Abstract
Introduction
Cardiometabolic syndrome (CMS), as a bunch of metabolic disorders mainly characterized by type 2 diabetes mellitus (T2DM), hypertension, atherosclerosis, central adiposity, and abdominal obesity triggering androgen deficiency, is one of the most critical threats to men. Although many significant preclinical and clinical findings explain CMS, new approaches toward common pathophysiological mechanisms and reasonable therapeutic targets are lacking.
Aim
To gain a further understanding of the role of androgen levels in various facets of CMS such as the constellation of cardiometabolic risk factors including central adiposity, dyslipidemia, insulin resistance, diabetes, and arterial hypertension and to define future directions for development of effective therapeutic modalities.
Methods
Clinical and experimental data were searched through scientific literature databases (PubMed) from 2009 to October 2019.
Main Outcome Measure
Evidence from basic and clinical research was gathered with regard to the causal impact and therapeutic roles of androgens on CMS.
Results
There are important mechanisms implicated in androgen levels and the risk of CMS. Low testosterone levels have many signs and symptoms on cardiometabolic and glycometabolic risks as well as abdominal obesity in men.
Clinical Implications
The implications of the findings can shed light on future improvements in androgen levels and add potentially predictive risk for CMS, as well as T2DM, abdominal obesity to guide clinical management in the early stage.
Strengths & Limitations
This comprehensive review refers to the association between androgens and cardiovascular health. A limitation of this study is the lack of large, prospective population-based studies that analyze the effects of testosterone treatment on CMS or mortality.
Conclusion
Low testosterone levels have several common features with metabolic syndrome. Thus, testosterone may have preventive role in the progress of metabolic syndrome and subsequent T2DM, abdominal obesity, and cardiovascular disease and likely affect aging men's health mainly through endocrine and vascular mechanisms. Further studies are necessary to evaluate the therapeutic interventions directed at preventing CMS in men.
Kirlangic OF, Yilmaz-Oral D, Kaya-Sezginer E, et al. The Effects of Androgens on Cardiometabolic Syndrome: Current Therapeutic Concepts. Sex Med 2020;8:132–155.
Key Words: Testosterone, Cardiometabolic Syndrome, Androgen Receptors, Type 2 Diabetes Mellitus, Hypogonadism, Androgen Replacement Therapy
Introduction
Cardiometabolic syndrome (CMS) is a group of metabolic disorders that occur together, increasing the risk of obesity, type 2 diabetes mellitus (T2DM), hypertension, hyperlipidemia, atherosclerosis.1,2 The most common cardiometabolic factors are hypertension, insulin resistance (IR), abdominal obesity, hypercholesterolemia, and low high-density lipoprotein (HDL)-cholesterol levels.3 CMS is an essential cause of the formation and progression of cardiovascular diseases (CVDs).4,5 CVDs triggers are primarily malnutrition, lack of physical activity, and metabolic syndromes (MetS) such as hypertension, obesity, T2DM, and risk factors to cardiometabolic disease.6 The prevalence of MetS was 17% among people over 40 years old, 29.7% for people between 40 and 49 years old, 37.5% between 50 and 59 years old, and over 44% among people aged ≥60 years.7 Definitions of MetS and its components have widely varied depending on several organizations.8 The “World Health Organization” and the most commonly used criteria “International Diabetes Federation” were adopted in 2005,8 and the “National Cholesterol Education Program Adult Treatment Panel III” was adopted in 2005 (Table 1).9 In the determination of MetS, the appearance of 3 of 5 risk factors is required: 1) abdominal obesity (measured by waist circumference), 2) fasting glucose (≥5.6 mmol/L), 3) HDL cholesterol (<1.0 mmol/L), 4) triglycerides (≥1.7 mmol/L, 5) blood pressure (≥130/≥85 mmHg) (Table 1).8 Based on “The National Health and Nutrition Examination Survey” data from 2001 to 2012, the prevalence of MetS in men increased with advanced age.10
Table 1.
Parameters defining metabolic syndrome
| Source | Parameters defining MetS | Ref. |
|---|---|---|
| International Diabetes Federation | • Central obesity and at least 4 of the following: • TG level > 150 mg/dL (1.7 mmol/L) • HDL cholesterol < 40 mg/dL (1.0 mmol/L) • Systolic BP ≥ 130 • Plasma glucose ≥ 100 mg/dL |
8 |
| World Health Organization | • Central obesity: waist/hip ratio >0.9 • TG level ≥150 mg/dL • HDL cholesterol <40 mg/dL • BP ≥140/90 mmHg • Plasma glucose: impaired glucose tolerance—impaired fasting glucose—T2DM |
8 |
| National Cholesterol Education Program Adult Treatment Panel III | • Central obesity: waist circumference ≥102 cm • TG level: specific treatment for lowering TG or ≥150 mg/dL • HDL cholesterol <40 mg/dL • BP ≥130/80 mmHg • Plasma glucose ≥100 ml/dL |
9 |
BP = blood pressure; HDL = high-density lipoprotein; MetS = metabolic syndromes; TG = triglycerides; T2DM = type 2 diabetes mellitus.
Because a rise in the incidence of CVDs in men has been indicated, androgens are thought of as vital hormones to clarify the underlying mechanisms of CVDs.11 Prospective data showed that men with low testosterone (T) levels were 40% more likely to die from CVD than with increased T concentrations.12 In addition, the protective role of androgens for the vascular system has also been reported.13 In other studies, it has been suggested that decreased androgen levels linked to hypogonadism or androgen deprivation therapy (ADT) enhance cardiovascular risk factors producing remarkable side effects, and T replacement therapy (TRT) caused amelioration in CV systems.14, 15, 16 1 meta-analysis reported that treatment with T reduced mortality and morbidity in CVD patients.17 On the contrary, a total of 93 randomized placebo-controlled studies revealed that T therapy did not reduce CV risk without an increase in CV risk.17 Meta-analysis trials showed a small increment in the risk of cardiovascular events linked to exogenous T.18
The National Institute of Health has issued policies mandating research data by investigators to focus on the understanding of androgen hormones on the role of metabolic processes in clinical trials.19 In addition, a longer duration of treatment with injectable T was associated with significant weight loss and metabolic improvements in an observational study.20 Besides, long-term T therapy was generally well tolerated with good adherence and improved urinary and sexual function with a high level of patient satisfaction scores.21
In this review, we focused on recent discoveries in the relationship between androgens (T and dihydrotestosterone [DHT]) and androgen receptors (ARs), their contribution to cardiometabolic health, and their potential role on CMS. The relationship between all these important factors and T deficit, as well as the mutual association among CMS, CVD, and hypogonadism was highlighted.
Androgen hormones and their receptors
Androgens represent a class of steroid hormones that regulate body development and sexual function in men.22, 23, 24 The actions of androgens are mediated through the AR in a DNA binding–dependent manner to manage target gene transcription.25 The major circulating androgen, T, is produced from cholesterol in the testicular Leydig cells.26 The AR has several and crucial biological roles in the development and maintenance of the reproductive function, CV, musculoskeletal, neural, immune, and hematopoietic systems.27 Androgens also are needed for the development of the male reproductive system and secondary sexual characteristics.28 T was demonstrated to play a modulatory role in the regulation of the male sexual response cycle. Especially, penile structure, regulation of nitric oxide pathway, and contractile pathways involved in the regulation of erection and detumescence are under androgen control.29
T can be converted to its more biologically active form, DHT, by 5α reductase and to estradiol by aromatase.25 The AR consists of 3 main functional domains: the N-terminal transcriptional regulation domain, the DNA binding domain, and the ligand-binding domain.30
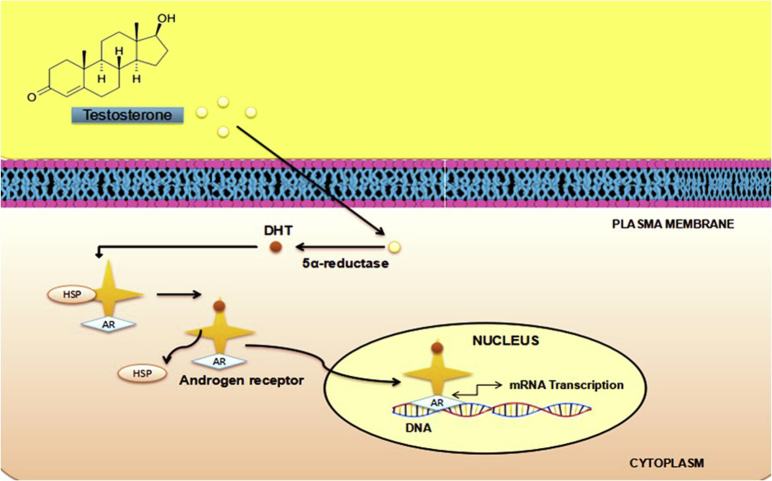
The DNA binding–dependent actions of the AR are also commonly referred to as “genomic” AR signaling.26 Upon entry of T into the target cell, it binds to the AR either directly or after its transformation to 5α-DHT (Figure 1).26 Binding to the receptor is accompanied by the change of the receptor configuration after a transformation and a translocation to the nucleus.26 Upon binding in the nucleus to specific DNA sequences, the receptor dimerizes with a second molecule and the homodimer interacts with further additional proteins (coregulators).31 This eventually causes transactivation of specific proteins or suppression of specific androgen-responsive genes.32 Crosstalk can occur at all stages of signaling cascades involving the DNA binding–independent or nongenomic actions of the AR.26
Figure 1.
The actions of androgens via androgen receptors (ARs). DHT = dihydrotestosterone; HSP = heat shock protein.
Androgens with the genomic and nongenomic signaling pathways regulate most of the intracellular transduction pathways concerned to glucose and lipid metabolism, including essential metabolic enzymes/proteins, nuclear transcription factors (peroxisome proliferator–activated receptor gamma, liver X receptor alpha, and forkhead box O1), inflammation, leptin sensitivity of hypothalamus, proliferation, differentiation of adipocytes, mitochondrial function, and vascular endothelial function.33, 34, 35 AR is crucial for male metabolism by regulating the energy balance.36
AR signaling pathways are potential targets for the prevention of androgen-related metabolic disorders.37 AR sensitivity differs between individuals and races.38 Men with more sensitive ARs have lower circulating T requirements than men with less sensitive ARs who require higher normal range T for receptor activation.39 Understanding the structure and function of the ligand-binding domain of the AR and its interaction with coregulators are important for the design of new AR antagonists and agonists.26 As such, understanding the role of androgen action mediated via both the DNA binding–dependent and non–DNA binding–dependent activity of the AR, in addition to the potential ligand-independent actions of the AR, in normal physiology as well as in different pathological conditions is crucial for the future therapeutic targets toward a wide range of AR-related clinical conditions.26
Androgens in CMS
T levels have established risk for CMS.40,41 T deficiency has detrimental effects on the health of men, such as cardiometabolic and glycometabolic functions, body composition and bone mineral density, sexual function, and the quality of life.42 Furthermore, Almehmadi et al showed a close relation between hypogonadism and CVD risk factors.43 T was conversely related to insulin, high-sensitivity C-reactive protein (hs-CRP), abnormal waist circumference, and HDL-cholesterol levels in males.44 Similarly, long-term T therapy for up to 12 years significantly improved sexual function, cardiometabolic risk factors such as anthropometric obesity measures, and metabolic risk factors.45 Numerous studies have indicated that both normal T levels and the increase of low T levels with TRT have preventive effects against CVD and cardiovascular mortality in older men with high cardiovascular risk.46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56 In hypogonadal men, the treatment of T is effective in the management of all cardiometabolic risk factors and may be an option as an add-on measure and secondary prevention strategies in hypogonadal men with a story of CVD.57 In contrast to previous studies, some issues have raised concerning the cardiovascular safety of exogenous T therapy, especially in older men.58 ADT is frequently used in the combination method for the therapy of prostate cancer and then can raise mortality from cardiovascular events. It was suggested that cardiometabolic risk due to ADT can be decreased by diet and lifestyle modification.59 However, further research is required to comprehend the impact of TRT on cardiometabolic health and to determine the beneficial or harmless dose, route, and duration time of T administration in clinical conditions.
Late-onset hypogonadism is linked to hyperglycemia, increased waist circumference, hypertriglyceridemia, hyperlipidemia, as well as diabetes mellitus.43 The connection between endogenous T levels and CV mortality and morbidity has been explained observationally in late-onset hypogonadism men. However, it has only been elucidated in randomized, placebo-controlled studies with low numbers of subjects and insufficient duration in men aged ≥60 years. A recent meta-analysis by Corona et al showed that low levels of T in aging men are a sign of CV risk.60 They also emphasized that the potential advantages of T treatment in the reduction of CV risk should be examined in long-term, specially designed studies.60
Hypogonadism is likely to happen due to the pathological process in aging men with physical disabilities, such as spinal cord injury.61 Young men with chronic spinal cord injury61 have a higher percentage of T deficiency compared with an age-matched control group.62 In young men with chronic spinal cord injury and an accelerated aging process after injury, hypogonadism is linked to an adverse cardiometabolic measure.63 Therefore, high-quality randomized controlled studies are required to investigate the efficacy and safety of TRT in this population with an increased risk of cardiometabolic diseases.64 Long-term T therapy reduces the risk of CVD with improvement in cardiometabolic function in men with hypogonadism.54 In addition, mortality associated with CVD was remarkably reduced in the T-group.54
The association between erectile dysfunction, hypogonadism, CVD, and T2DM is well documented.65 In a long-term (up to 12 years) clinical trial, T treatment induced management of erectile dysfunction, treated cardiometabolic risk factors, and reduced prostate cancer.45 Thus, T treatment should be given consistently for a long time, concerning the achievement of the maximum benefits.45 Although TRT in a physiological dose improved left ventricular function, some studies reported that it increased the risk of myocardial infarction in men with preexisting heart disease and hypogonadism, respectively.66,67 Low-dose TRT combined with vildagliptin (dipeptidyl peptidase 4 inhibitor) which increases the half-life of incretins may be an alternative for a physiological dose of TRT in conditions of obesity-IR with ADT.68
Higher circulating androgens and physical activity were linked to lower central adiposity at baseline and fewer CVD deaths in the recent follow-up study.69 A potential synergic effect of androgens and physical activity on cardiometabolic outcomes in aging males is consistent with previous data.69 Males with both hypogonadism and moderate-to-severe lower urinary tract symptoms possess a more severe cardiometabolic risk profile and benefit more from T treatment than males with mild lower urinary tract symptoms.70 T therapy is a highly effective treatment for ameliorating lower urinary tract symptoms in hypogonadal men.70 Treatment with T might also promote the possibility of adverse cardiovascular events.70,71
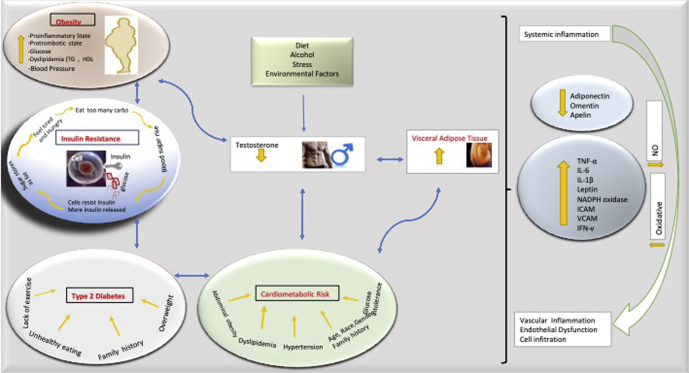
Fetal growth has a role in the programming of adult cardiometabolic disorders, which are linked to decreased T levels in males.72 Fetal growth and fetal androgen exposure could predetermine T levels in men, although how is unknown, as the adult Leydig cells do not differentiate until puberty.72 The data underline how a major component of male reproductive progress can essentially reprogram adult hormone production (through an epigenetic change), which may affect lifetime CVD risk.72 The results strongly suggest that T treatment in men with hypogonadism might prove useful in decreasing the risk of cardiometabolic diseases.73 The androgen hormones which are associated directly with CMS including abdominal obesity, hypertension, dyslipidemia, IR or T2DM, atherosclerosis, and inflammation in preclinical and clinical studies are summarized in Tables 2 and 3 and Figure 2.
Table 2.
