Abstract
Introduction
Type 2 diabetes mellitus erectile dysfunction (T2DMED) is one of the common complications of type 2 diabetes mellitus (T2DM). Icariside II (ICA II), a flavonoid derived from Epimedium, has been shown to improve erectile function in T2DMED rats.
Aim
To investigate the effect of ICA II and metformin (MET) on penile erectile function, mitochondrial autophagy, glucose-lipid metabolism in rats with T2DMED.
Methods
In the control and T2DMED groups, rats were administered normal saline. In the MET group, rats were administered MET for 0.2 g/kg/day. In the ICA II+MET group, rats were administered ICA II for 10 mg/kg/day and MET for 0.2 g/kg/day.
Results
The number of mating rats, number of erectile rats, erection rate, erection frequency, intracorneal pressure, and intracorneal pressure/mean arterial pressure in the ICA II+MET group and control group were significantly higher than corresponding values in than T2DMED group. The absolute values of fasting plasma glucose, glycated haemoglobin in the ICA II+MET group, MET group, and control group were significantly lower than in the T2DMED group. The advanced glycation end product (AGE) values in the ICA II+MET group and the MET group were lower than in the T2DMED group. The receptors for the AGE values and angiotensin II values in the ICA II+MET group were lower than in the T2DMED and MET groups. The high-density lipoprotein values, testosterone values, nitric oxide synthase activity, and cyclic guanosine monophosphate content in the ICA II+MET and control groups were higher than in the T2DMED group. The low-density lipoprotein values, triglyceride values, estradiol values, and total cholesterol values in the ICA II+MET and control groups were lower than in the T2DMED group.
Conclusion
ICA II could increase erectile function and smooth muscle cell/collagen fibril proportions, decreased mitochondrial autophagy, and AGE concentrations and improve lipid metabolism, nitric oxide synthase activity, cyclic guanosine monophosphate content, testosterone, estradiol, and Ang II in rat with T2DMED.
Zhang J, Li S, Zhang S, et al. Effect of Icariside II and Metformin on Penile Erectile Function, Histological Structure, Mitochondrial Autophagy, Glucose-Lipid Metabolism, Angiotensin II and Sex Hormone in Type 2 Diabetic Rats With Erectile Dysfunction. J Sex Med 2020;8:168–177.
Key Words: Icariside Ⅱ, Sexual function, Mitochondrial Autophagy, Glucose-lipid Metabolism, Sex Hormone
Introduction
With development of the social economy, an aging society, and changing lifestyles in humans, non-communicable chronic diseases have become the main cause of mortality and disease burden throughout the world.1 The incidence of diabetes mellitus (DM) is growing rapidly around the world, and DM has become the third serious chronic disease threatening human health after cancer and cardiovascular disease.2 Type 2 diabetes mellitus (T2DM) accounts for 90%–95% of DM. T2DM erectile dysfunction (T2DMED) is one of the common complications of T2DM.3 Non-enzymatic advanced glycation end product (AGE) formation increases during the hyperglycemic condition, and AGE clearance is obstructed to some extent in patients with T2DM.4,5 AGEs can induce tissue damage through the receptors for the AGE (RAGE) pathway.5 The partial effect of AGEs is mediated by the RAGE pathway; the interaction between AGEs and RAGE may be involved in the pathogenesis of complications of T2DM, such as diabetic microvascular complications, diabetic nephropathy, and diabetic retinopathy.5,6 Angiotensin II (Ang II) is one of the important active mediators in the renin angiotensin system (RAS), which has a vasoconstrictive effect and is closely related to the proliferation of smooth muscle cells.3,6 Testosterone (T) is the main component of androgen, which plays an essential role in male sexual differentiation and the maintenance of penile erectile function.3,7 Estradiol (E2) is the main component of estrogens, and estrogens are converted from androgens in male animals. E2 can resist the effect of androgen in vivo.3 The nitric oxide-cyclic guanosine monophosphate (NO-cGMP) signaling pathway plays a major regulatory role in penile erections.8 Nitric oxide synthase (NOS) is the enzyme that catalyzes the synthesis of NO from L-arginine in biological systems.8
Mitochondrial quality is a crucial determinant of cell viability. Mitochondrial autophagy plays a central role in preventing increased levels of reactive oxygen species and cell death. Metformin (MET) is one of the prescribed hypoglycemic drugs of first-line treatment for patients with T2DM. The natural medicine epimedium is a commonly used ingredient for nourishing and strengthening prescriptions in the traditional medical community.7,8 Icariside II (ICA II) is considered to be the main active monomeric component of epimedium in vivo.8 ICA II was synthesized artificially owing to the low content of epimedium in natural drugs in the study. In our study, we investigated the effect of ICA II and MET on penile erectile function, performed morphological observation, and determined the potential mechanism via the assessment of glucose-lipid metabolism the content of AGEs, RAGE, Ang II, T, and E2 in rats with T2DMED.
Materials and methods
Materials
A blood glucose meter (Roche Diagnostic, Basel, Switzerland), fluorescence spectrophotometer (Hitachi, Tokyo, Japan), streptozotocin (STZ) (Sigma Chemical Co, St. Louis, MO, USA), a rat insulin (INS) ELISA kit (Crystal Chem. Inc, USA), AGEs Kit (R&D Systems, Inc, United States), rat advanced glycation end product receptor (RAGE) ELISA kit (R&D Systems, Inc, United States), Ang II kit (R&D Systems, Inc, United States), HbA1c kit (Cayman Chemicals, Ann Arbor, MI, USA), MET tablets (Hangzhou Merck East Pharmaceutical Co, Ltd, standard 0.85 g/granule, national drug standard J20171012), M100 Multi Conductive Physiometer (Biopac Systems, USA), microsurgical instruments, thermostatic animal operating table, bipolar electrodes (silver, 0.1 mm diameter, 1 mm between positive and negative electrodes), and 26G needle were used in the experiments.
