ± 1.3 | 3.4 ± 1.4/4.7 ± 1.8 | 4.5 ± 1.33/4.5 ± 1.33 | 4.70 ± 0.51/5.30 ± 0.55 | 4.9 ± 0.9/4.9 ± 0.9 |
| Orgasm | 3.2 ± 0.49/5.4 ± 0.12 | 3.95 ± 1.23/4.91 ± 0.95/5.46 ± 0.51 | 3.5 ± 1.1/4.8 ± 0.6 | 3.9 ± 1.9/4.5 ± 1.7 | 4.0 ± 1.19/4.0 ± 1.19 | 4.38 ± 0.60/4.80 ± 0.65 | 4.3 ± 0.9/4.4 ± 0.8 |
| Satisfaction | 3.2 ± 0.41/5.0 ± 0.21 | 4.06 ± 0.96/4.51 ± 0.73/5.4 ± 0.54 | 3.3 ± 0.9/4.4 ± 1.0 | 4.0 ± 1.9/4.8 ± 1.1 | 4.0 ± 0.89/4.0 ± 0.89 | 4.92 ± 0.68/5.48 ± 0.53 | 4.6 ± 0.9/4.6 ± 0.8 |
| Pain/Dyspareunia | 3.3 ± 0.53/5.3 ± 0.24 | 4.37 ± 1.14/5.37 ± 1.17/5.5 ± 0.56 | 3.9 ± 1.0/5.1 ± 0.8 | 3.1 ± 1.4/3.2 ± 1.8 | 5.2 ± 1.26/5.2 ± 1.48 | 4.82 ± 0.65/5.42 ± 0.61 | 4.6 ± 1.0/4.5 ± 0.9 |
| Total FSFI | 18.2 ± 2.41/30.3 ± 0.76 | 23.92 ± 5.81/29.2 ± 3.65/32.31 ± 3.5 | N/A | 20.7 ± 6.9/25.7 ± 5.2 | 24.4 ± 5.19/23.8 ± 5.41 | 27.87 ± 3.62/31.52 ± 2.75 | 25.8 ± 3.9/25.7 ± 3.9 |
FSD = female sexual dysfunction; FSFI = Female Sexual Function Index; FT4 = free tetraiodothyronine; HC = healthy controls; NOS = Newcastle-Ottawa scale; TSF = thyroid-stimulating hormone.
Quality rated out of 9: 0–3 = low quality, 4–6 = medium quality, and 7–9 = high quality.
0 = undefined sampling strategy, 1 = consecutive sampling strategy.
0 = not stated; 1 = outpatient clinic; 2 = health promotion center; 3 = community-based healthcare.
Median (IQR = interquartile range).
Provided data on clinic hypothyroidism and health controls.
Provided data on subclinical hypothyroidism and health controls.
Provided data on clinic hypothyroidism, subclinical hypothyroidism, and health controls.
The mean NOS of the 7 studies was 6, of which 2 studies were 7, 3 studies were 6, and 2 studies were 5 (Table 1). The results indicated that the included studies had moderate quality at least, which could ensure the reliability of the meta-analysis.
Dimensions Analysis
Because of the heterogeneity among aspects, they were determined using the random effect model. Four studies compared the scores of the FSFI for patients with hypothyroidism and HC.7,16, 17, 18 In patients with SCH,7,9,19,20 except for Orgasm (fixed effect model), the other 5 dimensions and total FSFI score were analyzed with the random effect model.
