Abstract
Mutations in the genes encoding the highly conserved Ca2+-sensing protein calmodulin (CaM) cause severe cardiac arrhythmias, including catecholaminergic polymorphic ventricular tachycardia or long QT syndrome and sudden cardiac death. Most of the identified arrhythmogenic mutations reside in the C-terminal domain of CaM and mostly affect Ca2+-coordinating residues. One exception is the catecholaminergic polymorphic ventricular tachycardia–causing N53I substitution, which resides in the N-terminal domain (N-domain). It does not affect Ca2+ coordination and has only a minor impact on binding affinity toward Ca2+ and on other biophysical properties. Nevertheless, the N53I substitution dramatically affects CaM's ability to reduce the open probability of the cardiac ryanodine receptor (RyR2) while having no effect on the regulation of the plasmalemmal voltage-gated Ca2+ channel, Cav1.2. To gain more insight into the molecular disease mechanism of this mutant, we used NMR to investigate the structures and dynamics of both apo- and Ca2+-bound CaM-N53I in solution. We also solved the crystal structures of WT and N53I CaM in complex with the primary calmodulin-binding domain (CaMBD2) from RyR2 at 1.84–2.13 Å resolutions. We found that all structures of the arrhythmogenic CaM-N53I variant are highly similar to those of WT CaM. However, we noted that the N53I substitution exposes an additional hydrophobic surface and that the intramolecular dynamics of the protein are significantly altered such that they destabilize the CaM N-domain. We conclude that the N53I-induced changes alter the interaction of the CaM N-domain with RyR2 and thereby likely cause the arrhythmogenic phenotype of this mutation.
Keywords: calmodulin (CaM), calcium-binding protein, ryanodine receptor, protein structure, protein-protein interaction, structure-function, CaM-N53I, cardiac arrhythmia, CPVT, NMR, X-ray crystallography
Introduction
Calmodulin (CaM)6 is a ubiquitous protein that serves as an intracellular Ca2+ sensor with more than 300 interaction partners (1). In its mature processed form, CaM consists of 148 amino acid residues and is composed of two globular domains (N- and C-domain) connected by a flexible linker region (2). Each domain contains two EF-hand motifs that can bind one Ca2+ each, and the Ca2+ affinity is higher for the C-domain than the N-domain, giving CaM a wide Ca2+-sensing range. This wide range is used to conduct the Ca2+ signal in a variety of pathways because Ca2+-binding induces large conformational changes in CaM that alter its affinity for other protein targets (3, 4). CaM is an extremely conserved protein: all vertebrate species have the exact same protein sequence (2, 5). Selection pressure against CaM mutations is further underlined by the fact that humans have three different genes (CALM1–3) all coding the exact same CaM sequence (6).
In the cardiac context, CaM interacts with several ion channels (4, 7–10). This includes the L-type voltage-gated calcium channel (CaV1.2), for which CaM serves as the Ca2+ sensor mediating the Ca2+ signal that accelerates the inactivation of the channel in a process termed Ca2+-dependent inactivation (CDI) (8, 11). CaM also interacts with the cardiac ryanodine receptor (RyR2), which governs the release of Ca2+ stored in the sarcoplasmic reticulum in cardiomyocytes. RyR2 is activated by Ca2+ entering the myocyte through CaV1.2 or by a high sarcoplasmic reticulum luminal Ca2+ concentration, while it is inhibited by CaM at both diastolic and systolic Ca2+ concentrations (12, 13).
Mutations in RyR2 and some of its auxiliary proteins have been linked to catecholaminergic polymorphic ventricular tachycardia (CPVT), a condition that can lead to ventricular arrhythmias and sudden cardiac death (14–17). Recently, two individual CALM1 mutations were found in patients suffering from CPVT (N53I and N97S; CaM numbering throughout this article is according to the mature protein sequence without the initial Met). Subsequently, additional mutations in CaM-encoding genes have been reported. Some of these are linked to CPVT, some cause long QT syndrome, and some cause both. Others cause idiopathic ventricular fibrillation. Several other CaM variants have been identified through genome database searches but without being linked to any cardiac phenotype (18–23).
Many CaM mutations reduce CDI of CaV1.2, inhibition of RyR2, or both. Several mutations within the C-domain lead to long QT syndrome and have been shown to either obliterate or decrease the extent of CDI. In stark contrast, CaM-N53I shows a negligible effect on CDI (22, 24, 25), but a strong effect on RyR2, where different studies show either no inhibitory effect or an activating effect on RyR2 at different cellular Ca2+ concentrations. This is in sharp contrast to the RyR2 inhibition observed by CaM-WT at the same Ca2+ concentration (26–30).
However, the mechanistic details that explain how CaM mutations lead to cardiac arrhythmia are not always understood. It is puzzling that one mutated allele can cause a dominant phenotype in the presence of five healthy alleles. All of the reported arrhythmogenic mutations are observed in the CaM C-domain except for E45K, which was discovered very recently, and N53I (23). The Asn53 residue is involved neither in Ca2+ coordination nor in the packing of the hydrophobic core of the N-domain. It is situated in one of the helices, protruding out into the solvent (18–20, 22). Previous structural investigations of CaM mutants have often shown large structural changes, but the structure of CaM-N53I in complex with the CaV1.2 IQ domain did not significantly differ from CaM-WT in complex with the CaV1.2 IQ domain (31, 32). Although this result is expected, because the function of CaV1.2 is not perturbed by CaM-N53I (24), it does not explain the strong effect of N53I on RyR2 function, and the mechanism of action thus remains elusive.
Studies on CaM-N53I have shown how this variant causes increased RyR2 activity in murine ventricular myocytes, HEK293 cells, and single-channel RyR2 recordings (24, 26, 28, 29). The mutation also increases the heart rate in β-adrenergic stimulated zebrafish, similar to the tachycardia phenotype observed in the human index case discovered 8 years ago (5, 30). Although the effect of CaM-N53I in a cellular setting is evident, only small differences have been observed when looking at CaM-N53I biophysically. The Ca2+ affinity of both CaM-N53I domains, along with the Ca2+ affinity in the presence of a peptide representing the CaM-binding domain of RyR2 (CaMBD2), shows only small differences compared with CaM-WT (27, 29, 30). Based on the cellular effects described above, it seems likely that the N53I mutation interferes with the interaction between CaM and RyR2. However, a recent high-resolution cryo-EM structure of the CaM-WT in complex with full-length RyR2 does not support this hypothesis because amino acid Asn53 seems not to interact with RyR2 under any of the investigated conditions (33).
To investigate the structural effect of the N53I mutation, we determined the solution structures and dynamics of CaM-N53I both in the apo- and Ca2+-loaded state by NMR, along with solving the crystal structures of both Ca2+/CaM-WT and -N53I in complex with CaMBD2 of RyR2. The affinity of CaM-N53I for RyR2–CaMBD2 was also determined. The overall goal of this work is to find the differences that can explain the arrhythmogenic nature of CaM-N53I.
Results
Throughout this paper, the CaM numbering is for mature CaM, meaning that the initial methionine is excluded. Both apoCaM and Ca2+/CaM possess eight α-helices, which will be named A–H, of which A–D are in the N-domain, whereas E–H are in the C-domain. Structures of other CaM variants have already been solved in which it has been shown that a mutation can give rise to a structural change (31). We therefore started out by solving the structures of both apoCaM-N53I and Ca2+/CaM-N53I by NMR to investigate for structural differences compared with CaM-WT.
NMR structure of apoCaM-N53I
The two CaM domains are connected by a flexible linker that causes them to sample a lot of different spatial conformations, making a simultaneous alignment of both domains in an ensemble impossible. Therefore, an ensemble of each domain of apoCaM-N53I was compared with its corresponding domain from apoCaM-WT (PDB code 1CFD) (34) (Fig. 1, A and B). In the N-domain (Fig. 1A), differences are observed in the Ca2+-binding loops in both EF-hands but also in the flexible loop connecting the two EF-hands between helix B and C. No structural changes are observed in helix C harboring Ile53, which protrudes into the solvent like Asn53 in the WT. The variation in the 20 apoCaM-N53I structures is largest in the aforementioned loop regions. Comparing the apoCaM-N53I and apoCaM-WT C-domains (Fig. 1B), similar loop flexibility is observed with both interhelical loops and the EF-hand–connecting loop being flexible. Overall, the apoCaM-N53I C-domain is less well-defined than the N-domain because of a lower density of NOEs in the C-domain, but the same observation holds true for apoCaM-WT (35).
Figure 1.
Ensemble of 20 NMR structures of apoCaM-N53I (PDB code 6Y95) backbone only (carbon in cyan and nitrogen in blue) overlaid with apoCaM-WT in magenta (PDB code 1CFD). The residue at position 53 is in stick representation and labeled, whereas the other numbers refer to the position of selected residues. A, N-domain superposed on C, Cα, and N of residues 5–72. B, C-domain superposed on C, Cα, and N of residues 87–145.
Because of the structural similarity between apoCaM-WT and apoCaM-N53I, we analyzed chemical shift differences in the [1H-15N]-HSQCs to see which other residues were affected by the mutation (Fig. S1). As expected, the residues near the mutated Ile53 residue are affected because the chemical environment changes by substituting an amino acid. Notably, the two Ca2+-coordinating residues Asn60 and Thr62 are also affected, even though they are 10–12 Å away from Ile53.