Animals
All experimental protocols were approved by the Institutional Animal Care and Use Committee at Capital Medical University (Beijing, China). Male Wistar rats (SCXK [Jing] 2014-0010) were obtained from the Animal Breeding Center at the Capital Medical University Health Science Center. After 1 week of adaptive rearing, 96 healthy 8-week-old male Wistar rats were screened if they had normal sexual function by tail artery pressure (TAP) and apomorphine (APO) tests and mating experiments. The T2DM rat model was built with a single intraperitoneal (i.p.) injection of 25 mg/kg streptozotocin (STZ, Sigma Chemical Co, St. Louis, MO, USA) combined with a high-fat, high-sugar, and high-calorie diet. Total body weight, TAP, and fasting plasma glucose (FPG) were measured every week. At 8 weeks, all rats were tested for insulin tolerance. The insulin sensitivity index (ISI) was calculated as Ln[1/(FPG × FINS)]. Homeostasis model assessment was used to calculate the insulin resistance index (IRI) = FINS × FPG/22.5. Rats with FPG >16.7 mmol/L, glycated haemoglobin (HbA1c) > 12%, and ISI reduction were confirmed as T2DM rats. The T2DMED rats were selected by TAP and APO tests and mating experiments from T2DM rats. The rats were randomly divided into a control group, ICA II+MET group, MET group, and T2DMED group, and there were 16 rats in each group. The drug doses and treatment times were based on prestudy experiments. T2DMED rats were given MET (0.2 g kg−1 day−1; the MET group and ICA II+MET group) or ICA II (10 mg kg−1; the ICA II+MET group) by gavage feeding for 12 weeks. After a 1-week washout period without ICA II, erectile function was evaluated by intracorneal pressure/mean arterial pressure (ICP/MAP).
Erectile Function
The mating experiments were conducted under dim red light at the onset of the lights-off period. The male rat was placed in the test arena for more than 5 min. We then introduced a hormonally ready female rat. The test continued for 30 min or ended with an ejaculation occurrence. The female rat was swopped to a different female rat 3 times at a maximum if no intromission occurred after 30 min. The mount latency, intromission latency, and interintromission interval were recorded. The mount latency, intromission latency, and interintromission interval were used to evaluate erectile function. The number of mating rats, number of erectile rats, erection frequency, and erection rate were recorded. ED was diagnoses if the male rats failed to mate with different female rats 3 times within 1 week.
The rats from each group were anesthetized with 5% sodium pentobarbital. The pelvic ganglion, cavernous nerve, and cavernous arteries were exposed. Two 24-gauge needles connected to PE-50 tubes with heparinized saline were inserted into the corpus cavernosum and carotid arteries. A pressure transducer (Utah Medical Products, Midvale, UT, USA) was connected to monitor MAP. The cavernous nerve near the major pelvic ganglion was identified for ICP/MAP measurements (3 volt, 12 hertz, duration 60 s, interval time 30 min). A value of ICP/MAP lower than 0.45 was considered representative of ED.
Masson Trichrome Staining of Cavernous Tissue
The corpus cavernosum smooth muscle cells/collagen fibrils (CCSMCs/CFs) ratio was used to evaluate the cavernous tissue via Masson trichrome staining. The cavernous tissue specimens from each group were fixed in 2% paraformaldehyde and embedded in paraffin. The sections (3 mm in thickness) were then stained using a Masson trichrome staining kit (Sigma-Aldrich, St. Louis, MO, USA).
Autophagosome Observations By Transmission Electron Microscopy
Freshly dissected penis samples were fixed with 2% formaldehyde and 0.002% picric acid in 0.1 M phosphate buffer for 4 h. This was followed by overnight immersion in 30% sucrose. Tissues were stored at −80°C until use. Then sections were rinsed in phosphate buffer saline, postfixed in 1% osmium tetroxide with 1% potassium ferricyanide, dehydrated through a graded series of ethanol (30–90% and 100% ethanol 200 proof) and embedded in Eponate. The sections were then stained with uranyl acetate/lead citrate (Sigma-Aldrich) and viewed with transmission electron microscopy (TEM) (JEOL, Tokyo, Japan).
Measurement of Metabolic Variables
Blood glucose was measured using an Accu-Chek Active blood glucose meter (Roche Diagnostics, Indianapolis, IN, USA). Glucose levels are presented as percentages of baseline plasma glucose. The levels of total cholesterol (TC), triglycerides (TG), high-density lipoprotein (HDL), and low-density lipoprotein (LDL) were measured using an automatic biochemical analyzer. The measurements of HbA1c, AGEs, RAGE, and Ang II were made as per the standard protocols.
Testosterone, E2, NOS Activity and cGMP Concentration Assays
The rat corpus cavernosum tissue was used to detect the cGMP concentration and NOS activity using the corresponding enzyme immunoassay kits (Cayman Chemical, USA), as per the manufacturer's instructions. The concentrations of T and E2 were measured using a BS-300 automatic biochemistry analyzer (Mindray Biomedical Electronic Co, Ltd, Shenzhen, China).
Statistical Analysis
All statistical data entry work was the responsibility of other staff members in the department who did not participate in the study. The SPSS 17.0 software package (SPSS Inc, Chicago, IL, USA) was used for statistical analyses. Measurement data were expressed as the mean ± standard deviation (SD) and were analyzed using one-way ANOVA. P < .05 was considered as a statistical difference.
