Abstract
Background
The Aveed Risk Evaluation and Mitigation Strategy program was instituted because of potential risk of pulmonary oil microembolism (POME) and/or anaphylaxis after intramuscular injection of Aveed (testosterone undecanoate), indicated for treatment of adult male patients with congenital or acquired primary hypogonadism or hypogonadotropic hypogonadism.
Aim
To analyze the reporting rate of POME associated with testosterone undecanoate administration (750 mg/3 mL) during postmarketing surveillance.
Methods
The Endo Pharmaceuticals Inc database was searched for POME reports occurring from testosterone undecanoate approval on March 5, 2014, through June 30, 2018. Each case was reviewed and adjudicated by a drug safety physician to confirm the reported event had predefined clinical characteristics consistent with POME.
Outcomes
Annual rate and clinical features of spontaneously reported POME cases were characterized.
Results
During the 4.3-year period, 90,092 doses of intramuscular testosterone undecanoate were distributed via an Aveed Risk Evaluation and Mitigation Strategy program to health-care professionals for patient treatment. Of 633 individual case safety reports in the Endo Pharmaceuticals Inc safety database, 28 spontaneously reported adverse events were classified as POME, for a yearly spontaneously reported adverse event per-injection rate of <0.1%. Most (21/22) events resolved, and of those with a resolution time reported, most (13/17) resolved in ≤30 minutes. More than 60% (13/21) of patients required no medical intervention (ie, the POME event resolved spontaneously). One fatality was reported 18 months after a documented POME event and appeared unrelated to the reported testosterone undecanoate injection or subsequent injections after the POME event. In 3 out of 4 POME cases with symptoms serious enough to require an emergency room visit, issues with injection technique or dosing were identified as a potential contributing factor.
Clinical Implications
Injection technique and proper product usage are key elements in the prevention of POME events.
Strengths & Limitations
The reported rate of POME events was determined from a real-world clinical practice patient population; however, postmarketing safety data typically are underreported and retrospective in nature.
Conclusion
POME events appear to be rare, with resolution occurring quickly without medical intervention in most cases.
Pastuszak AW, Hu Y, Freid JD. Occurrence of Pulmonary Oil Microembolism After Testosterone Undecanoate Injection: A Postmarketing Safety Analysis. Sex Med 2020;8:237–242.
Key Words: Cough, Hypogonadism, Product Surveillance, Pulmonary Embolism, Safety, Testosterone
Introduction
Testosterone undecanoate (Aveed; Endo Pharmaceuticals Inc, Malvern, PA) was approved by the US Food and Drug Administration (FDA) in 2014 for treatment of adult male patients diagnosed with congenital or acquired primary hypogonadism or hypogonadotropic hypogonadism that resulted in a deficiency in or absence of endogenous testosterone.1 Some meta-analyses have supported that testosterone treatment is generally safe and does not increase cardiovascular or venous thromboembolism risk in patients diagnosed with late-onset hypogonadism.2,3 However, a 2019 case-crossover study (6-month case period) reported that testosterone therapy increased the risk of venous thromboembolism in men with or without hypogonadism (n = 39,622).4
In contrast to venous thromboembolism, pulmonary oil microembolism (POME) is an acute (eg, <60 minute onset) reaction to injection of an oil-based compound and has been reported as an adverse event related to testosterone preparations formulated in oil, including castor oil (eg, testosterone undecanoate), to provide a longer half-life than non–oil-based preparations.5 For injectable testosterone in an oil vehicle, the adverse events related to drug administration methods in clinical trials have included injection-site pain and POME.6, 7, 8, 9, 10 Testosterone undecanoate (750 mg/3 mL) is injected into the gluteus medius muscle with a dosing schedule of administration at treatment initiation (baseline), week 4, and then every 10 weeks.1 Steady-state concentrations of serum testosterone are achieved after the third injection in hypogonadal male patients.6 Of 130 hypogonadal male patients injected with testosterone undecanoate 750 mg, 1 patient experienced POME immediately after the third injection (week 14).6,10 This patient experienced a mild, nonproductive cough that resolved without intervention within 10 minutes. A 2016 meta-analysis supported that testosterone therapy in hypogonadal men had an acceptable safety profile, although studies included were of short duration.11