Desire
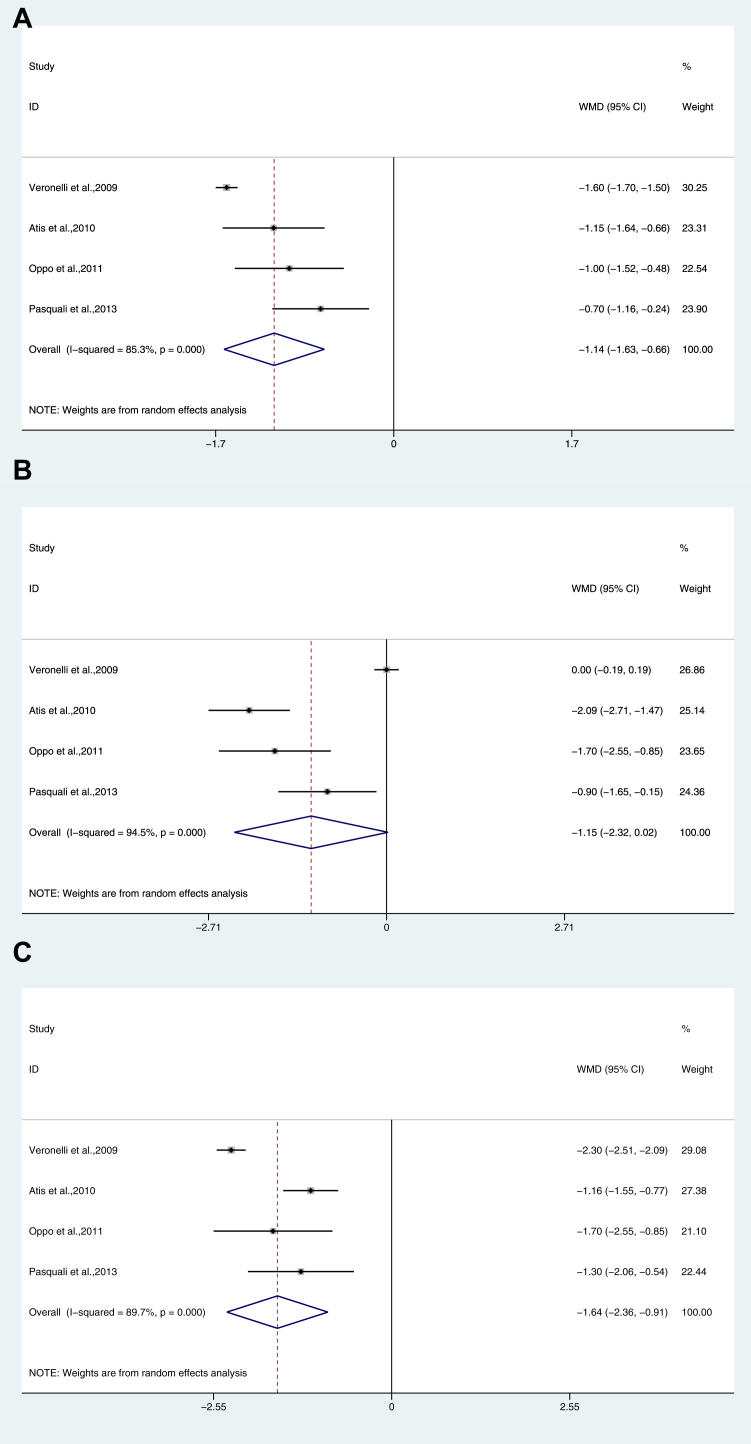
Female patients with hypothyroidism had a significantly lower score (MD: −1.145; 95% CI: -1.625, −0.664; P < .001), with heterogeneity among studies (P = .000) and I2 = 85.3%, Figure 2A).
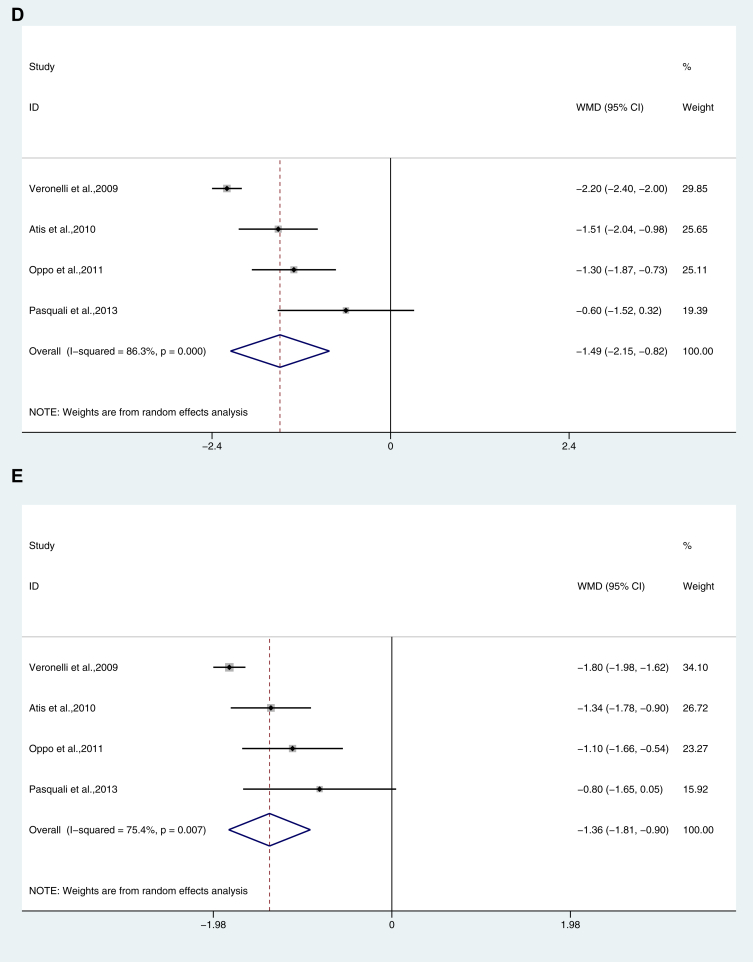
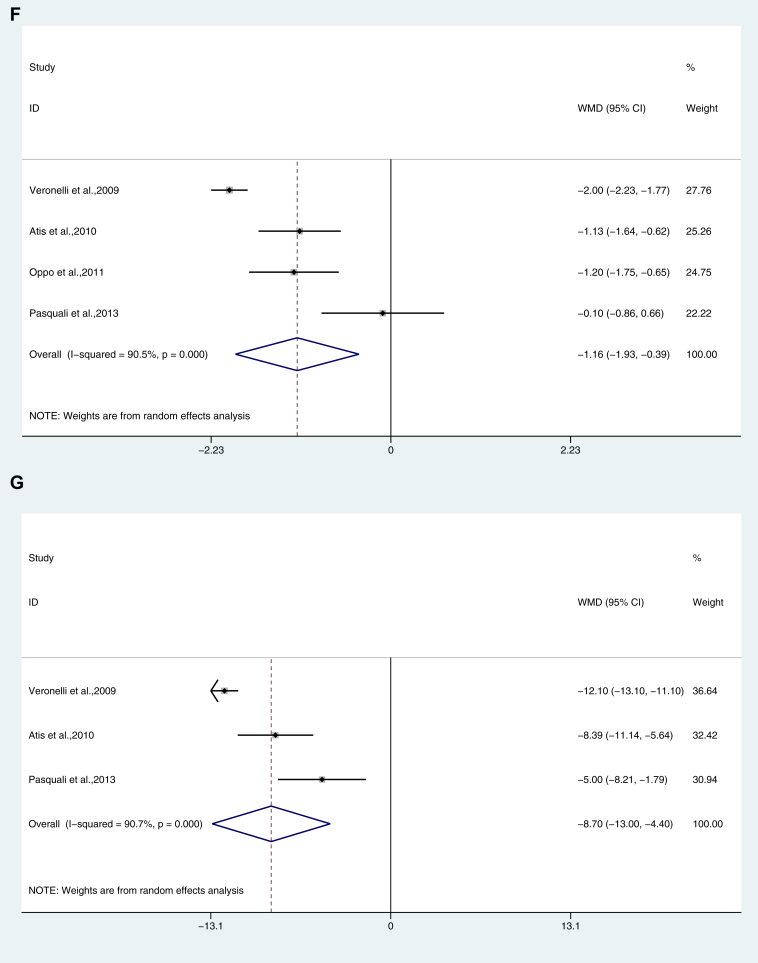
Figure 2.
Dimension analysis of female sexual function in hypothyroidism. (A) Desire; (B) Arousal; (C) Lubrication; (D) Orgasm; (E) Satisfaction; (F) Pain/Dyspareunia; (G) Total FSFI score. FSFI = Female Sexual Function Index.
There was no significant difference in Desire score between SCH and HC (P = .422, Table 2).
Table 2.
Dimensions analysis of FSFI in subclinical hypothyroidism
| SCH/HC | WMD | I2 | 95% CI | P | |
|---|---|---|---|---|---|
| Desire | 337/1937 | −0.231 | 95.60% | −0.795, 0.333 | .422 |
| Arousal | 337/1937 | −0.446 | 88.50% | −0.859, −0.033 | .034 |
| Lubrication | 337/1937 | −0.155 | 70.40% | −0.395, 0.085 | .205 |
| Orgasm | 337/1937 | −0.115 | 47.30% | −0.227, −0.002 | .046 |
| Satisfaction | 337/1937 | −0.286 | 85.00% | −0.594, 0.021 | .068 |
| Pain | 337/1937 | −0.104 | 68.50% | −0.374, 0.166 | .451 |
| Total FSFI | 337/1937 | −1.112 | 84.10% | −2.686, 0.462 | .166 |
Bold values indicate P < .05.
CI = confidence interval; FSFI = Female Sexual Function Index; SCH= subclinical hypothyroidism; WMD = nonstandard mean difference.