Results
Effect of ICA II on Improving Penile Erectile Function in T2DMED Rats
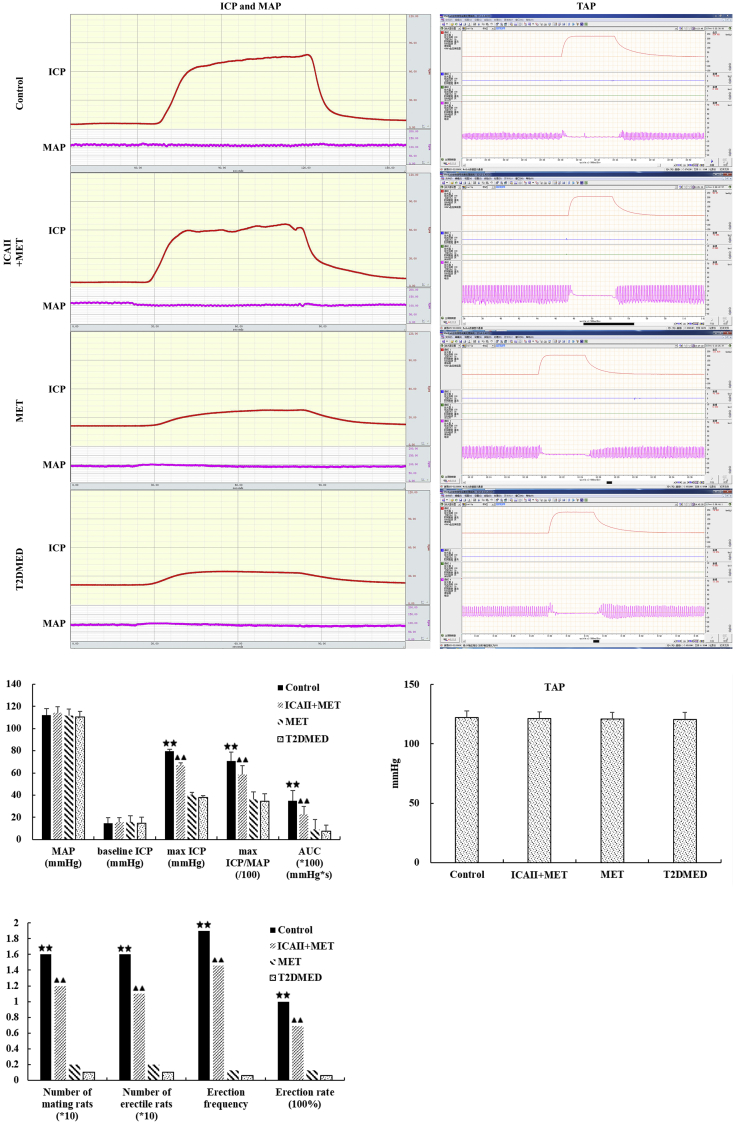
As illustrated in Figure 1, there was no statistical difference in the number of mating rats, number of erectile rats, erection frequency, erection rate, maximal ICP, maximal ICP/MAP, and area under the curve values between the MET and T2DMED groups (P > .05). However, the number of mating rats, number of erectile rats, erection rate, erection frequency, maximal ICP, maximal ICP/MAP and area under the curve values in the ICA II+MET group was higher than that in the T2DMED group (12 vs 1, 1.10 vs 0.10, 1.46 vs 0.06, 0.69 vs 0.06, 66.80 ± 8.03 vs 37.93 ± 6.88, 0.59 vs 0.34, and 2,281.14 ± 259.72 vs 731.47 ± 210.80, respectively, P < .01).There was no statistical difference in TAP, MAP, and baseline ICP among the 4 groups (P > .05).
Figure 1.
ICA II improved erectile function upon cavernous nerve electrostimulation in rats. Comparison of penile erectile function among the control, ICA II+MET, MET, and T2DMED groups (±s, n = 16). ★★: (P < .01), control group vs T2DMED group, ▴▲: (P < .01), ICA+MET group vs T2DMED group. AUC = area under the curve; ICA II = icariside II; ICP = intracorneal pressure; MAP = mean arterial pressure; MET = metformin; T2DMED = type 2 diabetes mellitus erectile dysfunction.
Effects of ICA II on Body Weight and FPG in T2DMED Rats
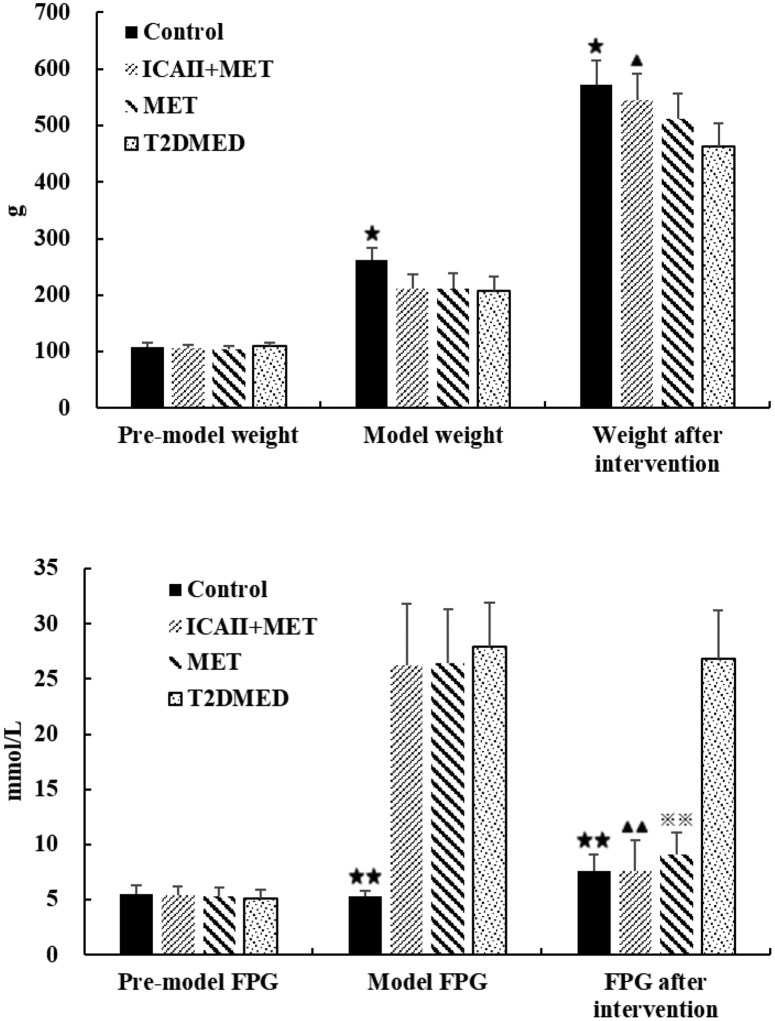
As shown in Figure 2, there was no statistical difference in the premodel weight among the 4 groups (P > .05). There was no statistical difference in the rat model weights among the MET group, ICA II+MET group, and T2DMED group (P > .05). The model weights in the MET group, ICA II+MET group, and T2DMED group were lower than in the control group (212.31 ± 26.96 vs 207.50 ± 25.06, 211.56 ± 24.26 vs 207.50 ± 25.06, P < .05). After intervention, the body weights in the ICA II+MET and control groups were higher than in the T2DMED group (543.13 ± 46.57 vs 462.31 ± 41.50, 571.50 ± 42.57 vs 462.31 ± 41.50, respectively, P < .05).