NMR structure of Ca2+/CaM-N53I
The ensemble of Ca2+/CaM-N53I structures is compared with the first structure of the Ca2+/CaM-WT ensemble (PDB code 1X02) (36) (Fig. 2, A and B). Compared with apoCaM, a higher degree of similarity is observed in the Ca2+/CaM comparison because the difference in the flexible loop regions are smaller. Ile53 is located right at the end of helix C in Ca2+/CaM-N53I, and no major changes are present compared with Ca2+/CaM-WT (Fig. 2A). The side chain of residue 53 is not within the hydrophobic binding pocket that is exposed when CaM binds Ca2+, so it is not at the typical interface whereby CaM binds amphipathic helices.
Figure 2.
Ensemble of 20 NMR structures of the Ca2+/CaM-N53I (PDB code 6Y94) backbone (carbon in cyan and nitrogen in blue) overlaid with the first structure of the Ca2+/CaM-WT ensemble (PDB code 1X02) in magenta. The yellow spheres indicate Ca2+ for both WT and N53I. The residue at position 53 is displayed as stick and labeled with N53I, whereas the other numbers refer to the position of selected residues. A, N-domain superposed on C, Cα, and N of residues 5–72. B, C-domain superposed on C, Cα, and N of residues residue 87–145.
Because of the high degree of similarity between the two Ca2+-bound structures, we investigated chemical shift differences in the [1H-15N]HSQC spectra of Ca2+/CaM-N53I and Ca2+/CaM-WT (Fig. S2). Not surprisingly, the differences are largest in residues 49–57 on α-helix C near the mutation site. However, as opposed to the apo structure, minor changes are also observed in residues 28–33 on α-helix B, which is part of the first EF-hand in the N-domain). In summary, small structural changes are observed both for the apoCaM- and Ca2+/CaM-N53I structures compared with their respective WT structures, along with some chemical shift changes propagating to residues not in the immediate vicinity of Ile53.
Crystal structure of Ca2+/CaM-WT bound to CaMBD2 of RyR2
Because the CaM-N53I variant is believed to cause dysregulation of RyR2 in arrhythmia patients, we investigated the CaM–RyR2 interaction. CaM interacts with RyR2 through CaMBD2 encompassing residues 3581–3611, and a deletion of this domain abolishes CaM binding to RyR2 (37). Because the resolution of Ca2+/CaM bound to full-length RyR2 is limited, with many side chains in CaM unresolved, we first solved the crystal structure CaMBD2 in complex with Ca2+/CaM-WT at 1.8 Å resolution. Three residues differ between CaMBD2 from RyR1 and RyR2 (RyR1/RyR2: Lys3614/Arg3581, Arg3629/Lys3596, and Thr3639/Ala3606). CaM exerts different actions on these two RyR isoforms, where RyR1 is activated and inhibited in the presence of low and high Ca2+ concentration, respectively, whereas RyR2 is inhibited during both low and high Ca2+ concentration (12, 37). A comparison of Ca2+/CaM-WT bound to the RyR1 and RyR2 CaMBD2 indicates that CaM-WT binds similarly to both RyR CaMBD2 isoforms with the characteristic 1–17 spacing between the Trp and Phe hydrophobic anchor residues in the CaMBD2s, shown by a root-mean-square deviation (RMSD) of 0.259 Å (Fig. 3A). Of the three residues that differ between the two CaMBD2s, only Lys3596 is resolved in the complex with CaMBD2 from RyR2.
Figure 3.
Superposition of the Ca2+/CaM-WT:RyR1-CaMBD2 (PDB code 2BCX) (gray) and Ca2+/CaM-WT:RyR2–CaMBD2 (PDB code 6Y4O) (blue) structures, based on Ca2+/CaM-WT. A, Ca2+/CaM-WT is shown in cartoon, CaMBD2 of RyR1 and RyR2 are shown as sticks, and Ca2+ is shown as yellow spheres. Labelled are the two aromatic anchor residues Trp3620/Phe3636 and Trp3587/Phe3603 in CaMBD2 for RyR1 and RyR2, respectively. The green circle highlights the only amino acid substitution (Arg3629/Lys3596) that is resolved in the RyR2 CaMBD2 structure. B, close-up of the area containing the Arg3629/Leu3629 difference between the two CaMBD2s. Distances mark bond length where it is seen how Arg3629 from CaMBD2 in RyR1 is capable of making hydrogen bonds with both Leu18 and through water Ser38 in CaM, whereas the distance is too long for hydrogen bonds from Lys3629 from RyR2 CaMBD2.
Arg3629 in RyR1 interacts with both Leu18 and Ser38 from CaM through two hydrogen bonds and through a water-mediated hydrogen bond, respectively. The Lys3596 lysyl group in RyR2 is smaller than the guanidino group in Arg3629, thereby increasing the distance to Leu18 and Ser38 from CaM. Lys3596 is also angled slightly more toward the peptide backbone than Arg3629, increasing the distance to CaM residues Leu18 and Ser38 even further (Fig. 3B). The two unresolved residues Arg3581 and Ala3606 in the RyR2 complex correspond to Lys3614 and Thr3639 in the RyR1 complex, respectively. In the RyR1 complex, Lys3614 does not make any interactions, but Thr3639 forms hydrogen bonds from its side chain to the carbonyl groups of Cys3635 and Phe3636. This interaction is not possible for the Ala3606 side chain in the RyR2 complex and could lead to instability and an altered structure. However, the structures are still highly similar in this C-terminal part of the CaMBD2s. Despite these differences in binding, we previously found the affinities of Ca2+/CaM for the RyR1 and RyR2 CaMBD2 segments to be very similar, with a Kd ∼45 nm (38).
Crystal structure of Ca2+/CaM-N53I bound to CaMBD2 of RyR2
We next solved the structure of the Ca2+/CaM-N53I in complex with CaMBD2 from RyR2 to a resolution of 1.9 Å. When the structure of Ca2+/CaM-WT and Ca2+/CaM-N53I bound to CaMBD2 from RyR2 are superposed, subtle conformational changes are observed in the vicinity of residue 53 (Fig. 4A). When zooming in on the area harboring EF-hand 2, subtle changes in both the position of the Ca2+ and the Ca2+-coordinating residues are seen (Fig. 4B). The conformation of the two CaM-N53I domains is also highly similar to the CaM-WT domains, with an RMSD of 0.384 Å. For the individual domains, RMSD values of 0.221 Å for the N-domain (residue 4–73) and 0.190 Å for the C-domain (residue 85–145) are obtained. In the crystal structure of Ca2+/CaM-WT bound to CaMBD2 from RyR1 (PDB code 2BCX), Asn53 forms hydrogen bonds with CaM residues Gln49 and Asp56, the latter through a water molecule. We therefore investigated the hydrogen bond network in helix C in both the Ca2+/CaM-WT and -N53I:RyR2–CaMBD2 crystal structures (Fig. 4, C and D). In the Ca2+/CaM-WT:RyR2–CaMBD2 complex, Asn53 Oδ1 forms a hydrogen bond with Gln49 Hϵ2, but the water-mediated hydrogen bond to Asp56 is absent (Fig. 4C). For the Ca2+/CaM-N53I:RyR2–CaMBD2 complex, the side chain of Gln49 is not resolved, probably because of the lost hydrogen bond because the side chain of Ile53 cannot participate in a hydrogen bond. The chemical shift of Gln49 also changes strongly upon mutation of Asn53 in the absence of CaMBD2 (Fig. S9). It is, however, not possible to conclude whether this is due to a disruption of its H-bond with Asn53 or merely due to a change in the chemical environment. Another hydrogen bond, formed between the backbone atoms of Asp50 and Glu54 in the WT structure, is absent in the mutant Ile53 structure because the distance between the two atoms has increased from 2.19 to 2.48 Å (Fig. 4D).
Figure 4.
Comparison of the two RyR2 CaMBD2 complexes with Ca2+/CaM-WT (PDB code 6Y4O) and Ca2+/CaM-N53I (PDB code 6Y4P), respectively. A, superposition of Ca2+/CaM-WT:RyR2–CaMBD2 (blue) and Ca2+/CaM-N53I:RyR2–CaMBD2 (orange, CaMBD2 in green). The superposition is based on CaMDB2. CaM is shown in cartoon representation, CaMBD2 as sticks, Ca2+ is shown as yellow spheres, and the amino acid at the N53I mutation site is shown as sticks. B, close-up of the mutation site with the backbone, the Ca2+-coordinating amino acids, and the site of mutation, represented as sticks, where the superposition is based on CaM rather than CaMBD2. The yellow spheres represent Ca2+, and the red spheres are water participating in the coordination of the Ca2+. C and D, close-up of the hydrogen bond (yellow dashed lines) network in helix C of Ca2+/CaM-WT (C) and Ca2+/CaM-N53I (D) in complex with CaMBD2 with arrows indicating two hydrogen bonds missing in the Ca2+/CaM-N53I:RyR2–CaMBD2 complex.
Asn53 is found on the solvent-exposed side of CaM, where it interacts neither with Ca2+ nor with CaMBD2. When looking at a surface hydrophobicity plot of the two structures, it is clear that the N53I substitution turns the surface from polar to hydrophobic (Fig. 5). The solvent-accessible area for residues Asn53 and Ile53 is 89 and 190 Å2, respectively. This exposed hydrophobic area may promote additional interactions with RyR2 that are not observed for WT CaM.
Figure 5.

Comparison of the Ca2+/CaM-WT:RyR2–CaMBD2 (PDB code 6Y4O) and Ca2+/CaM-N53I:RyR2–CaMBD2 (PDB code 6Y4P) structures. CaM is shown in surface representation, with a color gradient from white to green indicating the hydrophobicity based on the Kyte–Doolittle scale (39) (green is hydrophobic). CaMBD2 (gray) is in cartoon representation, and the black circles indicate the areas of the Asn53 and Ile53 residues.