Arousal
Female patients with hypothyroidism (MD: −1.147; 95% CI: −2.316, 0.023; P = .000, I2 = 94.5%, Figure 2B) and SCH (MD, −0.446; 95% CI, −0.859, −0.033; P = .034, I2 = 88.5%, Table 2) had a significantly lower score.
Lubrication
Female patients with hypothyroidism had a significantly lower score (MD: −1.637; 95% CI: −2.361, −0.913; P = .000, I2 = 89.7%, Figure 2C).
There was no significant difference in the Lubrication score between SCH and HC (P = .205, Table 2).
Orgasm
Female patients with hypothyroidism (MD: −1.487; 95% CI: −2.152, −0.822; P = .000, I2 = 86.3%, Figure 2D) and SCH (MD: −0.115; 95% CI: −0.227, −0.002; P = .046, I2 = 47.3%, Table 2) had a significantly lower score.
Satisfaction
Female patients with hypothyroidism had a significantly lower score (MD: −1.355; 95% CI: -1.808, 0.902; P = .007, I2 = 75.4%, Figure 2E).
There was no significant difference in the Satisfaction score between SCH and HC (P = .068, Table 2).
Pain/Dyspareunia
Female patients with hypothyroidism had a significantly lower score (MD: −1.160; 95% CI: -1.925, −0.394; P = .000, I2 = 90.5%, Figure 2F).
There was no significant difference in the Pain score between SCH and HC (P = .451, Table 2).
Total FSFI Score
Female patients with hypothyroidism had a significantly lower score (MD: −8.701; 95% CI: −12.996, −4.405; P = .000, I2 = 90.7%, Figure 2G).
There was no significant difference in the total FSFI score between SCH and HC (P = .166, Table 2).
Sensitivity Analysis
Sensitivity analysis was performed to assess the influence of an individual study on the overall weighted mean difference (WMD) of female sexual function. After exclusion of any study, there was no significant change in the overall effect except for the following aspects. In female patients with hypothyroidism, if the study16 was removed from the Lubrication dimension, the combined WMD changed from -1.64 (95% CI: −2.36, −0.91) to −1.26 (95% CI: −1.59, −0.94).
In women with SCH, if the study20 was removed from the Lubrication dimension, the combined WMD changed from −0.16 (95% CI: −0.39, 0.08) to −0.02 (95% CI: −0.13, 0.10). And, if the study20 was removed from the Pain dimension, the combined WMD changed from -0.10 (95% CI: −0.37, 0.17) to 0.06 (95% CI: −0.07, 0.19).
Publication Bias Assessment
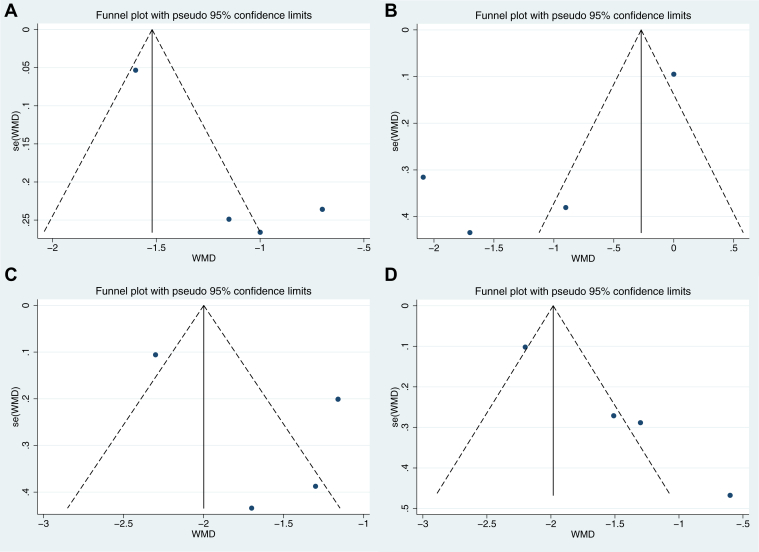
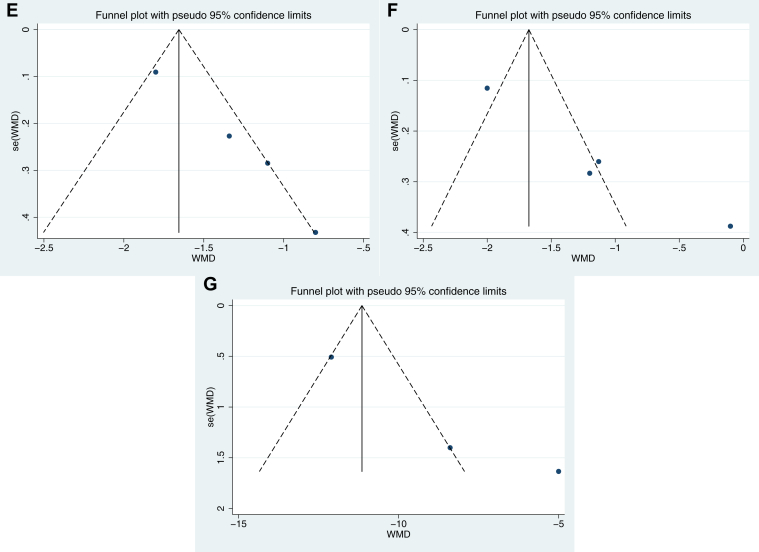
In female patients with hypothyroidism, we used funnel plots and Egger's test to explain publication bias, and the results showed that there was no obvious publication bias for desire (P = .059, Figure 3A), arousal (P = .104, Figure 3B), lubrication (P = .311, Figure 3C), and total FSFI (P = .139, Figure 3G), except orgasm (P = .003, Figure 3D), satisfaction (P = .005, Figure 3E), and pain (P = .016, Figure 3F).