Figure 2.
Comparisons of body weight and FPG in the control, ICA II+MET, MET, and T2DMED groups at different times (±s, n = 16). ★: (P < .05), control group vs T2DMED group, ▲: (P < .05), ICA+MET group vs T2DMED group. ICA II = icariside II; MET = metformin; T2DMED = type 2 diabetes mellitus erectile dysfunction.
As shown in Figure 2, there was no statistical difference in premodel FPG among the 4 groups (P > .05). There was no statistical difference in model FPG among the MET group, ICA II+MET group, and T2DMED group (P > .05). The model FPG in the MET group, ICA II+MET group, and T2DMED group was higher than in the control group (26.45 ± 4.88 vs 27.91 ± 4.00, 26.26 ± 5.59 vs 27.91 ± 4.00, respectively, P < .01). After experimental intervention, there was no statistical difference in FPG among the MET group, ICA II+MET group, and control group (P > .05). After intervention, FPG in the MET group, ICA II+MET group, and control group was significantly lower than that in the T2DMED group (9.08 ± 1.97 vs 7.58 ± 1.49, 7.56 ± 2.82 vs 7.58 ± 1.49, respectively, P < .01).
Effect of ICA II on the CCSMCs/CFs Ratio in the Corpus Cavernosum
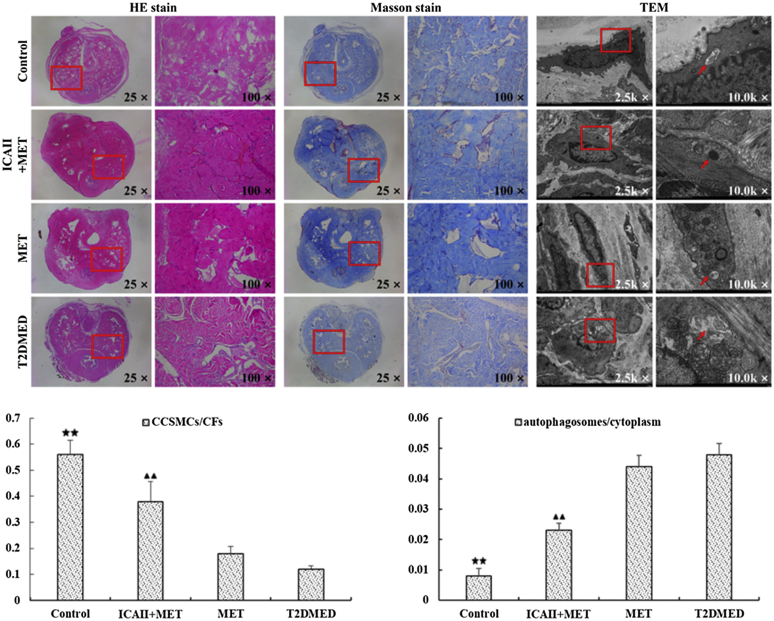
Penile interstitial collagen deposition was evaluated by Masson's trichrome staining. As shown in Figure 3, the content of smooth muscle in the ICA II+MET treatment group was significantly higher than in the T2DMED and MET groups. The collagen deposition of penile cavernous tissue was significantly increased in the T2DMED and MET groups. The ICA II+MET treatment group had increased content of smooth muscle and reduced cavernous fibrosis. The CCSMCs/CFs ratio in the ICA II+MET group was significantly higher than in the T2DMED group (0.38 ± 0.08 vs 0.12 ± 0.01, P < .01).
Figure 3.
Comparisons of the CCSMCs/CFs ratio and autophagosomes/cytoplasmic rates in the control, ICA II+MET, MET, and T2DMED groups (±s, n = 16). ★★: (P < .01), control group vs T2DMED group, ▲▲: (P < .01), ICA II+MET group vs T2DMED group. CCSMC = corpus cavernosum smooth muscle cell; CF = collagen fibril; ICA II = icariside II; MET = metformin; T2DMED = type 2 diabetes mellitus erectile dysfunction.
Effect of ICA II on Autophagosomes By TEM
As shown in Figure 3, compared with the T2DMED group, autophagosome quantities were significantly lower in the ICA II+MET group and control groups (0.023 ± 0.0023 vs 0.048 ± 0.0036, P < .01). However, there was no statistical difference in autophagosome quantities between the MET and T2DMED groups (P > .05).
