Abstract
Members of the interleukin (IL)-1 family are key determinants of inflammation. Despite their role as intercellular mediators, most lack the leader peptide typically required for protein secretion. This lack is a characteristic of dozens of other proteins that are actively and selectively secreted from living cells independently of the classical endoplasmic reticulum-Golgi exocytic route. These proteins, termed leaderless secretory proteins (LLSPs), comprise proteins directly or indirectly involved in inflammation, including cytokines such as IL-1β and IL-18, growth factors such as fibroblast growth factor 2 (FGF2), redox enzymes such as thioredoxin, and proteins most expressed in the brain, some of which participate in the pathogenesis of neurodegenerative disorders. Despite much effort, motifs that promote LLSP secretion remain to be identified. In this review, we summarize the mechanisms and pathophysiological significance of the unconventional secretory pathways that cells use to release LLSPs. We place special emphasis on redox regulation and inflammation, with a focus on IL-1β, which is secreted after processing of its biologically inactive precursor pro-IL-1β in the cytosol. Although LLSP externalization remains poorly understood, some possible mechanisms have emerged. For example, a common feature of LLSP pathways is that they become more active in response to stress and that they involve several distinct excretion mechanisms, including direct plasma membrane translocation, lysosome exocytosis, exosome formation, membrane vesiculation, autophagy, and pyroptosis. Further investigations of unconventional secretory pathways for LLSP secretion may shed light on their evolution and could help advance therapeutic avenues for managing pathological conditions, such as diseases arising from inflammation.
Keywords: monocyte, Toll-like receptor (TLR), stress, lysosome, reactive oxygen species (ROS), oxidation-reduction (redox), inflammation, innate immunity, interleukin 1 (IL-1), secretion, cryopyrin-associated periodic syndrome (CAPS), gasdermin D, leaderless secretory proteins, misfolding, unconventional protein secretion, secretory lysosomes, innate immune response
Introduction
How secreted proteins are selected from the crowded interior of cells is a key question in biology. In the early 1950s, by integrating both morphological and biochemical findings, George Palade along with other researchers established the role of the endoplasmic reticulum (ER) and the Golgi apparatus in the transport and release of secreted proteins (1). Günter Blobe, César Milstein, and others further demonstrated that an N-terminal stretch of hydrophobic amino acids (called signal peptide or leader sequence) is characteristic of proteins destined to enter the ER and begin their voyage toward the extracellular space (2, 3). Thus, when in 1984 Charles Dinarello's laboratory cloned the gene of the soluble inflammatory cytokine interleukin-1β (IL-1β) (4), the absence of a signal peptide in this protein raised an intriguing question for cell biologists. How can a protein devoid of a secretory signal find its way out of the cell? At first, given its proinflammatory role, it was thought that IL-1β is released by dying cells at sites of injury or infection. However, many lines of evidence argued against this hypothesis. Intrigued by this problem, we and others investigated IL-1β secretion in human monocytes and observed the following.
(i) The release of IL-1β is selective: upon being triggered by lipopolysaccharide (LPS), human monocytes release mature IL-1β, but little if any of other abundant proteins, as are released upon necrosis (5).
(ii) Monocytes accumulate the biologically inactive precursor pro-IL-1β (33 kDa) in the cytosol and secrete active IL-1β (17 kDa). Dying cells release only pro-IL-1β, implying that its processing requires living cells and is coupled to secretion.
(iii) Drugs that block ER-to-Golgi traffic (e.g. brefeldin A) do not inhibit IL-1β secretion (5).
(iv) Human IL-1β lacks post-translational modifications that occur along the ER-Golgi route. However, glycosylated IL-1β is secreted when it is appended with a leader sequence (6).
Together, these observations implied the existence of a novel pathway for IL-1β secretion (5) and possibly for other proteins devoid of a signal peptide (7). The existence of secretory pathways alternative to the ER-Golgi route had been shown in yeast for a-mating factor (8). The family of leaderless secretory proteins (LLSPs) grew 2 years later with the discovery of the secretion of the cytosolic oxidoreductase thioredoxin (Trx) (9) and basic fibroblast growth factor (bFGF, now FGF2) (10), prompting us to introduce the term “leaderless secretion” (9). Many LLSPs were later found (Table 1). More recently, the term “unconventional protein secretion” (UPS) was introduced to include the transport of some transmembrane proteins that are translocated co-translationally into the ER but bypass the Golgi to reach the plasma membrane (Fig. 1A). Readers are referred to a special issue of Seminars in Cell and Developmental Biology entirely devoted to the UPS (11).
Table 1.