Figure 3.
Publication bias assessment of female sexual function in hypothyroidism. (A) Desire; (B). Arousal; (C) Lubrication; (D) Orgasm; (E) Satisfaction; (F) Pain/Dyspareunia; (G) Total FSFI score. FSFI = Female Sexual Function Index.
In women with SCH, we used Egger's test to explain publication bias, and the results showed that there was no obvious publication bias for desire (P = .273), arousal (P = .452), lubrication (P = .273), orgasm (P = .187), pain (P = .266), and total FSFI (P = .127), except satisfaction (P = .015).
Odds Ratio of FSD in Hypothyroidism and SCH
Hypothyroidism could cause FSD (odds ratio [OR] = 3.912, 95%CI: 1.680, 9.112, P = .002, I2 = 15.40%). However, SCH may not be a risk factor for FSD (OR = 1.036, 95%CI: 0.639, 1.678, P = .886, I2 = 51.80%), as shown in Table 3.
Table 3.
OR of FSD in patients with hypothyroidism and SCH
| CASE/HC | OR | I2 | 95% CI | P | |
|---|---|---|---|---|---|
| SCH | 348/1937 | 1.036 | 51.80% | 0.639, 1.678 | .886 |
| Hypothyroidism | 47/73 | 3.912 | 15.40% | 1.680, 9.112 | .002 |
Bold value indicates P < .05.
CI = confidence interval; FSD=Female sexual dysfunction; HC = healthy controls; OR = odds ratio; SCH = subclinical hypothyroidism.
Discussion
The purpose of this systematic review was to evaluate the effect of hypothyroidism on female sexual function by the FSFI and to compare it with that of the general population. This review showed that the sexual function of women with hypothyroidism is worse than that of healthy subjects without hypothyroidism. The FSFI scores of women with hypothyroidism are lower in all dimensions, with lubrication being the worst. However, in SCH, only sexual arousal and orgasm decreased. These results are consistent with previous studies having shown that thyroid failure is associated with FSD. Our findings are significant and can help professionals dealing with sexual and reproductive health. Drugs used in the diagnosis, treatment, and treatment of hypothyroidism may lead to increased sexual dysfunction.10,21 However, despite the importance and prevalence of the disease, clinicians and patients ignore it. In fact, only a small percentage of patients consult their doctors about sex, and their doctors do not often ask them these questions.22,23
Long-term primary hypothyroidism can lead to hyperprolactinemia. In women, hyperprolactinemia is closely related to hypoactive sexual desire disorder.17 Hyperprolactinemia may be a factor of sexual dysfunction in women with clinical hypothyroidism and SCH.7 Increased TSH may lead to hyperprolactinemia, which can lead to decreased libido, lubrication, and orgasm failure by reducing gonadotropin-releasing hormone production.24 In pathophysiology, the decrease of sexual desire, lubrication, and sexual satisfaction may be related to sexual dysfunction of angiogenic women. Hypoandrogen leads to inadequate vaginal acceptance, resulting in difficulty in sexual intercourse, affecting the hypothalamic limbic structure, where it arouses sensation and pleasure.25 Menopausal FSD also involves many other factors, such as estrogen reduction and vaginal dryness.26,27 These syndromes should be considered when evaluating women with sexual arousal disorder.