The pathophysiology of POME is not well understood and appears to involve accidental delivery of the oil vehicle into the venous circulation, where it subsequently moves to the lungs and results in coughing.12, 13, 14 During a 3.5-year prospective observational Australian study (N = 347) of 3,022 injections of testosterone undecanoate 1,000 mg in 4 mL of castor oil, POME was observed after 56 injections (66% very mild or mild, 19% severe; 40% with onset during injection) in 43 patients, with an estimated rate of 19 POME events (95% confidence interval, 14–24) per 1,000 injections.15 During a multicenter contraceptive study in China of monthly injections with testosterone undecanoate 500 mg in 4 mL of tea seed oil, 2.1% of 1,045 healthy men reported severe cough lasting minutes after injection, although no serious adverse events were reported during the study period.8 A published analysis of clinical trial data, as well as European postmarketing information from testosterone undecanoate 1,000 mg/4 mL (Nebido; Bayer Pharma AG, Berlin, Germany), observed a lower per-patient rate of POME, at 0.7% (1.24 cases per 1,000 injections).16 An integrated safety analysis of 18 clinical trials identified 9 adjudicated cases of POME events among 3,556 patients treated with either 750-mg or 1,000-mg testosterone undecanoate intramuscular injections.1
However, because a review of European data led to concerns of serious POME events,1 the FDA required a boxed warning in the US prescribing information for testosterone undecanoate injection. The boxed warning identifies serious POME reactions as urge to cough, dyspnea, throat tightening, chest pain, dizziness, and syncope. The US prescribing information also indicates that POME has occurred during or immediately after the administration of testosterone undecanoate and can occur after any injection of the drug during the course of therapy. The guidance instructs that patients be observed for 30 minutes after the injection to provide medical treatment in the event of POME. In addition, the FDA required Endo Pharmaceuticals Inc to institute an Aveed Risk Evaluation and Mitigation Strategy program to monitor POME and anaphylaxis adverse events.1
The objective of this article is to report the occurrence of POME in real-world clinical practice, as determined via spontaneously reported adverse events received by Endo Pharmaceuticals Inc, and to calculate a reporting rate (ie, the approximate occurrence of POME in relation to all events reported and recorded in the Endo Pharmaceuticals safety database for testosterone undecanoate).
Materials and methods
Adverse event cases identified as POME using predefined criteria (discussed in the following sections; developed in collaboration between Endo Pharmaceuticals Inc and the FDA) that occurred since the US launch of testosterone undecanoate on March 5, 2014, through June 30, 2018, were retrieved from a search of the Endo Pharmaceuticals Inc safety database (Argus Version 8.0.1) and collated. In real time, to confirm the diagnosis of POME during the relevant timeframe, drug safety physicians manually reviewed each case considered to meet POME criteria. These physicians adjudicated cases and identified them as POME if the reported event(s) occurred during or within approximately 30 minutes after testosterone undecanoate injection and the reported symptoms were (1) cough or dyspnea (with or without other symptoms such as throat irritation, malaise, hyperhidrosis, chest pain, dizziness, or paresthesia) or (2) described and conveyed by a medically qualified reporter using the Medical Dictionary for Regulatory Activities term “pulmonary oil microembolism.”17
Results
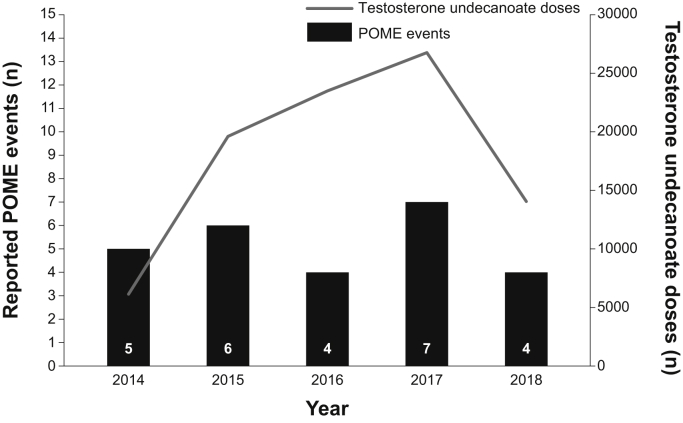
During the 4.3-year review period, 90,092 doses of testosterone undecanoate injection were distributed via the Aveed Risk Evaluation and Mitigation Strategy program to health-care professionals for patient treatment. The manufacturer received 633 MedWatch reports related to the product during this time period, 28 of which were adjudicated as POME. The rate of spontaneously reported adverse events of POME was <0.1% per injection annually (Figure 1), excluding 2 cases with no POME event date reported. Of the 28 cases of POME (Table 1), patient age was reported for 18 cases, with the mean age being 51 years (range, 33‒75 years). 6 of the 28 cases did not report a POME event outcome. Of the 22 cases for which the outcome of the POME event was captured as of the last report date, most (n = 21; 95.5%) were reported as resolved and 1 (4.5%) was reported as not recovering. In 19 cases for which the time to onset of the POME event after injection was reported, 94.7% (n = 18) of the cases were evident in ≤30 minutes. In 17 cases for which the resolution time of the event was reported, POME events were transient and resolved in ≤30 minutes (n = 13) or by 3 hours after onset (n = 4); 13 of these cases required no medical intervention. After the reported POME event, 64.3% (9/14) of the patients continued treatment with testosterone undecanoate.