Cellular functions and locations of leaderless secretory proteins
| Protein | Extracellular function | Intracellular localization and function | References |
|---|---|---|---|
| Cytokines | |||
| IL-1α | Mediator of inflammation and immune response | Nucleus, regulation of transcription | 68, 119 (see also Ref. 66 and references therein) |
| IL-1β | Mediator of inflammation and immune response | Cytosol, function NDa | 5 (see also Ref. 66 and references therein |
| IL-18 | Mediator of inflammation and immune response | Cytosol, function ND | 66 (and references therein), 120 |
| IL-33 | Mediator of inflammation and immune response | Nucleus, function ND | 69 (see also Ref. 66 and references therein) |
| IL-37 | Inhibitor of innate immunity | Cytosol and nucleus, inhibitor of innate immunity | 70 |
| Caspase-1 | Extracellular processing of pro-IL-1? | Cytosol, IL-1β, and IL-18 convertase; mediator of secretion of other LLSPs | 119, 121 |
| FGF-1/acidic FGF | Growth, survival, and differentiating factor | Nucleus, anti-apoptotic, neurotrophic factor | 122 (and references therein) |
| FGF-2/basic FGF | Growth, survival, and differentiating factor | Nuclear, anti-apoptotic factor | 10 (see also Ref. 123 and references therein) |
| Annexin A1, A2, A4, A5 | Modulators of coagulation and anti-inflammatory mediator; soluble or bound to the outer leaflet of the plasma membrane | Cytosol: membrane trafficking and organization; nucleus: pro-apoptotic | 124 (and references therein) |
| Members of the galectin protein family | Mediator of innate immunity | Cytosol, pro-apoptotic | 7 (see also Ref. 124 and references therein) |
| Members of the S100 protein family (S100A8, S100A9) | Antimicrobial and chemotactic activities | Cytosol, EF-hand domain--containing Ca2+-binding proteins | 125 |
| MIF-1 | Pro-inflammatory mediator | Cytosol, function ND | 48, 126 |
| Endothelial monocyte--activating polypeptide (EMAP)-2 | Pro-inflammatory mediator with anti-angiogenic properties | Cytosol, protein translation (tRNA multisynthetase complex) | 127 |
| HMGB1 | Mediator of inflammation and immune response | Nucleus, transcriptional regulation, DNA replication and repair, and nucleosome assembly | 30 (see also Ref. 128 and references therein) |
| Phosphoglucose isomerase/autocrine motility factor (PGI/AMF) | Inducer of cell migration | Cytosol, glycolytic enzyme (phosphoglucose isomerase) | 129 |
| Redox enzymes | |||
| Trx | Cytokine-like inflammatory mediator, possibly through thiol-disulfide exchange | Cytosol, oxidoreductase | 9 |
| Trx reductase (TrxR) | Regeneration of oxidized Trx? | Cytosol, Trx reductase | 130 |
| Peroxiredoxin (PRDX)1, PRDX2 | Cytokine-like inflammatory mediator, possibly through peroxidation reactions | Cytosol, peroxidases | 85, 131 |
| SOD-1 | Prion-like properties, involved in familial ALS | Cytosol; converts superoxide radicals to molecular oxygen and hydrogen peroxide | 75, 132 |
| Brain proteins | |||
| APE1/Ref-1 | Cytokine-like anti-inflammatory mediator, through thiol-disulfide exchange | Nucleus, antioxidant | 49, 73 |
| CNTF | Cytokine, member of the IL-6 family; neuroprotective, anti-inflammatory | Cytosol, function ND | 72, 134 |
| Tau | Interneuronal signaling, trans-synaptic transmission | Cytosol, stabilization of microtubules | 135 (see also Ref. 33 and references therein) |
| α-Synuclein | Internalized by neurons, modulator of neuronal death | Presynaptic vesicle, functioning as modulator of synaptic vesicle mobility | 136–138 |
| Engrailed 2 | Secreted by tumor cells, modifies tumor microenvironment | Nucleus, homeodomain-containing transcription factor functioning in early development | 60, 139 |
| Others | |||
| Transglutaminase | Cross-linking enzyme with pleiotropic activity; regulates the cellular interactions with the extracellular matrix | Cytosol, nucleus, mitochondria; cross-linking enzyme with pleiotropic activity; NF-KB activator | 140, 141 |
| Insulin-degrading enzyme (IDE) | Zn2+ peptidase, amyloid peptide degradation | Cytosol, Zn2+ peptidase | 142 |
a ND, not determined.
Figure 1.
Different protein secretion routes. A, the left section of the panel depicts the classical secretory pathway. Secretory and membrane proteins endowed with a signal peptide are co-translationally translocated into the ER and transported to the Golgi and downstream organelles of the exocytic pathway. Secretory vesicles eventually fuse with the plasma membrane, releasing secretory proteins into the extracellular environment and exposing membrane-bound proteins (1–3). Routes different from Classical exocytosis grouped under the name Unconventional protein secretion (UPS) (11) are shown on the right. A few leader sequence–bearing transmembrane proteins translocate co-translationally into the ER but bypass the Golgi en route to the plasma membrane. Many cytosolic proteins lacking the secretory signal peptide (i.e. LLSPs) are secreted via direct translocation through the plasma membrane (pore-mediated) or via intracellular vesicles that fuse with the membrane. Some LLSPs can be released via exosomes or microvesicles. B, DAMPs are released upon rupture of the cell membrane (92). LLSPs instead selectively leave living cells via membrane translocation or via vesicles that pinch off the cell membrane (20). The latter mechanism requires selective accumulation at the membrane that will give rise to the vesicle. Translocation may occur at the plasma membrane or into intracellular vesicles, such as secretory lysosomes. Likewise, pinching off may occur directly at the plasma membrane, followed by shedding of microvesicles, or indirectly via multivesicular bodies that release exosomes. Upon translocation, LLSPs are immediately accessible, whereas, after pinching off, they are protected by a membrane until the vesicles break up.