The relationship between androgen levels and the components of cardiometabolic syndrome in different animal models
| Animal model | Results and conclusion | Ref. |
|---|---|---|
| Androgen deficiency induced by gonadectomy in middle-aged rats | -Lower plasma T concentration -No effect on blood pressure, plasma concentration of insulin, high- and low-density lipoprotein cholesterol, insulin resistance -Higher liver triacylglycerol concentration |
268 |
| Male Wistar rats fed with HFD/high-sucrose diet + orchiectomy | -Increased subcutaneous and visceral adiposity, circulating triglycerides, cholesterol, and insulin and low circulating T -T protected against subcutaneous fat accumulation and hypercholesterolemia in rats with HFD/high-sucrose diet and orchiectomy |
269 |
| Rats drinking a 10% fructose solution or fed with HFD (35%) for 10 weeks as a model of metabolic syndrome | -Higher plasma levels of luteinizing hormone and lower plasma levels of T -A significant increase in body weight, systolic blood pressure, plasma low-density lipoprotein cholesterol, cholesterol, triglycerides |
270 |
| Castrated rats fed with high-energy diet | -Slightly lower body weight, increased subcutaneous fat area, fasting glucose, and hemoglobin A1c -Unaffected fasting levels of insulin, triglycerides, total cholesterol, and high-density lipoprotein cholesterol levels |
271 |
| Hypogonadal aged male rats treated with T | -Decreased visceral fat cell size in T-treated group | 272 |
| Prenatal treatment with T or DHT in adult male rats | -Altered body composition by T and DHT -Increased subcutaneous fat depots and glucose levels in T males -No differences in insulin sensitivity, circulating lipid, and leptin levels by T and DHT |
273 |
| Male rabbits fed a HFD, with or without T supplementation and rabbits made hypogonadal by a single injection of a long-acting gonadotropin-releasing hormone analog, triptorelin |
-Normalized fasting glucose levels, glucose tolerance, and dramatically decreased visceral adipose tissue accumulation by T -A negative correlation between visceral fat accumulation and T plasma level -The highest amount of visceral fat in gonadotropin-releasing hormone-treated rabbits |
274 |
| Preadipocytes isolated from visceral adipose tissue of regular diet, HFD, and T-treated HFD rabbits | -Restored insulin sensitivity in visceral adipose tissue -Normalization of reduced insulin-stimulated triglyceride synthesis, glucose uptake in preadipocytes of HFD rats |
275 |
DHT = dihydrotestosterone; HFD = high-fat diet; T = testosterone.
Table 3.
Effects of testosterone replacement therapy in patients with cardiometabolic syndrome
| Ref. | Design (n) | Cohort | TRT method/duration | Results |
|---|---|---|---|---|
| 45 | Observational prospective (n = 850) | Hypogonadal men | T treatment for 12 years | • Improvements in cardiometabolic risk factors, erectile dysfunction, urinary function |
| 54 | Observational prospective (n = 656) | Hypogonadal men | T undecanoate (1,000 mg/12w) for 10 years | • Decreased systolic and diastolic blood pressure, levels of triglycerides, LDL and HDL, HbA1c levels, blood glucose levels, and body weight |
| 57 | Observational (n = 77) | Hypogonadal men with CVD | T undecanoate (1,000 mg/12w) for 8 years | • Decreased body weight, waist circumference, and BMI • Improved cardiometabolic parameters such as lipid pattern, glycemic control, blood pressure, heart rate, and pulse pressure |
| 70 | Observational prospective (n = 850) | Hypogonadal men | T undecanoate (1,000 mg/12w) for 8 years | • Considerable improvements in anthropometric parameters, lipids and glycemic control, blood pressure, C-reactive protein, and quality of life |
| 131 | Multicenter DBPC-RT (n = 220) | Hypogonadal men with T2DM and/or MetS | T gel 2%, TTS, for 12 months | • Reduced insulin resistance • Improvements in glycemic control, total and LDL cholesterol, body composition, libido, and sexual function |
| 137 | Multicenter DBPC-RT | Obese men with T2DM and serum T ≤ 14 nmol/L | T undecanoate (1,000 mg/12w) for 2 years | • Normalization in blood glucose and improved body composition. • Decrease in total and or abdominal fat mass and increase in lean mass and muscle strength |
| 225 | Crossover DBPC-RT (n = 24) | Hypogonadal men with T2DM | Intramuscular T injections (200 mg/3w) for 3 months | • Reduced HOMA-IR, glycated hemoglobin, and fasting blood glucose, visceral adiposity, waist circumference, total cholesterol, and no changes in blood pressure |
| 258 | DBPC-RT (n = 788) | Men ≥65 y and serum T levels <275 ng/dL | T gel 1%, for 12 months | • Decrease in total cholesterol, HDL, and LDL, fasting insulin, and HOMA-IR, and no alterations in triglycerides, d-dimer, C-reactive protein, interleukin 6, troponin, glucose, or HbA1c levels |
| 276 | RCT (n = 80) | Hypogonadal men with T2DM | T-gel (50 mg/day) for 9 months | • Significant decrease in waist circumference, HOMA-IR and HbA1c, concentrations of resistin, ICAM-1, p-selectin and C-reactive protein, leptin |
| 277 | CT (n = 102) | Hypogonadal men with T2DM & ischemic stroke | T undecanoate (1,000 mg/12w) for 2 years, re-evaluation at 5 years | • Reductions in BMI, the levels of cholesterol, triglycerides, LDL, and HDL and systolic and diastolic arterial pressures |
| 278 | DBPC-RT (n = 55) | Hypogonadal men with T2DM and obesity | T undecanoate (1,000 mg/10w) for 1 year | • Reductions in HOMA-IR and HbA1c • An increase in flow-mediated dilatation |
| 279 | CT (n = 42) | Hypogonadal men >40 years, with chronic heart failure and BMI>30 kg/m2 | T undecanoate (1,000 mg/2 injections), evaluation after 24 w | • Decline in insulin and serum glucose and a slight increase in LDL cholesterol and a decrease in triglycerides • No changes in other variables of metabolic syndrome and other biochemical variables, as well as echocardiographic variables, blood pressure |
| 280 | DBPC-RT (n = 39) | 50- to 70 year-old men with T2DM and T levels <7.3 nmol/L | T gel for 24 | • Decrease in high subcutaneous fat area, levels of adiponectin, leptin, leptin/adiponectin ratio, and HDL cholesterol and no change in hepatic fat content and visceral adipose tissue |
| 281 | Observational (n = 120) | Men with late-onset hypogonadism | T undecanoate (1,000 mg/10-14w) for 8 years | • Decreased waist circumference, percentage of body fat, glycated hemoglobin, cholesterol, LDL and no changes in BMI, HDL, triglyceride |
| 282 | Observational prospective (n = 115) | Hypogonadal men | T undecanoate (1,000 mg/10-14w) for up to 10 years | • A decrease in WC, body weight and BMI, fasting glucose, insulin resistance and HbA1c levels, the ratio of triglycerides: HDL, total cholesterol: HDL ratio and non–HDL cholesterol, systolic and diastolic blood pressure, C-reactive protein, and an increase in HDL levels |
| 283 | Observational (n = 58) | Men with mild symptoms of T deficiency and subnormal T levels (<2.35 ng/ml) | T undecanoate (1,000 mg/12 w) | • A reduction in total cholesterol, components of metabolic syndrome • Increase in whole blood viscosity, hemoglobin, and hematocrit levels |
| 284 | RCT (n = 857) | Men with T2DM | TRT | • TRT was not associated with improvements in cardiovascular disease risk factors. |
| 267 | Meta-analysis of observational studies (n = 4,513) | Men receiving TS in 32 observational studies which evaluate body mass composition and glycometabolic parameters | TS | • Body mass composition: decline in body fat, increase in lean mass • T2DM parameters: decline in fasting glycemia, HOMA-IR index • Obesity parameters: decline in BW, WC, and BMI • Blood pressure: decline in systolic and diastolic BP • Lipid profile: decline in total cholesterol, triglyceride and increase in HDL |
| 266 | Meta-analysis of RCTs (n = 5,078) | Men in TS and control groups of 59 RCTs which evaluate body mass composition and glycometabolic parameters | TS | • Body mass composition: decline in body fat, increase in lean mass • T2DM parameters: decline in fasting glycemia, HOMA-IR index • Obesity parameters: no changes in BW, WC, and BMI • Blood pressure: no changes in systolic and diastolic BP • Lipid profile: no changes in total cholesterol, triglyceride, HDL |
BMI = body mass index; BW = body weight; CT = controlled trial; DBPC-RT = double-blind placebo-controlled randomized trial; HbA1c = hemoglobin A1c; HDL = high-density lipoprotein HOMA-IR = homeostatic model assessment insulin resistance; LDL = low-density lipoprotein; RCT = randomized controlled trial; T = testosterone; T2DM = type 2 diabetes mellitus; BP = blood pressure; TRT = testosterone replacement therapy; TS = testosterone supplementation; WC = waist circumference; HOMA-IR = homeostatic model assessment insulin resistance.
Figure 2.
Interactions between cardiometabolic syndrome (CMS) components and testosterone (T).
For cardiologists and diabetologists, a vascular test using penile color Doppler procedure is used to predict erectile dysfunction and helps for better management of patients, their comorbidities, and complications. Further study is required to evaluate both a causal connection between hypogonadism and cardiometabolic risk in men and routine screening for T deficiency. Furthermore, clinical practice guidelines are necessary to develop for the specific dose and duration of TRT in patients with CMS.
Abdominal Obesity
Obesity is a rapidly growing problem due to its prevalence, costs, and health effects.74 Obesity is an abnormal accumulation of body fat and classified as a disease in 1990 and described as a body mass index (BMI) of 30 kg/m2 or more.75 Obesity is a probable risk even for patients without hypertension, high blood cholesterol, and T2DM.76 According to the WHO, there are around 2 billion overweight adults. It means that 39% of adults aged 18 years or over are obese (39% of men and 40% of women).74 In 1975, more than 1% of children and adolescents aged 5-19 years were obese, while more than 124 million children and adolescents were obese in 2016.77 The worldwide prevalence of obesity nearly tripled over the last 30 years.78 CVD risk was greater in obese individuals without MetS than in metabolically healthy normal-weight participants.76 Currently, hypogonadism in obese men is associated with the failure of male gonadal function.79 Few detailed clinical studies are available to define the exact role of androgens in the management of metabolism and body fat in men.80 Low T levels could cause increased visceral abdominal depots in the lack of inhibitory signals in adipogenesis and lipid uptake.80 In some men, the clinical signs of obesity and dysglycemia that include IR, MetS, and T2DM are consistent with androgen deficiency.81 Men with obesity commonly show low serum T levels that were usually below 10.5 nM of total T, comparing with the normal T levels which are around 20 nM in healthy men (Figure 3).82 Interestingly, the first signs of IR only can be observed when circulating T falls below 6 to 8 nM; however, patients with serum total T in the range 8 to 12 nM often have symptom of obesity-associated hypogonadism.83,84 Thus, careful assessment of T levels in men with obesity to diagnose hypogonadism is required to prevent severe adverse metabolic effects of lower T levels.79 Obese men also have fertility-related outcomes together with signs and symptoms caused by decreased circulating T levels.79
Figure 3.
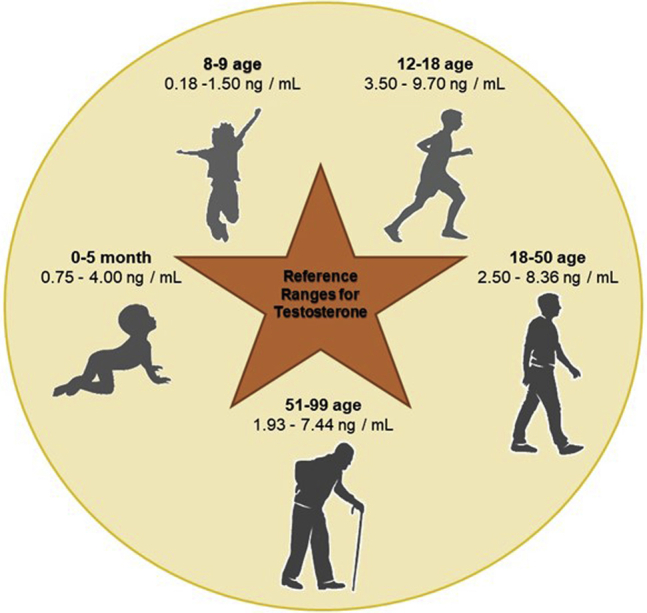
The age-specific reference ranges for testosterone (T).
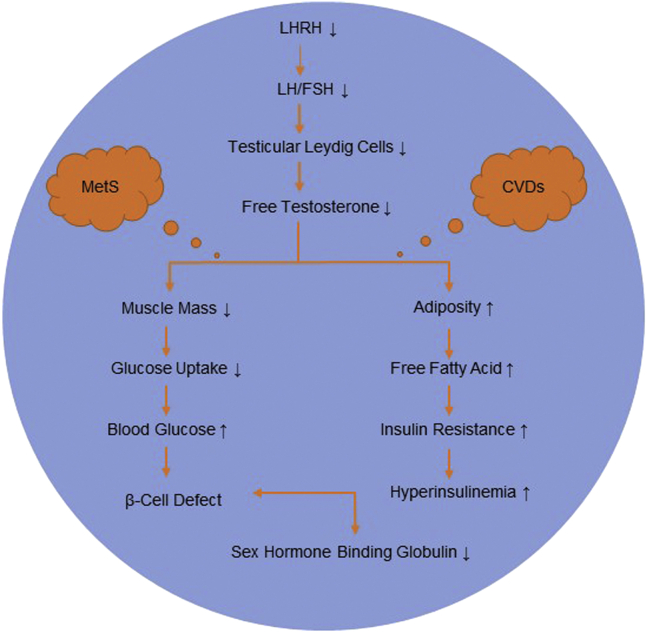
Obesity is related to increased inflammatory cytokine synthesis and aromatization of T to estradiol in peripheral adipose tissue.85 Fat cells also produce leptin and inflammatory cytokines, which have been conversely related to T levels, perhaps via an inhibitory effect on luteinizing hormone (LH) synthesis.86 High levels of leptin in men may be thought of as a direct prohibitory impact on Leydig cell function.87 Low total T and sex hormone–binding globulin (SHBG) concentrations have been associated with the MetS in men, but this association varies according to BMI and the number of MetS components.88 In addition, the pathophysiological mechanisms linking low T and SHBG concentrations to cardiometabolic risk should be clarified (Figure 4).88 Furthermore, visceral adiposity decreased SHBG levels and LH amplitude and affected bioavailable T in obese males.89 The diminished androgen response to LH stimulus has been caused by a deficiency in the enzymatic transformation of 17 OH-progesterone to T, which is explained by a leptin-related rise in 17 OH-progesterone level.90 The data have demonstrated that basal and LH-induced T levels in men were decreased in obesity and are inversely related to the circulating concentration of leptin.91 Increased levels of leptin in obese individuals may inhibit the stimulation of androgen synthesis by LH/human chorionic gonadotropin, thereby reducing T levels.86 These results provide evidence to suggest that leptin can make a major contribution to the pathogenesis of decreased androgens in obese men.91 Previous clinical studies have shown that TRT decreased levels of leptin and insulin, but no alterations were observed in serum glucose or lipids with a decrease in tumor necrosis factor-alpha (TNF-α), interleukin-1 beta, and CRP.87
Figure 4.
The pathophysiological mechanisms linking low testosterone (T) and sex hormone–binding globulin (SHBG) concentrations to cardiometabolic syndrome (CMS).
Central obesity leads to a rise in aromatase activity which promotes the one-direction transformation of T to estradiol. By decreasing estradiol levels, there would be a correlative increment in T levels.92 Moreover, low T levels also contribute to the accumulation of excessive fat, when obese males with BMI> 35 kg/m2 have considerably higher plasma estradiol and less T levels than healthy males.93 In preclinical studies, T deficiency reduced lipolysis.36,94 T induces lipolysis and reduces fatty acid synthesis.95,96 T usually inhibits adipocyte development, but there is a greater fat mass in states of low T levels.97
Obesity has been related to low T levels in numerous studies.89,93,98 In cross-sectional and longitudinal studies, low T concentration is linked with higher visceral fat accumulation.89 Previous data demonstrated that a reduction in BMI with an increase in total T showed a negative correlation between T and body fat in men.48,52,99 Triglyceride uptake and lipoprotein lipase activity can be inhibited by T and that triglycerides in the abdominal adipose tissue lead to faster turnover and induce visceral fat storage.100,101
TRT can reduce obesity, fat mass, waist circumference, morbidity, and mortality, as well as improve glycemic control and overall cardiometabolic status, compared with placebo in men with low T and high prevalence of CVD and MetS.102,103 It is widely accepted that TRT reduces fat mass as well as enhances muscle body mass.104 TRT for 60 months caused a significant decrease in body weight, waist circumference, and improvements in insulin sensitivity, lipid profile, systolic and diastolic blood pressure in hypogonadal men with the MetS.105 According to the facts, clinicians should be aware of the effects of TRT on body composition and parameters of MetS to improve clinical outcomes and the patient's quality of life.106 Long-term T treatment ameliorated MetS components with a high level of patient satisfaction.54,73 For instance, TRT should be restricted to men with low T levels related to central obesity and the resulting IR.106 The relationship between obesity and late-onset hypogonadism is bidirectional. Accordingly, weight loss can improve obesity-associated comorbidities as well as T levels.107,108 The effects of nonsurgical weight loss on T levels sometimes showed contradictory results, whereas an increase in T levels by the weight loss after bariatric surgery is greater than obtained with only lifestyle interventions, suggesting bariatric surgery for the management of hypogonadism in obese males.109 Although surgery did not improve sperm quality and function, bariatric surgery induced an increase in male sex hormones, a decrease in female sex hormones, and sustained weight loss in male patients with obesity.110 Obesity-associated functional hypogonadism can be controlled by weight loss and physical exercise.111 The meta-analysis of 13 published studies showed that the mean diet-induced weight reduction of 9.8% was associated with an increase in total T of 2.8 nmol/L and 2.05 nmol/L.111 The results of trials that enrolled 567 patients showed that a low-calorie diet leads to a significant increase in total T levels at the end point. Meta-regression analysis showed that each 5 kg of weight reduction causes a 1 nmol/L increase.111 A significant, sustained increase in total and free T after bariatric surgery was observed when compared to the presurgical values in 5 systematic meta-analyses.107,110,112, 113, 114 Combining the results of 8 trials, physical exercise increased total T levels at end point.115, 116, 117, 118, 119, 120, 121, 122 In summary, it has been suggested that lifestyle modifications with weight loss and exercise as well as bariatric surgery are significantly associated with a significant improvement in serum T levels.107,110,111
The identification of high cardiometabolic risk among overweight or obese patients can be managed by measuring waist circumference in addition to BMI. The usage of T supplementation as an anti-obesity drug is rising due to its effect through the reduction of visceral adipose tissue and an increase in muscle mass for men with hypogonadism. T can have numerous benefits for men with late-onset hypogonadism, but the exact role of T in the regulation of body composition is still unclear. The presence of MetS is correlated with reductions in T levels in the aging males. The use of clinical and biochemical criteria, such as the number of MetS components, is necessary for the diagnosis and management of late-onset hypogonadism.