Effects of ICA II on Metabolic Variables in T2DMED Rats
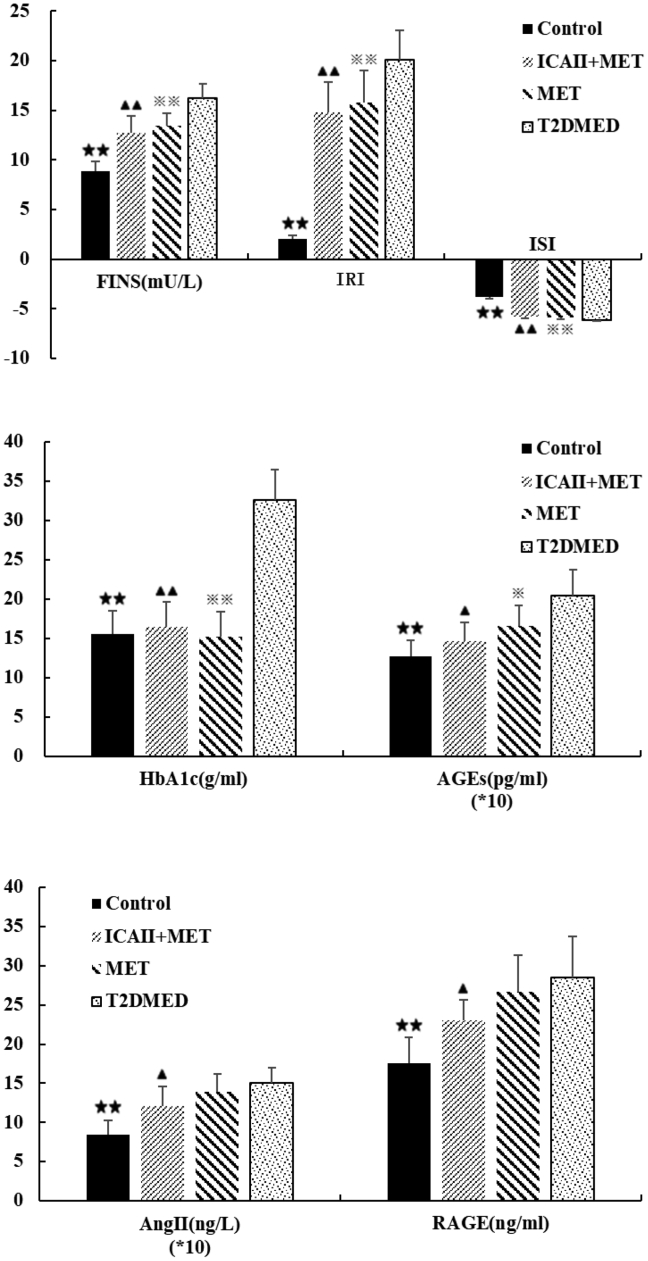
As shown in Figure 4, there was no statistical difference in the absolute values of FINS, IRI, and ISI between the ICA II+MET and MET groups (P > .05). The absolute values of FINS, IRI, and ISI in the control group, ICA II+MET group, and MET group were lower than that in the T2DMED group (8.82 ± 1.01, 2.08 ± 0.31, −3.83 ± 0.16 vs 16.27 ± 1.36, 20.12 ± 2.96, −6.10 ± 0.15. 12.76 ± 1.69, 14.75 ± 3.11, −5.78 ± 0.22 vs 16.27 ± 1.36, 20.12 ± 2.96, −6.10 ± 0.15. 13.43 ± 1.26, 15.78 ± 3.19, −5.85 ± 0.22 vs 16.27 ± 1.36, 20.12 ± 2.96, −6.10 ± 0.15, respectively, P < .01). There was no statistical difference in absolute value of HbA1c among the MET group, ICA II+MET group, and control group (P > .05). The absolute values of HbA1c in the MET group, ICA II+MET group, and control group were significantly lower than in the T2DMED group (15.20 ± 3.22 vs 32.62 ± 3.82, 16.42 ± 3.17 vs 32.62 ± 3.82, 15.59 ± 2.89 vs 32.62 ± 3.82, respectively, P < .01). The AGE value in the control group was significantly lower than in the T2DMED group (127.04 ± 20.04 vs 204.59 ± 32.61, P < .01).
Figure 4.
Comparisons of metabolic variables among the control, ICA II+MET, MET, and T2DMED (±s, , n = 16). ★★: (P < .01), control group vs T2DMED group, ▲▲: (P < .01), , ▲: (P < .05), ICA+MET group vs T2DMED group, ※※:(P < .01), ※:(P < .05), MET group vs T2DMED group. ICA II = icariside II; MET = metformin; T2DMED = type 2 diabetes mellitus erectile dysfunction.
The AGE values in the ICA II+MET and MET groups were lower than in the T2DMED group (146.32 ± 24.20 vs 204.59 ± 32.61, 165.15 ± 26.89 vs 204.59 ± 32.61, respectively, P < .05), and there was no statistical difference in AGE values between the MET group and ICA II+MET group (P > .05).
The value of RAGE in the control group was significantly lower than in the T2DMED group (17.57 ± 3.24 vs 28.55 ± 5.18, P < .01). There was no statistical difference in the values of RAGE between the MET and T2DMED groups (P > .05), but the value of RAGE in the ICA II+MET group was lower than that in the T2DMED group (23.01 ± 2.61 vs 28.55 ± 5.18, P < .05).
The value of Ang II in the control group was significantly lower than in the T2DMED group (8.48 ± 1.79 vs 15.02 ± 1.99, P < .01). There was no statistical difference in the value of Ang II between the MET and T2DMED groups (P > .05), but the value of Ang II in the ICA II+MET group was lower than that in the T2DMED and MET groups (12.08 ± 2.57 vs 15.02 ± 1.99, 12.08 ± 2.57 vs 13.95 ± 2.28, respectively, P < .05).
Effects of ICA II on Lipid Metabolism in T2DMED Rats
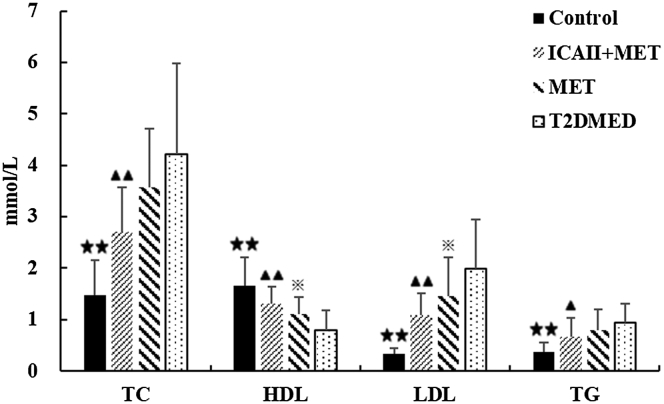
As shown in Figure 5, ICA II treatment improved lipid metabolism in T2DMED rats. The HDL values in the ICA II+MET and control groups were significantly higher than in the T2DMED group (1.31 ± 0.32 vs 0.79 ± 0.40, 1.67 ± 0.54 vs 0.79 ± 0.40, respectively, P < .01). The LDL values and TC values in the ICA II+MET group were significantly lower than in the T2DMED group (1.09 ± 0.42 vs 1.99 ± 0.96, 2.70 ± 0.87 vs 4.21 ± 1.77, respectively, P < .01). The TG values in the ICA II+MET group were lower than in the T2DMED group (0.67 ± 0.37 vs 0.94 ± 0.36, P < .05). There was no statistical difference in TC values and TG values between the MET group and T2DMED group (P > .05).