In this review, we summarize the current state of knowledge of the mechanistic features of LLSP secretion by mammalian cells and highlight the interplays among LLSPs, redox mechanisms, and inflammation, focusing on the prototypic cytokine, IL-1β.
Key open questions concerning LLSPs
How are LLSPs recognized?
Despite numerous efforts (12–15), the signals or motifs shared among LLSPs that mediate their secretion remained unidentified for many years. Sequence analyses revealed two features associated with LLSP subsets (Table 1) (12): (i) basic amino acid stretches in FGF1 and FGF2, IL-1α, high-mobility group box 1 (HMGB1), histone 4, and engrailed-2 (i.e. in LLSPs with a nuclear localization) and (ii) the redox-active CXXC in oxidoredoxins (e.g. Trx and macrophage migration–inhibitory factor (MIF)). In FGF1 (16) and FGF2 (17), the basic stretch is indeed required for their secretion. In other LLSPs, however, the role of the basic sequence in secretion has not been addressed. Moreover, many redox enzymes carrying the CXXC motif are not secreted, and mutations of the redox active site in Trx do not block its export (18). Very recently, a study by Zhang et al. (19) proposed that a motif present on various IL-1 family members and other LLSPs drives the secretion of these proteins and showed that this motif is sufficient to direct a leaderless cargo to the route of secretion.
How do LLSPs exit from living cells?
To exit cells without jeopardizing the cells' integrity, LLSPs must either translocate through a membrane or localize to selected membrane areas that pinch off as vesicles (20) (Fig. 1B). Translocation can involve the plasma membrane or intracellular vesicles, such as secretory lysosomes, that eventually fuse with the plasma membrane. Likewise, vesicular mechanisms can exploit release of microvesicles from the plasma membrane or of exosomes after exocytosis of multivesicular bodies. Additional or different routes are used by different cell types (11). For instance, autophagy has been proposed for secretion of the acyl CoA-binding protein Acb1 in yeast (21) and IL-1β in mammals (22) (Fig. 2A). However, the role of autophagy remains controversial, as findings from other studies suggest that autophagy prevents IL-1β secretion (23–25) and that autophagic failure promotes α-synuclein release (26).
Figure 2.
LLSPs that translocate into autophagic or endolysosomal vesicles can be partially degraded. A, through still largely undefined mechanisms, LLSPs may end up in secretory lysosomes or else in the lumen or the intermembrane space of double-membrane autophagosomes (thick arrows). Both vesicle types can fuse with the cell membrane and release their contents into the extracellular space. If they fuse with lysosomes, however, their contents are degraded. B, cytosolic proteins may translocate to late endocytic vesicles following recognition by HSP chaperones. In MAPS, misfolded substrates are first recognized by HSP70 and enriched at the ER surface by USP19 (31, 55). They are then transferred to late endosomes by DNAJC5 for translocation that is followed by exocytosis. C, in CMA, HSC70 recognizes substrates with KFERQ-like motifs and targets them to lysosomes for LAMP2a-dependent translocation (52, 53). Although the “classical” CMA is not part of the pathways for LLSP secretion, it has many features common to other UPS pathways. Interestingly, some CMA cargoes are not degraded but are secreted upon fusion with the plasma membrane (54).
In yeast, nutrient starvation favors the appearance of Acb1-containing vesicles that lack the double membrane typical of autophagosomes (27). These structures, belonging to the so-called compartment for UPS (CUPS) are then released as exosomes (27). The relationships among LLSP-containing mammalian autophagic vesicles (22), secretory lysosomes (28–30), and yeast CUPS remain to be investigated.
An interesting secretion route is the so-called misfolding-associated protein secretion (MAPS) pathway (31), which mediates the release of potentially cytotoxic polypeptides such as α-synuclein and Tau and other cytosolic misfolded proteins. These proteins are delivered to late endosomes that, like secretory lysosomes, eventually fuse with the plasma membrane, releasing their contents (31, 32) (Fig. 2B).
How many unconventional LLSP secretory pathways are there?
In some cases, a single LLSP can exploit more than one secretion pathway (33) (Table 1). For instance, Tau can use MAPS but can also directly translocate through the plasma membrane (34, 35) or be released via endo-lysosomal vesicles or exosomes (33). Multiple mechanisms have been described also for IL-1β secretion and have possible implications in tuning inflammation (see below).
A common feature of the LLSP pathways is their induction by stress. Our initial observation that stress promotes IL-1β secretion (5) has gained strong support. Lysosome exocytosis, exosome formation, membrane vesiculation, autophagy, and pyroptosis are all tightly regulated by stresses, especially redox stress (24, 36–39).