The decrease of FSD in hypothyroidism is due to infiltration of thyroiditis rather than thyroid failure.20 On the other hand, hypothyroidism and SCH are related to mood disorders, fatigue, and depression.8,20 Depression is associated with hypoactive sexual desire disorder,28 which may lead to FSD.8 Hence, SCH may have a potential indirect effect on FSD. At the same time, it is believed that long-term excess or insufficient thyroid hormones can lead to mental disorders such as irritability, depression, and changes in sexual behavior.8 In addition, in modern society, sexual relationship is also considered as an important part of people's life. Therefore, sexual dysfunction in turn has a negative impact on social relations and self-esteem.19
Sexual response in women is adversely affected both by aging and menopause.27 The incidence of hypothyroidism is mostly concentrated in menopause, and the symptoms of perimenopause may overlap with hypothyroidism, leading to FSD independently. Therefore, screening for hypothyroidism in perimenopausal women is usually recommended.29 FSD is still a multifactor problem. Cayan et al30 detected the presence of lower educational level, unemployment status, chronic diseases, multiparity, and menopause status as important risk factors for FSD. Hypothyroidism is also associated with female metabolic syndrome, which promotes the development of cardiovascular disease and type 2 diabetes, both of which independently drive the development of FSD.31 In view of the complexity of the results of the aforementioned studies, it is worthwhile to design a good prospective control study in the future.
There are 2 points to emphasize about the FSFI. This psychological test was initially used only for the evaluation of sexual desire. Neijenhuijs et al32 also suggested that researchers use FSFI-19 for confirmatory factor analysis and report the factor structure found in their samples. However, from a practical point of view, FSFI-19 and FSFI-6 are good screening tools for the definition of FSD. FSFI-6, which appeared in 2010, is a valuable tool for screening women who may have FSD and can help any doctor to disclose FSD quickly and effectively.33 Unfortunately, through literature search, FSFI-6 is rarely used for hypothyroidism.
In addition, Castelo-Branco et al34 have conducted 2 consecutive cross-sectional studies in 5 European countries, using interviews and online surveys for postmenopausal women. This study revealed the importance of defining the profile of postmenopausal women to develop interventions to help them overcome barriers to the diagnosis, management, and treatment of vulvovaginal atrophy. Among 526 female subjects, Mollaioli D et al35 verified a visual analog scale, called Orgasmometer-F, which can be used as a new psychological measurement tool to measure the orgasm intensity of the female population, indicating that SD damage orgasm intensity. We have explored the literature and have not found these tests for sexual behavior in women with thyroid disease, which may be a major limitation of current research.
The study is not free from some limitations. Most of the studies in this review recruited patients from hospital outpatient clinics, who may be patients with more severe hypothyroidism, and the sample size of cases included in the study was small. In the recruitment areas of each study, the ethnic, cultural background, and way of thinking of the recruitment objects will be different, which may be the reason for the high heterogeneity of some parts of this study. In addition, there is no disease-specific scale to more accurately assess female sexual function in patients with hypothyroidism. Therefore, in choosing the FSFI to investigate the sexual function of women with hypothyroidism, we may not have considered the effect of the disease itself on the outcome, and the conclusion of the effect of hypothyroidism on female sexual function is limited. However, owing to the small number of articles involved, the asymmetry of the funnel chart cannot be denied, so we cannot deny the publication bias. These limitations should be mentioned and discussed in future studies.
As recently suggested, the investigation of sexual dysfunction should be part of the diagnostic work, which can help physicians in different clinical settings assess the sexual problems of female patients with specific clinical conditions, so as to facilitate identification and possible treatment.
Conclusion
Paying attention to the sexual dysfunction of hypothyroid women and putting forward treatment strategies are helpful to improve the sexual and emotional relationship of couples and improve the quality of life. In a word, hypothyroidism has different degrees of damage to all aspects of female sexual function, while SCH may not cause FSD. Longitudinal studies with a larger sample size are needed in different countries and regions to assess the sexual function of the population in order to achieve clinical utility.
Statement of authorship
Category 1
-
(a)Conception and Design
- Yilin Wang; Hongli Wang
-
(b)Acquisition of Data
- Yilin Wang; Hongli Wang
-
(c)Analysis and Interpretation of Data
- Yilin Wang; Hongli Wang
Category 2
-
(a)Drafting the Article
- Yilin Wang; Hongli Wang
-
(b)Revising It for Intellectual Content
- Yilin Wang; Hongli Wang
Category 3
-
(a)Final Approval of the Completed Article
- Yilin Wang
Footnotes
Yilin Wang and Hongli Wang contributed equally to this work.