Figure 5.
Comparisons of lipid metabolism among the control, ICA II+MET, MET, and T2DMED groups (±s, n = 16). ★★: (P < .01), control group vs T2DMED group, ▲▲: (P < .01), ▲: (P < .05), ICA+MET group vs T2DMED group, ※: (P < .05), MET group vs T2DMED group. ICA II = icariside II; MET = metformin; T2DMED = type 2 diabetes mellitus erectile dysfunction.
Effects of ICA II on Serum T, Serum E2, NOS Activity, and cGMP Content in T2DMED Rats
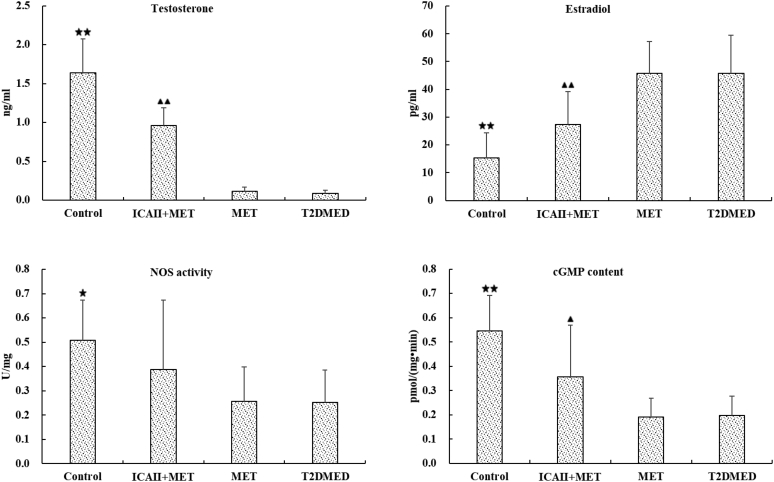
As shown in Figure 6, There was no statistical difference in serum T and serum E2 between the MET and T2DMED groups (P > .05). Serum T levels in the ICA II+MET and control groups were significantly higher than in the T2DMED group (0.96 ± 0.23 vs 0.09 ± 0.04, 1.64 ± 0.44 vs 0.09 ± 0.04, respectively, P < .01). The serum E2 values in the ICA II+MET and control groups were significantly lower than in the T2DMED group (0.27 ± 0.12 vs 0.46 ± 0.14, 0.15 ± 0.09 vs 0.46 ± 0.14, respectively, P < .01). There was no statistical difference in the NOS activity and cGMP content between the MET and T2DMED groups (P > .05). However, the NOS activity and cGMP content in the ICA II+MET groups were significantly higher than in the T2DMED group (0.39 ± 0.32 vs 0.25 ± 0.13, 0.36 ± 0.21 vs 0.20 ± 0.08, respectively, P < .01).
Figure 6.
Comparisons of serum T, serum E2, NOS activity, and cGMP content among the control, ICA II+MET, MET. and T2DMED groups (±s, n = 16). ★★: (P < .01), control group vs T2DMED group, ▲▲: (P < .01), ▲: (P < .05), ICA+MET group vs T2DMED group. ★: (P < .05), control group vs T2DMED group. cGMP = cyclic guanosine monophosphate; E2 = estradiol; ICA II = icariside II; MET = metformin; NOS = nitric oxide synthase; T = testosterone; T2DMED = type 2 diabetes mellitus erectile dysfunction.
Discussion
T2DM is a chronic disease that can have many complications, and it can seriously affect the quality of life of patients.1,2 ED is a sexual dysfunction described as the inability to develop or maintain an erection of the penis that is adequate for sexual intercourse.9, 10, 11 The incidence of ED in patients with T2DM is significantly higher than the incidence of ED in healthy people. The incidence rate of ED in 40-year-old patients with T2DM is 35–90%, and the incidence rate of ED in patients older than 40 years with T2DM is more than 65%.9 Phosphodiesterase type 5 (PDE5) inhibitors that can enhance the NO–cGMP pathway are currently the first-line treatment method for ED. However, PDE5 inhibitors have weak effects on patients with DM.4 Therefore, there is a great need to develop more effective novel strategies aimed at improving erectile function for DM-induced ED (DMED). Our previous studies have confirmed that ICA II can increase erectile function and smooth muscle cell proliferation in diabetic ED rats.8 The impact of ICA II on autophagy, glucose-lipid metabolism, AGEs, RAGE, Ang II, T, E2, and NO–cGMP pathway in the corpus cavernosum of diabetic rats has not been studied until now.
One of the clinical manifestations of T2DM is glucose metabolism disorder, which is related to insulin resistance (IR) and a relative deficiency of insulin.12 The risk of T2DM in persistently long-term obese individuals is significantly higher than that in normal-weight individuals.10,12 Fat cell hypertrophy can lead to low or high expression of adipocyte hormones in patients with T2DM, which can affect their IR and insulin sensitivity (IS) levels.12 IR plays a major role in the pathogenesis of T2DM, and the insulin levels may be higher than in the control group during the early stages of DM, but the ability of insulin to bind to insulin receptors and the postreceptor effect are reduced.9,10,12 The index of IR and IS are key factors that determine the success of a T2DM animal model. A high-fat and high-sugar diet can lead to IR extensively, and a high-calorie diet can lead to obesity in rats. With the single i.p. injection of STZ, there is damage to pancreatic β cells, which could impair glucose tolerance in rats. In the study, the T2DM rat model was developed with the single i.p. injection of 25 mg kg− STZ and a high-fat, high-sugar, and high-calorie diet for 20 weeks. We used diabetic rats to demonstrate ED as measured by decreased maximal ICP, maximal ICP/MAP and mating experiments.