Type 2 Diabetes Mellitus
Diabetes is described by hyperglycemia caused by defects in insulin action, insulin secretion, or both and defined as a fasting plasma glucose ≥7 mmol/L, a glycated hemoglobin ≥6.5%, or a 2-hour plasma glucose ≥11.1 mmol/L after 75 g oral glucose tolerance test. In addition, nephropathy, retinopathy, and neuropathy are known as microvascular complications of diabetes, and macrovascular complications are more prevalent in several CVDs with the rise in diabetes.123
One of the major factors that contribute to T2DM in male patients is the decrease in T levels, which has been demonstrated to be a marker for insulin resistance and the development of CVDs.124, 125, 126 A meta-analysis demonstrated that men with T2DM have significant reductions in total T, free T, and SHBG levels.127 The risk of T2DM enhanced in males with serum SHBG <40 nmol/L and serum T < 7 nmol/L.126 There are many mechanisms for the relationship between low serum T levels and T2DM with IR as well as central obesity.128,129 Furthermore, IR, and not hyperglycemia and weight per se, seems to be the main determinant of low SHBG related to low free T levels in obese men.130 In addition, previous data demonstrated the importance of IR as a mediating factor for the association between low T level and T2DM.131 Similarly, AR knockout mice revealed a greater IR.128 In mouse models, T regulated differentiation of pluripotent stem cells to the myogenic lineage but inhibited their commitment to adipocytes via T.97 In addition, T reduced IR by rising catecholamine-induced lipolysis in vitro, decreasing lipoprotein lipase activity and triglyceride uptake in human abdominal tissue in vivo.132
Many clinical trials in hypogonadal men with T2DM defined that TRT decreased body fat, glycated hemoglobin, fasting glucose, and triglyceride and did not affect BMI, blood pressure, total cholesterol, HDL-cholesterol levels.133, 134, 135, 136, 137 The treatment with injectable T undecanoate decreased fasting glucose and HbA1c levels in hypogonadal men.70,138 Also, T improved IR by promoting lipolysis and myogenesis.139 The results defined the effects of T treatment on body composition in male patients, involving an increase in muscle mass and a decrease in fat mass as well as a reduction in IR.97 Acute withdrawal of T in hypogonadal male patients for 2 weeks decreased insulin sensitivity without alterations in body composition, indicating that sex steroids directly regulate IR.140 In men with T2DM and low total T and high SHBG, a higher risk of mortality was observed without depending on age.141 Thus, the combination of SHBG and T assays may allow establishment of diagnostic and treatment thresholds in men with T2DM.141
MetS, which has a role in T2DM, also elevates the risk for CVD which ultimately results as the most common reason of death in diabetic patients.142 Similarly, a previous clinical study showed that low T and DHT levels have been related to high risk for CV events/death in T2DM men.143 A low total T level increases the risk of MetS,144 while it is an independent risk factor for T2DM.145 TRT showed beneficial impacts on metabolic factors and insulin sensitivity in men with MetS and T2DM.131 Also, systematic review and meta-analysis detected constructive impacts of TRT on the decline in waist circumference, IR, fasting glucose, and the increase in HDL levels in hypogonadal patients with MetS.133,145,146
Hypertension
Hypertension affects 25% of the population in the United States and is the most prevalent CVDs.147 From the preclinical and human epidemiological studies, blood pressure is modulated by androgens and estrogens.148
Men have mostly higher blood pressure than women based on human data.147,149, 150, 151 In preclinical studies, including several hypertensive mouse and rat studies, the male group has a higher blood pressure than the female group.148,152, 153, 154, 155 Preclinical data indicated that androgens were likely to cause hypertension via inducing sodium reabsorption156 or via enhancing angiotensinogen synthesis in the kidney.157 In hypertensive nonobese rats, it has been demonstrated that androgens cause an enhancement in blood pressure.158 Interestingly, the AR is also a nuclear factor kappa B target gene.159 Wu et al noted that 20-hydroxy-5,8,11,14-eicosatetraenoic acid, which contributed to oxidative stress, endothelial dysfunction, and inflammation via activation of nuclear factor kappa B, induced androgen-mediated hypertension.160 In individual cell types, the AR may play independently significant roles in hypertension progress. In a previous study, the use of mouse strain with a floxed AR exon 3 mice for the deletion of AR in selective cells has seemed interesting to study the role of AR in hypertension.161 In addition, a hybrid rat model lacking functional AR represented decreased blood pressure levels,162 showing that androgen and AR signaling can play a crucial role in hypertension. However, controversial observations show that AR knockout mice had high blood pressure,163 and castration failed to prevent prenatally programmed hypertension.164 Also, AR-deficient mice had higher blood pressure than control rats.159 It was showed that androgens probably induced oxidative stress and elevated endothelin synthesis, resulting in hypertension due to renal vasoconstriction in men.165,166
Reduced adiponectin/leptin ratio suggested as a marker of dysfunctional adipose tissue, is associated with increased number of cardiometabolic risk factors.167 Thus, adipocyte dysfunction induced endothelial alterations that seem to be the primarily involved mechanism in the association between androgen deficiency and hypertension.89,168 Furthermore, high levels of leptin may mainly promote hypertension by overactivity of the sympathetic nervous system.89,169
Treatment with androgen exacerbated hypertension and induced a high risk of CVDs.170 In fact, the androgen-induced direct effect may promote hypertension, which has been hypothesized.171 Recent clinical data showed that subcutaneous T treatment increased mean systolic and diastolic blood pressure after 26 weeks.172 Thus, androgen/AR signaling increases hypertension and anti-androgen treatment might inhibit hypertension. However, few epidemiological studies have shown opposite data that T has an inverse correlation with blood pressure in the male population.173,174 Furthermore, hypertensive men had higher SHBG levels and lower free T than normotensive men, and free T was inversely associated with systolic and diastolic blood pressure following adjustment for covariates such as age, smoking, alcohol consumption, and physical activity.175 Androgen deficiency increases the prevalence of hypertension and CVD.176,177 In aged males, total T levels are inversely involved in systolic blood pressure independently from other risk factors and preexisting health conditions.178,179 Indeed, hypogonadal men had a 24% greater risk of mortality related to CVDs.178 In middle-aged and older male patients, lower T levels are linked to CVD, such as hypertension.168 A previous study demonstrated that in men with hypertension, total T is independently and conversely related to central pulse pressure, wave reflections, and left ventricular mass.180 Several researchers have indicated that lower T levels lead to chronic CVDs and disease progression.181 Previous clinical studies demonstrated that T therapy caused considerable improvement in blood pressure and quality of life.45,70 Furthermore, TRT induced a reduction in diastolic and systolic blood pressure,182,183 but TRT exacerbates hypertension and CVD risk in men without T deficiency.184
Androgen deficiency, especially hypoandrogenism is related to the increased prevalence of hypertension.89 When aged males with hypertension have reduced levels of androgens, androgen supplementation decreased the blood pressure of these patients. Molecular mechanisms linking androgen dysregulation to hypertension are almost unknown, but they are likely to be associated with enhanced visceral fat and chronic inflammatory state via different mechanisms. There is a need to elucidate the crosstalk between AR and nuclear factor kappa B signaling pathway, and the pathophysiological mechanism of androgen dysregulation leads to the development and progression of MetS and hypertension. Consequently, further study is needed to explore the relationship between androgen/AR signaling and hypertension.
Atherosclerosis
In the vascular wall, an inflammatory disease characterized by a chronic accumulation of lipids into arterial intima attributed to a response-to-injury is known as atherosclerosis.185 Atherosclerosis progression rarely leads to major symptoms due to the preservation of the arterial lumen. However, the expansion of the sclerotic area eventually causes progressive destruction of vascular smooth muscle cells and secretion of proteases in the plaque leading to rupture, which can result in thrombotic occlusion.185
The relationship between androgens and atherogenesis, which is one of the major significant issues in the determination of impacts related to androgens on CVDs, is poorly understood. Based on emerging data about the association between male gender and high death incidence from atherosclerosis, the hypothesis of androgens that promoted the development of atherosclerosis has gained importance.186,187
In an epidemiological study, an inverse correlation was shown between atherosclerosis and T levels in men indicating that atherosclerosis development can be suppressed by the physiological levels of androgens.188 In addition, there was an inverse relation between T levels and apolipoprotein B, a biomarker implicated in subclinical atherosclerosis.189 Also, ADT showed an increase in atherosclerosis in prostate cancer patients,190 and DHT administration inhibited foam cell formation and thus suppressed atherosclerosis.191 A study by Basaria et al indicated that alterations in intima-media thickness or calcium scores were not linked to alterations in T levels between patients receiving T treatment.47 On the contrary, among aging males with hypogonadism, treatment with T gel for 1 year was linked to enhanced total plaque volume, but without alterations in coronary artery calcium score.192 Similarly, Alamir et al indicated a trend toward higher values of T and lower plaque volume, but this association was not statistically significant.193 Also, the increased incidence of CVDs, including atherosclerosis, has been shown in the abuse of androgens in athletes.181 In the interpretation of these results, the conversion of androgen into estrogens and nongenomic actions of androgens should be considered as an influencing factor on atherosclerosis development.194, 195, 196 Besides the conversion into estradiol, potential atheroprotective effects of T could be a direct action of ARs. Some beneficial effects were observed by targeting AR in specific cell types without suppressing androgen levels in atherosclerotic mice.197
In summary, studies indicated that there is a complex role for androgen action in atherosclerosis. Targeting AR in selective cells rather than targeting androgen levels might offer a better therapy in atherosclerosis. Furthermore, the role of the AR on atherosclerosis is still required to be addressed.
Dyslipidemia
Dyslipidemia is known as increased low-density lipoprotein (LDL), triglycerides, total cholesterol levels, or low HDL levels.198 Obese people commonly have dyslipidemia as a threatening factor for CVD. Imbalance of lipid profile, dysfunctional fat metabolism, fat deposition, obesity, and cardiovascular risk is increased in dyslipidemia. In patients with coronary artery disease, dyslipidemia is estimated to account for more than half of the worldwide cases.198 Dyslipidemic state is prospectively related to low T levels in men, and in reverse, a correlation in the same direction has been reported for total and free T with HDL.174,199 Low T levels are likely to have harmful effects on CVD by negatively affecting dyslipidemia.200 Also, the promotion of lipolysis and reduction of fatty acid synthesis are controlled by T.96 A strict bidirectional connection between fat accumulation and T deficiency in men has been shown.201 In men, to elucidate the potential risk for atherosclerosis and CVD is importantly involved in the impact of T on the lipid profile.
In multiple cross-sectional studies, endogenous T levels affect lipid metabolism in the existence of a less atherogenic lipid profile with higher T levels.202 Low androgen levels are connected to dyslipidemia in older diabetic male patients.203 Low T levels showed a tendency to a higher prevalence of dyslipidemia in controlled hypertensive patients.204
The effects of T on lipid levels in circulation are inconsistent. In several studies, deficiency of T is accompanied by enhanced LDL and triglyceride levels,39,199,205, 206, 207 as well as reduced HDL in men with and without T2DM208, 209, 210 or atherogenic lipid profiles with all these indicators.211 In some cross-sectional studies, it was not found any association between endogenous T and serum lipid measurements212,213 or even raised LDL in patients in high endogenous T profiles. In the many series of reviews and meta-analyses of clinical studies that related TRT in men with eugonadism and hypogonadism, significant declines of LDL-C and total cholesterol have been reported.214, 215, 216 TRT led to decreased LDL, total cholesterol, triglycerides, and increased HDL.216, 217, 218, 219 In hypogonadal and normal men with T treatment, lipoprotein was additionally lowered. This is a particle of LDL-like possessing atherogenic and thrombotic properties and is notable as an independent risk factor for atherosclerosis.131,220, 221, 222 The declines in LDL levels after different doses of atorvastatin treatment did not induce any significant differences in adrenal hormone levels in men with hypercholesterolemia.223 Furthermore, treatment with rosuvastatin decreased free T profiles but did not affect sexual function in T2DM patients.224
In most of the studies on T on HDL effects, there were inconsistent results such as no change87,225, 226, 227 or a decrease.131,226,228 The differences between the studies are still not apparent. It has been suggested that reverse cholesterol transport by T stimulation may increase HDL consumption rather than T which has negatively any effect on HDL.39 The differences in most of these studies are due to discrepancies in patient age, T-preparation, and administration route, given dose and treatment duration. Also, some previous studies do not consider the various changes in these differences and how they additionally lower lipoprotein subfractions.229
The meta-analysis showed that TRT decreased total-cholesterol levels in the presence of low baseline T levels, while it did not affect normal baseline T levels.215 Also, a previous study revealed the exact contrary relation between TRT and its effectiveness on total cholesterol levels regarding baseline T levels.230 Therefore, conclusive statements cannot be made based on the effect of TRT on overall cholesterol levels. TRT led to a decline in the levels of both HDL and LDL and did not change triglyceride levels.214 On the other hand, long-term T therapy significantly improved triglycerides, triglyceride-glucose index, lipid accumulation, total cholesterol, HDL ratio, LDL, HDL, and non-HDL.138
High cholesterol levels were shown to be one of the most critical risk factors for the development of atherosclerosis.231 Many types of research have examined the correlation between levels of T and lipoprotein profile. However, extensive-prospective population-based studies linking between T and lipid levels are not available. There are uncertainty and inconsistent results that are available in earlier studies.