Figure 1.
Number of reported pulmonary oil microembolism events and testosterone undecanoate doses by year. 2 cases that did not have POME event dates were excluded. POME = pulmonary oil microembolism.
Table 1.
Summary of pulmonary oil microembolism adverse events
| Case | Event year | Age, y | Event injection number | Event description as reported | Onset of POME event after injection, min | Duration of POME event, min | Medical intervention | Outcome of event |
|---|---|---|---|---|---|---|---|---|
| 1 | 2014 | NR | 1 | POME | NR | 120 | Yes, oxygen and steroid therapy in the office | NR |
| 2 | 2014 | 52 | 2 | 1) Cough | 0∗ | 20 | No | Recovered/resolved |
| 2) Sweat | ||||||||
| 3) Dizziness | ||||||||
| 3 | 2014 | 51 | 2 | POME with coughing fit and sweating | 1 | 2–3 | No | Recovered/resolved |
| 4 | 2014 | 55 | 3 | Coughing fits, uncontrollable coughing attacks | >30 | NR | NR | NR |
| 5 | 2014 | NR | 3† | Coughed a couple of times | 2 | 4 | No | Recovered/resolved |
| 6 | 2015 | NR | 4 | Coughing during an injection; urge to cough | <2 | 120–180 | No | Recovered/resolved |
| 7 | 2015 | 62 | 4 | Bad cough after leaving HCP office | NR | NR | No | NR |
| 8 | 2015 | 45 | 2 | POME | 0 | 60 | No | Recovered/resolved |
| 9 | 2015 | 53 | 2 | 1) Hard time breathing especially at night when trying to sleep | NR | NR | NR | Not recovered/resolved |
| 2) Nonstop coughing | ||||||||
| 10 | 2015 | 52 | 2 | 1) Felt tingling in mouth/pins in mouth sensation | 0∗ | 30 | No | Recovered/resolved |
| 2) Urge to cough | ||||||||
| 3) Began to sweat | ||||||||
| 4) Possible POME | ||||||||
| 11 | 2015 | 48 | 2 | Coughing immediately postinjection | <1 | 10 | No | Recovered/resolved |
| 12 | 2016 | NR | NR | Coughing postinjection | NR | NR | NR | Recovered/resolved |
| 13 | 2016 | 33 | 10 or 11 | Few minutes postinjection experienced a cough | Few | NR | Yes, ED visit | Recovered/resolved |
| 14 | 2016 | NR | NR | Possible POME | NR | NR | NR | NR |
| 15 | 2016 | 41 | NR | 1) Hemoptysis | NR | 10 | Yes | Resolved/subsequent mortality |
| 2) Coughing | ||||||||
| 16 | 2017 | NR | 4 | Dry cough | 0 | 5 | No | Recovered/resolved |
| 17 | 2017 | NR | NR‡ | Dry cough | 25 | 30 | No | Recovered/resolved |
| 18 | 2017 | 47 | 9 | POME | <1 | 5 | No | Recovered/resolved |
| 19 | 2017 | NR | 4 | Coughing constantly | 0 | 10 | No | Recovered/resolved |
| 20 | 2017 | 62 | 3 | Microembolism | <1 | NR | Yes, ED visit | Recovered/resolved |
| 21 | 2017 | 48 | 7 | 1) Difficulty breathing and catching breath | 15 | 60 | Yes, “supportive measures” in the office | Recovered/resolved |
| 2) Chest tightness | ||||||||
| 22 | 2017 | 35 | 7 | 1) Sensation to cough | NR | “A couple” | No | Recovered/resolved |
| 2) Flushed | ||||||||
| 23 | 2018 | 67 | 1 | 1) Coughing | 0 | 10 | Yes, oxygen in office | Recovered/resolved |
| 2) Clammy | ||||||||
| 24 | 2018 | 75 | NR | Slight cough at the end of injection procedure and a more aggressive cough postinjection | NR | NR | NR | Recovered/resolved |
| 25 | 2018 | 47 | NR | POME (shortness of breath and asthma-like symptoms) | 15 | NR | Yes, ED visit | Recovered/resolved |
| 26 | 2018 | NR | 2 | POME after receiving second injection (coughing, red eyes, diaphoresis) | 30 | NR | Yes, ED visit | NR |
| 27 | NR | NR | NR | POME | NR | NR | NR | NR |
| 28 | NR | 46 | 6 | Coughed for 4–5 minutes | 0∗ | 4–5 | No | Recovered/resolved |
ED = emergency department; HCP = health-care provider; NR = not reported; POME = pulmonary oil microembolism.