The specific mechanism of DMED is unknown and still under investigation. Long-term disorders of glycolipid metabolism in patients with DM can cause pathological changes in the penile erectile tissue physiological functions and structure.9,11,13 Decreased smooth muscle cell/collagen fibril proportions, endothelial dysfunction, increased apoptosis of CCSMCs and hypogonadism has been considered as the common pathological changes for T2DMED.10,14 A long-term hyperglycemia state can accelerate the formation of HbA1c in patients with T2DM.7 HbA1c can chronically cause damage to blood vessels and tissues, and it can also accelerate AGE and Ang II formation.11 AGE is known to interact with its specific receptors, RAGE, and results in oxidant stress and autophagy. There is evidence that the accumulation of AGEs and RAGE is associated with diabetic complications and is responsible for cavernoma endothelial dysfunction in T2DMED.7,11 In the study, it was found that ICA II could decrease the levels of RAGE and AGEs in T2DMED rats.
The reduction of the CCSMCs content in the corpus cavernosum and the cavernous fibrosis are important pathological features of ED.8 Ang II is involved in the control of cell proliferation, fibrosis, and oxidative stress at the cell and tissue levels. In patients with T2DM, the concentration of Ang II in penile tissue is significantly increased.3,6,8 Angiotensin-converting enzyme inhibitors and Ang II receptor blockers can ameliorate CCSMC fibrosis and extend the life span of CCSMCs in animal models.3,6,8 Ang II can also cause thickening of the penile vessel wall and damage vasomotor function, which results in ED.6,8 In our study, we evaluated whether the CCSMCs/CFs ratio in the ICA II+MET group and control group were significantly higher than in the T2DMED group. It was concluded that the decrease in erectile function may be related the downregulation of CCSMCs/CFs ratio in the T2DMED rats and ICA II can ameliorate these effects.
It had been reported that autophagy is a basic physiological process that helps maintain cell homeostasis, which has been shown to be involved in the pathological process of DMED.13 Autophagy is at a low level under physiological conditions, and the degraded intracellular components can provide nutrients for cell recycling.13,15 Mitochondrial autophagy is an adaptive metabolic response that is necessary to prevent increased levels of reactive oxygen species.15, 16, 17 However, autophagy acts on mitochondrial dysfunction when it is abnormally upregulated under pathological conditions.13,15,16 TEM is considered the gold standard for the detection of autophagy. In the corpus cavernosum of the diabetic penis, increased levels of both glycolipid metabolism and oxidative stress lead to excessive mitochondrial autophagy. In the study, it was found that ICA II can reduce the level of mitochondrial autophagy in the penile tissue of diabetic rats. The mechanism of ICA II in autophagy in the penile tissue of T2DMED rats may be related to the regulation of AGEs, RAGE, and glucose-lipid metabolism.
Increases in TC, TG, and LDL levels and decreases in HDL levels are the main manifestation of lipid metabolism disorders.17, 18, 19 Hyperlipidemia not only causes damage to the vascular smooth muscle tissue of the penis but it also can induce ED by causing damage to the peripheral cavernous nerve that innervates a penile erection.19,20 It has been extensively studied that autophagy was shown to regulate lipid droplet degradation directly.13 In our study, it was found, on the basis of MET, that there was improvement in FPG, HbA1c, and AGEs and that ICA II could regulate lipid metabolism by increasing HDL levels and decreasing LDL, TC, and TG levels in T2DMED rats.
During sexual stimulation, NO released from the corpus cavernosum endothelial cells diffuses into CCSMCs, activates cGMP, increases cGMP concentrations in the cells, decreases calcium ion release, and then causes relaxation of the CCSMCs, congestion of the cavernous sinus of the penis, oppression of the penis, and then penile erection.8,15,17 NO is known to act as an important neural mediator of penile erection. NOS is the key rate-limiting enzyme in NO synthesis, which is very important in NO-dependent functional regulation.17 It has been shown that downregulation of the NOS and cGMP-phosphodiesterase activities are an important occurrence in the pathogenesis of ED.20,21
Serum T is the main component of androgen and plays a key role in maintaining male sexual function.22 E2 can resist androgen action in vivo. The plasma levels of T are closely related to glycolipid metabolism.21,22 T2DM leads to a state of non-bacterial inflammation in the organism, reducing the signal from the hypothalamic axis insulin receptor, resulting in a decrease in the level of T.22,23 MET is a first-line hypoglycemic drug that is used for T2DM treatment. Traditionally, epimedii herb has been used as a tonic drug for centuries in traditional Chinese medicine. ICA II is the main active monomeric component of epimedium in vivo. Chemically synthesized ICA II has fewer adverse reactions and is more convenient than natural epimedium. In the study, it was found that ICA II could improve NOS activity, the cGMP content, and the level of T and E2 in T2DMED rats, which may be related to the improvement of penile erectile function in T2DMED rats.
Conclusion
This study has shown that ICA II could increase erectile function and smooth muscle cell/collagen fibril proportions, decreased mitochondrial autophagy, and AGE concentrations and improve lipid metabolism, NOS activity, cGMP content, T, E2, and Ang II in rat with T2DMED, which may be a promising experimental theoretical basis for T2DMED clinical treatment.
Statement of authorship
Category 1
-
(a)Conception and Design
- Jian Zhang; Shuang Li; Yuexin Liu
-
(b)Acquisition of Data
- Jian Zhang; Shuang Li; Shiqing Zhang; Yonghui Wang
-
(c)Analysis and Interpretation of Data
- Jian Zhang; Shuang Li; Shipeng Jin; Wenzeng Yang
Category 2
-
(a)Drafting the Article
- Jian Zhang; Shuang Li; Shiqing Zhang; Yonghui Wang; Shipeng Jin
-
(b)Revising It for Intellectual Content
- Jian Zhang; Shuang Li; Chunli Zhao; Wenzeng Yang,
Category 3
-
(a)Final Approval of the Completed Article
- Jian Zhang; Shuang Li; Yuexin Liu; Guangqi Kong
Footnotes
Yuexin Liu and Guangqi Kong are contributed equally.
Conflict of Interest: The authors report no conflicts of interest.
Funding: This study was funded by Beijing Municipal Natural Science Foundation (7172110).
Contributor Information
Yuexin Liu, Email: doctorlyx@126.com.
Guangqi Kong, Email: summerharvest11@163.com.