Inflammation
The regulation of inflammation is controlled by T, and thus, several metabolic pathways are regulated indirectly.232 The immune system is also affected by androgens, and various parts of the immune system, including mast cells, neutrophils, macrophages, T-cells, and B-cells, are reported to express AR suggesting immunoregulatory activities of androgens.233, 234, 235, 236 The AR is likely to be a connection with many components having a significant role in the control of immune and inflammatory response and cell growth and differentiation.233, 234, 235, 236 Epidemiological and clinical data reported elevated inflammatory biomarkers in response to androgen deficiency and reduced levels of such biomarkers by T therapy.70,237, 238, 239, 240, 241, 242, 243 The evidence mentioned previously suggests that there is an inverse relationship between androgen concentration and proinflammatory cytokines. Androgens have anti-inflammatory effects, and androgen replacement treatment in hypogonadal men induces a considerable decrease in inflammatory cytokine levels.227
The source of inflammatory cytokines in adipose tissues is linked to androgen actions through stimulation of lipolysis244 and inhibition of adipose tissue lipoprotein lipase activity.245 Low T levels have been found to be associated with enhanced adipocytes-derived inflammatory markers such as leptin,246,247 adiponectin.104 The exogenous T suppressed serum leptin and adiponectin concentrations in young men.90,248 Increases in other proinflammatory chemokines such as monocyte chemoattractant protein-1 that are essential to control monocyte/macrophage infiltration into adipose tissue could be a reason for the decrease in androgen levels.249,250 Besides, the significant inverse relationship between interleukin-6 and T displays a considerable role of low visceral fat inflammation in the hypogonadism linked to MetS.251 A meaningful reverse relation between soluble interleukin-6 receptor and T was reported in 473 older males252 and between T and TNF-α and macrophage inflammatory protein 1-α levels in young males.253 Anti-inflammatory treatment with an antagonist of interleukin-1 induced an increment in T levels in obese male patients with T deficiency.254 The increased circulating and cavernosal concentrations of TNF-α, CRP, intercellular adhesion molecule-1, and monocyte chemoattractant protein-1, as well as decreased T levels in aged rats, were normalized by the TNF-α inhibitor, etanercept.255 T deficiency increased inflammation in castrated rats, which can cause ED.256
However, the effects of T on the inflammatory markers are controversial. Bianchi et al conducted a systematic literature review to investigate the potential effect of T on the modulation of the proinflammatory cytokines secretion.257 According to this study, an inverse relationship between T level and inflammatory markers has been found among the 17 studies except one that assessed men with low T levels.257 However, a wide discrepancy has been found among the results of 18 studies that evaluated the impact of T administration on inflammatory markers.257 Exogenous T had no effect on inflammatory markers in 6 studies.257 In a previous trial, T treatment did not change levels of inflammatory markers such as interleukin-6 and CRP levels compared with placebo in men (aged ≥65 years old with an average of serum T levels <275 ng/Dl).258 In a similar manner, supplementation of oral T undecanoate (dosage of 160 mg daily for 26 weeks) did not enhance hs-CRP levels in men (n = 237, aged 60 to 80 years).259 It has been reported that low T level and high hs-CRP levels are independent predictors of MetS.260
Diabetic men with low T levels have impairments of metabolic profile and leukocyte-endothelium cell interaction, inflammation enhancement, and mitochondrial function, which are the harmful factors for cardiovascular events.261 Pancreatic β cells can be protected by T with the binding to the AR in these cells resulting in ameliorated insulin sensitivity. Increasing lipolysis and suppressing the following inflammation by the activation of several hormones as well as enzymes make it a potent tool for the management of obesity and T2DM.232 T leads to a reduction of adipose tissues linked to inflammation and corrects insulin sensitivity instead of primary role on a glucose disposition such as insulin and other T2DM treatments.232 TRT is commonly used in hypogonadal patients. However, it is not widely used for T2DM therapy although hypogonadal patients show many common symptoms like T2DM patients, such as insulin insensitivity, increased inflammation, obesity, reduction in muscle strength and mass.232 Moderate elevation of T concentrations with obesity involved in inflammatory factors can control glucose homeostasis via raising IR and earlier insulin secretion.262 T treatment in men with T2DM and hypogonadotropic hypogonadism increases insulin sensitivity and together decreases circulating levels of inflammation markers.263 T directly and indirectly (via growth hormone stimulation) decreases inflammation levels in adipose tissue and thus protecting the role against IR.263 The β-cell islets from AR knockout male mice have different gene expression of insulin secretion and inflammation, demonstrating a significant role of androgens in the regulation of β-cell functions.264 Therefore, androgen action in β-cell health in males with implications for T2DM development is essential.264 TRT also might benefit with regard to heart health leading to enhanced cardiac output, anti-inflammatory, and vasodilation properties.263
Androgen deficiency might support inflammatory and immune responses inside adipose tissue by alternative mechanisms. Consequently, it is evident that T has a significant regulatory role in inflammatory response processes. Androgen replacement decreases inflammation state in men via protection by MetS progression. However, contradictory data concerning the impact of T treatment on inflammatory cytokines have emerged as a consequence of different dose, route, T therapy duration time, and features of study design. Furthermore, the conversion of T to the estrogen may interfere with the inflammatory state and cause misinterpretation of the T effects on inflammation. Further investigations that are defining the impact of T therapy are needed to elucidate the exact mechanism between androgens and the inflammatory processes.
Future Perspectives
Androgens present either positive or negative effects on human cardiovascular health in normal and pathological conditions. In healthy men, endogenous T may be preservative in males and decrease CVD risk. However, the benefits of exogenous TRT on CVD in male patients with low levels of T remain controversial, and tangible evidence concerning the effects of T therapy on CVD-related mortality is lacking. Many studies are needed to clarify the effects of androgens on CV health and to shed light on the fundamental mechanisms in the bidirectional interplay between androgens and CMS.
Obesity is a condition of low-grade inflammation, and adipose tissue produces considerable quantities of proinflammatory markers as obesity develops. In addition, there is a bidirectional relation between levels of T and obesity as well as inflammatory markers. Estradiol-T imbalance resulting from an increase in aromatase activity by obesity is also an area that needs further studies to understand the effects of estradiol on the T homeostasis. Low T levels enhance the risk of visceral obesity, MetS and CVDs, and conversely, visceral obesity also induces low T levels. Furthermore, TRT has a favorable effect on obesity, fat mass, waist circumference, as well as cardiometabolic status.
Abnormal lipid profiles, the increment of proinflammatory markers, IR, and hypertension are widespread symptoms in males with androgen deficiency, so it is hard to separate the pathophysiology of each constituent without the consideration of these subjects in a comprehensive framework. From a clinical perspective, androgen levels add potentially predictive risk beyond obesity. Furthermore, androgen deficiency by raising visceral obesity and inducing adipocyte and endothelial dysfunction is likely to be a critical factor for the enhanced prevalence of hypertension in men. In addition, nuclear factor kappa B regulates androgen receptor expression, and its enhanced activation might lead to the development and progression of hypertension and MetS in a state of decreased AR occupation. The connection between hypertension and androgen levels is multifaceted related to chronic arterial diseases because of other common risk factors. Further studies are required to elucidate the underlying molecular mechanisms of androgen deficiency that is related to the increased risk of hypertension.
Atherosclerosis is a primary risk factor for CVDs. In epidemiological studies, an inverse correlation was shown between atherosclerosis and T levels in men, so the development of atherosclerosis can be suppressed under the physiological levels of androgens. In addition, a direct effect of T on plaque progress is likely to be mediated by the vascular ARs. Furthermore, T and long-term administration of T causes relaxations of the isolated aorta and can have a favorable effect on the blood flow of coronary arteries in men with chronic arterial disease. Therefore, men receiving or planning to start ADT with a personal or family history of CVDs might benefit from screening for various cardiovascular risks.
High-concentration T supposed to be related to the increased risk of CVD in men, but this assumption is uncertain for different reasons. Some studies investigating the association between T concentration and CVD show a different relation between T and the risk of CVD. In a meta-analysis, T is associated indirectly with CVD among men over age 70 years, which proposes that an age-related decline in T may be liable for the indirect relation between T concentration and the risk of CVD.
Some investigations have suggested that T deficiency is a potential risk factor for CVD. TRT has various outcomes on the risk of CVD based on T concentration before therapy. For example, TRT reduces the risk of CVD in hypogonadal men and raises the risk of CVD in healthy men. MetS, which is a significant risk factor for CVD, is related to T concentration. T deficiency linked to T2DM together with raised fasting glucose and fasting insulin. In men with T deficiency, TRT increases insulin sensitivity and decreases glucose and insulin and the risk of T2DM. Besides, TRT decreases obesity and promotes lean tissue mass, both of which would provide to lowering the risk of T2DM in the hypogonadal men. Thus, the “advantage” of T may be associated more with advancements in IR and altered muscle glucose metabolism than advances in CVD, particularly when considering the effect of T in hypogonadal men.
Recent studies in AR knockout animal models have enabled a more detailed description of androgen signaling, lipid metabolism, and involvement in atherosclerosis. AR knockout mice models demonstrated increased weight, plasma cholesterol, liver TG content, and disrupted glucose metabolism that was reversed by a nonaromatizable AR agonist, DHT. Consequently, the AR signal reduces atherosclerosis and improves the lipid profile as well as risk factors for CVD.
There is one interesting observation which we think should be considered during the translation of literature regarding the impact of TRT on CMS-related parameters. Also addressed in a recent review by Rastrelli et al,265 discrepancy exists between the results of meta-analyses from RCTs and observational studies regarding some of the CMS parameters.266,267 While a meta-analysis of observational studies demonstrated significant improvements in almost all outcomes (fat mass, lean mass, body weight, waist circumference, BMI, fasting glycemia, HOMA-IR index, lipid profile, and systolic and diastolic blood pressure),267 there was no impact of TRT on body weight, waist circumference, BMI, blood pressure and lipid profile in a meta-analysis of RCTs.266 Possible overall explanations proposed for this discordance include the higher severity of hypogonadism and the longer follow-up periods in observational studies as compared to RCTs.265 Specifically for body weight parameters (body weight, waist circumference, and BMI), decreasing and increasing effects of TRT on fat and lean mass, respectively, may eradicate their opposite impact preventing a net change in body weight and related parameters.
Despite many investigations regarding the cardiac, metabolic, and hormonal effects of androgens, there is still a need for better understanding of precise mechanisms of androgens for improving all components of the CMS. Ongoing research might provide the knowledge required to fully understand the different responses to androgens in the CMS.
Statement of authorship
Category 1
-
(a)Conception and Design
- Omer Faruk Kirlangic; Serap Gur
-
(b)Acquisition of Data
- Omer Faruk Kirlangic; Gamze Toktanis; Aybuke Suveyda Tezgelen; Ekrem Sen; Armagan Khanam; Serap Gur
-
(c)Analysis and Interpretation of Data
- Omer Faruk Kirlangic; Didem Yilmaz-Oral; Ecem Kaya-Sezginer; Gamze Toktanis; Aybuke Suveyda Tezgelen; Ekrem Sen; Armagan Khanam; Cetin Volkan Oztekin; Serap Gur
Category 2
-
(a)Drafting the Article
- Omer Faruk Kirlangic; Didem Yilmaz-Oral; Ecem Kaya-Sezginer; Serap Gur
-
(b)Revising It for Intellectual Content
- Omer Faruk Kirlangic; Didem Yilmaz-Oral; Ecem Kaya-Sezginer; Cetin Volkan Oztekin; Serap Gur
Category 3
-
(a)Final Approval of the Completed Article
- Omer Faruk Kirlangic; Didem Yilmaz-Oral; Ecem Kaya-Sezginer; Gamze Toktanis; Aybuke Suveyda Tezgelen; Ekrem Sen; Armagan Khanam; Cetin Volkan Oztekin; Serap Gur
Footnotes
Conflict of Interest: The author(s) report no conflicts of interest.
Funding: None.
References
- 1.Kloner R.A. Testosterone and cardiovascular health: safety of treatment of hypogonadism. Sex Med Rev. 2015;3:56–62. doi: 10.1002/smrj.36. [DOI] [PubMed] [Google Scholar]
- 2.Kloner R.A., Carson C., 3rd, Dobs A. Testosterone and cardiovascular disease. J Am Coll Cardiol. 2016;67:545–557. doi: 10.1016/j.jacc.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Ruiz-Fernandez N., Espinoza M., Barrios E. [Cardiometabolic factors in a comunity located at Valencia city, Venezuela] Rev Salud Publica (Bogota) 2009;11:383–394. doi: 10.1590/s0124-00642009000300007. [DOI] [PubMed] [Google Scholar]
- 4.Srivastava A.K. Challenges in the treatment of cardiometabolic syndrome. Indian J Pharmacol. 2012;44:155. doi: 10.4103/0253-7613.93579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grundy S.M., Cleeman J.I., Daniels S.R. Diagnosis and management of the metabolic syndrome: an American heart association/National heart, Lung, and blood Institute scientific statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 6.Greenfield D.M., Snowden J.A. Cardiovascular diseases and metabolic syndrome. In: Carreras E., Dufour C., Mohty M., editors. The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies. Springer; Cham, Switzerland: 2019. p. 415. [PubMed] [Google Scholar]
- 7.Jahangiry L., Shojaeezadeh D., Montazeri A. Health-related quality of life among people Participating in a metabolic syndrome E-screening program: a Web-based study. Int J Prev Med. 2016;7:27. doi: 10.4103/2008-7802.174893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lakka H.-M., Laaksonen D.E., Lakka T.A. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. Jama. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 9.Ford E.S., Giles W.H., Dietz W.H. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. Jama. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 10.Shin D., Kongpakpaisarn K., Bohra C. Trends in the prevalence of metabolic syndrome and its components in the United States 2007-2014. Int J Cardiol. 2018;259:216–219. doi: 10.1016/j.ijcard.2018.01.139. [DOI] [PubMed] [Google Scholar]
- 11.Kelly D.M., Jones T.H. Testosterone and cardiovascular risk in men. Front Horm Res. 2014;43:1–20. doi: 10.1159/000360553. [DOI] [PubMed] [Google Scholar]
- 12.Kato D., Tabuchi H., Uno S. Safety, efficacy, and Persistence of long-term Mirabegron treatment for overactive Bladder in the daily clinical setting: interim (1-year) report from a Japanese post-Marketing Surveillance study. Low Urin Tract Symptoms. 2019;11:14–23. doi: 10.1111/luts.12188. [DOI] [PubMed] [Google Scholar]