Ensemble refinement of CaM-WT and -N53I bound to CaMBD2 of RyR2
We performed an ensemble refinement (40, 41) on the two crystal structures of the Ca2+/CaM-WT:RyR2–CaMBD2 and Ca2+/CaM-N53I:RyR2–CaMBD2 complexes to investigate whether the N53I mutation could lead to instability of helix C in CaM (Fig. 6). The ensemble shows that the linker connecting the two domains is highly flexible together with the loop regions between helix B-C and F-G and the N and C termini in both CaM-WT and CaM-N53I (Fig. 6, A–C). This is in agreement with {1H}-15N-NOE values recorded by NMR on Ca2+/CaM-WT and -N53I, although these data are recorded in the absence of CaMBD2 (Fig. S6). Interestingly, a detailed investigation of the stability of helix C in CaM-N53I reveals an extended distance between Asp50 O and Glu54 N on the backbone, compared with CaM-WT (Fig. 6D). This result correlates with the missing hydrogen bond illustrated in Fig. 4 (C and D).
Figure 6.
Ensemble refinement reveals plausible α-helix instability. A and B, representative ensembles of structures describing the electron density for Ca2+/CaM-WT:RyR2–CaMBD2 (gray) and Ca2+/CaM-N53I:RyR2–CaMBD2 (wheat). The highly mobile linker region of CaM is shown in orange, and CaMBD2 is shown in blue. C, root-mean-square fluctuations (RMSF) for Ca2+/CaM-WT:RyR2–CaMBD2 and Ca2+/CaM-N53I:RyR2–CaMBD2 with placement of the four EF-hands and the eight helices. Fully drawn lines are the means of five ensemble runs with standard deviations shown as lightly colored regions. D, in-detail investigation of the hydrogen bonds of helix C in both Ca2+/CaM-WT:RyR2–CaMBD2 and Ca2+/CaM-N53I:RyR2–CaMBD2. Distances are shown as frequencies calculated from all five ensemble runs, with Asp50–Glu54 highlighted in red.
Effect of CaM-N53I on binding to CaMBD2 of RyR2
We have previously reported the affinity of CaM-WT and several other CaM mutants toward the RyR2 CaMBD2 under both apo and Ca2+-saturating conditions, using isothermal titration calorimetry (ITC) (28). These data show significant changes in CaMBD2 affinity for some of the mutants. By the same procedure, we determined the affinity of both apo- and Ca2+/CaM-N53I toward CaMBD2 of RyR2 (RyR2 Arg3581–Leu3607) (Fig. 7).
Figure 7.
ITC thermogram for the titration of CaMBD2 peptide with CaM-WT and CaM-N53I under both Ca2+-free (apo) and Ca2+-bound (Ca2+) conditions. Isotherms were fit to a one-site binding model. DP is the measured power difference between the reference and the sample cell to maintain a zero temperature between the cells.
The binding of apoCaM to CaMBD2 is entropy-driven, whereas the binding of Ca2+/CaM to CaMBD2 is enthalpy-driven for both CaM-WT and -N53I, in agreement with previous results for CaM-WT (28, 38). For CaM-N53I, the affinities of the apo- and Ca2+ interaction with the RyR2 CaMBD2 are low (28.7 μm) and high (0.015 μm), respectively, showing only minor differences when compared with CaM-WT binding to the RyR2 CaMBD2 (apo 27.8 μm, Ca2+ 0.012 μm) (Table S4).
Effect of the N53I mutation on CaM dynamics
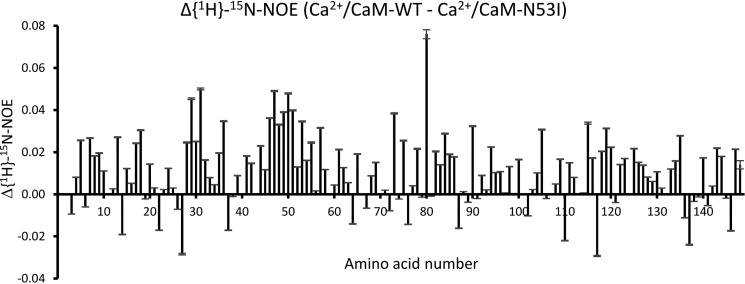
In addition to the missing hydrogen bonds in the Ca2+/CaM-N53I:RyR2–CaMBD2 crystal structure, another significant difference caused by the N53I mutation is an increased Ca2+ dissociation rate from the N-domain in CaM-N53I, reported previously both in the absence and presence of RyR2–CaMBD2 (30, 42). We therefore investigated the dynamics of both CaM-WT and CaM-N53I in the absence and presence of Ca2+ by NMR. In the apo form, no significant differences in the {1H}-15N-NOE spectra can be observed between CaM-WT and -N53I (Fig. S3). Comparing the {1H}-15N-NOE spectra for Ca2+/CaM-WT and Ca2+/CaM-N53I, a mobility difference is observed in amino acids 29–31 and 47–51 situated on α-helices B and C, respectively (Fig. 8 and Fig. S6). This result correlates well with the chemical shift perturbations observed between the Ca2+-bound forms (Fig. S2).
Figure 8.
Difference between {1H}-15N-NOE for Ca2+/CaM-WT and Ca2+/CaM-N53I (Δ{1H}-15N-NOE) where N53I is subtracted from WT. Positive bars indicate higher mobility in N53I compared with WT, and vice versa for negative bars. Large differences are observed in residues 29–31 and 47–51 corresponding to helix B and helix C, respectively. A plot of the {1H}-15N-NOE values for WT and N53I can be seen in Fig. S6.
We then recorded 15N-T1 and 15N-T2 relaxation for both CaM-WT and -N53I in the absence and presence of Ca2+, but there were no significant differences in those parameters between CaM-WT and -N53I (Fig. S4, S5, S7, and S8). Because these experiments only probe the motions on the picosecond to nanosecond time scale, hydrogen deuterium exchange (HDX) experiments were performed to examine the slow hydrogen backbone amide exchange, which is on the time scale of 103–107 s. For both apoCaMs, the exchange took place too fast to be observable, but for Ca2+/CaM-WT and Ca2+/CaM-N53I, HDX was observable for residues mainly found in the core of each CaM domain (Fig. 9). HDX could be observed for many of the same residues in the two proteins, but the exchange rates of Ile27, Ile52, and Glu67 HN could only be determined in CaM-WT, whereas an exchange rate of residue 53 HN was only obtained for CaM-N53I. This means Ile53 HN has a slower exchange rate compared with Asn53 HN in the WT. At the same time Ile52 HN has a slower exchange rate in the WT compared with Ile52 HN in N53I. The majority of the HN in the CaM-N53I residues exchange faster than the HN in the residues in CaM-WT.
Figure 9.
Hydrogen deuterium exchange data for Ca2+/CaM-WT (black) and Ca2+/CaM-N53I (red). Protection factors p = krc/kex are determined by comparing measured hydrogen–deuterium exchange rates, kex, with the rate in a random coil model in which the residues are completely unprotected, krc (43). A larger value indicates a slower exchange. Some amino acids are missing a value because of the exchange rate being too fast to measure with HDX.
To investigate intramolecular mobility on the millisecond time scale, T2-relaxation dispersion was measured for both CaM-WT and -N53I. A significant difference was observed here. In apoCaM-WT, conformational exchange rates (kex) for backbone nitrogen atoms in the N-domain are much lower than those in the C-domain (Fig. 10A), indicating a less stable fold of the C-domain, a well-known characteristic of CaM (44). Strikingly, in apoCaM-N53I, kex of backbone nitrogen atoms in the N-domain are similar to the residues in the C-domain (Fig. 10B). This points to a destabilization of the N-domain of apoCaM-N53I. The relaxation dispersion of both Ile52 and Asn53/Ile53 is shown in Fig. 10 (C and D). For Ca2+/CaM-WT and Ca2+/CaM-N53I, a completely different result is obtained because nearly all residues in both variants have either a much lower or a nonmeasurable kex (Fig. 10, E and F) indicating more stable domains. This higher stability of Ca2+/CaM compared with apoCaM has already previously been reported (45) and is corroborated by the results presented here.
Figure 10.
Rates of intrinsic exchange obtained from T2-relaxation dispersion experiments with τCPMG = 50 ms. A and B, apoCaM-WT (A) and apoCaM-N53I (B) with kex versus amino acid sequence for those resonances that could be assigned. The absence of a bar indicates either kex < 30 s−1 or a nonmeasurable kex. C and D, relaxation dispersion curves for residue Ile52 (C) and N53I (D) with CaM-WT in black and CaM-N53I in red. The lines indicate fits to an exchange process. E and F, as A and B but for Ca2+/CaM-WT (E) and Ca2+/CaM-N53I (F).
Discussion
Mutations in the highly conserved Ca2+-sensing protein CaM can lead to cardiac arrhythmia and cardiac death. The first human CaM mutation, CaM-N53I, was identified by genetic linkage in a large family with dominantly inherited CPVT. Importantly, the large sarcoplasmic Ca2+-release channel RyR2 is the only other protein in which mutations are dominantly linked to the same phenotypical expression, and CaM is an important regulator of RyR2 gating properties. Studies have demonstrated an effect of CaM-N53I on zebrafish heart rhythm and intracellular Ca2+-release events in ventricular myocytes (25, 30). Also, the regulation of RyR2 by CaM-N53I was shown to be aberrant because CaM-N53I displays an activating effect on the channel compared with CaM-WT, which shows an inhibitory effect (26, 27). There are four putative regions of CaM interaction with RyR2, of which CaMBD2 is required for CaM-regulation (37, 38, 46–48). Even though cryo-EM structures of CaM-WT bound to full-length RyR2 are now available, these structures suffer from poor local resolution around CaM, and a mechanistic explanation for the disease mechanism underlying the N53I variant is still lacking.