Conflict of Interest: The authors declare no conflict of interest in this work.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Contributor Information
Yilin Wang, Email: wyl_1206@126.com.
Hongli Wang, Email: 15150822468@163.com.
References
- 1.Chaker L., Bianco A.C., Jonklaas J. Hypothyroidism. Lancet. 2017;390:1550–1562. doi: 10.1016/S0140-6736(17)30703-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biondi B., Cappola A.R., Cooper D.S. Subclinical hypothyroidism: a review. Jama. 2019;322:153–160. doi: 10.1001/jama.2019.9052. [DOI] [PubMed] [Google Scholar]
- 3.Basson R., Berman J., Burnett A. Report of the international consensus development conference on female sexual dysfunction: definitions and classifications. J Urol. 2000;163:888–893. [PubMed] [Google Scholar]
- 4.Hatzimouratidis K., Hatzichristou D. Sexual dysfunctions: classifications and definitions. J Sex Med. 2007;4:241–250. doi: 10.1111/j.1743-6109.2007.00409.x. [DOI] [PubMed] [Google Scholar]
- 5.Carle A., Pedersen I.B., Knudsen N. Hypothyroid symptoms Fail to predict thyroid insufficiency in Old people: a population-based case-control study. Am J Med. 2016;129:1082–1092. doi: 10.1016/j.amjmed.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Carosa E., Lenzi A., Jannini E.A. Thyroid hormone receptors and ligands, tissue distribution and sexual behavior. Mol Cell Endocrinol. 2018;467:49–59. doi: 10.1016/j.mce.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Atis G., Dalkilinc A., Altuntas Y., Ergenekon EJJoSM Sexual dysfunction in women with clinical hypothyroidism and subclinical hypothyroidism. J Sex Med. 2010;7:2583–2590. doi: 10.1111/j.1743-6109.2010.01815.x. [DOI] [PubMed] [Google Scholar]
- 8.Bhasin S., Enzlin P., Coviello A. Sexual dysfunction in men and women with endocrine disorders. Lancet. 2007;369:597–611. doi: 10.1016/S0140-6736(07)60280-3. [DOI] [PubMed] [Google Scholar]
- 9.Luo H., Zhao W., Yang H. Subclinical hypothyroidism would not lead to female sexual dysfunction in Chinese women. BMC Women's Health. 2018;18 doi: 10.1186/s12905-017-0465-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gabrielson A.T., Sartor R.A., Hellstrom W.J.G. The impact of thyroid disease on sexual dysfunction in men and women. Sex Med Rev. 2019;7:57–70. doi: 10.1016/j.sxmr.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Rosen R., Brown C J., Leiblum S. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 12.Bates J.N., Kohn T.P., Pastuszak A.W. Effect of thyroid hormone Derangements on sexual function in men and women. Sex Med Rev. 2020;8:217–230. doi: 10.1016/j.sxmr.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 14.Higgins J.P.T., Thompson S.G., Deeks J.J. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moher D., Liberati A., Tetzlaff J. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. w264. [DOI] [PubMed] [Google Scholar]
- 16.Veronelli A., Mauri C., Zecchini B. Sexual dysfunction is frequent in premenopausal women with diabetes, obesity, and hypothyroidism, and correlates with markers of increased cardiovascular risk. A preliminary report. J Sex Med. 2009;6:1561–1568. doi: 10.1111/j.1743-6109.2009.01242.x. [DOI] [PubMed] [Google Scholar]
- 17.Oppo A., Franceschi E., Atzeni F. Effects of hyperthyroidism, hypothyroidism, and thyroid autoimmunity on female sexual function. J Endocrinological Invest. 2011;34:449–453. doi: 10.1007/BF03346712. [DOI] [PubMed] [Google Scholar]
- 18.Pasquali D., Maiorino M.I., Renzullo A. Female sexual dysfunction in women with thyroid disorders. J Endocrinological Invest. 2013;36:729–733. doi: 10.3275/8933. [DOI] [PubMed] [Google Scholar]
- 19.Hong H., Lee H.J., Kim S.M. Subclinical hypothyroidism is not a risk factor for female sexual dysfunction in Korean Middle-aged women. Thyroid. 2015;25:784–788. doi: 10.1089/thy.2015.0015. [DOI] [PubMed] [Google Scholar]