- 13.Huang C.K., Lee S.O., Chang E. Androgen receptor (AR) in cardiovascular diseases. J Endocrinol. 2016;229:R1–R16. doi: 10.1530/JOE-15-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elsherbiny A., Tricomi M., Bhatt D. State-of-the-Art: a review of cardiovascular effects of testosterone replacement therapy in adult males. Curr Cardiol Rep. 2017;19:35. doi: 10.1007/s11886-017-0838-x. [DOI] [PubMed] [Google Scholar]
- 15.Traish A.M., Guay A., Feeley R. The dark side of testosterone deficiency: I. Metabolic syndrome and erectile dysfunction. J Androl. 2009;30:10–22. doi: 10.2164/jandrol.108.005215. [DOI] [PubMed] [Google Scholar]
- 16.Tsujimura A. The relationship between testosterone deficiency and men's health. World J Mens Health. 2013;31:126–135. doi: 10.5534/wjmh.2013.31.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corona G., Rastrelli G., Di Pasquale G. Testosterone and cardiovascular risk: meta-analysis of interventional studies. J Sex Med. 2018;15:820–838. doi: 10.1016/j.jsxm.2018.04.641. [DOI] [PubMed] [Google Scholar]
- 18.Onasanya O., Iyer G., Lucas E. Association between exogenous testosterone and cardiovascular events: an overview of systematic reviews. Lancet Diabetes Endocrinol. 2016;4:943–956. doi: 10.1016/S2213-8587(16)30215-7. [DOI] [PubMed] [Google Scholar]
- 19.Morselli E., Frank A.P., Santos R.S. Sex and gender: critical variables in pre-clinical and clinical Medical research. Cell Metab. 2016;24:203–209. doi: 10.1016/j.cmet.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 20.Cunningham G.R. Testosterone and metabolic syndrome. Asian J Androl. 2015;17:192–196. doi: 10.4103/1008-682X.148068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haider K.S., Haider A., Doros G. Long-term testosterone therapy improves urinary and sexual function, and quality of life in men with hypogonadism: results from a Propensity matched Subgroup of a controlled registry study. J Urol. 2018;199:257–265. doi: 10.1016/j.juro.2017.07.039. [DOI] [PubMed] [Google Scholar]
- 22.Morales A. The long and tortuous history of the discovery of testosterone and its clinical application. J Sex Med. 2013;10:1178–1183. doi: 10.1111/jsm.12081. [DOI] [PubMed] [Google Scholar]
- 23.Oettel M. The endocrine pharmacology of testosterone therapy in men. Naturwissenschaften. 2004;91:66–76. doi: 10.1007/s00114-003-0494-4. [DOI] [PubMed] [Google Scholar]
- 24.Bhasin S. Regulation of body composition by androgens. J Endocrinol Invest. 2003;26:814–822. doi: 10.1007/BF03345230. [DOI] [PubMed] [Google Scholar]
- 25.Chang C., Saltzman A., Yeh S. Androgen receptor: an overview. Crit Rev Eukaryot Gene Expr. 1995;5:97–125. doi: 10.1615/critreveukargeneexpr.v5.i2.10. [DOI] [PubMed] [Google Scholar]
- 26.McEwan I.J., Brinkmann A.O. Androgen physiology: receptor and metabolic disorders. In: Feingold K.R., Anawalt B., Boyce A., editors. Endotext. MDText. com, Inc.; South Dartmouth, MA: 2000. [Google Scholar]
- 27.Davey R.A., Grossmann M. Androgen receptor structure, function and Biology: from Bench to Bedside. Clin Biochem Rev. 2016;37:3–15. [PMC free article] [PubMed] [Google Scholar]
- 28.MacLean H.E., Chu S., Warne G.L. Related individuals with different androgen receptor gene deletions. J Clin Invest. 1993;91:1123–1128. doi: 10.1172/JCI116271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rastrelli G., Guaraldi F., Reismann Y. Testosterone replacement therapy for sexual symptoms. Sex Med Rev. 2019;7:464–475. doi: 10.1016/j.sxmr.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 30.MacLean H.E., Warne G.L., Zajac J.D. Localization of functional domains in the androgen receptor. J Steroid Biochem Mol Biol. 1997;62:233–242. doi: 10.1016/s0960-0760(97)00049-6. [DOI] [PubMed] [Google Scholar]
- 31.Heemers H.V., Tindall D.J. Androgen receptor (AR) coregulators: a diversity of functions converging on and regulating the AR transcriptional complex. Endocr Rev. 2007;28:778–808. doi: 10.1210/er.2007-0019. [DOI] [PubMed] [Google Scholar]
- 32.Wang Q., Li W., Zhang Y. Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell. 2009;138:245–256. doi: 10.1016/j.cell.2009.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bagchi G., Wu J., French J. Androgens transduce the G alphas-mediated activation of protein kinase A in prostate cells. Cancer Res. 2008;68:3225–3231. doi: 10.1158/0008-5472.CAN-07-5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gatson J.W., Kaur P., Singh M. Dihydrotestosterone differentially modulates the mitogen-activated protein kinase and the phosphoinositide 3-kinase/Akt pathways through the nuclear and novel membrane androgen receptor in C6 cells. Endocrinology. 2006;147:2028–2034. doi: 10.1210/en.2005-1395. [DOI] [PubMed] [Google Scholar]
- 35.Carrero J., Qureshi A., Parini P. Vol. 10. CH-4009 BASEL; Switzerland: 2008. Endogenous testosterone and mortality due to all-causes and cardiovascular disease in men undergoing hemodialysis. (Blood Purification: Karger Allschwilerstrasse). 435-35. [Google Scholar]
- 36.Yanase T., Fan W., Kyoya K. Androgens and metabolic syndrome: lessons from androgen receptor knock out (ARKO) mice. J Steroid Biochem Mol Biol. 2008;109:254–257. doi: 10.1016/j.jsbmb.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 37.Navarro G., Allard C., Xu W. The role of androgens in metabolism, obesity, and diabetes in males and females. Obesity (Silver Spring) 2015;23:713–719. doi: 10.1002/oby.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ackerman C.M., Lowe L.P., Lee H. Ethnic variation in allele distribution of the androgen receptor (AR) (CAG)n repeat. J Androl. 2012;33:210–215. doi: 10.2164/jandrol.111.013391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu F.C., von Eckardstein A. Androgens and coronary artery disease. Endocr Rev. 2003;24:183–217. doi: 10.1210/er.2001-0025. [DOI] [PubMed] [Google Scholar]
- 40.Ren J., Kelley R.O. Cardiac health in women with metabolic syndrome: clinical aspects and pathophysiology. Obesity (Silver Spring) 2009;17:1114–1123. doi: 10.1038/oby.2009.8. [DOI] [PubMed] [Google Scholar]
- 41.Tamate K., Charleton M., Gosling J.P. Direct colorimetric monoclonal antibody enzyme immunoassay for estradiol-17 beta in saliva. Clin Chem. 1997;43:1159–1164. [PubMed] [Google Scholar]
- 42.Traish A.M. Benefits and health implications of testosterone therapy in men with testosterone deficiency. Sex Med Rev. 2018;6:86–105. doi: 10.1016/j.sxmr.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Almehmadi Y., Yassin D.J., Yassin A.A. Erectile dysfunction is a prognostic indicator of comorbidities in men with late onset hypogonadism. Aging Male. 2015;18:186–194. doi: 10.3109/13685538.2015.1046044. [DOI] [PubMed] [Google Scholar]
- 44.Chrysohoou C., Panagiotakos D., Pitsavos C. Low total testosterone levels are associated with the metabolic syndrome in elderly men: the role of body weight, lipids, insulin resistance, and inflammation; the Ikaria study. Rev Diabet Stud. 2013;10:27–38. doi: 10.1900/RDS.2013.10.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saad F., Caliber M., Doros G. Long-term treatment with testosterone undecanoate injections in men with hypogonadism alleviates erectile dysfunction and reduces risk of major adverse cardiovascular events, prostate cancer, and mortality. Aging Male. 2020;23:81–92. doi: 10.1080/13685538.2019.1575354. [DOI] [PubMed] [Google Scholar]
- 46.Anderson J.L., May H.T., Lappe D.L. Impact of testosterone replacement therapy on myocardial infarction, stroke, and death in men with low testosterone concentrations in an Integrated health care system. Am J Cardiol. 2016;117:794–799. doi: 10.1016/j.amjcard.2015.11.063. [DOI] [PubMed] [Google Scholar]
- 47.Basaria S., Harman S.M., Travison T.G. Effects of testosterone administration for 3 Years on subclinical atherosclerosis progression in older men with low or low-normal testosterone levels: a randomized clinical trial. JAMA. 2015;314:570–581. doi: 10.1001/jama.2015.8881. [DOI] [PubMed] [Google Scholar]
- 48.Cheetham T.C., An J., Jacobsen S.J. Association of testosterone replacement with cardiovascular outcomes among men with androgen deficiency. JAMA Intern Med. 2017;177:491–499. doi: 10.1001/jamainternmed.2016.9546. [DOI] [PubMed] [Google Scholar]
- 49.Gagliano-Juca T., Icli T.B., Pencina K.M. Effects of testosterone replacement on Electrocardiographic parameters in men: findings from two randomized trials. J Clin Endocrinol Metab. 2017;102:1478–1485. doi: 10.1210/jc.2016-3669. [DOI] [PubMed] [Google Scholar]
- 50.Haring R., Volzke H., Steveling A. Low serum testosterone levels are associated with increased risk of mortality in a population-based cohort of men aged 20-79. Eur Heart J. 2010;31:1494–1501. doi: 10.1093/eurheartj/ehq009. [DOI] [PubMed] [Google Scholar]
- 51.Muraleedharan V., Marsh H., Kapoor D. Testosterone deficiency is associated with increased risk of mortality and testosterone replacement improves survival in men with type 2 diabetes. Eur J Endocrinol. 2013;169:725–733. doi: 10.1530/EJE-13-0321. [DOI] [PubMed] [Google Scholar]
- 52.Ohlsson C., Barrett-Connor E., Bhasin S. High serum testosterone is associated with reduced risk of cardiovascular events in elderly men. The MrOS (Osteoporotic Fractures in Men) study in Sweden. J Am Coll Cardiol. 2011;58:1674–1681. doi: 10.1016/j.jacc.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 53.Sharma R., Oni O.A., Gupta K. Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur Heart J. 2015;36:2706–2715. doi: 10.1093/eurheartj/ehv346. [DOI] [PubMed] [Google Scholar]
- 54.Traish A.M., Haider A., Haider K.S. Long-term testosterone therapy improves cardiometabolic function and reduces risk of cardiovascular disease in men with hypogonadism: a real-life observational registry study setting comparing treated and Untreated (control) groups. J Cardiovasc Pharmacol Ther. 2017;22:414–433. doi: 10.1177/1074248417691136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wallis C.J., Lo K., Lee Y. Survival and cardiovascular events in men treated with testosterone replacement therapy: an intention-to-treat observational cohort study. Lancet Diabetes Endocrinol. 2016;4:498–506. doi: 10.1016/S2213-8587(16)00112-1. [DOI] [PubMed] [Google Scholar]
- 56.Yeap B.B., Alfonso H., Chubb S.A. In older men, higher plasma testosterone or dihydrotestosterone is an independent predictor for reduced incidence of stroke but not myocardial infarction. J Clin Endocrinol Metab. 2014;99:4565–4573. doi: 10.1210/jc.2014-2664. [DOI] [PubMed] [Google Scholar]
- 57.Haider A., Yassin A., Haider K.S. Men with testosterone deficiency and a history of cardiovascular diseases benefit from long-term testosterone therapy: observational, real-life data from a registry study. Vasc Health Risk Manag. 2016;12:251–261. doi: 10.2147/VHRM.S108947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Al-Khazaali A., Arora R., Muttar S. Controversial effects of exogenous testosterone on cardiovascular diseases. Am J Ther. 2016;23:e1504–e1513. doi: 10.1097/MJT.0000000000000212. [DOI] [PubMed] [Google Scholar]
- 59.Turner L., Poole K., Faithfull S. Current and future strategies for the nutritional management of cardiometabolic complications of androgen deprivation therapy for prostate cancer. Nutr Res Rev. 2017;30:220–232. doi: 10.1017/S0954422417000087. [DOI] [PubMed] [Google Scholar]
- 60.Corona G., Rastrelli G., Di Pasquale G. Endogenous testosterone levels and cardiovascular risk: meta-analysis of observational studies. J Sex Med. 2018;15:1260–1271. doi: 10.1016/j.jsxm.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 61.Guarasci G.R., Kline R.L. Pressure natriuresis following acute and chronic inhibition of nitric oxide synthase in rats. Am J Physiol. 1996;270:R469–R478. doi: 10.1152/ajpregu.1996.270.2.R469. [DOI] [PubMed] [Google Scholar]
- 62.Sullivan S.D., Nash M.S., Tefera E. Prevalence and Etiology of hypogonadism in young men with chronic spinal cord injury: a cross-sectional analysis from two University-based Rehabilitation Centers. PM R. 2017;9:751–760. doi: 10.1016/j.pmrj.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sullivan S.D., Nash M.S., Tefara E. Relationship between gonadal function and cardiometabolic risk in young men with chronic spinal cord injury. PM R. 2018;10:373–381. doi: 10.1016/j.pmrj.2017.08.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nightingale T.E., Moore P., Harman J. Body composition changes with testosterone replacement therapy following spinal cord injury and aging: a mini review. J Spinal Cord Med. 2018;41:624–636. doi: 10.1080/10790268.2017.1357917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ryan J.G., Gajraj J. Erectile dysfunction and its association with metabolic syndrome and endothelial function among patients with type 2 diabetes mellitus. J Diabetes Complications. 2012;26:141–147. doi: 10.1016/j.jdiacomp.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 66.Finkle W.D., Greenland S., Ridgeway G.K. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One. 2014;9:e85805. doi: 10.1371/journal.pone.0085805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vigen R., O'Donnell C.I., Baron A.E. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829–1836. doi: 10.1001/jama.2013.280386. [DOI] [PubMed] [Google Scholar]
- 68.Arinno A., Apaijai N., Kaewtep P. Combination of low-dose testosterone and vildagliptin confers cardioprotection in castrated obese rats. J Endocrinol. 2019;240:467–481. doi: 10.1530/JOE-18-0673. [DOI] [PubMed] [Google Scholar]
- 69.Chasland L.C., Knuiman M.W., Divitini M.L. Higher circulating androgens and higher physical activity levels are associated with less central adiposity and lower risk of cardiovascular death in older men. Clin Endocrinol (Oxf) 2019;90:375–383. doi: 10.1111/cen.13905. [DOI] [PubMed] [Google Scholar]
- 70.Saad F., Doros G., Haider K.S. Hypogonadal men with moderate-to-severe lower urinary tract symptoms have a more severe cardiometabolic risk profile and benefit more from testosterone therapy than men with mild lower urinary tract symptoms. Investig Clin Urol. 2018;59:399–409. doi: 10.4111/icu.2018.59.6.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaminetsky J.C., McCullough A., Hwang K. A 52-week study of dose adjusted subcutaneous testosterone Enanthate in Oil Self-Administered via disposable Auto-Injector. J Urol. 2019;201:587–594. doi: 10.1016/j.juro.2018.09.057. [DOI] [PubMed] [Google Scholar]
- 72.Kilcoyne K.R., Smith L.B., Atanassova N. Fetal programming of adult Leydig cell function by androgenic effects on stem/progenitor cells. Proc Natl Acad Sci U S A. 2014;111:E1924–E1932. doi: 10.1073/pnas.1320735111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Traish A.M., Haider A., Doros G. Long-term testosterone therapy in hypogonadal men ameliorates elements of the metabolic syndrome: an observational, long-term registry study. Int J Clin Pract. 2014;68:314–329. doi: 10.1111/ijcp.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Centre for Public Health Excellence at NICE (UK) National Collaborating Centre for Primary Care (UK) National Institute for Health and Clinical Excellence (UK); London: 2006. Obesity: the prevention, identification, assessment and management of overweight and obesity in adults and children. (NICE Clinical Guidelines, No. 43) [PubMed] [Google Scholar]
- 75.Ogden C.L., Carroll M.D., Kit B.K. Prevalence of childhood and adult obesity in the United States, 2011-2012. Jama. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ettehad D., Emdin C.A., Kiran A. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. The Lancet. 2016;387:957–967. doi: 10.1016/S0140-6736(15)01225-8. [DOI] [PubMed] [Google Scholar]
- 77.Agha M., Agha R. The rising prevalence of obesity: part A: impact on public health. Int J Surg Oncol. 2017;2:e17. doi: 10.1097/IJ9.0000000000000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ogden C.L., Carroll M.D., Fryar C.D. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS Data Brief. 2015:1–8. [PubMed] [Google Scholar]
- 79.Carrageta D.F., Oliveira P.F., Alves M.G. Obesity and male hypogonadism: Tales of a vicious cycle. Obes Rev. 2019;20:1148–1158. doi: 10.1111/obr.12863. [DOI] [PubMed] [Google Scholar]
- 80.Tchernof A., Despres J.P. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93:359–404. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 81.Grossmann M., Ng Tang Fui M., Cheung A.S. Late-onset hypogonadism: metabolic impact. Andrology. 2019 doi: 10.1111/andr.12705. [DOI] [PubMed] [Google Scholar]