So how can our results help to understand the arrhythmogenic phenotype? First of all, our data show that the overall structure of CaM-N53I remains unaltered in the apo, the Ca2+, and the Ca2+ + CaMBD2-bound state. However, we discern two major differences that likely underlie the disease mechanism. First, the N53I mutation leads to an exposed large hydrophobic residue, which directly could drive interactions between the CaM N-domain and RyR2 that otherwise do not occur. Second, the mutation leads to a destabilization of the N-domain, resulting in an increase in the internal dynamics of the protein. In the apo-state, 15N-T2 relaxation occurs faster around the mutation site and around amino acids 27–31 (Fig. S5). Also, the heteronuclear {1H}-15N-NOE, a parameter sensitive to intramolecular mobility on the picosecond to nanosecond time scale, of amino acids in this region of the protein drops slightly (Fig. S3), hinting at slightly increased mobility. Most significantly, T2-relaxation dispersion of the CaM-N53I N-domain changes dramatically: although the CaM-WT N-domain displays almost no relaxation dispersion, the N53I mutation introduces T2-relaxation dispersion throughout the whole N-domain (Fig. 10, A and B). This means that the N-domain of apoCaM-N53I undergoes conformational exchange on the millisecond time scale contrary to the N-domain of apoCaM-WT, in which no conformational exchange could be detected. Taken together, the data suggest that there is a higher degree of relatively slow internal motions in the N-domain of CaM-N53I than in CaM-WT. This can be viewed as a constantly ongoing, slow conformational exchange between two or more states. There could be a single excited state, which we do not know much about, populated for a fraction of time, or there could be a more general destabilization of the fold of the N-domain. This is also reflected in the previously reported lower melting temperature for the N-domain of CaM-N53I compared with CaM-WT (30).
In the Ca2+-bound form, mobility changes are not as pronounced: the heteronuclear {1H}-15N-NOE values around amino acid 30 and throughout helix C are slightly decreased in CaM-N53I, but also other regions of the mutant display small changes (Fig. 8 and Fig. S6). The 15N-T1 values (Fig. S7) of Ca2+/CaM-N53I are generally lower and 15N-T2 values (Fig. S8) are generally higher than in the WT. This points to an increased overall mobility of the mutant. Native PAGE experiments reported earlier (30), however, do not support a significant change in hydrodynamic radius of CaM-N53I in solution. Neither Ca2+/CaM-N53I nor Ca2+/CaM-WT display significant T2-relaxation dispersion, probably because of Ca2+ binding which yields a strong contribution to the stability of the fold in both the N- and C-domains. Only few hydrogen–deuterium exchange rates could be obtained. They are generally slightly faster for Ca2+/CaM-N53I than for Ca2+/CaM-WT, also in helices B and C, with the notable exception of Ile52, whose HN exchanges much faster in the N53I mutant, and the mutated amino acid 53, whose HN exchanges much slower in the mutated protein (Fig. 9), suggesting an altered H-bonding network in the vicinity of the mutation site. Despite the absence of larger, detectable mobility changes, the N53I mutation was shown to also destabilize the N-domain of Ca2+/CaM-N53I by 4.7 kJ/mol, relative to Ca2+/CaM-WT (30). Chemical shift perturbation (Fig. S1 and Fig. S2) occurs not only around the mutation site, but also throughout helix C and also in parts of helix B, pointing at a change in the time-averaged chemical environment of the affected amino acid backbone atoms HN and N.
What might be the reason for the drastic destabilization of the N-domain in N53I? The X-ray structure of the Ca2+/CaM-N53I:RyR2–CaMBD2 complex provides a clue (Fig. 4, C and D): the side chains of Gln49 and Asn53 form a stable H-bond, which cannot be formed by the aliphatic side chain of Ile53. At the same time, a H-bond between Asp50 and Glu54 and a water-mediated H-bond between Asn53 and Asp56 are missing in the mutant protein. Although we cannot directly observe the H-bond in solution, the strong chemical shift changes in Gln49 Hδ2 and Nδ2 occurring upon mutation of Asn53 to Ile53 in both apoCaM and Ca2+/CaM could point at a change in hydrogen bonding to the Gln49 side chain (Fig. S9).
It is difficult to explain how a mutation on amino acid Asn53, which does not directly take part in Ca2+ binding and is remote to the CaMBD2-binding site of CaM (Figs. 4A and 5), could possibly influence the interaction of CaM with RyR2. Therefore, additional interaction sites in RyR2 apart from CaMBD2 were suggested (37, 38, 46–48) to hold the key to the dysregulation of RyR2 by CaM-N53I. Indeed, recent cryo-EM structures of the RyR2–CaM complex (33) revealed that the N-domain of CaM has additional interaction sites with RyR2, both in the apo and in the Ca2+-bound forms. In the apo-form (PDB code 6JI8), helix C of CaM is close to amino acid Lys2558 of RyR2, but because of unresolved amino acids in this region of RyR2, the exact interaction site with CaM helix C cannot be determined. Also, other parts of the N-domain show interactions with RyR2. Importantly, CaM EF-hand 2, a region where we can see chemical shift changes introduced by the N53I mutation, is not resolved in the cryo-EM structure but appears close enough to the RyR2 surface to make an interaction with this region possible. In the Ca2+-bound form of the RyR2–CaM complex (PDB code 6JV2), the side-chain carboxylate of Asp50, which is located very close to amino acid Asn53, is only 2.4 Å away from the guanidine group of Arg2209 in RyR2 (Fig. 11B). Superposition of our crystal structure of Ca2+/CaM-N53I:RyR2–CaMBD2 onto the cryo-EM structure (PDB code 6JV2) reveals slight differences especially in the Ca2+-binding EF-hands of CaM, and an overall RMSD of 1.83 Å is obtained for superposition on N, Cα, and C′ over all helix residues (Fig. 11A).
Figure 11.
Crystal structure of Ca2+/CaM-N53I:RyR2–CaMBD2 (orange, CaMBD2 in green) superposed on the cryo-EM structure of RyR2 with Ca2+/CaM-WT (gray) (PDB code 6JV2) (33), and Ca2+ as yellow spheres. The purple dots indicate unresolved residues. A, the two structures are superposed on CaM N, Cα, and C′. Differences are especially observed in the Ca2+-binding EF-hands (EF1–4). B, close-up on the region harboring N53I showing how Asn53 is too far away to participate in interactions with RyR2. Only Asp50 from helix C in CaM interacts with Arg2209 from RyR2. The two structures are superposed on N, Cα, and C′ of helix C of CaM.
These secondary interaction sites revealed by the cryo-EM structures allow us to propose that the destabilization of helix C in CaM-N53I disturbs the interaction of CaM with RyR2 not by altering the interaction with CaMBD2 but by altering the secondary interactions made by the N-domain of CaM. We show that the N53I mutation does not alter the affinity of CaM for CaMBD2, neither in the apo nor in the Ca2+-bound state (Fig. 7 and Table S4). However, even though the N53I mutation has only a small impact on the Ca2+ affinity of CaM alone (30), a reduced Ca2+-affinity for the CaM-N53I:CaMBD2 complex was reported (29), because of an increase in the dissociation rate of Ca2+ from the complex (42). Single-channel ion conductivity experiments demonstrated that CaM-N53I is not as efficient in closing RyR2 as CaM-WT (28). However, our results only show minute changes in protein structure and moderate changes in its intramolecular dynamics.
This demonstrates how finely tuned the regulation of the heartbeat is. Its correct regulation depends critically on very precise affinity and kinetics of the tripartite RyR2–CaM–Ca2+ interaction. It has already been established that RyR2 mutations can lead to spontaneous Ca2+ release and CPVT and that mutations in some RyR2-interacting proteins can lead to CPVT (49). Ca2+ binding to the N-domain of CaM is vital for RyR2 regulation, and it is believed that N-domain binding to CaMBD2, which only occurs at systolic Ca2+ concentrations, is at the heart of the feedback loop that closes RyR2 at high cytosolic Ca2+. CaM-N53I, with its higher Ca2+ dissociation rate, is not be able to keep RyR2 closed tightly enough (28), leading to a leaky RyR2 and consequently CPVT.
Ca2+ binding to the N-domain of the apoCaM:RyR2–CaMBD2 complex is accompanied by a large conformational rearrangement, both of the N-domain and of the interaction epitopes between CaM and RyR2. Any stabilization or destabilization of those interaction epitopes, both in the apo and in the Ca2+-bound states, will have an influence on the apparent Ca2+ affinity. We here demonstrate that the interaction epitopes of the CaM N-domain are affected by the N53I mutation in both states. It is worth bearing in mind that the CaM-N53I variant represents the largest known cohort of people living with an arrhythmogenic CaM variant because the gene has been carried over for generations, whereas most of the other arrhythmogenic CaM variants are de novo mutations. This marks the CaM-N53I variant as less severe compared with the other CaM variants. In light of that, it is not surprising that we observe only small changes in the protein structure. In conclusion, we have shown that the N53I mutation does not alter the overall structure of CaM, but the mutation does change the internal dynamic behavior and stability of the N-domain, and this disturbance in the N-domain is likely to cause the observed phenotype.