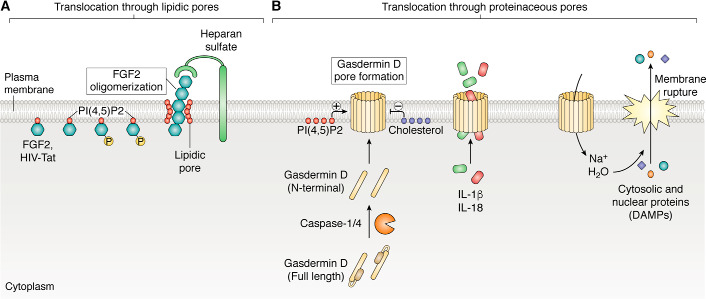
Although LLSP externalization remains poorly understood, some plausible mechanisms have emerged (Fig. 3). Phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2), a phospholipid enriched at the inner leaflet of the plasma membrane, is required for the translocation and secretion of HIV-1 Tat (40) and FGF2 (41). Both proteins accumulate at the plasma membrane via binding to PI(4,5)P2. This interaction induces oligomerization and translocation, possibly through lipidic pores. Heparan sulfate proteoglycans at the cell surface are required for FGF2 secretion (17), likely acting as a pulling ratchet (Fig. 3A).
Figure 3.
Secretion through membrane pores. A, direct translocation of LLSPs across the plasma membrane through lipidic pores has been described for HIV-Tat (40) and FGF (41). These proteins interact with a phosphoinositide, PI(4,5)P2, at the inner leaflet and with heparan sulfates at the outer leaflet of the plasma membrane. PI(4,5)P2 induces oligomerization of FGF2 that is phosphorylated by Tec kinase and promotes formation of a lipidic membrane pore through which the protein is externalized. B, LLSPs can be directly translocated across the plasma membrane through proteinaceous pores. Activated caspase-1 or -4 can cleave GSDMD, generating an N-terminal domain that oligomerizes and forms pores in the plasma membrane (42–44). PI(4,5)P2 favors membrane insertion, whereas cholesterol inhibits it (44). Initially, the pore size allows passage of small proteins, such as the mature form of IL-1β or IL-18, but not of larger cytoplasmic molecules. However, ion imbalances cause cell swelling and eventually membrane rupture, pyroptosis, and release of DAMPs and larger complexes (39, 45, 46).
Proteinaceous pores can be formed by gasdermin D (GSDMD) (42, 43) (Fig. 3B). Upon activation of inflammatory caspase-4 (caspase-11 in mice) or caspase-1 by intracellular LPS or intense extracellular inflammatory stimuli (39, 42), GSDMD undergoes proteolytic cleavage. The unleashed N-terminal domains insert into the plasma membrane lipids, oligomerize, and form pores that mediate the exit of small cytokines, such as IL-1β and IL-18, before pyroptotic cell death (39, 44, 45). PI(4,5)P2 increases and cholesterol reduces membrane insertion of GSDMD (46).
ABC subfamily A member 1 (ABCA1) is responsible for cholesterol efflux and has been implicated in the release of IL-1β (47), MIF-1 (48), and apurinic/apyrimidinic endonuclease 1/redox effector factor-1 (APE1/Ref-1), a nuclear factor controlling the responses to oxidative stress (49). Interestingly, ABCA1 is a mammalian homolog of yeast STE6 (50) required for secretion of the leaderless protein a-factor (8).
LLSPs secreted through exosomes or microvesicles (Fig. 2) or autophagic vesicles (Fig. 3A) are likely to accumulate where the membrane bends to form the vesicles with no need for translocation. However, KFERQ-like motifs have been proposed for IL-1β translocation into vesicle intermediates in autophagy-mediated secretion (51). These motifs mediate the stress-dependent translocation of cytosolic proteins into lysosomes through lysosomal-associated membrane protein 2A (LAMP2A), in a process named chaperone-mediated autophagy (CMA) (52, 53). Although the main fate of these proteins is their degradation, some CMA cargoes may be externalized intact, including cytoplasmic antigens destined for major histocompatibility complex class II presentation (54). Thus, it is tempting to speculate that this mechanism might mediate secretion of other LLSPs containing KFERQ-like motifs. Different signals may then trigger degradation or secretion of proteins that reach lysosomes via CMA (Fig. 4B), a feature that, in the case of leaderless cytokines, can allow for tuning of inflammation.
Figure 4.
Redox-dependent control of inflammation. A, in all cell types, Trx reduces protein disulfide bonds by interchange reactions (143). Its reductive power is restored by Trx reductase in a reaction that depends on NADPH. B, owing to the presence of Trx and other systems, thiols in the cytosol are mostly reduced (SH>S-S). In contrast, disulfides predominate outside the cell (S-S>SH). LLSPs and DAMPs are externalized with reduced cysteines, switching or losing their bioactivity upon oxidation that occurs in a time- and distance-dependent manner in the oxidizing extracellular milieu (91). Thus, the levels of Trx, other reductases, and NADPH in the extracellular space control the strength and duration of LLSP and DAMP activities (91, 107). Especially during stress, Trx, Trx reductase, glutaredoxins, peroxiredoxins, SOD-1, APE1/Ref-1, and MIF-1 are secreted through unconventional pathways, impacting the outcome of inflammatory responses (86) via thiol-disulfide exchange reactions (61). Extracellular LLSP redoxins reduce regulatory disulfides, modulating the activity of target receptors (61, 84, 90) as well as of soluble ligands secreted through the classical exocytic route. For instance, IL-4 is inactivated when a disulfide is reduced by Trx (133).