- 82.Travison T.G., Vesper H.W., Orwoll E. Harmonized reference ranges for circulating testosterone levels in men of Four cohort studies in the United States and Europe. J Clin Endocrinol Metab. 2017;102:1161–1173. doi: 10.1210/jc.2016-2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Singh A.B., Hsia S., Alaupovic P. The effects of varying doses of T on insulin sensitivity, plasma lipids, apolipoproteins, and C-reactive protein in healthy young men. J Clin Endocrinol Metab. 2002;87:136–143. doi: 10.1210/jcem.87.1.8172. [DOI] [PubMed] [Google Scholar]
- 84.Grossmann M. Testosterone and glucose metabolism in men: current concepts and controversies. J Endocrinol. 2014;220:R37–R55. doi: 10.1530/JOE-13-0393. [DOI] [PubMed] [Google Scholar]
- 85.Vermeulen A., Kaufman J., Goemaere S. Estradiol in elderly men. The Aging Male. 2002;5:98–102. [PubMed] [Google Scholar]
- 86.Isidori A.M., Caprio M., Strollo F. Leptin and androgens in male obesity: evidence for leptin contribution to reduced androgen levels. J Clin Endocrinol Metab. 1999;84:3673–3680. doi: 10.1210/jcem.84.10.6082. [DOI] [PubMed] [Google Scholar]
- 87.Kalinchenko S.Y., Tishova Y.A., Mskhalaya G.J. Effects of testosterone supplementation on markers of the metabolic syndrome and inflammation in hypogonadal men with the metabolic syndrome: the double-blinded placebo-controlled Moscow study. Clin Endocrinol (Oxf) 2010;73:602–612. doi: 10.1111/j.1365-2265.2010.03845.x. [DOI] [PubMed] [Google Scholar]
- 88.Brand J.S., Rovers M.M., Yeap B.B. Testosterone, sex hormone-binding globulin and the metabolic syndrome in men: an individual participant data meta-analysis of observational studies. PLoS One. 2014;9:e100409. doi: 10.1371/journal.pone.0100409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moretti C., Lanzolla G., Moretti M. Androgens and hypertension in men and women: a Unifying View. Curr Hypertens Rep. 2017;19:44. doi: 10.1007/s11906-017-0740-3. [DOI] [PubMed] [Google Scholar]
- 90.Adan L., Bussieres L., Trivin C. Effect of short-term testosterone treatment on leptin concentrations in boys with pubertal delay. Horm Res. 1999;52:109–112. doi: 10.1159/000023445. [DOI] [PubMed] [Google Scholar]
- 91.Caprio M., Isidori A., Isidori A. Leptin actions in rat Leydig cells. J Endocrinol Invest. 1999;22:14. [Google Scholar]
- 92.Raven G., de Jong F.H., Kaufman J.M. In men, peripheral estradiol levels directly reflect the action of estrogens at the hypothalamo-pituitary level to inhibit gonadotropin secretion. J Clin Endocrinol Metab. 2006;91:3324–3328. doi: 10.1210/jc.2006-0462. [DOI] [PubMed] [Google Scholar]
- 93.Vermeulen A., Kaufman J.M., Deslypere J.P. Attenuated luteinizing hormone (LH) pulse amplitude but normal LH pulse frequency, and its relation to plasma androgens in hypogonadism of obese men. J Clin Endocrinol Metab. 1993;76:1140–1146. doi: 10.1210/jcem.76.5.8496304. [DOI] [PubMed] [Google Scholar]
- 94.Taschler U., Radner F.P., Heier C. Monoglyceride lipase deficiency in mice impairs lipolysis and attenuates diet-induced insulin resistance. J Biol Chem. 2011;286:17467–17477. doi: 10.1074/jbc.M110.215434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.De Pergola G. The adipose tissue metabolism: role of testosterone and dehydroepiandrosterone. Int J Obes. 2000;24:S59. doi: 10.1038/sj.ijo.0801280. [DOI] [PubMed] [Google Scholar]
- 96.Dubois V., Laurent M.R., Jardi F. Androgen deficiency exacerbates high-fat diet-induced metabolic alterations in male mice. Endocrinology. 2016;157:648–665. doi: 10.1210/en.2015-1713. [DOI] [PubMed] [Google Scholar]
- 97.Singh R., Artaza J.N., Taylor W.E. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology. 2003;144:5081–5088. doi: 10.1210/en.2003-0741. [DOI] [PubMed] [Google Scholar]
- 98.Haffner S., Valdez R., Stern M. Obesity, body fat distribution and sex hormones in men. Int J Obes Relat Metab Disord J Int Assoc Study Obes. 1993;17:643–649. [PubMed] [Google Scholar]
- 99.Kapoor D., Malkin C., Channer K. Androgens, insulin resistance and vascular disease in men. Clin Endocrinol. 2005;63:239–250. doi: 10.1111/j.1365-2265.2005.02299.x. [DOI] [PubMed] [Google Scholar]
- 100.Allan C.A., McLachlan R.I. Androgens and obesity. Curr Opin Endocrinol Diabetes Obes. 2010;17:224–232. doi: 10.1097/MED.0b013e3283398ee2. [DOI] [PubMed] [Google Scholar]
- 101.Cheng J., Han B., Li Q. Testosterone: Relationships with metabolic disorders in men-an observational study from SPECT-China. Int J Endocrinol. 2017;2017:4547658. doi: 10.1155/2017/4547658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gururani K., Jose J., George P.V. Testosterone as a marker of coronary artery disease severity in middle aged males. Indian Heart J. 2016;68(Suppl 3):S16–S20. doi: 10.1016/j.ihj.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Basaria S., Coviello A.D., Travison T.G. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109–122. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Host C., Gormsen L.C., Hougaard D.M. Acute and short-term chronic testosterone fluctuation effects on glucose homeostasis, insulin sensitivity, and adiponectin: a randomized, double-blind, placebo-controlled, crossover study. J Clin Endocrinol Metab. 2014;99:E1088–E1096. doi: 10.1210/jc.2013-2807. [DOI] [PubMed] [Google Scholar]
- 105.Francomano D., Lenzi A., Aversa A. Effects of five-year treatment with testosterone undecanoate on metabolic and hormonal parameters in ageing men with metabolic syndrome. Int J Endocrinol. 2014;2014:527470. doi: 10.1155/2014/527470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bassil N., Alkaade S., Morley J.E. The benefits and risks of testosterone replacement therapy: a review. Ther Clin Risk Manag. 2009;5:427–448. doi: 10.2147/tcrm.s3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Corona G., Rastrelli G., Monami M. Body weight loss reverts obesity-associated hypogonadotropic hypogonadism: a systematic review and meta-analysis. Eur J Endocrinol. 2013;168:829–843. doi: 10.1530/EJE-12-0955. [DOI] [PubMed] [Google Scholar]
- 108.Rastrelli G., Lotti F., Reisman Y. Metabolically healthy and unhealthy obesity in erectile dysfunction and male infertility. Expert Rev Endocrinol Metab. 2019;14:321–334. doi: 10.1080/17446651.2019.1657827. [DOI] [PubMed] [Google Scholar]
- 109.Di Vincenzo A., Busetto L., Vettor R. Obesity, male reproductive function and bariatric surgery. Front Endocrinol (Lausanne) 2018;9:769. doi: 10.3389/fendo.2018.00769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee Y., Dang J.T., Switzer N. Impact of bariatric surgery on male sex hormones and sperm quality: a systematic review and meta-analysis. Obes Surg. 2019;29:334–346. doi: 10.1007/s11695-018-3557-5. [DOI] [PubMed] [Google Scholar]
- 111.Corona G., Rastrelli G., Morelli A. Treatment of functional hypogonadism besides Pharmacological substitution. World J Mens Health. 2019 doi: 10.5534/wjmh.190061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Corona G., Vignozzi L., Sforza A. Obesity and late-onset hypogonadism. Mol Cell Endocrinol. 2015;418 Pt 2:120–133. doi: 10.1016/j.mce.2015.06.031. [DOI] [PubMed] [Google Scholar]
- 113.Escobar-Morreale H.F., Santacruz E., Luque-Ramirez M. Prevalence of 'obesity-associated gonadal dysfunction' in severely obese men and women and its resolution after bariatric surgery: a systematic review and meta-analysis. Hum Reprod Update. 2017;23:390–408. doi: 10.1093/humupd/dmx012. [DOI] [PubMed] [Google Scholar]
- 114.Xu J., Wu Q., Zhang Y. Effect of bariatric surgery on male sexual function: a meta-analysis and systematic review. Sex Med. 2019;7:270–281. doi: 10.1016/j.esxm.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Armamento-Villareal R., Aguirre L.E., Qualls C. Effect of lifestyle intervention on the hormonal profile of Frail, obese older men. J Nutr Health Aging. 2016;20:334–340. doi: 10.1007/s12603-016-0698-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hiruntrakul A., Nanagara R., Emasithi A. Effect of endurance exercise on resting testosterone levels in sedentary subjects. Cent Eur J Public Health. 2010;18:169–172. doi: 10.21101/cejph.a3589. [DOI] [PubMed] [Google Scholar]
- 117.Arazi H., Ghiasi A., Afkhami M. Effects of different rest Intervals between Circuit resistance exercises on post-exercise blood pressure responses in normotensive young males. Asian J Sports Med. 2013;4:63–69. [PMC free article] [PubMed] [Google Scholar]
- 118.Hayes L.D., Sculthorpe N., Herbert P. Six weeks of conditioning exercise increases total, but not free testosterone in lifelong sedentary aging men. Aging Male. 2015;18:195–200. doi: 10.3109/13685538.2015.1046123. [DOI] [PubMed] [Google Scholar]
- 119.Kumagai H., Zempo-Miyaki A., Yoshikawa T. Lifestyle modification increases serum testosterone level and decrease central blood pressure in overweight and obese men. Endocr J. 2015;62:423–430. doi: 10.1507/endocrj.EJ14-0555. [DOI] [PubMed] [Google Scholar]
- 120.Kumagai H., Zempo-Miyaki A., Yoshikawa T. Increased physical activity has a greater effect than reduced energy intake on lifestyle modification-induced increases in testosterone. J Clin Biochem Nutr. 2016;58:84–89. doi: 10.3164/jcbn.15-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hayes L.D., Herbert P., Sculthorpe N.F. Exercise training improves free testosterone in lifelong sedentary aging men. Endocr Connect. 2017;6:306–310. doi: 10.1530/EC-17-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kumagai H., Yoshikawa T., Zempo-Miyaki A. Vigorous physical activity is associated with regular Aerobic exercise-induced increased serum testosterone levels in overweight/obese men. Horm Metab Res. 2018;50:73–79. doi: 10.1055/s-0043-117497. [DOI] [PubMed] [Google Scholar]
- 123.Beckman J.A., Creager M.A., Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287:2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 124.Hernandez-Mijares A., Garcia-Malpartida K., Sola-Izquierdo E. Testosterone levels in males with type 2 diabetes and their relationship with cardiovascular risk factors and cardiovascular disease. J Sex Med. 2010;7:1954–1964. doi: 10.1111/j.1743-6109.2010.01705.x. [DOI] [PubMed] [Google Scholar]
- 125.Liu X., Jiang J., Liu X. Association of serum testosterone with different classes of glucose metabolism and the mediation effect of obesity: the Henan Rural Cohort Study. Diabetes Metab Res Rev. 2019;35:e3133. doi: 10.1002/dmrr.3133. [DOI] [PubMed] [Google Scholar]
- 126.O'Reilly M.W., Glisic M., Kumarendran B. Serum testosterone, sex hormone-binding globulin and sex-specific risk of incident type 2 diabetes in a retrospective primary care cohort. Clin Endocrinol (Oxf) 2019;90:145–154. doi: 10.1111/cen.13862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang J., Li X., Cai Z. Association between testosterone with type 2 diabetes in adult males, a meta-analysis and trial sequential analysis. Aging Male. 2019:1–12. doi: 10.1080/13685538.2018.1557139. [DOI] [PubMed] [Google Scholar]
- 128.Lin H.Y., Xu Q., Yeh S. Insulin and leptin resistance with hyperleptinemia in mice lacking androgen receptor. Diabetes. 2005;54:1717–1725. doi: 10.2337/diabetes.54.6.1717. [DOI] [PubMed] [Google Scholar]
- 129.Mohammed M., Al-Habori M., Abdullateef A. Impact of metabolic syndrome factors on testosterone and SHBG in type 2 diabetes mellitus and metabolic syndrome. J Diabetes Res. 2018;2018:4926789. doi: 10.1155/2018/4926789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Souteiro P., Belo S., Oliveira S.C. Insulin resistance and sex hormone-binding globulin are independently correlated with low free testosterone levels in obese males. Andrologia. 2018;50:e13035. doi: 10.1111/and.13035. [DOI] [PubMed] [Google Scholar]
- 131.Jones T.H., Arver S., Behre H.M. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome (the TIMES2 study) Diabetes Care. 2011;34:828–837. doi: 10.2337/dc10-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xu X.F., De Pergola G., Bjorntorp P. Testosterone increases lipolysis and the number of beta-adrenoceptors in male rat adipocytes. Endocrinology. 1991;128:379–382. doi: 10.1210/endo-128-1-379. [DOI] [PubMed] [Google Scholar]
- 133.Corona G., Rastrelli G., Maggi M. Diagnosis and treatment of late-onset hypogonadism: systematic review and meta-analysis of TRT outcomes. Best Pract Res Clin Endocrinol Metab. 2013;27:557–579. doi: 10.1016/j.beem.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 134.Corona G., Monami M., Rastrelli G. Type 2 diabetes mellitus and testosterone: a meta-analysis study. Int J Androl. 2011;34:528–540. doi: 10.1111/j.1365-2605.2010.01117.x. [DOI] [PubMed] [Google Scholar]
- 135.Cai X., Tian Y., Wu T. Metabolic effects of testosterone replacement therapy on hypogonadal men with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Asian J Androl. 2014;16:146–152. doi: 10.4103/1008-682X.122346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dimitriadis G.K., Randeva H.S., Aftab S. Metabolic phenotype of male obesity-related secondary hypogonadism pre-replacement and post-replacement therapy with intra-muscular testosterone undecanoate therapy. Endocrine. 2018;60:175–184. doi: 10.1007/s12020-017-1516-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wittert G., Atlantis E., Allan C. Testosterone therapy to prevent type 2 diabetes mellitus in at-risk men (T4DM): design and implementation of a double-blind randomized controlled trial. Diabetes Obes Metab. 2018 doi: 10.1111/dom.13601. [DOI] [PubMed] [Google Scholar]
- 138.Yassin A., Haider A., Haider K.S. Testosterone therapy in men with hypogonadism prevents progression from Prediabetes to type 2 diabetes: eight-year data from a registry study. Diabetes Care. 2019;42:1104–1111. doi: 10.2337/dc18-2388. [DOI] [PubMed] [Google Scholar]
- 139.Ma R.C., Tong P.C. Testosterone levels and cardiovascular disease. Heart. 2010;96:1787–1788. doi: 10.1136/hrt.2010.207068. [DOI] [PubMed] [Google Scholar]
- 140.Yialamas M.A., Dwyer A.A., Hanley E. Acute sex steroid withdrawal reduces insulin sensitivity in healthy men with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2007;92:4254–4259. doi: 10.1210/jc.2007-0454. [DOI] [PubMed] [Google Scholar]
- 141.Ramachandran S., Hackett G.I., Strange R.C. Sex hormone binding globulin: a review of its interactions with testosterone and age, and its impact on mortality in men with type 2 diabetes. Sex Med Rev. 2019;7:669–678. doi: 10.1016/j.sxmr.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 142.Benjamin E.J., Virani S.S., Callaway C.W. Heart disease and stroke statistics-2018 update: a report from the American heart association. Circulation. 2018;137:e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 143.Malipatil N.S., Yadegarfar G., Lunt M. Male hypogonadism: 14-year prospective outcome in 550 men with type 2 diabetes. Endocrinol Diabetes Metab. 2019;2:e00064. doi: 10.1002/edm2.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cephus J.Y., Stier M.T., Fuseini H. Testosterone attenuates group 2 innate Lymphoid cell-mediated Airway inflammation. Cell Rep. 2017;21:2487–2499. doi: 10.1016/j.celrep.2017.10.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Corona G., Monami M., Rastrelli G. Testosterone and metabolic syndrome: a meta-analysis study. J Sex Med. 2011;8:272–283. doi: 10.1111/j.1743-6109.2010.01991.x. [DOI] [PubMed] [Google Scholar]
- 146.Zhang K.S., Zhao M.J., An Q. Effects of testosterone supplementation therapy on lipid metabolism in hypogonadal men with T2DM: a meta-analysis of randomized controlled trials. Andrology. 2018;6:37–46. doi: 10.1111/andr.12425. [DOI] [PubMed] [Google Scholar]
- 147.Burt V.L., Whelton P., Roccella E.J. Prevalence of hypertension in the US adult population. Results from the third National health and Nutrition Examination Survey, 1988-1991. Hypertension. 1995;25:305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- 148.Rowland N.E., Fregly M.J. Role of gonadal hormones in hypertension in the Dahl salt-sensitive rat. Clin Exp Hypertens A. 1992;14:367–375. doi: 10.3109/10641969209036195. [DOI] [PubMed] [Google Scholar]
- 149.Khoury S., Yarows S.A., O'Brien T.K. Ambulatory blood pressure monitoring in a nonacademic setting. Effects of age and sex. Am J Hypertens. 1992;5:616–623. doi: 10.1093/ajh/5.9.616. [DOI] [PubMed] [Google Scholar]
- 150.Wiinberg N., Hoegholm A., Christensen H.R. 24-h ambulatory blood pressure in 352 normal Danish subjects, related to age and gender. Am J Hypertens. 1995;8:978–986. doi: 10.1016/0895-7061(95)00216-2. [DOI] [PubMed] [Google Scholar]