Experimental procedures
Protein expression and purification for NMR
For uniformly 13C- and 15N-labeled CaM-N53I, Escherichia coli Rosetta-2 (DE3) cells (Novagen) were transformed with a plasmid from a previous study (30) containing the gene for the CaM-N53I variant. Cells were first grown in LB medium containing no isotope labeled compounds. At A600 ∼0.5, the medium was centrifuged, the supernatant was discarded, and a portion of the pelleted cells was resuspended in standard M9 minimal medium containing 15N-ammonium sulfate and [U-13C] d-glucose to an A600 of ∼0.05. At an A600 of ∼0.5, the expression was induced by 1 mm isopropyl β-d-1-thiogalactopyranoside and incubated for 18 h at 25 °C. The cells were harvested by centrifugation and resuspended in 5 ml of lysis buffer (20 mm Tris-HCl, 50 mm NaCl, 2 mm MgSO4, and 1 mm β-mercaptoethanol (BME) at pH 7.5) per g of cell mass. The cells were lysed by three cycles of freeze-thawing followed by sonication. The lysate was centrifuged at 30,000 × g at 4 °C for 45 min, and the supernatant was applied to a 120-ml amylose column (New England Biolabs), washed with 5 column volumes (CV) of buffer A (20 mm Tris-HCl, 200 mm NaCl, pH 7.4), and eluted with 100% (v/v) buffer B (buffer A and 10 mm maltose). The fusion protein was cleaved with recombinant tobacco etch virus (TEV) protease overnight at 4 °C. The sample was then applied to a 50-ml Q-Sepharose anion-exchange column equilibrated in buffer C (20 mm Tris-HCl, 50 mm NaCl, pH 7.5), washed with 3 CV of 30% (v/v) buffer D (20 mm Tris-HCl, 500 mm NaCl, pH 7.5), and then eluted with a linear gradient from 30 to 100% (v/v) buffer D. The eluted protein was concentrated to 2 ml. The sample was applied to a HiLoad 16/60 Superdex200 column (GE Healthcare) using buffer E (2 mm HEPES, 100 mm KCl, pH 6.5). The molecular weight of the purified protein was verified by MALDI-TOF MS, and concentration was determined by absorption at 280 nm.
NMR samples, assignment, structure calculation, energy refinement, and validation
NMR samples for structure determination were as follows: apoCaM-N53I: 1.9 mm 13C,15N-labeled CaM-N53I, 20 mm HEPES, 100 mm KCl, 1 mm EDTA, 2 mm NaN3, and 0.1 mm TSP-d4 (sodium 2,2,3,3-tetradeutero, 3-(TMS) propionate) at pH 6.3 dissolved in 95% H2O, 5% D2O; and Ca2+/CaM-N53I: 0.56 mm 13C 15N labeled CaM-N53I, 2 mm HEPES, 100 mm KCl, 10 mm CaCl2, 50 mm NaOAc-d3, 2 mm NaN3, and 0.1 mm TSP-d4 at pH 6.57 dissolved in 95% H2O, 5% D2O.
NMR samples for relaxation measurements were as follows: apoCaM-WT: 1.2 mm 13C 15N labeled CaM-WT, 2 mm HEPES, 10 mm KCl, 10 mm EDTA, 2 mm NaN3, and 0.1 mm TSP-d4 at pH 6.53 dissolved in 95% H2O, 5% D2O; Ca2+/CaM-WT: 1.0 mm 13C 15N labeled CaM-WT, 2 mm HEPES, 10 mm KCl, 10 mm CaCl2, 2 mm NaN3, and 0.1 mm TSP-d4 at pH 6.57 dissolved in 95% H2O, 5% D2O; apoCaM-N53I: 1.2 mm 15N labeled CaM-N53I, 2 mm HEPES, 10 mm KCl, 10 mm EDTA, 2 mm NaN3, and 0.1 mm TSP-d4 at pH 6.56 dissolved in 95% H2O, 5% D2O; and Ca2+/CaM-N53I: 1.0 mm 15N labeled CaM-N53I, 2 mm HEPES, 10 mm KCl, 10 mm CaCl2, 2 mm NaN3, and 0.1 mm TSP-d4 at pH 6.57 dissolved in 95% H2O, 5% D2O.
For apoCaM-N53I, all NMR spectra were recorded on a BRUKER AVIII-600 MHz spectrometer equipped with an RT-TXI probe (including a 3D 15N-edited NOESY with 60 ms of mixing time), except for (H)CCH-TOCSY, H(C)CH-TOCSY, 3D 13C-edited NOESY for aliphatic carbon atoms (75-ms mixing time) and 3D 13C-edited NOESY for aromatic carbon atoms (120-ms mixing time), which were recorded on a BRUKER AVI-800 MHz spectrometer. For Ca2+/CaM-N53I, all spectra were recorded on a BRUKER AVIII-600 MHz spectrometer equipped with a CPP-TCI probe, except for a 3D 15N-edited NOESY TROSY (70-ms mixing time), 3D 13C-edited NOESY for aliphatic carbon atoms (70-ms mixing time), and 3D 13C-edited NOESY TROSY for aromatic carbon atoms (80-ms mixing time) which were recorded on a BRUKER AVIII-HD-900 MHz spectrometer. Spectra were acquired at 298.1 K (structure of apoCaM-N53I and all relaxation measurements both on apo- and Ca2+/CaM-WT and -N53I) and 310.1 K (structure of Ca2+/CaM-N53I) and BRUKER TopSpin 3.2 was used for recording and processing. The following spectra were acquired: 2D-15N-HSQC, 2D-13C HSQC (constant-time for aliphatic and constant-time TROSY for aromatic carbon atoms), 3D 15N-edited TOCSY, CBCA(CO)NH, CBCANH, HBHA(CBCACO)NH, HC(C)H-TOCSY, (H)CCH-TOCSY, HNCA, HNCO, HN(CA)CO, (HB)CB(CGCD)HD, (HB)CB(CGCDCE)HE, and experiments for determining 15N-T1, 15N-T2, and the {1H}-15N NOE (50). T2-relaxation dispersion was measured using the BRUKER standard pulse program hsqcrexetf3gpsi3d (51) with a constant relaxation delay τCPMG of 50 ms (2 × 25 ms) and CPMG field strengths ranging from 0 to 1200 Hz (20, 40, 60, 80, 100, 120, 140, 160, 200, 260, 320, 500, 600, 700, 800, 900, 1000, and 1200 Hz with repetitions at 40, 80, 120, 160, 260, 500, 700, 900, and 1200 Hz) for apoCaM and field strengths ranging from 0 to 1125 Hz (125, 250, 375, 500, 625, 750, 875, 1000 and 1125 Hz with repetitions of all field strengths) for Ca2+/CaM, acquired in random order in an interleaved manner recording a full cycle of field strengths for each individual scan at a time.
The chemical shift assignments for 1H, 13C, and 15N of the stereo array isotope-labeled Ca2+/CaM -WT (52) were used as a template for the assignment of Ca2+/CaM-N53I, whereas the chemical shift assignment for 1H, 13C, and 15N of apoCaM-WT7 was used as template for apoCaM-N53I. Both assignments were performed manually using the software CARA 1.8.4.2 (53) with their respective templates used. The NEASY module was used to collect and integrate NOESY cross-peaks. Based on the chemical shift assignments from HN, Hα, Cα, CO, and N, TALOS+ (54) was used for a preliminary empirically prediction of the protein backbone dihedral angles ϕ and ψ. The backbone dihedral angles from TALOS+ and the integrated and assigned NOESY peak lists were used to calculate an ensemble of 20 structures with CYANA 3.0 (55–58). YASARA 14.12.2 (59) was used to perform an energy refinement both in vacuo and water on the ensemble of 20 model structures, with the NOVA (59) and YASARA (60) force fields, respectively. Finally, the two ensembles were validated using the protein structure validation software suite (61) to determine the quality of the structures. A summary of distance restraints and the details from the CYANA calculations can be seen in Table S1.
Cloning, expression, and purification for crystallography
The pEGST_CaM was coexpressed with CaMBD2 in a modified pET28a vector containing in tandem, a His tag, maltose-binding protein, and a cleavage site for the TEV protease in front of CaMBD2 (62) in E. coli Rosetta (DE3) pLacI (Novagen) at 37 °C. The expression was induced at A600 ∼0.6 with 0.4 mm isopropyl β-d-1-thiogalactopyranoside for 4 h. The cells were lysed by sonication in buffer A (250 mm KCl, 10 mm HEPES, pH 7.4, 10 mm CaCl2) and supplemented with 2 ml of glycerol, 25 mg ml−1 lysozyme, 25 mg ml−1 DNase, and 1 mm phenylmethanesulfonyl fluoride. The lysate was applied to a 25-ml PorosMC (Tosoh Biosep) column, washed with 5 CV of buffer A followed by 5 CV of 30% buffer B (250 mm KCl, 500 mm imidazole). Protein was eluted with 5 CV of 100% buffer B. The eluate was 2-fold diluted with buffer A supplemented with 10 mm BME, applied to a 40-ml amylose column, and washed with 3.5 CV buffer A containing 10 mm BME. The protein was eluted with 3.5 CV of buffer C (buffer A with 10 mm maltose and 10 mm BME). The sample was dialyzed against buffer A overnight at 4 °C and simultaneously cleaved with recombinant TEV protease.
The cleaved sample was 2-fold diluted in buffer A and applied to a PorosMC column collecting the flow-through along with 2 CV of buffer A wash. The protein was concentrated and supplemented with 10 mm BME and applied to a Superdex200 column (GE Healthcare) in buffer A containing 10 mm BME. To remove remaining fusion protein, the sample was 2-fold diluted in buffer A and applied onto a 5-ml gravity Co2+-affinity column, collecting flow-through and 1 CV of buffer A wash. The sample was then concentrated in a spin filter, while the buffer was changed into a crystallization buffer containing (25 mm KCl, 10 mm HEPES, pH 7.4, 2 mm CaCl2, 5 mm DTT). Protein crystals were grown by the hanging-drop method at room temperature. The Ca2+/CaM-WT:RyR2–CaMBD2 and Ca2+/CaM-N53I:RyR2–CaMBD2 complexes were crystallized in 0.1 m sodium acetate, pH 4.70, and 23% PEG 550 monomethyl ether, with Ca2+/CaM-N53I:RyR2–CaMBD2 crystals used for seeding of Ca2+/CaM-WT:RyR2–CaMBD2.