As depicted in Fig. 2 (B and C), CMA and MAPS have similar characteristics. However, in CMA, heat shock cognate 71-kDa protein (HSC70) targets substrates carrying suitable motifs to LAMP2A for lysosomal translocation (Fig. 2B). In contrast, the deubiquitinase USP19 recruits misfolded proteins at the ER surface for the subsequent, DNAJC5-dependent translocation into late endosomes (55) in MAPS (Fig. 3C).
Yet another pathway similar to CMA has been recently reported to mediate the translocation of leaderless proteins from the cytosol into intracellular vesicles (19). The transmembrane protein TMED10/TMP21 is proposed to directly facilitate the translocation of a broad spectrum of LLSPs into the ER-Golgi intermediate compartment (ERGIC). The process is enhanced by chaperones of the HSP90 family (19). It remains to be investigated whether and how this pathway is related to the previously described pathways involving autophagosomes (22), multivesicular bodies, and secretory endolysosomes (28, 39, 51).
How did different pathways for LLSP externalization evolve?
An intriguing possibility is that UPS pathways initially served to remove mutated, overexpressed, or aged proteins that are potentially toxic to cells lacking efficient proteolytic mechanisms. Examples of such proteins have been documented in yeast and in mammalian cells that overexpress rhodanese or GFP (56–58). Such pathways are advantageous for unicellular organisms, providing a mechanism allowing the expulsion of toxic molecules, but they could be detrimental in metazoans. Indeed, MAPS (31, 55) can mediate the release of protein aggregates that contribute to the pathogenesis of human neurogenerative disease, such as Tau and α-synuclein aggregates (32). LLSP molecules fulfilling a defined intracellular function (e.g. FGF2, annexins, galectins, and redoxins) might have been expelled from stressed cells as a primitive mechanism of protein down-regulation. Extruded proteins that can activate cell-surface receptors (e.g. IL-1 and FGF family members), enter vicinal cells (e.g. FGF2 and engrailed-2) (59, 60), or modulate the bioactivity of receptors or ligands (e.g. Trx) (61) might have been selected as players in intercellular communications. More recently, viral proteins such as HIV-Tat (62) have been reported to exploit the same mechanisms. Secreted HIV-Tat interacts with membrane receptors and enters vicinal cells, contributing to both nonimmune and immune dysfunctions in AIDS.
During evolution, some LLSPs, such as IL-1β, lost their intracellular function, whereas others retained key functions. For example, IL-1α is a transcriptional regulator inside the cell and functions as a pro-inflammatory cytokine when externalized. Trx and other LLSPs play similar intra- and extracellular roles (Table 1).
Why should LLSPs lack a signal sequence?
Additional regulatory possibilities or different stress sensitivity could make a particular secretion path better-suited than the classical secretory route involving leader peptides, especially for cargoes impacting inflammation. Transit along the ER-Golgi route may result in posttranslational modifications that interfere with a secreted protein's activity and function. For example, when dispatched to the ER, IL-1β undergoes N-glycosylation and loses bioactivity (63). Galectin and other LLSPs with lectin activity might be trapped in secretory organelles containing abundant glycoproteins, glycolipids, and polysaccharides. Formation of disulfides, which increase the stability of classical secretory proteins, may change the conformation of LLSPs and impair their function. Remarkably, avoiding classical exocytosis may prevent dangerous autocrine circuits, as is the case for FGF2: expression of a chimeric signal peptide-containing FGF2—but not of leaderless FGF2—constitutively activates FGF receptors and induces cell transformation (64).
LLSP or DAMP?
Damage-associated molecular patterns (DAMPs) are molecules with defined intracellular functions that, when released upon loss of membrane integrity, sustain sterile inflammation (65). Unlike DAMPs, which are passively released, LLSPs undergo active and regulated secretion. In this respect, IL-1β is a prototypic LLSP, active only if secreted upon processing of inactive pro-IL-1β precursors. In between lie proteins such as HMGB1, IL-1α, IL-33, and IL-37 (reviewed in Ref. 66). Being active in their unprocessed form, they act as DAMP when released by injured or dying cells (67). However, they can also be actively secreted by living cells, especially upon stress (30, 68–71).
The capability of cells to release pro-inflammatory signals before losing membrane integrity provides survival advantages presumably selected during the evolution of cellular defenses. To that end, the selective release of LLSPs does not entail DAMP efflux and is key for limiting inflammatory responses.