During injection; other “0” values represent onset of symptoms immediately after injection completion.
Patient had 1 additional injection with no symptoms.
Patient had multiple injections with no symptoms.
There were 4 reports of patients who received testosterone undecanoate, were assessed as having experienced POME, and were subsequently evaluated in the emergency department (Table 1). One report involved a 33-year-old male (case 13) who experienced a cough after a nurse used an inappropriate technique to inject the medication (ie, did not follow US prescribing information for administration1). The patient recovered and was discharged from the emergency department. No information was reported regarding administration of treatment for POME.
A second report involved a 62-year-old male (case 20) who received a reduced dose of testosterone undecanoate from 2 different vials (improper injection/dosing [ie, did not follow US prescribing information for administration1]) that resulted in cough within 20 seconds of administration, followed by respiratory distress, increased blood pressure and heart rate, and severe pain. The patient was treated with oxygen in the physician's office and transported to the emergency department, where he received 4 low-dose aspirin tablets (total dose, 324 mg). His symptoms were reported to have resolved. The patient was later diagnosed with both hypersensitivity to a component of the testosterone undecanoate injection formulation and POME.
The third report, for a male patient of unspecified age (case 26), stated that, during the injection procedure, the physician aspirated the syringe 3 times before administration of testosterone undecanoate (ie, inappropriate injection technique; did not follow US prescribing information for administration1), and the patient experienced cough, red eyes, and diaphoresis within 30 minutes of the injection. He was evaluated in the emergency department and diagnosed with POME. The report did not detail the treatment for POME, whether the patient was hospitalized, or if the symptoms resolved.
The fourth report involved a 47-year-old male (case 25) with a medical history of childhood asthma who developed shortness of breath and “asthma-like symptoms” (not otherwise specified) 15 minutes after receiving a testosterone undecanoate injection. He was monitored in the physician's office for 2 hours. The dyspnea did not resolve, and he was sent to an urgent care center. The staff at the center recommended that he be evaluated in the emergency department because they were unable to perform a chest x-ray. It is not known if he presented to the emergency department. Follow-up by the physician's office noted that the patient's symptoms had resolved.
There was 1 reported fatality (case 15) among the 28 cases of POME. This patient had experienced POME related to incorrect injection technique (ie, dose was administered during a shorter time period than stated in US prescribing information1) in October 2016, and the incident of POME resolved. The patient continued to receive testosterone undecanoate injection every 8 weeks without further suspected POME events reported until his death in June 2018 (more than 18 months after the event of POME) from an unspecified cause.
Discussion
The US spontaneous reporting rate for POME from March 5, 2014 (US approval date for testosterone undecanoate) through June 30, 2018, was <0.1% per injection annually. The reporting rate provides a useful estimate of the occurrence of POME in patients treated with testosterone undecanoate in real-world clinical practice. The reports received indicate that POME is an event that most often spontaneously resolves within 30 minutes of symptom onset. Incorrect injection technique or incorrect product usage was a common element in 3 out of the 4 POME cases for which symptoms required an emergency room visit. The 1 reported fatality appeared unrelated to POME, as the death occurred approximately 18 months after the POME event and the patient had received an unknown number of subsequent testosterone undecanoate injections during that time period. Overall, these data support the conclusion that POME is a rare event that occurs as the result of intramuscular injection of testosterone undecanoate and typically resolves spontaneously and that injection technique and proper product usage are key elements in the prevention of POME events.