X-ray data collection and structure determination
Crystals were harvested and flash-frozen in solutions containing the original growth conditions supplemented with 20% PEG 400. Diffraction data were collected at the Canadian Macromolecular Crystallography Facility 08B1-1 and the Advanced Photon Source at Argonne National Laboratory. The data were processed with XDS (63) and scaled with Aimless (CCP4 package). The structures of both RyR2 CaMBD2 complexes were solved by molecular replacement with Phaser (Phenix package) (64) using the 2.0 Å crystal structure of the Ca2+/CaM-WT:RyR1-CaMBD2 complex as a model (Lys3614–Asn3643, RyR1 CaMBD2) (PDB code 2BCX) (65). The model was subsequently manually built with COOT (66) and refined with phenix.refine (67). The final maps contain electron density for residues 3582–3605 of CaMBD2 from RyR2, residues 4–76 and 81–145 for CaM-WT, and residues 4–77 and 82–146 for CaM-N53I, as well as four well-ordered Ca2+. Because the linker region of CaM and CaMBD2 N and C termini are poorly represented in the electron density maps, these residues are omitted from the final model of both complexes. The statistics are shown in Table S2. Structures were deposited in the PDB with codes 6Y4O and 6Y4P.
Time-averaged X-ray restrained ensemble refinement
The two crystal structures solved here for Ca2+/CaM-WT:RyR2–CaMBD2 (PDB code 6Y4O) and Ca2+/CaM-N53I:RyR2–CaMBD2 (PDB code 6Y4P) were used for Ensemble refinement. A time-averaged electron density restrained molecular dynamics simulation was applied to generate a grouping or ensemble of structures that describe the structural dynamics inherent in the electron density in a quantifiable manner. Manual addition of residues not found in the density, addition of poorly resolved side chains, and removal of conformers were performed using COOT, and the final PDB code files were subjected to Phenix.ready_set to generate ligand restraints and add explicit hydrogen atoms. Ensemble refinement was performed using the implementation in Phenix version 1.10.1-2155 (67) according to Refs. 40 and 41. A grid search was performed for optimal values of pTLS (0.6–0.9), Tbath (2.5, 5, and 10), and Tx (0.3, 0.6, and 1.2× the resolution-dependent value) scoring for the lowest final Rfree. Selected optimal parameters were pTLS of 0.8, T_bath of 2.5, and Tx of 0.6 for both structures with average Rfree/Rwork of 0.1639/0.2234 for the WT and 0.1684/0.2509 for the N53I structure. These parameters were used to perform five random seed repeats for each structure. Root-mean-square fluctuation values for the five repeated ensembles were calculated using PyMOL (PyMOL Molecular Graphics System, version 1.8, Schrödinger) for all residues together using a custom Python script ens_tool.py (40). N–O distances for residues in α-helix C, harboring the N53I mutation, were calculated using residues and frequency histograms created using GraphPad Prism v7.0. Statistics are shown in Table S3.
Isothermal titration calorimetry
The ITC experiments were performed as described previously (28).
Data availability
The atomic coordinates and structure factors or NMR restraints, respectively, have been deposited in the Protein Data Bank (PDB codes 6Y4O for Ca2+/CaM-WT bound to RyR2-CAMBD2, 6Y4P for Ca2+/CaM-N53I bound to RyR2-CAMBD, 6Y94 for Ca2+/CaM-N53I, and 6Y95 for apoCaM-N53I). The NMR chemical shifts have been deposited in the BioMagResBank (accession nos. 34497 for apoCaM-N53I and 34496 for Ca2+/CaM-N53I). All other data are contained within the manuscript.
Author contributions
C. H., F. V. P., M. T. O., and R. W. conceptualization; C. H., L. H., K. L., M. B., K. T. L., C. S., F. V. P., M. T. O., and R. W. data curation; C. H., L. H., K. L., M. B., A. B. S., K. T. L., C. S., F. V. P., M. T. O., and R. W. formal analysis; C. H., K. L., M. B., F. V. P., M. T. O., and R. W. supervision; C. H., F. V. P., M. T. O., and R. W. funding acquisition; C. H., L. H., F. V. P., M. T. O., and R. W. validation; C. H., L. H., K. L., M. B., A. B. S., K. T. L., C. S., F. V. P., M. T. O., and R. W. investigation; C. H., L. H., A. B. S., F. V. P., M. T. O., and R. W. visualization; C. H., L. H., F. V. P., M. T. O., and R. W. methodology; C. H., L. H., K. L., K. T. L., C. S., F. V. P., M. T. O., and R. W. writing-original draft; C. H., F. V. P., M. T. O., and R. W. project administration; C. H., L. H., K. L., M. B., A. B. S., K. T. L., C. S., F. V. P., M. T. O., and R. W. writing-review and editing.
Supplementary Material
Acknowledgments
We thank the Centre for Biomolecular Magnetic Resonance, Frankfurt, Germany for access to NMR equipment (supported by the project Bio-NMR, funded by European Union Contract 261863) and Dr. Frank Löhr for expert assistance. We also thank Dr. Hideo Iwai from the University of Helsinki for access to NMR equipment and expert technical assistance. We thank Ad Bax for a chemical shift list of apoCaM-WT. We also thank the support staff at the Advanced Photon Source (Chicago) GM/CA-CAT Beamline 23-ID-D and at the Canadian Light Source (Saskatoon, Saskatchewan, Canada), which is supported by the Natural Sciences and Engineering Research Council of Canada, the National Research Council Canada, the Canadian Institutes of Health Research, the Province of Saskatchewan, Western Economic Diversification Canada, and the University of Saskatchewan.
This work was supported by Danish Council for Independent Research Grant DFF-1323-00344 and Novo Nordisk Foundation Grants NNF18OC0053032 and NNF16OC0023344. The medical biotechnology group at Aalborg University is supported by the Obel Family Foundation, the SparNord Foundation, and the Carlsberg Foundation. This work is also supported by Operating Grant PJT-148632 from the Canadian Institutes of Health Research (to F. V. P.). The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Tables S1–S4 and Figs. S1–S9.
The atomic coordinates and structure factors (codes 6Y94, 6Y95, 6Y4P, and 6Y4O) have been deposited in the Protein Data Bank (http://wwpdb.org/).
The NMR chemical shift data of this paper are available from the Biological Magnetic Resonance Data Bank under BMRB accession numbers 34496 and 34497.
A. Bax, personal communication.
- CaM
- calmodulin
- RyR2
- ryanodine receptor 2
- CaMBD2
- calmodulin binding domain 2
- CPVT
- catecholaminergic polymorphic ventricular tachycardia
- CaV1.2
- L-type voltage-gated calcium channel
- CDI
- Ca2+-dependent inactivation
- N-domain
- N-terminal domain
- C-domain
- C-terminal domain
- RMSD
- root-mean-square deviation
- HDX
- hydrogen deuterium exchange
- NMR
- nuclear magnetic resonance
- CV
- column volumes
- PDB
- Protein Data Bank
- HSQC
- heteronuclear single quantum coherence
- ITC
- isothermal titration calorimetry
- BME
- β-mercaptoethanol
- TEV
- tobacco etch virus
- TOCSY
- total correlation spectroscopy
- TROSY
- transverse relaxation optimized spectroscopy.