LLSPs can be classified into cytokines, redox enzymes, and brain proteins (Table 1), three groups that largely overlap and impact inflammation. Cytokines are direct inflammatory mediators, whereas redoxins determine the duration and intensity of the responses (see Fig. 4 and next paragraph). The third group comprises members with clear inflammatory roles, including ciliary neurotrophic factor (CNTF), a cytokine with tissue-reparative properties (72), and APE1/Ref-1, a redoxin that inhibits tumor necrosis factor α (TNFα)-mediated inflammation (73). Moreover, the LLSPs Tau, α-synuclein, and superoxide dismutase 1 (SOD-1), when secreted, cause neurodegenerative disorders with relevant inflammatory components (32, 34, 74–78).
LLSPs, redox regulation, and inflammation
Inflammation is tightly linked to redox remodeling. Stressed cells activate responses that entail reactive oxygen species (ROS) production (79). ROS control many steps in inflammatory responses, from the anti-pathogen oxidative burst to wound healing (80). ROS production is balanced with antioxidant responses that can affect the extracellular redox poise (81) (Fig. 4). The extracellular space is normally oxidizing, with high cystine/cysteine ratios, and both soluble and membrane-bound proteins externalized through the classical pathway contain many stabilizing disulfide bonds. In contrast, the cysteines in cytosolic proteins (including LLSPs) are mainly in the reduced state (Fig. 4B). Secreted small thiols and redox enzymes can alter the extracellular redox state, thereby modulating inflammation (82), blood clotting (83), and virus infection (84). Some secreted redox enzymes normally reside in the ER (e.g. protein-disulfide isomerase, P5, peroxiredoxin 4, and ERp44). Others, such as Trx (40) (Fig. 4), Trx reductase, and peroxiredoxins 1 and 2 (85), are LLSPs. Stress-associated molecular patterns (SAMPs) are present on redox modulators released from stressed cells and influence inflammatory responses (86). SAMPs such as cysteine are key, for instance, in antigen presentation, when dendritic cells release cysteine to feed T cells, which lack transporters for oxidized cystine, the molecular form of the amino acid that predominates extracellularly. Cysteine feeding allows antigen-specific T cell proliferation. Upon robust stimulation, dendritic cells also secrete Trx that maintains cysteines in their reduced form, prolonging T cell feeding (87). Also ROS-activated monocytes (9, 88) and B lymphocytes (9, 89) secrete Trx. Of note, serum Trx is in the low nanomolar range in healthy individuals but is dramatically increased in diseases having a strong inflammatory component (reviewed in Ref. 82). Secreted redoxins impact inflammatory circuits, by reshuffling disulfides in ligand and receptor proteins (61) (Fig. 4B). For instance, reduction of an allosteric disulfide in CD4 facilitates HIV entry into T cells (84). Similarly, Trx-dependent reduction of disulfides in the extracellular domains of TRPC5 (90) and CD30 (61) regulates their activities. Secreted APE1/Ref-1, a nuclear redoxin with cytokine-like activity, modifies the redox state of TNFα receptors, thereby inhibiting TNFα–mediated inflammation in endothelial cells (73).
When released in the extracellular microenvironment, LLSPs and DAMPs may undergo cysteine oxidation, a modification that often alters their bioactivities (91). A classic example is HMGB1, which, upon oxidation of its three cysteines, shifts from chemo-attractant to cytokine inducer and, ultimately, to regenerative factor (92). However, the presence of extracellular glutaredoxin, Trx, or other SAMPs may counteract HMGB1 oxidation, prolonging its chemokine activity (91). Accordingly, higher Trx activity, which correlates with aggressive rheumatoid arthritis, causes accumulation of reduced HMGB1-CXCL12 complexes in the synovial fluid. Such complexes are transient in healthy individuals, because the fast HMGB1 oxidation in the absence of inflammation-induced SAMPs impairs their stability (93). Also, tissue transglutaminase 2 (TG2), which can deamidate gluten-derived peptides, is rapidly oxidized in the extracellular milieu but can be reactivated by Trx. Interferon-γ, a key cytokine in celiac disease, induces Trx secretion, supporting a pathogenic circuit (94).
A feverish topic: IL-1β secretion
Models for IL-1β externalization
As fever is one of the few afflictions that affect all human beings, the cloning of the gene encoding IL-1β, previously called the “endogenous pyrogen” (4), generated huge interest in the biomedical research community. In the following years, a number of models explaining how this leaderless protein is secreted were suggested. The first model proposed that IL-1β is secreted through secretory lysosomes that fuse with the plasma membrane (28, 29, 39, 95). This model implicates translocation across the endolysosomal membrane.
Other vesicular pathways were then proposed. Solid evidence indicates that autophagic vesicles mediate protein release (22) but has also shown that inhibition of autophagic degradation promotes IL-1β secretion (23–25). Microvesicles (96) and exosomes (97) are also suggested as carriers of IL-1β, raising the question of how pro-IL-1β is recognized and included in these vesicles.