- 151.Collier A., Ghosh S., McGlynn B. Prostate cancer, androgen deprivation therapy, obesity, the metabolic syndrome, type 2 diabetes, and cardiovascular disease: a review. Am J Clin Oncol. 2012;35:504–509. doi: 10.1097/COC.0b013e318201a406. [DOI] [PubMed] [Google Scholar]
- 152.Reckelhoff J.F., Roman R.J. Androgens and hypertension: role in both males and females? Hypertension. 2011;57:681–682. doi: 10.1161/HYPERTENSIONAHA.110.162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ouchi Y., Share L., Crofton J.T. Sex difference in the development of deoxycorticosterone-salt hypertension in the rat. Hypertension. 1987;9:172–177. doi: 10.1161/01.hyp.9.2.172. [DOI] [PubMed] [Google Scholar]
- 154.Ashton N., Balment R.J. Sexual dimorphism in renal function and hormonal status of New Zealand genetically hypertensive rats. Acta Endocrinol (Copenh) 1991;124:91–97. doi: 10.1530/acta.0.1240091. [DOI] [PubMed] [Google Scholar]
- 155.Chen Y.F., Meng Q.C. Sexual dimorphism of blood pressure in spontaneously hypertensive rats is androgen dependent. Life Sci. 1991;48:85–96. doi: 10.1016/0024-3205(91)90428-e. [DOI] [PubMed] [Google Scholar]
- 156.Kienitz T., Quinkler M. Testosterone and blood pressure regulation. Kidney Blood Press Res. 2008;31:71–79. doi: 10.1159/000119417. [DOI] [PubMed] [Google Scholar]
- 157.Chen Y.F., Naftilan A.J., Oparil S. Androgen-dependent angiotensinogen and renin messenger RNA expression in hypertensive rats. Hypertension. 1992;19:456–463. doi: 10.1161/01.hyp.19.5.456. [DOI] [PubMed] [Google Scholar]
- 158.Yanes L.L., Sartori-Valinotti J.C., Iliescu R. Testosterone-dependent hypertension and upregulation of intrarenal angiotensinogen in Dahl salt-sensitive rats. Am J Physiol Ren Physiol. 2009;296:F771–F779. doi: 10.1152/ajprenal.90389.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhang L., Altuwaijri S., Deng F. NF-kappaB regulates androgen receptor expression and prostate cancer growth. Am J Pathol. 2009;175:489–499. doi: 10.2353/ajpath.2009.080727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wu C.C., Cheng J., Zhang F.F. Androgen-dependent hypertension is mediated by 20-hydroxy-5,8,11,14-eicosatetraenoic acid-induced vascular dysfunction: role of inhibitor of kappaB Kinase. Hypertension. 2011;57:788–794. doi: 10.1161/HYPERTENSIONAHA.110.161570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lin T.H., Yeh S., Chang C. Tissue-specific knockout of androgen receptor in mice. Methods Mol Biol. 2011;776:275–293. doi: 10.1007/978-1-61779-243-4_16. [DOI] [PubMed] [Google Scholar]
- 162.Ely D.L., Salisbury R., Hadi D. Androgen receptor and the testes influence hypertension in a hybrid rat model. Hypertension. 1991;17:1104–1110. doi: 10.1161/01.hyp.17.6.1104. [DOI] [PubMed] [Google Scholar]
- 163.Huang C.K., Luo J., Lai K.P. Androgen receptor promotes abdominal aortic aneurysm development via modulating inflammatory interleukin-1alpha and transforming growth factor-beta1 expression. Hypertension. 2015;66:881–891. doi: 10.1161/HYPERTENSIONAHA.115.05654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Woods L.L., Morgan T.K., Resko J.A. Castration fails to prevent prenatally programmed hypertension in male rats. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1111–R1116. doi: 10.1152/ajpregu.00803.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hestiantoro A., Swaab D.F. Changes in estrogen receptor-alpha and -beta in the infundibular nucleus of the human hypothalamus are related to the occurrence of Alzheimer's disease neuropathology. J Clin Endocrinol Metab. 2004;89:1912–1925. doi: 10.1210/jc.2003-030862. [DOI] [PubMed] [Google Scholar]
- 166.Sartori-Valinotti J.C., Iliescu R., Fortepiani L.A. Sex differences in oxidative stress and the impact on blood pressure control and cardiovascular disease. Clin Exp Pharmacol Physiol. 2007;34:938–945. doi: 10.1111/j.1440-1681.2007.04643.x. [DOI] [PubMed] [Google Scholar]
- 167.Fruhbeck G., Catalan V., Rodriguez A. Adiponectin-leptin ratio: a promising index to estimate adipose tissue dysfunction. Relation with obesity-associated cardiometabolic risk. Adipocyte. 2018;7:57–62. doi: 10.1080/21623945.2017.1402151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Moulana M., Lima R., Reckelhoff J.F. Metabolic syndrome, androgens, and hypertension. Curr Hypertens Rep. 2011;13:158–162. doi: 10.1007/s11906-011-0184-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hall J.E., da Silva A.A., do Carmo J.M. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J Biol Chem. 2010;285:17271–17276. doi: 10.1074/jbc.R110.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tangredi J.F., Buxton I.L. Hypertension as a complication of topical testosterone therapy. Ann Pharmacother. 2001;35:1205–1207. doi: 10.1345/aph.1A020. [DOI] [PubMed] [Google Scholar]
- 171.Himmelmann A., Svensson A., Hansson L. Influence of sex on blood pressure and left ventricular mass in adolescents: the Hypertension in Pregnancy Offspring Study. J Hum Hypertens. 1994;8:485–490. [PubMed] [Google Scholar]
- 172.Gittelman M., Jaffe J.S., Kaminetsky J.C. Safety of a new subcutaneous testosterone Enanthate Auto-Injector: results of a 26-week study. J Sex Med. 2019;16:1741–1748. doi: 10.1016/j.jsxm.2019.08.013. [DOI] [PubMed] [Google Scholar]
- 173.Svartberg J., von Muhlen D., Schirmer H. Association of endogenous testosterone with blood pressure and left ventricular mass in men. The Tromso Study. Eur J Endocrinol. 2004;150:65–71. doi: 10.1530/eje.0.1500065. [DOI] [PubMed] [Google Scholar]
- 174.Barrett-Connor E., Khaw K.T. Endogenous sex hormones and cardiovascular disease in men. A prospective population-based study. Circulation. 1988;78:539–545. doi: 10.1161/01.cir.78.3.539. [DOI] [PubMed] [Google Scholar]
- 175.Yang Q., Li Z., Li W. Association of total testosterone, free testosterone, bioavailable testosterone, sex hormone-binding globulin, and hypertension. Medicine (Baltimore) 2019;98:e15628. doi: 10.1097/MD.0000000000015628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Saad F. Androgen therapy in men with testosterone deficiency: can testosterone reduce the risk of cardiovascular disease? Diabetes Metab Res Rev. 2012;28(Suppl 2):52–59. doi: 10.1002/dmrr.2354. [DOI] [PubMed] [Google Scholar]
- 177.Traish A.M., Saad F., Feeley R.J. The dark side of testosterone deficiency: III. Cardiovascular disease. J Androl. 2009;30:477–494. doi: 10.2164/jandrol.108.007245. [DOI] [PubMed] [Google Scholar]
- 178.Vikan T., Johnsen S.H., Schirmer H. Endogenous testosterone and the prospective association with carotid atherosclerosis in men: the Tromso study. Eur J Epidemiol. 2009;24:289–295. doi: 10.1007/s10654-009-9322-2. [DOI] [PubMed] [Google Scholar]
- 179.Laughlin G.A., Barrett-Connor E., Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008;93:68–75. doi: 10.1210/jc.2007-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Vlachopoulos C., Pietri P., Ioakeimidis N. Inverse association of total testosterone with central haemodynamics and left ventricular mass in hypertensive men. Atherosclerosis. 2016;250:57–62. doi: 10.1016/j.atherosclerosis.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 181.Liu P.Y., Death A.K., Handelsman D.J. Androgens and cardiovascular disease. Endocr Rev. 2003;24:313–340. doi: 10.1210/er.2003-0005. [DOI] [PubMed] [Google Scholar]
- 182.Mah P.M., Wittert G.A. Obesity and testicular function. Mol Cell Endocrinol. 2010;316:180–186. doi: 10.1016/j.mce.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 183.Zitzmann M. Mechanisms of disease: pharmacogenetics of testosterone therapy in hypogonadal men. Nat Clin Pract Urol. 2007;4:161–166. doi: 10.1038/ncpuro0706. [DOI] [PubMed] [Google Scholar]
- 184.Reckelhoff J.F., Yanes L.L., Iliescu R. Testosterone supplementation in aging men and women: possible impact on cardiovascular-renal disease. Am J Physiol Ren Physiol. 2005;289:F941–F948. doi: 10.1152/ajprenal.00034.2005. [DOI] [PubMed] [Google Scholar]
- 185.Falk E., Shah P.K., Fuster V. Coronary plaque disruption. Circulation. 1995;92:657–671. doi: 10.1161/01.cir.92.3.657. [DOI] [PubMed] [Google Scholar]
- 186.Roger V.L., Go A.S., Lloyd-Jones D.M. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Shaw L.J., Bugiardini R., Merz C.N. Women and ischemic heart disease: evolving knowledge. J Am Coll Cardiol. 2009;54:1561–1575. doi: 10.1016/j.jacc.2009.04.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Svartberg J., von Muhlen D., Mathiesen E. Low testosterone levels are associated with carotid atherosclerosis in men. J Intern Med. 2006;259:576–582. doi: 10.1111/j.1365-2796.2006.01637.x. [DOI] [PubMed] [Google Scholar]
- 189.Rezanezhad B., Borgquist R., Willenheimer R. Association between serum levels of testosterone and biomarkers of subclinical atherosclerosis. Aging Male. 2018;21:182–186. doi: 10.1080/13685538.2017.1412422. [DOI] [PubMed] [Google Scholar]
- 190.Shahani S., Braga-Basaria M., Basaria S. Androgen deprivation therapy in prostate cancer and metabolic risk for atherosclerosis. J Clin Endocrinol Metab. 2008;93:2042–2049. doi: 10.1210/jc.2007-2595. [DOI] [PubMed] [Google Scholar]
- 191.Qiu Y., Yanase T., Hu H. Dihydrotestosterone suppresses foam cell formation and attenuates atherosclerosis development. Endocrinology. 2010;151:3307–3316. doi: 10.1210/en.2009-1268. [DOI] [PubMed] [Google Scholar]
- 192.Budoff M.J., Ellenberg S.S., Lewis C.E. Testosterone treatment and coronary artery plaque volume in older men with low testosterone. JAMA. 2017;317:708–716. doi: 10.1001/jama.2016.21043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Abd Alamir M., Ellenberg S.S., Swerdloff R.S. The Cardiovascular Trial of the Testosterone Trials: rationale, design, and baseline data of a clinical trial using computed tomographic imaging to assess the progression of coronary atherosclerosis. Coron Artery Dis. 2016;27:95–103. doi: 10.1097/MCA.0000000000000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kushwaha R.S., Hazzard W.R. Exogenous estrogens attenuate dietary hypercholesterolemia and atherosclerosis in the rabbit. Metabolism. 1981;30:359–366. doi: 10.1016/0026-0495(81)90116-5. [DOI] [PubMed] [Google Scholar]
- 195.Haarbo J., Christiansen C. The impact of female sex hormones on secondary prevention of atherosclerosis in ovariectomized cholesterol-fed rabbits. Atherosclerosis. 1996;123:139–144. doi: 10.1016/0021-9150(96)05798-x. [DOI] [PubMed] [Google Scholar]
- 196.Nettleship J.E., Jones T.H., Channer K.S. Physiological testosterone replacement therapy attenuates fatty streak formation and improves high-density lipoprotein cholesterol in the Tfm mouse: an effect that is independent of the classic androgen receptor. Circulation. 2007;116:2427–2434. doi: 10.1161/CIRCULATIONAHA.107.708768. [DOI] [PubMed] [Google Scholar]
- 197.Huang C.K., Pang H., Wang L. New therapy via targeting androgen receptor in monocytes/macrophages to battle atherosclerosis. Hypertension. 2014;63:1345–1353. doi: 10.1161/HYPERTENSIONAHA.113.02804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Liu H.H., Li J.J. Aging and dyslipidemia: a review of potential mechanisms. Ageing Res Rev. 2015;19:43–52. doi: 10.1016/j.arr.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 199.Barud W., Palusinski R., Beltowski J. Inverse relationship between total testosterone and anti-oxidized low density lipoprotein antibody levels in ageing males. Atherosclerosis. 2002;164:283–288. doi: 10.1016/s0021-9150(02)00069-2. [DOI] [PubMed] [Google Scholar]
- 200.Greenfield D.M., Snowden J.A. Springer; 2019. Cardiovascular diseases and metabolic syndrome. The EBMT Handbook; pp. 415–420. [PubMed] [Google Scholar]
- 201.Salam R., Kshetrimayum A.S., Keisam R. Testosterone and metabolic syndrome: the link. Indian J Endocrinol Metab. 2012;16(Suppl 1):S12–S19. doi: 10.4103/2230-8210.94248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Haring R., Baumeister S.E., Volzke H. Prospective association of low total testosterone concentrations with an adverse lipid profile and increased incident dyslipidemia. Eur J Cardiovasc Prev Rehabil. 2011;18:86–96. doi: 10.1097/HJR.0b013e32833c1a8d. [DOI] [PubMed] [Google Scholar]
- 203.Barrett-Connor E. Lower endogenous androgen levels and dyslipidemia in men with non-insulin-dependent diabetes mellitus. Ann Intern Med. 1992;117:807–811. doi: 10.7326/0003-4819-117-10-807. [DOI] [PubMed] [Google Scholar]
- 204.Zamorano-Leon J.J., Segura A., Lahera V. Relationship between erectile dysfunction, diabetes and dyslipidemia in hypertensive-treated men. Urol J. 2018;15:370–375. doi: 10.22037/uj.v0i0.4068. [DOI] [PubMed] [Google Scholar]
- 205.Haffner S.M., Mykkanen L., Valdez R.A. Relationship of sex hormones to lipids and lipoproteins in nondiabetic men. J Clin Endocrinol Metab. 1993;77:1610–1615. doi: 10.1210/jcem.77.6.8263149. [DOI] [PubMed] [Google Scholar]
- 206.Dockery F., Bulpitt C.J., Agarwal S. Testosterone suppression in men with prostate cancer leads to an increase in arterial stiffness and hyperinsulinaemia. Clin Sci (Lond) 2003;104:195–201. doi: 10.1042/CS20020209. [DOI] [PubMed] [Google Scholar]
- 207.Yannucci J., Manola J., Garnick M.B. The effect of androgen deprivation therapy on fasting serum lipid and glucose parameters. J Urol. 2006;176:520–525. doi: 10.1016/j.juro.2006.03.057. [DOI] [PubMed] [Google Scholar]
- 208.Simon D., Charles M.A., Nahoul K. Association between plasma total testosterone and cardiovascular risk factors in healthy adult men: the Telecom Study. J Clin Endocrinol Metab. 1997;82:682–685. doi: 10.1210/jcem.82.2.3766. [DOI] [PubMed] [Google Scholar]
- 209.Van Pottelbergh I., Braeckman L., De Bacquer D. Differential contribution of testosterone and estradiol in the determination of cholesterol and lipoprotein profile in healthy middle-aged men. Atherosclerosis. 2003;166:95–102. doi: 10.1016/s0021-9150(02)00308-8. [DOI] [PubMed] [Google Scholar]
- 210.Stanworth R.D., Kapoor D., Channer K.S. Dyslipidaemia is associated with testosterone, oestradiol and androgen receptor CAG repeat polymorphism in men with type 2 diabetes. Clin Endocrinol (Oxf) 2011;74:624–630. doi: 10.1111/j.1365-2265.2011.03969.x. [DOI] [PubMed] [Google Scholar]
- 211.Haidar A., Yassin A., Saad F. Effects of androgen deprivation on glycaemic control and on cardiovascular biochemical risk factors in men with advanced prostate cancer with diabetes. Aging Male. 2007;10:189–196. doi: 10.1080/13685530701653538. [DOI] [PubMed] [Google Scholar]
- 212.Kiel D.P., Baron J.A., Plymate S.R. Sex hormones and lipoproteins in men. Am J Med. 1989;87:35–39. doi: 10.1016/s0002-9343(89)80480-2. [DOI] [PubMed] [Google Scholar]
- 213.Denti L., Pasolini G., Sanfelici L. Aging-related decline of gonadal function in healthy men: correlation with body composition and lipoproteins. J Am Geriatr Soc. 2000;48:51–58. doi: 10.1111/j.1532-5415.2000.tb03028.x. [DOI] [PubMed] [Google Scholar]
- 214.Whitsel E.A., Boyko E.J., Matsumoto A.M. Intramuscular testosterone esters and plasma lipids in hypogonadal men: a meta-analysis. Am J Med. 2001;111:261–269. doi: 10.1016/s0002-9343(01)00833-6. [DOI] [PubMed] [Google Scholar]
- 215.Isidori A.M., Giannetta E., Greco E.A. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin Endocrinol (Oxf) 2005;63:280–293. doi: 10.1111/j.1365-2265.2005.02339.x. [DOI] [PubMed] [Google Scholar]
- 216.Jones T.H., Saad F. The effects of testosterone on risk factors for, and the mediators of, the atherosclerotic process. Atherosclerosis. 2009;207:318–327. doi: 10.1016/j.atherosclerosis.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 217.Heufelder A.E., Saad F., Bunck M.C. Fifty-two-week treatment with diet and exercise plus transdermal testosterone reverses the metabolic syndrome and improves glycemic control in men with newly diagnosed type 2 diabetes and subnormal plasma testosterone. J Androl. 2009;30:726–733. doi: 10.2164/jandrol.108.007005. [DOI] [PubMed] [Google Scholar]
- 218.Saad F., Gooren L., Haider A. An exploratory study of the effects of 12 month administration of the novel long-acting testosterone undecanoate on measures of sexual function and the metabolic syndrome. Arch Androl. 2007;53:353–357. doi: 10.1080/01485010701730880. [DOI] [PubMed] [Google Scholar]
- 219.Zitzmann M., Nieschlag E. Androgen receptor gene CAG repeat length and body mass index modulate the safety of long-term intramuscular testosterone undecanoate therapy in hypogonadal men. J Clin Endocrinol Metab. 2007;92:3844–3853. doi: 10.1210/jc.2007-0620. [DOI] [PubMed] [Google Scholar]
- 220.Ozata M., Yildirimkaya M., Bulur M. Effects of gonadotropin and testosterone treatments on Lipoprotein(a), high density lipoprotein particles, and other lipoprotein levels in male hypogonadism. J Clin Endocrinol Metab. 1996;81:3372–3378. doi: 10.1210/jcem.81.9.8784099. [DOI] [PubMed] [Google Scholar]