References
- 1. Yap K. L., Kim J., Truong K., Sherman M., Yuan T., and Ikura M. (2000) Calmodulin target database. J. Struct. Funct. Genomics 1, 8–14 10.1023/A:1011320027914 [DOI] [PubMed] [Google Scholar]
- 2. Friedberg F., and Rhoads A. R. (2001) Evolutionary aspects of calmodulin. IUBMB Life. 51, 215–221 10.1080/152165401753311753 [DOI] [PubMed] [Google Scholar]
- 3. Tidow H., and Nissen P. (2013) Structural diversity of calmodulin binding to its target sites. FEBS J. 280, 5551–5565 10.1111/febs.12296 [DOI] [PubMed] [Google Scholar]
- 4. Sorensen A. B., Søndergaard M. T., and Overgaard M. T. (2013) Calmodulin in a heartbeat. FEBS J. 280, 5511–5532 10.1111/febs.12337 [DOI] [PubMed] [Google Scholar]
- 5. Nyegaard M., Overgaard M. T., Søndergaard M. T., Vranas M., Behr E. R., Hildebrandt L. L., Lund J., Hedley P. L., Camm A. J., Wettrell G., Fosdal I., Christiansen M., and Børglum A. D. (2012) Mutations in calmodulin cause ventricular tachycardia and sudden cardiac death. Am. J. Hum. Genet. 91, 703–712 10.1016/j.ajhg.2012.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fischer R., Koller M., Flura M., Mathews S., Strehler-Page M. A., Krebs J., Penniston J. T., Carafoli E., and Strehler E. E. (1988) Multiple divergent mRNAs code for a single human calmodulin. J. Biol. Chem. 263, 17055–17062 [PubMed] [Google Scholar]
- 7. Bers D. M. (2002) Cardiac excitation-contraction coupling. Nature 415, 198–205 10.1038/415198a [DOI] [PubMed] [Google Scholar]
- 8. Zühlke R. D., Pitt G. S., Deisseroth K., Tsien R. W., and Reuter H. (1999) Calmodulin supports both inactivation and facilitation of L-type calcium channels. Nature 399, 159–162 10.1038/20200 [DOI] [PubMed] [Google Scholar]
- 9. Sarhan M. F., Tung C.-C., van Petegem F., and Ahern C. A. (2012) Crystallographic basis for calcium regulation of sodium channels. Proc. Natl. Acad. Sci. U.S.A. 109, 3558–3563 10.1073/pnas.1114748109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ben-Johny M., Yang P. S., Niu J., Yang W., Joshi-Mukherjee R., and Yue D. T. (2014) Conservation of Ca2+/calmodulin regulation across Na and Ca2+ channels. Cell. 157, 1657–1670 10.1016/j.cell.2014.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ben-Johny M., and Yue D. T. (2014) Calmodulin regulation (calmodulation) of voltage-gated calcium channels. J. Gen. Physiol. 143, 679–692 10.1085/jgp.201311153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Balshaw D. M., Xu L., Yamaguchi N., Pasek D. A., and Meissner G. (2001) Calmodulin binding and inhibition of cardiac muscle calcium release channel (ryanodine receptor). J. Biol. Chem. 276, 20144–20153 10.1074/jbc.M010771200 [DOI] [PubMed] [Google Scholar]
- 13. Tian X., Tang Y., Liu Y., Wang R., and Chen S. R. (2013) Calmodulin modulates the termination threshold for cardiac ryanodine receptor-mediated Ca2+ release. Biochem. J. 455, 367–375 10.1042/BJ20130805 [DOI] [PubMed] [Google Scholar]
- 14. Györke I., Hester N., Jones L. R., and Györke S. (2004) The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys. J. 86, 2121–2128 10.1016/S0006-3495(04)74271-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Priori S. G., Napolitano C., Tiso N., Memmi M., Vignati G., Bloise R., Sorrentino V., and Danieli G. A. (2001) Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation 103, 196–200 10.1161/01.CIR.103.2.196 [DOI] [PubMed] [Google Scholar]
- 16. Laitinen P. J., Brown K. M., Piippo K., Swan H., Devaney J. M., Brahmbhatt B., Donarum E. A., Marino M., Tiso N., Viitasalo M., Toivonen L., Stephan D. A., and Kontula K. (2001) Mutations of the cardiac ryanodine receptor (RyR2) gene in familial polymorphic ventricular tachycardia. Circulation 103, 485–490 10.1161/01.CIR.103.4.485 [DOI] [PubMed] [Google Scholar]
- 17. Coumel P., Fidelle J., Lucet V., Attuel P., and Bouvrain Y. (1978) Catecholamine-induced severe ventricular arrhythmias with Adams–Stokes syndrome in children: report of four cases. Br. Heart J. 40, 28–37 [Google Scholar]
- 18. Crotti L., Johnson C. N., Graf E., De Ferrari G. M., Cuneo B. F., Ovadia M., Papagiannis J., Feldkamp M. D., Rathi S. G., Kunic J. D., Pedrazzini M., Wieland T., Lichtner P., Beckmann B.-M., Clark T., et al. (2013) Calmodulin mutations associated with recurrent cardiac arrest in infants. Circulation 127, 1009–1017 10.1161/CIRCULATIONAHA.112.001216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marsman R. F., Barc J., Beekman L., Alders M., Dooijes D., van den Wijngaard A., Ratbi I., Sefiani A., Bhuiyan Z. A., Wilde A. A., and Bezzina C. R. (2014) A mutation in CALM1 encoding calmodulin in familial idiopathic ventricular fibrillation in childhood and adolescence. J. Am. Coll. Cardiol. 63, 259–266 10.1016/j.jacc.2013.07.091 [DOI] [PubMed] [Google Scholar]
- 20. Makita N., Yagihara N., Crotti L., Johnson C. N., Beckmann B.-M., Roh M. S., Shigemizu D., Lichtner P., Ishikawa T., Aiba T., Homfray T., Behr E. R., Klug D., Denjoy I., Mastantuono E., et al. (2014) Novel calmodulin mutations associated with congenital arrhythmia susceptibility. Circ. Cardiovasc. Genet. 7, 466–474 10.1161/CIRCGENETICS.113.000459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boczek N. J., Gomez-Hurtado N., Ye D., Calvert M. L., Tester D. J., Kryshtal D., Hwang H. S., Johnson C. N., Chazin W. J., Loporcaro C. G., Shah M., Papez A. L., Lau Y. R., Kanter R., Knollmann B. C., et al. (2016) Spectrum and prevalence of CALM1-, CALM2-, and CALM3-encoded calmodulin variants in long QT syndrome and functional characterization of a novel long QT syndrome-associated calmodulin missense variant, E141G. Circ. Cardiovasc. Genet. 9, 136–146 10.1161/CIRCGENETICS.115.001323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gomez-Hurtado N., Boczek N. J., Kryshtal D. O., Johnson C. N., Sun J., Nitu F. R., Cornea R. L., Chazin W. J., Calvert M. L., Tester D. J., Ackerman M. J., and Knollmann B. C. (2016) Novel CPVT-associated calmodulin mutation in CALM3 (CALM3-A103V) activates arrhythmogenic Ca waves and sparks. Circ. Arrhythm. Electrophysiol. 9, e004161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Crotti L., Spazzolini C., Tester D. J., Ghidoni A., Baruteau A. E., Beckmann B. M., Behr E. R., Bennett J. S., Bezzina C. R., Bhuiyan Z. A., Celiker A., Cerrone M., Dagradi F., De Ferrari G. M., Etheridge S. P., et al. (2019) Calmodulin mutations and life-threatening cardiac arrhythmias: insights from the International Calmodulinopathy Registry. Eur. Heart J. 40, 2964–2975 10.1093/eurheartj/ehz311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Limpitikul W. B., Dick I. E., Joshi-Mukherjee R., Overgaard M. T., George A. L. Jr., and Yue D. T. (2014) Calmodulin mutations associated with long QT syndrome prevent inactivation of cardiac L-type Ca2+ currents and promote proarrhythmic behavior in ventricular myocytes. J. Mol. Cell. Cardiol. 74, 115–124 10.1016/j.yjmcc.2014.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yin G., Hassan F., Haroun A. R., Murphy L. L., Crotti L., Schwartz P. J., George A. L., and Satin J. (2014) Arrhythmogenic calmodulin mutations disrupt intracellular cardiomyocyte Ca2+ regulation by distinct mechanisms. J. Am. Heart Assoc. 3, e000996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hwang H. S., Nitu F. R., Yang Y., Walweel K., Pereira L., Johnson C. N., Faggioni M., Chazin W. J., Laver D., George A. L. Jr., Cornea R. L., Bers D. M., and Knollmann B. C. (2014) Divergent regulation of ryanodine receptor 2 calcium release channels by arrhythmogenic human calmodulin missense mutants. Circ. Res. 114, 1114–1124 10.1161/CIRCRESAHA.114.303391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vassilakopoulou V., Calver B. L., Thanassoulas A., Beck K., Hu H., Buntwal L., Smith A., Theodoridou M., Kashir J., Blayney L., Livaniou E., Nounesis G., Lai F. A., and Nomikos M. (2015) Distinctive malfunctions of calmodulin mutations associated with heart RyR2-mediated arrhythmic disease. Biochim. Biophys. Acta 1850, 2168–2176 10.1016/j.bbagen.2015.07.001 [DOI] [PubMed] [Google Scholar]
- 28. Søndergaard M. T., Liu Y., Larsen K. T., Nani A., Tian X., Holt C., Wang R., Wimmer R., van Petegem F., Fill M., Chen S. R., and Overgaard M. T. (2017) The arrhythmogenic calmodulin p.Phe142Leu mutation impairs C-domain Ca2+ binding but not calmodulin-dependent inhibition of the cardiac ryanodine receptor. J. Biol. Chem. 292, 1385–1395 10.1074/jbc.M116.766253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Søndergaard M. T., Tian X., Liu Y., Wang R., Chazin W. J., Chen S. R., and Overgaard M. T. (2015) Arrhythmogenic calmodulin mutations affect the activation and termination of cardiac ryanodine receptor–mediated Ca2+ release. J. Biol. Chem. 290, 26151–26162 10.1074/jbc.M115.676627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Søndergaard M. T., Sorensen A. B., Skov L. L., Kjaer-Sorensen K., Bauer M. C., Nyegaard M., Linse S., Oxvig C., and Overgaard M. T. (2015) Calmodulin mutations causing catecholaminergic polymorphic ventricular tachycardia confer opposing functional and biophysical molecular changes. FEBS J. 282, 803–816 10.1111/febs.13184 [DOI] [PubMed] [Google Scholar]