The identities, functions, and interrelationships of these vesicles remain to be clarified. From a functional viewpoint, a unifying explanation could be that pro-IL-1β–containing vesicles can fuse either with the plasma membrane or with degradation-competent lysosomes, depending on the type of signal received. In this scenario, lysosomal degradation could be a way to halt IL-1β release from cells exposed to weak or ambiguous pro-inflammatory signals.
Recently, the Schekman laboratory reconstructed in vitro translocation of pro-IL-1β into LAMP2A-expressing vesicles. This translocation requires heat shock protein 90 (HSP90) and pentapeptide motifs in IL-1β that are loosely related to KFERQ (51). Interestingly, caspase-1 contains a similar motif, and IL-1β–containing lysosomes are positive for LAMP2A (39), suggesting that secretion of IL-1β and caspase-1 (and possibly other LLSPs) through secretory lysosomes may represent a variant of CMA (Fig. 2C).
As detailed above, IL-1β can be secreted also by direct translocation through GSDMD pores at the plasma membrane (Fig. 3B) that allow passive release of cytosolic proteins in a size-dependent manner (42–44, 46, 39).
The mystery of IL-1β processing
As noted above, IL-1β is produced as an inactive precursor, pro-IL-1β, that requires processing by caspase-1 (98). This enzyme, also called IL-1–converting enzyme (ICE) is activated by the inflammasome (99). Human monocytes activated by inflammatory stimuli such as Toll-like receptor (TLR) agonists contain abundant pro-IL-1β but little if any processed molecules. The reverse is true in the extracellular space (5, 27), implying that pro-IL-1β cleavage is coupled to exocytosis or takes place extracellularly, immediately upon release. During efficient pore-mediated secretion (39), only traces of pro-IL-1β and lactate dehydrogenase are found extracellularly, suggesting that the size of the pore restricts pro-IL-1β and lactate dehydrogenase egress, until pyroptosis releases DAMPs and other large molecules (100). A tempting possibility is that active ICE is recruited near pores, ensuring the preferential release of bioactive IL-1β.
It is unclear how human monocytes activate IL-1β processing during the fusion of secretory lysosomes with the plasma membrane. Lysosomes at the plasma membrane have a higher pH than the juxtanuclear ones (101). It is hence possible that this pH shift couples vesicular exocytosis (102) and pro-IL-1β processing.
Physiologic significance of different secretory mechanisms for IL-1β
Why this redundancy in transport mechanisms? Are all of these pathways physiologically relevant? A first technical constraint in providing definitive answers is that only a few cell types, mainly myelomonocytic cells and microglia, are professional IL-1β–secreting cells. These cells are short-lived and difficult to manipulate genetically. Thus, most studies have aimed at reconstituting the IL-1β secretory pathways in vitro in cell lines that do not normally secrete IL-1β. However, discrepant results can be generated by the use of different model systems (5, 28, 103–107). For example, secretion of mature IL-1β by mouse macrophages and human monocytic cell lines requires two signals (Fig. 5). Signal 1 (i.e. TLR agonists) triggers pro-IL-1β synthesis; signal 2 (extracellular ATP (105–106) or other triggers (108)) is needed for inflammasome activation and IL-1β secretion. By contrast, signal 2 is dispensable in primary human monocytes (Fig. 5), which are true professional IL-1β–secreting cells (5, 39, 109). These differences reflect mainly differences in stress sensitivity and redox defenses among these cells (104). In monocytes that are stress-hypersensitive, TLR agonists induce accumulation of ROS that enhance pro-IL-1β biosynthesis and trigger release of endogenous ATP. Autocrine activation of purinergic P2X7 receptors (109, 110) causes further ROS production, K+ efflux, Ca2+ entry, inflammasome activation, and IL-1β secretion (111). The strength and mechanism of administration of the two different signals also impact the rate and mode of secretion. TLR4 activation by extracellular LPS induces mild secretion through secretory lysosomes (28, 39, 95). However, when injected intracellularly, LPS leads to massive IL-1β release via GSDMD pores (42–44). Likewise, the simultaneous activation of multiple TLR agonists results in a switch from lysosome-mediated to pore-mediated secretion (39). Intercellular circuits are also important: release of ATP and other DAMPs by injured vicinal cells could sustain inflammasome activation and IL-1β secretion. Thus, the intensity and type of the pro-inflammatory stimuli, and the context in which they are perceived by monocytes, dictate pathway selection and impact the efficacy of IL-1β antagonistic therapies (112). For instance, use of neutralizing antibodies or of soluble decoy receptors to intercept IL-1β would be less active against microvesicles or exosomes than reagents blocking IL-1 receptor on target cells.
Figure 5.

Different cell types have different redox sensitivity that determines the requirements for IL-1β secretion. In murine macrophages and myelomonocytic cell lines (A), two signals are required for secretion of mature IL-1β. Signal 1 induces pro-IL-1β and ROS production and leads to release of only low ATP levels that are unable to productively activate P2X7 receptors. Therefore, exogenous ATP (signal 2) is required to activate P2X7 receptors and downstream pathways, leading to IL-1β processing and secretion. In human monocytes instead (B), a single signal (i.e. TLR activation) induces pro-IL-1β that triggers the release of ATP at levels sufficient to autocrinally activate P2X7 receptors, inflammasomes, and eventually abundant secretion of mature IL-1β.