References
- 1.Hunter D.J., Reddy K.S. Noncommunicable diseases. N Engl J Med. 2013;369:1336–1343. doi: 10.1056/NEJMra1109345. [DOI] [PubMed] [Google Scholar]
- 2.Lagani V., Chiarugi F., Manousos D. Realization of a service for the long-term risk assessment of diabetes-related complications. J Diabetes Its Complications. 2015;29:691–698. doi: 10.1016/j.jdiacomp.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Corona G., Giorda C.B., Cucinotta D. Sexual dysfunction at the onset of type 2 diabetes: the interplay of depression, hormonal and cardiovascular factors. J Sex Med. 2014;11:2065–2073. doi: 10.1111/jsm.12601. [DOI] [PubMed] [Google Scholar]
- 4.Goswami S.K., Gangadarappa S.K., Vishwanath M. Antioxidant potential and ability of Phloroglucinol to decrease formation of advanced glycation end products increase Efficacy of Sildenafil in diabetes-induced sexual dysfunction of rats. Sex Med. 2016;4:e104–e112. doi: 10.1016/j.esxm.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu J., Chen L.J., Yu J. Involvement of advanced glycation end products in the pathogenesis of diabetic retinopathy. Cell Physiol Biochem. 2018;48:705–717. doi: 10.1159/000491897. [DOI] [PubMed] [Google Scholar]
- 6.Xu H.Z., Wang W.N., Zhang Y.Y. Effect of angiotensin II type 1 receptor blocker on 12-lipoxygenase activity and slit diaphragm protein expression in type 2 diabetic rat glomeruli. J Nephrol. 2016;29:775–782. doi: 10.1007/s40620-016-0296-3. [DOI] [PubMed] [Google Scholar]
- 7.Musicki B., Burnett A.L. Constitutive NOS uncoupling and NADPH oxidase upregulation in the penis of type 2 diabetic men with erectile dysfunction. Androl. 2017;5:294–298. doi: 10.1111/andr.12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J., Wang Y.B., Ma C.G. Icarisid II, a PDE5 inhibitor from Epimedium wanshanense, increases cellular cGMP by enhancing NOS in diabetic ED rats corpus cavernosum tissue. Andrologia. 2012;44(Suppl 1):87–93. doi: 10.1111/j.1439-0272.2010.01144.x. [DOI] [PubMed] [Google Scholar]
- 9.Pattanaik S., Kaundal P., Mavuduru R.S. Endothelial dysfunction in patients with erectile dysfunction: a Double-Blind, Randomized-control trial using Tadalafil. Sex Med. 2019;7:41–47. doi: 10.1016/j.esxm.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phe V., Roupret M. Erectile dysfunction and diabetes: a review of the current evidence-based medicine and a synthesis of the main available therapies. Diabetes Metab. 2012;38:1–13. doi: 10.1016/j.diabet.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Zhang C., Luo D., Li T. Transplantation of human Urine-derived Stem cells ameliorates erectile function and cavernosal endothelial function by Promoting autophagy of corpus cavernosal endothelial cells in diabetic erectile dysfunction rats. Stem Cells Int. 2019;2019:2168709. doi: 10.1155/2019/2168709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arner P. Fatty acids, obesity and insulin resistance. Obes Facts. 2015;8:147–155. doi: 10.1159/000381224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung K.W., Kim K.M., Choi Y.J. The critical role played by endotoxin-induced liver autophagy in the maintenance of lipid metabolism during sepsis. Autophagy. 2017;13:1113–1129. doi: 10.1080/15548627.2017.1319040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ouyang X., Han X., Chen Z. MSC-derived exosomes ameliorate erectile dysfunction by alleviation of corpus cavernosum smooth muscle apoptosis in a rat model of cavernous nerve injury. Stem Cell Res Ther. 2018;9:246. doi: 10.1186/s13287-018-1003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J., Wu X.J., Zhuo D.X. Effect of tankyrase 1 on autophagy in the corpus cavernosum smooth muscle cells from ageing rats with erectile dysfunction and its potential mechanism. Asian J Androl. 2010;12:744–752. doi: 10.1038/aja.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao J., Deng Y., Yin C. Icariside II, a novel phosphodiesterase 5 inhibitor, protects against H2 O2 -induced PC12 cells death by inhibiting mitochondria-mediated autophagy. J Cell Mol Med. 2017;21:375–386. doi: 10.1111/jcmm.12971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lei H., Li H., Tian L. Icariside II ameliorates endothelial dysfunction by regulating the MAPK pathway via miR-126/SPRED1 in diabetic human cavernous endothelial cells. Drug Des Development Ther. 2018;12:1743–1751. doi: 10.2147/DDDT.S166734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin D., Qi Y., Huang C. Associations of lipid parameters with insulin resistance and diabetes: a population-based study. Clin Nutr. 2018;37:1423–1429. doi: 10.1016/j.clnu.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 19.Ben Khedher M.R., Bouhajja H., Haj Ahmed S. Role of disturbed fatty acids metabolism in the pathophysiology of diabetic erectile dysfunction. Lipids Health Dis. 2017;16:241. doi: 10.1186/s12944-017-0637-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Durmus N., Toylu A., Evcim S. Time-course changes of nLDL-induced erectile dysfunction. Int J Impot Res May. 2017;29:115–119. doi: 10.1038/ijir.2017.5. [DOI] [PubMed] [Google Scholar]
- 21.Assaly-Kaddoum R., Giuliano F., Laurin M. Low Intensity Extracorporeal Shock Wave Therapy improves erectile function in a model of type II diabetes Independently of NO/cGMP pathway. J Urol Sep. 2016;196:950–956. doi: 10.1016/j.juro.2016.03.147. [DOI] [PubMed] [Google Scholar]
- 22.Seftel A.D. Re: testosterone treatment and sexual function in older men with low testosterone levels. J Urol Dec. 2016;196:1721–1724. doi: 10.1016/j.juro.2016.09.058. [DOI] [PubMed] [Google Scholar]
- 23.Dhindsa S., Ghanim H., Batra M. Hypogonadotropic hypogonadism in men with Diabesity. Diabetes Care. 2018;41:1516–1525. doi: 10.2337/dc17-2510. [DOI] [PMC free article] [PubMed] [Google Scholar]