- 31. Wang K., Holt C., Lu J., Brohus M., Larsen K. T., Overgaard M. T., Wimmer R., and van Petegem F. (2018) Arrhythmia mutations in calmodulin cause conformational changes that affect interactions with the cardiac voltage-gated calcium channel. Proc. Natl. Acad. Sci. U.S.A. 115, E10556–E10565 10.1073/pnas.1808733115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang K., Brohus M., Holt C., Overgaard M. T., Wimmer R., and van Petegem F. (2020) Arrhythmia mutations in calmodulin can disrupt cooperativity of Ca2+ binding and cause misfolding. J. Physiol. 598, 1169–1186 10.1113/JP279307 [DOI] [PubMed] [Google Scholar]
- 33. Gong D., Chi X., Wei J., Zhou G., Huang G., Zhang L., Wang R., Lei J., Chen S. R. W., and Yan N. (2019) Modulation of cardiac ryanodine receptor 2 by calmodulin. Nature 572, 347–351 10.1038/s41586-019-1377-y [DOI] [PubMed] [Google Scholar]
- 34. Kuboniwa H., Tjandra N., Grzesiek S., Ren H., Klee C. B., and Bax A. (1995) Solution structure of calcium-free calmodulin. Nat. Struct. Biol. 2, 768–776 10.1038/nsb0995-768 [DOI] [PubMed] [Google Scholar]
- 35. Zhang M., Tanaka T., and Ikura M. (1995) Calcium-induced conformational transition revealed by the solution structure of apo calmodulin. Nat. Struct. Biol. 2, 758–767 10.1038/nsb0995-758 [DOI] [PubMed] [Google Scholar]
- 36. Chattopadhyaya R., Meador W. E., Means A. R., and Quiocho F. A. (1992) A calmodulin structure refined at 1.7 A resolution. J. Mol. Biol. 228, 1177–1192 10.1016/0022-2836(92)90324-D [DOI] [PubMed] [Google Scholar]
- 37. Yamaguchi N., Xu L., Pasek D. A., Evans K. E., and Meissner G. (2003) Molecular basis of calmodulin binding to cardiac muscle Ca2+ release channel (ryanodine receptor). J. Biol. Chem. 278, 23480–23486 10.1074/jbc.M301125200 [DOI] [PubMed] [Google Scholar]
- 38. Lau K., Chan M. M. Y., and van Petegem F. (2014) Lobe-specific calmodulin binding to different ryanodine receptor isoforms. Biochemistry 53, 932–946 10.1021/bi401502x [DOI] [PubMed] [Google Scholar]
- 39. Kyte J., and Doolittle R. F. (1982) A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157, 105–132 10.1016/0022-2836(82)90515-0 [DOI] [PubMed] [Google Scholar]
- 40. Burnley B. T., and Gros P. (2013) phenix.ensemble_refinement: a test study of apo and holo BACE1. Comput. Crystallogr. Newsl. 4, 51–58 [Google Scholar]
- 41. Burnley B. T., Afonine P. V., Adams P. D., and Gros P. (2012) Modelling dynamics in protein crystal structures by ensemble refinement. Elife 1, e00311 10.7554/eLife.00311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu B., Walton S. D., Ho H. T., Belevych A. E., Tikunova S. B., Bonilla I., Shettigar V., Knollmann B. C., Priori S. G., Volpe P., Radwański P. B., Davis J. P., and Györke S. (2018) Gene transfer of engineered calmodulin alleviates ventricular arrhythmias in a calsequestrin-associated mouse model of catecholaminergic polymorphic ventricular tachycardia. J. Am. Heart Assoc. 7, e008155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bai Y., Milne J. S., Mayne L., and Englander S. W. (1993) Primary structure effects on peptide group hydrogen exchange. Proteins 17, 75–86 10.1002/prot.340170110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sorensen B. R., and Shea M. A. (1998) Interactions between domains of Apo calmodulin alter calcium binding and stability. Biochemistry 37, 4244–4253 10.1021/bi9718200 [DOI] [PubMed] [Google Scholar]
- 45. Tsalkova T. N., and Privalov P. L. (1985) Thermodynamic study of domain organization troponin C and calmodulin. J. Mol. Biol. 181, 533–544 10.1016/0022-2836(85)90425-5 [DOI] [PubMed] [Google Scholar]
- 46. Yuchi Z., Lau K., and van Petegem F. (2012) Disease mutations in the ryanodine receptor central region: crystal structures of a phosphorylation hot spot domain. Structure 20, 1201–1211 10.1016/j.str.2012.04.015 [DOI] [PubMed] [Google Scholar]
- 47. Søndergaard M. T., Liu Y., Guo W., Wei J., Wang R., Brohus M., Overgaard M. T., and Chen S. R. W. (2019) Role of cardiac ryanodine receptor calmodulin-binding domains in mediating the action of arrhythmogenic calmodulin N-domain mutation N54I. FEBS J. 15147 10.1111/febs.15147 [DOI] [PubMed] [Google Scholar]
- 48. Brohus M., Søndergaard M. T., Wayne Chen S. R., van Petegem F., and Overgaard M. T. (2019) Ca2+-dependent calmodulin binding to cardiac ryanodine receptor (RyR2) calmodulin-binding domains. Biochem. J. 476, 193–209 10.1042/BCJ20180545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Priori S. G., and Chen S. R. (2011) Inherited dysfunction of sarcoplasmic reticulum Ca2+ handling and arrhythmogenesis. Circ. Res. 108, 871–883 10.1161/CIRCRESAHA.110.226845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Farrow N. A., Muhandiram R., Singer A. U., Pascal S. M., Kay C. M., Gish G., Shoelson S. E., Pawson T., Forman-Kay J. D., and Kay L. E. (1994) Backbone dynamics of a free and phosphopeptide-complexed Src homology 2 domain studied by 15N NMR relaxation. Biochemistry 33, 5984–6003 10.1021/bi00185a040 [DOI] [PubMed] [Google Scholar]
- 51. Tollinger M., Skrynnikov N. R., Mulder F. A., Forman-Kay J. D., and Kay L. E. (2001) Slow dynamics in folded and unfolded states of an SH3 domain. J. Am. Chem. Soc. 123, 11341–11352 10.1021/ja011300z [DOI] [PubMed] [Google Scholar]
- 52. Kainosho M., Torizawa T., Iwashita Y., Terauchi T., Mei Ono A., and Güntert P. (2006) Optimal isotope labelling for NMR protein structure determinations. Nature 440, 52–57 10.1038/nature04525 [DOI] [PubMed] [Google Scholar]
- 53. Keller R. (2004) The Computer Aided Resonance Assignment Tutorial, Cantina, Goldau, Switzerland [Google Scholar]
- 54. Shen Y., Delaglio F., Cornilescu G., and Bax A. (2009) TALOS+: a hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J. Biomol. NMR 44, 213–223 10.1007/s10858-009-9333-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Güntert P., and Buchner L. (2015) Combined automated NOE assignment and structure calculation with CYANA. J. Biomol. NMR 62, 453–471 10.1007/s10858-015-9924-9 [DOI] [PubMed] [Google Scholar]
- 56. Güntert P. (1997) Calculating protein structures from NMR data. Methods Mol. Biol. 60, 157–194 [DOI] [PubMed] [Google Scholar]
- 57. Güntert P., Mumenthaler C., and Wüthrich K. (1997) Torsion angle dynamics for NMR structure calculation with the new program DYANA. J. Mol. Biol. 273, 283–298 10.1006/jmbi.1997.1284 [DOI] [PubMed] [Google Scholar]
- 58. Herrmann T., Güntert P., and Wüthrich K. (2002) Protein NMR structure determination with automated NOE-identification in the NOESY spectra using the new software ATNOS. J. Biomol. NMR. 24, 171–189 10.1023/A:1021614115432 [DOI] [PubMed] [Google Scholar]
- 59. Krieger E., Koraimann G., and Vriend G. (2002) Increasing the precision of comparative models with YASARA NOVA: a self-parameterizing force field. Proteins 47, 393–402 10.1002/prot.10104 [DOI] [PubMed] [Google Scholar]
- 60. Krieger E., Joo K., Lee J., Lee J., Raman S., Thompson J., Tyka M., Baker D., and Karplus K. (2009) Improving physical realism, stereochemistry, and side-chain accuracy in homology modeling: four approaches that performed well in CASP8. Proteins 77, 114–122 10.1002/prot.22570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rezácová P., Borek D., Moy S. F., Joachimiak A., and Otwinowski Z. (2008) Crystal structure and putative function of small Toprim domain-containing protein from Bacillus stearothermophilus. Proteins 70, 311–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lau K., and van Petegem F. (2014) Crystal structures of wild type and disease mutant forms of the ryanodine receptor SPRY2 domain. Nat. Commun. 5, 5397 10.1038/ncomms6397 [DOI] [PubMed] [Google Scholar]
- 63. Kabsch W. (2010) XDS. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 10.1107/S0907444909047337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. McCoy A. J., Grosse-Kunstleve R. W., Adams P. D., Winn M. D., Storoni L. C., and Read R. J. (2007) Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 10.1107/S0021889807021206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Maximciuc A. A., Putkey J. A., Shamoo Y., and MacKenzie K. R. (2006) Complex of calmodulin with a ryanodine receptor target reveals a novel, flexible binding mode. Structure 14, 1547–1556 10.1016/j.str.2006.08.011 [DOI] [PubMed] [Google Scholar]
- 66. Emsley P., Lohkamp B., Scott W. G., and Cowtan K. (2010) Features and development of COOT. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 10.1107/S0907444910007493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Adams P. D., Afonine P. V., Bunkóczi G., Chen V. B., Davis I. W., Echols N., Headd J. J., Hung L.-W., Kapral G. J., Grosse-Kunstleve R. W., McCoy A. J., Moriarty N. W., Oeffner R., Read R. J., Richardson D. C., et al. (2010) PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 10.1107/S0907444909052925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mulder F. A., Schipper D., Bott R., and Boelens R. (1999) Altered flexibility in the substrate-binding site of related native and engineered high-alkaline Bacillus subtilisins. J. Mol. Biol. 292, 111–123 10.1006/jmbi.1999.3034 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The atomic coordinates and structure factors or NMR restraints, respectively, have been deposited in the Protein Data Bank (PDB codes 6Y4O for Ca2+/CaM-WT bound to RyR2-CAMBD2, 6Y4P for Ca2+/CaM-N53I bound to RyR2-CAMBD, 6Y94 for Ca2+/CaM-N53I, and 6Y95 for apoCaM-N53I). The NMR chemical shifts have been deposited in the BioMagResBank (accession nos. 34497 for apoCaM-N53I and 34496 for Ca2+/CaM-N53I). All other data are contained within the manuscript.