- 20.Krysiak R., Drosdzol-Cop A., Skrzypulec-Plinta V. Sexual function and depressive symptoms in young women with thyroid autoimmunity and subclinical hypothyroidism. Clin Endocrinol (Oxf) 2016;84:925–931. doi: 10.1111/cen.12956. [DOI] [PubMed] [Google Scholar]
- 21.Pakpour A.H., Mir Saeed Y., Isa Mohammadi Z., Andrea BJAoG, Obstetrics Prevalence and risk factors of the female sexual dysfunction in a sample of infertile Iranian women. Arch Gynecol Obstet. 2012;286:1589–1596. doi: 10.1007/s00404-012-2489-x. [DOI] [PubMed] [Google Scholar]
- 22.Moreira E.D., Kim S.C., Glasser D., Gingell CJJoSM Sexual activity, prevalence of sexual problems, and associated help-seeking patterns in men and women aged 40-80 years in Korea: data from the Global Study of Sexual Attitudes and Behaviors (GSSAB) J Sex Med. 2010;3:201–211. doi: 10.1111/j.1743-6109.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- 23.Choi J., Dong W.S., Lee S. Dose-response relationship between cigarette smoking and female sexual dysfunction. Obstet Gynecol Sci. 2015;58:302–308. doi: 10.5468/ogs.2015.58.4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buvat J. Hyperprolactinemia and sexual function in men: a short review. Int J impotence Res. 2003;15:373–377. doi: 10.1038/sj.ijir.3901043. [DOI] [PubMed] [Google Scholar]
- 25.Traish A.M., Kim N.K., Munarriz R., Goldstein IJF, Sterility Role of androgens in female genital sexual arousal: receptor expression, structure, and function. Fertil Steril. 2002;77:11–18. doi: 10.1016/s0015-0282(02)02978-3. [DOI] [PubMed] [Google Scholar]
- 26.Avis N.E., Green R. The perimenopause and sexual functioning. Obstet Gynecol Clin North Am. 2011;38:587–594. doi: 10.1016/j.ogc.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Genazzani A.R., Gambacciani M., Simoncini T. Menopause and aging, quality of life and sexuality. Climacteric. 2007;10:88–96. doi: 10.1080/13697130701297760. [DOI] [PubMed] [Google Scholar]
- 28.Fabre L.F., Brown C.S., Smith L.C. Gepirone-ER treatment of hypoactive sexual desire disorder (HSDD) associated with depression in women. J Sex Med. 2011;8:1411–1419. doi: 10.1111/j.1743-6109.2011.02216.x. [DOI] [PubMed] [Google Scholar]
- 29.Shifren J.L., Gass M.L. The North American Menopause Society recommendations for clinical care of midlife women. Menopause. 2014;21:1038–1062. doi: 10.1097/GME.0000000000000319. [DOI] [PubMed] [Google Scholar]
- 30.Cayan S., Akbay E., Bozlu M. The prevalence of female sexual dysfunction and potential risk factors that may impair sexual function in Turkish women. Urol Int. 2004;72:52–57. doi: 10.1159/000075273. [DOI] [PubMed] [Google Scholar]
- 31.Udenze I., Nnaji I., Oshodi T. Thyroid function in adult Nigerians with metabolic syndrome. Pan Afr Med J. 2014;18:352. doi: 10.11604/pamj.2014.18.352.4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neijenhuijs K.I., Hooghiemstra N., Holtmaat K. The female sexual function index (FSFI)-A systematic review of measurement properties. J Sex Med. 2019;16:640–660. doi: 10.1016/j.jsxm.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Isidori A.M., Pozza C., Esposito K. Development and validation of a 6-item version of the female sexual function index (FSFI) as a diagnostic tool for female sexual dysfunction. J Sex Med. 2010;7:1139–1146. doi: 10.1111/j.1743-6109.2009.01635.x. [DOI] [PubMed] [Google Scholar]
- 34.Castelo-Branco C., Biglia N., Nappi R.E. Characteristics of post-menopausal women with genitourinary syndrome of menopause: Implications for vulvovaginal atrophy diagnosis and treatment selection. Maturitas. 2015;81:462–469. doi: 10.1016/j.maturitas.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 35.Mollaioli D., Di Sante S., Limoncin E. Validation of a Visual Analogue Scale to measure the subjective perception of orgasmic intensity in females: the Orgasmometer-F. PLoS One. 2018;13:e0202076. doi: 10.1371/journal.pone.0202076. [DOI] [PMC free article] [PubMed] [Google Scholar]