- 221.Zmunda J.M., Thompson P.D., Dickenson R. Testosterone decreases lipoprotein(a) in men. Am J Cardiol. 1996;77:1244–1247. doi: 10.1016/s0002-9149(96)00174-9. [DOI] [PubMed] [Google Scholar]
- 222.Marcovina S.M., Lippi G., Bagatell C.J. Testosterone-induced suppression of lipoprotein(a) in normal men; relation to basal lipoprotein(a) level. Atherosclerosis. 1996;122:89–95. doi: 10.1016/0021-9150(95)05756-0. [DOI] [PubMed] [Google Scholar]
- 223.Baspinar O., Bayram F., Korkmaz S. The effects of statin treatment on adrenal and sexual function and nitric oxide levels in hypercholesterolemic male patients treated with a statin. J Clin Lipidol. 2016;10:1452–1461. doi: 10.1016/j.jacl.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 224.Hsieh C.J., Huang B. Rosuvastatin decreases testosterone levels but not sexual function in men with type 2 diabetes. Diabetes Res Clin Pract. 2016;120:81–88. doi: 10.1016/j.diabres.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 225.Kapoor D., Goodwin E., Channer K.S. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2006;154:899–906. doi: 10.1530/eje.1.02166. [DOI] [PubMed] [Google Scholar]
- 226.Bagatell C.J., Heiman J.R., Matsumoto A.M. Metabolic and behavioral effects of high-dose, exogenous testosterone in healthy men. J Clin Endocrinol Metab. 1994;79:561–567. doi: 10.1210/jcem.79.2.8045977. [DOI] [PubMed] [Google Scholar]
- 227.Malkin C.J., Pugh P.J., Jones R.D. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004;89:3313–3318. doi: 10.1210/jc.2003-031069. [DOI] [PubMed] [Google Scholar]
- 228.Thompson P.D., Cullinane E.M., Sady S.P. Contrasting effects of testosterone and stanozolol on serum lipoprotein levels. JAMA. 1989;261:1165–1168. [PubMed] [Google Scholar]
- 229.Kelly D.M., Jones T.H. Testosterone and obesity. Obes Rev. 2015;16:581–606. doi: 10.1111/obr.12282. [DOI] [PubMed] [Google Scholar]
- 230.Haddad R.M., Kennedy C.C., Caples S.M. Testosterone and cardiovascular risk in men: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc. 2007;82:29–39. doi: 10.4065/82.1.29. [DOI] [PubMed] [Google Scholar]
- 231.Godsland I.F., Wynn V., Crook D. Sex, plasma lipoproteins, and atherosclerosis: prevailing assumptions and outstanding questions. Am Heart J. 1987;114:1467–1503. doi: 10.1016/0002-8703(87)90552-7. [DOI] [PubMed] [Google Scholar]
- 232.Fink J., Matsumoto M., Tamura Y. Potential application of testosterone replacement therapy as treatment for obesity and type 2 diabetes in men. Steroids. 2018;138:161–166. doi: 10.1016/j.steroids.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 233.Chen W., Beck I., Schober W. Human mast cells express androgen receptors but treatment with testosterone exerts no influence on IgE-independent mast cell degranulation elicited by neuromuscular blocking agents. Exp Dermatol. 2010;19:302–304. doi: 10.1111/j.1600-0625.2009.00969.x. [DOI] [PubMed] [Google Scholar]
- 234.Mantalaris A., Panoskaltsis N., Sakai Y. Localization of androgen receptor expression in human bone marrow. J Pathol. 2001;193:361–366. doi: 10.1002/1096-9896(0000)9999:9999<::AID-PATH803>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 235.Viselli S.M., Reese K.R., Fan J. Androgens alter B cell development in normal male mice. Cell Immunol. 1997;182:99–104. doi: 10.1006/cimm.1997.1227. [DOI] [PubMed] [Google Scholar]
- 236.Lai J.J., Lai K.P., Zeng W. Androgen receptor influences on body defense system via modulation of innate and adaptive immune systems: lessons from conditional AR knockout mice. Am J Pathol. 2012;181:1504–1512. doi: 10.1016/j.ajpath.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Tremellen K., McPhee N., Pearce K. Endotoxin-initiated inflammation reduces testosterone production in men of reproductive age. Am J Physiol Endocrinol Metab. 2018;314:E206–E213. doi: 10.1152/ajpendo.00279.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Burney B.O., Hayes T.G., Smiechowska J. Low testosterone levels and increased inflammatory markers in patients with cancer and relationship with cachexia. J Clin Endocrinol Metab. 2012;97:E700–E709. doi: 10.1210/jc.2011-2387. [DOI] [PubMed] [Google Scholar]
- 239.Bobjer J., Naumovska M., Giwercman Y.L. High prevalence of androgen deficiency and abnormal lipid profile in infertile men with non-obstructive azoospermia. Int J Androl. 2012;35:688–694. doi: 10.1111/j.1365-2605.2012.01277.x. [DOI] [PubMed] [Google Scholar]
- 240.Ng M.K., Liu P.Y., Williams A.J. Prospective study of effect of androgens on serum inflammatory markers in men. Arterioscler Thromb Vasc Biol. 2002;22:1136–1141. doi: 10.1161/01.atv.0000022167.80130.a6. [DOI] [PubMed] [Google Scholar]
- 241.Nasser M., Haider A., Saad F. Testosterone therapy in men with Crohn's disease improves the clinical course of the disease: data from long-term observational registry study. Horm Mol Biol Clin Investig. 2015;22:111–117. doi: 10.1515/hmbci-2015-0014. [DOI] [PubMed] [Google Scholar]
- 242.Saad F., Haider A., Gooren L. Hypogonadal men with psoriasis benefit from long-term testosterone replacement therapy - a series of 15 case reports. Andrologia. 2016;48:341–346. doi: 10.1111/and.12452. [DOI] [PubMed] [Google Scholar]
- 243.Corrales J.J., Almeida M., Burgo R. Androgen-replacement therapy depresses the ex vivo production of inflammatory cytokines by circulating antigen-presenting cells in aging type-2 diabetic men with partial androgen deficiency. J Endocrinol. 2006;189:595–604. doi: 10.1677/joe.1.06779. [DOI] [PubMed] [Google Scholar]
- 244.Belanger C., Hould F.S., Lebel S. Omental and subcutaneous adipose tissue steroid levels in obese men. Steroids. 2006;71:674–682. doi: 10.1016/j.steroids.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 245.Marin P., Lonn L., Andersson B. Assimilation of triglycerides in subcutaneous and intraabdominal adipose tissues in vivo in men: effects of testosterone. J Clin Endocrinol Metab. 1996;81:1018–1022. doi: 10.1210/jcem.81.3.8772568. [DOI] [PubMed] [Google Scholar]
- 246.Grosman H., Fabre B., Lopez M. Complex relationship between sex hormones, insulin resistance and leptin in men with and without prostatic disease. Aging Male. 2016;19:40–45. doi: 10.3109/13685538.2015.1100600. [DOI] [PubMed] [Google Scholar]
- 247.Behre H.M., Simoni M., Nieschlag E. Strong association between serum levels of leptin and testosterone in men. Clin Endocrinol (Oxf) 1997;47:237–240. doi: 10.1046/j.1365-2265.1997.2681067.x. [DOI] [PubMed] [Google Scholar]
- 248.Luukkaa V., Pesonen U., Huhtaniemi I. Inverse correlation between serum testosterone and leptin in men. J Clin Endocrinol Metab. 1998;83:3243–3246. doi: 10.1210/jcem.83.9.5134. [DOI] [PubMed] [Google Scholar]
- 249.Ruige J.B., Bekaert M., Lapauw B. Sex steroid-induced changes in circulating monocyte chemoattractant protein-1 levels may contribute to metabolic dysfunction in obese men. J Clin Endocrinol Metab. 2012;97:E1187–E1191. doi: 10.1210/jc.2011-3069. [DOI] [PubMed] [Google Scholar]
- 250.Morooka N., Ueguri K., Yee K.K.L. Androgen-androgen receptor system improves chronic inflammatory conditions by suppressing monocyte chemoattractant protein-1 gene expression in adipocytes via transcriptional regulation. Biochem Biophys Res Commun. 2016;477:895–901. doi: 10.1016/j.bbrc.2016.06.155. [DOI] [PubMed] [Google Scholar]
- 251.Gautier A., Bonnet F., Dubois S. Associations between visceral adipose tissue, inflammation and sex steroid concentrations in men. Clin Endocrinol (Oxf) 2013;78:373–378. doi: 10.1111/j.1365-2265.2012.04401.x. [DOI] [PubMed] [Google Scholar]
- 252.Maggio M., Basaria S., Ceda G.P. The relationship between testosterone and molecular markers of inflammation in older men. J Endocrinol Invest. 2005;28:116–119. [PubMed] [Google Scholar]
- 253.Bobjer J., Katrinaki M., Tsatsanis C. Negative association between testosterone concentration and inflammatory markers in young men: a nested cross-sectional study. PLoS One. 2013;8:e61466. doi: 10.1371/journal.pone.0061466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Ebrahimi F., Urwyler S.A., Straumann S. IL-1 antagonism in men with metabolic syndrome and low testosterone: a randomized clinical trial. J Clin Endocrinol Metab. 2018;103:3466–3476. doi: 10.1210/jc.2018-00739. [DOI] [PubMed] [Google Scholar]
- 255.Demirtas Sahin T., Yazir Y., Utkan T. TNF-alpha antagonism with etanercept enhances penile NOS expression, cavernosal reactivity, and testosterone levels in aged rats. Can J Physiol Pharmacol. 2018;96:200–207. doi: 10.1139/cjpp-2017-0113. [DOI] [PubMed] [Google Scholar]
- 256.Kataoka T., Hotta Y., Maeda Y. Testosterone deficiency causes endothelial dysfunction via elevation of Asymmetric Dimethylarginine and oxidative stress in castrated rats. J Sex Med. 2017;14:1540–1548. doi: 10.1016/j.jsxm.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 257.Bianchi V.E. The anti-inflammatory effects of testosterone. J Endocr Soc. 2019;3:91–107. doi: 10.1210/js.2018-00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Mohler E.R., 3rd, Ellenberg S.S., Lewis C.E. The effect of testosterone on cardiovascular biomarkers in the testosterone trials. J Clin Endocrinol Metab. 2018;103:681–688. doi: 10.1210/jc.2017-02243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Nakhai-Pour H.R., Grobbee D.E., Emmelot-Vonk M.H. Oral testosterone supplementation and chronic low-grade inflammation in elderly men: a 26-week randomized, placebo-controlled trial. Am Heart J. 2007;154:1228.e1–1228.e7. doi: 10.1016/j.ahj.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 260.Wickramatilake C.M., Mohideen M.R., Pathirana C. Association of metabolic syndrome with testosterone and inflammation in men. Ann Endocrinol (Paris) 2015;76:260–263. doi: 10.1016/j.ando.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 261.Rovira-Llopis S., Banuls C., de Maranon A.M. Low testosterone levels are related to oxidative stress, mitochondrial dysfunction and altered subclinical atherosclerotic markers in type 2 diabetic male patients. Free Radic Biol Med. 2017;108:155–162. doi: 10.1016/j.freeradbiomed.2017.03.029. [DOI] [PubMed] [Google Scholar]
- 262.Luotola K., Piltonen T.T., Puurunen J. Testosterone is associated with insulin resistance index independently of adiposity in women with polycystic ovary syndrome. Gynecol Endocrinol. 2018;34:40–44. doi: 10.1080/09513590.2017.1342793. [DOI] [PubMed] [Google Scholar]
- 263.Dhindsa S., Ghanim H., Batra M. Insulin resistance and inflammation in hypogonadotropic hypogonadism and their reduction after testosterone replacement in men with type 2 diabetes. Diabetes Care. 2016;39:82–91. doi: 10.2337/dc15-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Xu W., Niu T., Xu B. Androgen receptor-deficient islet beta-cells exhibit alteration in genetic markers of insulin secretion and inflammation. A transcriptome analysis in the male mouse. J Diabetes Complications. 2017;31:787–795. doi: 10.1016/j.jdiacomp.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Rastrelli G., Filippi S., Sforza A. Metabolic syndrome in male hypogonadism. Front Horm Res. 2018;49:131–155. doi: 10.1159/000485999. [DOI] [PubMed] [Google Scholar]
- 266.Corona G., Giagulli V.A., Maseroli E. Therapy OF endocrine disease: testosterone supplementation and body composition: results from a meta-analysis study. Eur J Endocrinol. 2016;174:R99–R116. doi: 10.1530/EJE-15-0262. [DOI] [PubMed] [Google Scholar]
- 267.Corona G., Giagulli V.A., Maseroli E. Testosterone supplementation and body composition: results from a meta-analysis of observational studies. J Endocrinol Invest. 2016;39:967–981. doi: 10.1007/s40618-016-0480-2. [DOI] [PubMed] [Google Scholar]
- 268.Borbelyova V., Domonkos E., Babickova J. Does long-term androgen deficiency lead to metabolic syndrome in middle-aged rats? Exp Gerontol. 2017;98:38–46. doi: 10.1016/j.exger.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 269.Donner D.G., Elliott G.E., Beck B.R. Trenbolone improves cardiometabolic risk factors and myocardial tolerance to Ischemia-Reperfusion in male rats with testosterone-deficient metabolic syndrome. Endocrinology. 2016;157:368–381. doi: 10.1210/en.2015-1603. [DOI] [PubMed] [Google Scholar]
- 270.Bernasconi P.A., Cardoso N.P., Reynoso R. Melatonin and diet-induced metabolic syndrome in rats: impact on the hypophysial-testicular axis. Horm Mol Biol Clin Investig. 2013;16:101–112. doi: 10.1515/hmbci-2013-0005. [DOI] [PubMed] [Google Scholar]
- 271.Christoffersen B., Raun K., Svendsen O. Evalution of the castrated male Sprague-Dawley rat as a model of the metabolic syndrome and type 2 diabetes. Int J Obes (Lond) 2006;30:1288–1297. doi: 10.1038/sj.ijo.0803261. [DOI] [PubMed] [Google Scholar]
- 272.Abdelhamed A., Hisasue S., Shirai M. Testosterone replacement alters the cell size in visceral fat but not in subcutaneous fat in hypogonadal aged male rats as a late-onset hypogonadism animal model. Res Rep Urol. 2015;7:35–40. doi: 10.2147/RRU.S72253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Lazic M., Aird F., Levine J.E. Prenatal androgen treatment alters body composition and glucose homeostasis in male rats. J Endocrinol. 2011;208:293–300. doi: 10.1677/JOE-10-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Filippi S., Vignozzi L., Morelli A. Testosterone partially ameliorates metabolic profile and erectile responsiveness to PDE5 inhibitors in an animal model of male metabolic syndrome. J Sex Med. 2009;6:3274–3288. doi: 10.1111/j.1743-6109.2009.01467.x. [DOI] [PubMed] [Google Scholar]
- 275.Maneschi E., Morelli A., Filippi S. Testosterone treatment improves metabolic syndrome-induced adipose tissue derangements. J Endocrinol. 2012;215:347–362. doi: 10.1530/JOE-12-0333. [DOI] [PubMed] [Google Scholar]
- 276.Khripun I., Vorobyev S., Belousov I. Influence of testosterone substitution on glycemic control and endothelial markers in men with newly diagnosed functional hypogonadism and type 2 diabetes mellitus: a randomized controlled trial. Aging Male. 2019;22:241–249. doi: 10.1080/13685538.2018.1506918. [DOI] [PubMed] [Google Scholar]
- 277.Morgunov L.Y., Denisova I.A., Rozhkova T.I. Hypogonadism and its treatment following ischaemic stroke in men with type 2 diabetes mellitus. Aging Male. 2020;23:71–80. doi: 10.1080/13685538.2018.1487932. [DOI] [PubMed] [Google Scholar]
- 278.Groti K., Zuran I., Antonic B. The impact of testosterone replacement therapy on glycemic control, vascular function, and components of the metabolic syndrome in obese hypogonadal men with type 2 diabetes. Aging Male. 2018;21:158–169. doi: 10.1080/13685538.2018.1468429. [DOI] [PubMed] [Google Scholar]
- 279.Goncharov N., Katsya G., Gaivoronskaya L. Effects of short-term testosterone administration on variables of the metabolic syndrome, in particular aldosterone. Horm Mol Biol Clin Investig. 2012;12:401–406. doi: 10.1515/hmbci-2012-0023. [DOI] [PubMed] [Google Scholar]
- 280.Magnussen L.V., Andersen P.E., Diaz A. MR spectroscopy of hepatic fat and adiponectin and leptin levels during testosterone therapy in type 2 diabetes: a randomized, double-blinded, placebo-controlled trial. Eur J Endocrinol. 2017;177:157–168. doi: 10.1530/EJE-17-0071. [DOI] [PubMed] [Google Scholar]
- 281.Permpongkosol S., Khupulsup K., Leelaphiwat S. Effects of 8-year treatment of long-acting testosterone undecanoate on metabolic parameters, urinary symptoms, bone mineral density, and sexual function in men with late-onset hypogonadism. J Sex Med. 2016;13:1199–1211. doi: 10.1016/j.jsxm.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 282.Yassin A.A., Nettleship J., Almehmadi Y. Effects of continuous long-term testosterone therapy (TTh) on anthropometric, endocrine and metabolic parameters for up to 10 years in 115 hypogonadal elderly men: real-life experience from an observational registry study. Andrologia. 2016;48:793–799. doi: 10.1111/and.12514. [DOI] [PubMed] [Google Scholar]
- 283.Zhang L.T., Shin Y.S., Kim J.Y. Could testosterone replacement therapy in hypogonadal men ameliorate anemia, a cardiovascular risk factor? An observational, 54-week Cumulative registry study. J Urol. 2016;195:1057–1064. doi: 10.1016/j.juro.2015.10.130. [DOI] [PubMed] [Google Scholar]
- 284.Hackett G., Cole N., Mulay A. Long-term testosterone therapy in type 2 diabetes is associated with reduced mortality without improvement in conventional cardiovascular risk factors. BJU Int. 2019;123:519–529. doi: 10.1111/bju.14536. [DOI] [PubMed] [Google Scholar]