The rates of spontaneously reported POME observed in this postmarketing analysis of patients in real-world clinical practice are somewhat lower (<0.1%) but consistent with the registration studies and published clinical studies with more defined patient populations (0.8%‒2.1%).6,8,10,15,16 The reported incidence of POME was 0.8% in the pivotal clinical study for testosterone undecanoate 750 mg conducted in the United States.6,10 Focusing specifically on POME adverse events, Meyer and Mann conducted a review of safety data available for 2 formulations of testosterone undecanoate, a 750-mg formulation (during clinical development) and a 1,000-mg formulation (clinical development and postmarketing surveillance in Europe).16 The per-patient rate of acute POME was 0.7%.16 In another clinical study with testosterone undecanoate in oil, severe coughing was observed at a per-patient rate of 2.1%.8 The results of this clinical study are similar to a prospective study that demonstrated a per-injection rate of 2.0% for POME.15 In all these studies and analyses, POME was mild and resolved quickly.6,8,10,15,16
One potential consequence of long-term testosterone therapy is venous thromboembolism.2, 3, 4 Meta-analyses from 2017 and 2018 demonstrated no increased risk for venous thromboembolism in men taking testosterone therapy.2,3 However, the investigators from the 2018 study concluded that the analysis would not be able to reveal significant differences because of the limited number of study populations analyzed.3 A 2019 analysis of a larger study population, of men with and without hypogonadism (n = 39,622), concluded that testosterone use (6-month case period) increased the risk of venous thromboembolism events.4 In contrast to venous thromboembolism, POME is an acute (<60-minute duration) adverse event associated with injection of an oil-based compound such as testosterone.7,16
A key strength of this study is that the reported rate of POME events for testosterone undecanoate 750 mg was determined from a real-world clinical practice patient population. Limitations of the present study include the fact that postmarketing safety data are typically underreported and retrospective in nature and that documentation of these events tends to be incomplete and reliant upon spontaneous reporting and response to follow-up queries.18 In some cases, underreporting occurs because, unless the event is considered serious, it is not hypothesized to be drug-related.18 However, testosterone undecanoate is only available through the Aveed Risk Evaluation and Mitigation Strategy Program, which requires health-care providers to be certified with the program and have equipment and personnel trained to manage POME before they can administer the testosterone undecanoate injection.1 Furthermore, certified health-care providers are required to observe patients for 30 minutes after injection.1 This is considered a sufficient observation period for capturing POME events and, thus, may help to increase the likelihood of spontaneous POME event reporting.
Conclusions
Testosterone undecanoate is a safe and effective form of testosterone therapy. Postmarketing surveillance (March 5, 2014, through June 30, 2018) of testosterone undecanoate demonstrated that POME events were rare and resolved quickly without medical intervention in the majority of cases reported. Some events that led to further evaluation in the emergency department were associated with non–label-adherent injection techniques or dosing errors. This emphasizes the need for careful attention to correct injection technique, as detailed in the prescribing information, to minimize or avert the occurrence of serious adverse events such as POME.
Statement of authorship
Category 1
-
(a)Conception and Design
- Alexander W. Pastuszak; Yiqun Hu; Jeffrey D. Freid
-
(b)Acquisition of Data
- Jeffrey D. Freid
-
(c)Analysis and Interpretation of Data
- Alexander W. Pastuszak; Yiqun Hu; Jeffrey D. Freid
Category 2
-
(a)Drafting the Article
- Alexander W. Pastuszak; Yiqun Hu; Jeffrey D. Freid
-
(b)Revising It for Intellectual Content
- Alexander W. Pastuszak; Yiqun Hu; Jeffrey D. Freid
Category 3
-
(a)Final Approval of the Completed Article
- Alexander W. Pastuszak; Yiqun Hu; Jeffrey D. Freid
Acknowledgments
Technical editorial and medical writing assistance was provided by Mary Beth Moncrief, PhD, and Julie B. Stimmel, PhD, Synchrony Medical Communications, LLC, West Chester, PA, under the direction of the authors.
Footnotes
Conflicts of Interest: Alexander W. Pastuszak reports being a consultant for Bayer, Boston Scientific, and Endo Pharmaceuticals Inc and serving on the speakers' bureau for Endo Pharmaceuticals Inc. Yiqun Hu and Jeffrey D. Freid are employees of Endo Pharmaceuticals Inc.
Funding: The study was funded by Endo Pharmaceuticals Inc.
References
- 1.AVEED® (testosterone undecanoate) injection, for intramuscular use CIII [package insert] Endo Pharmaceuticals Inc; Malvern, PA: 2019. [Google Scholar]
- 2.Corona G., Dicuio M., Rastrelli G. Testosterone treatment and cardiovascular and venous thromboembolism risk: what is ‘new’? J Investig Med. 2017;65:964–973. doi: 10.1136/jim-2017-000411. [DOI] [PubMed] [Google Scholar]
- 3.Houghton D.E., Alsawas M., Barrioneuvo P. Testosterone therapy and venous thromboembolism: a systematic review and meta-analysis. Thromb Res. 2018;172:94–103. doi: 10.1016/j.thromres.2018.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker R.F., Zakai N.A., MacLehose R.F. Association of testosterone therapy with risk of venous thromboembolism among men with and without hypogonadism. JAMA Intern Med. 2019 doi: 10.1001/jamainternmed.2019.5135. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behre H.M., Abshagen K., Oettel M. Intramuscular injection of testosterone undecanoate for the treatment of male hypogonadism: phase I studies. Eur J Endocrinol. 1999;140:414–419. doi: 10.1530/eje.0.1400414. [DOI] [PubMed] [Google Scholar]
- 6.Wang C., Harnett M., Dobs A.S. Pharmacokinetics and safety of long-acting testosterone undecanoate injections in hypogonadal men: an 84-week phase III clinical trial. J Androl. 2010;31:457–465. doi: 10.2164/jandrol.109.009597. [DOI] [PubMed] [Google Scholar]
- 7.Mackey M.A., Conway A.J., Handelsman D.J. Tolerability of intramuscular injections of testosterone ester in oil vehicle. Hum Reprod. 1995;10:862–865. doi: 10.1093/oxfordjournals.humrep.a136051. [DOI] [PubMed] [Google Scholar]
- 8.Gu Y., Liang X., Wu W. Multicenter contraceptive efficacy trial of injectable testosterone undecanoate in Chinese men. J Clin Endocrinol Metab. 2009;94:1910–1915. doi: 10.1210/jc.2008-1846. [DOI] [PubMed] [Google Scholar]
- 9.Sartorius G., Fennell C., Spasevska S. Factors influencing time course of pain after depot oil intramuscular injection of testosterone undecanoate. Asian J Androl. 2010;12:227–233. doi: 10.1038/aja.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morgentaler A., Dobs A.S., Kaufman J.M. Long acting testosterone undecanoate therapy in men with hypogonadism: results of a pharmacokinetic clinical study. J Urol. 2008;180:2307–2313. doi: 10.1016/j.juro.2008.08.126. [DOI] [PubMed] [Google Scholar]
- 11.Elliott J., Kelly S.E., Millar A.C. Testosterone therapy in hypogonadal men: a systematic review and network meta-analysis. BMJ Open. 2017;7:e015284. doi: 10.1136/bmjopen-2016-015284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keats T.E. Pantopaque pulmonary embolism. Radiology. 1956;67:748–750. doi: 10.1148/67.5.748. [DOI] [PubMed] [Google Scholar]
- 13.Bron K.M., Baum S., Abrams H.L. Oil embolism in lymphangiography. Incidence, manifestations, and mechanism. Radiology. 1963;80:194–202. doi: 10.1148/80.2.194. [DOI] [PubMed] [Google Scholar]
- 14.Gough J.H., Gough M.H., Thomas M.L. Pulmonary complications following lymphography; with a note on technique. Br J Radiol. 1964;37:416–421. doi: 10.1259/0007-1285-37-438-416. [DOI] [PubMed] [Google Scholar]
- 15.Middleton T., Turner L., Fennell C. Complications of injectable testosterone undecanoate in routine clinical practice. Eur J Endocrinol. 2015;172:511–517. doi: 10.1530/EJE-14-0891. [DOI] [PubMed] [Google Scholar]
- 16.Meyer R.J., Mann M. Pulmonary oil micro-embolism (POME) syndrome: a review and summary of a large case series. Curr Med Res Opin. 2015;31:837–841. doi: 10.1185/03007995.2015.1012254. [DOI] [PubMed] [Google Scholar]
- 17.AVEEDTM REMS education program for healthcare providers [package insert] Endo Pharmaceuticals Inc; Malvern, PA: 2016. [Google Scholar]
- 18.Huang Y.L., Moon J., Segal J.B. A comparison of active adverse event surveillance systems worldwide. Drug Saf. 2014;37:581–596. doi: 10.1007/s40264-014-0194-3. [DOI] [PMC free article] [PubMed] [Google Scholar]