Stress dependence of IL-1β secretion
As alluded to in the foregoing, ROS-dependent events control IL-1β release at many key steps, including transcription via redox-sensitive NF-κB, processing by inflammasomes (113), secretion (88, 114, 115), lysosome exocytosis (38), GSDMD pore formation (39), and autophagy (24, 25).
The level of stress may then influence the choice of a pathway or the switch from one to another. A remarkable example is how monocytes adapt IL-1β secretion to environmental cues, shifting from a vesicular to a pore-dependent path. This decision depends on the strength of stimulation. If only TLR4 signaling is triggered, pro-IL-1β translocates to LAMP2A+ intracellular lysosomes that eventually fuse with the plasma membrane, thereby releasing IL-1β (39). Interestingly, inhibitors of lysosomal proteases increase overall IL-1β secretion (28), implying that, in the absence of inhibitors, some of the 33-kDa precursors are normally degraded in lysosomes. It is conceivable the there is a ratio between degradation and secretion that changes with the intensity and duration of the inflammatory stimulus. Thus, the lysosomal pathway mediates IL-1β secretion but also offers a point of return by allowing degradation to prevail over secretion when IL-1β secretion is no longer required. Furthermore, upon mild stimulation, monocytes die by undergoing apoptosis, limiting inflammation (39). However, when a strong pro-inflammatory stimulus is provided, stress increases, resulting in increased ROS production, ATP release, and P2X7R-mediated signaling (114), which eventually activate GSDMD cleavage (39). The result of this cascade of events is that IL-1β secretion rates increase and, importantly, monocytes undergo pyroptosis, releasing additional DAMPs and hence extending inflammation. Specific inhibition of ROS production prevents this cascade, highlighting the redox sensitivity of its many steps (39, 88).
Venturing into pathophysiological implications, we surmise that secretory lysosome-mediated mechanisms dominate in conditions of low pathogen load or small sterile injury, inducing a self-limiting redox response and allowing a controlled release of IL-1β to restore homeostasis. However, in severe conditions like multiple infections or severe trauma, GSDMD/pyroptosis-mediated secretion is activated. The explosive secretion through pores may initially benefit the host, but a vicious circle of release of IL-1β and additional DAMPs may soon start, possibly leading to cell death, as in sepsis and shock (116).
Which way to take? How pore-mediated IL-1β secretion predominates in cryopyrin-associated periodic syndromes
Cryopyrin-associated periodic syndromes (CAPS) are a group of severe autoinflammatory diseases linked to gain-of-function mutations in the inflammasome component NLRP3. Their devastating inflammatory manifestations are largely dependent on dysregulated IL-1β secretion due to inflammasome hyperactivation (117). Monocytes from individuals with CAPS are chronically stressed, possibly because of the presence of the mutant NLRP3, and display ROS levels that are constitutively higher than in unstressed monocytes (88, 110, 118). These features lower the threshold for inflammasome activation. As a consequence, low doses of LPS that are unable to activate and induce IL-1β secretion in healthy monocytes elicit exaggerated ATP release, inflammasome activation, and accelerated and abundant IL-1β secretion through GSDMD pores (116), followed by pyroptosis in CAPS monocytes (39). This phenotype is similar to that observed in healthy monocytes upon stimulation with three TLR agonists (39, 114). The activation of the GSDMD, IL-1β hypersecretion, and the subsequent release of DAMPs through pyroptosis in CAPS monocytes exposed to minor stimuli may explain the recurrent episodes of severe systemic inflammatory attacks in the absence of recognizable triggers (117).
In summary, in human monocytes, mild stimuli normally activate lysosome-dependent IL-1β secretion. Pore-mediated secretion is induced only when mild stimulation is matched by stress, either “acquired” following additional stimulation or “constitutive,” due to genetic alterations or metabolic disturbances. In constitutively stressed cells, such as monocytes from individuals with CAPS, even a mild stimulus can activate the pyroptotic pathway of secretion, with explosive release of IL-1β and possibly other DAMPs that further amply inflammation.
Concluding remarks
For their growth and survival, multicellular organisms depend on proper signaling among all of their components. Ligands and receptors must be released in appropriate quantities and at the right time and location. Thus, it is not surprising that multiple secretory pathways have evolved for optimal precision and timing of intercellular dialogues. These pathways include those involving LLSPs, and many open questions remain in the wide-ranging field of UPS. First, we do not have a complete picture of how LLSPs are recognized in the crowded cytosol. The export pathways have been characterized for only a few of them. In most cases, it remains largely undefined how their translocation across the plasma membrane or the membrane of intermediate vesicles occurs. A general question still awaiting an answer concerns the reason for additional pathways, given the many mechanisms that can regulate classical protein secretion. Are all unconventional pathways aimed at secreting LLSPs, or do some of them have a different primary role? This question is relevant in light of the hypothesis that some secretion pathways have evolved as mechanisms that protect cells against toxic or dysfunctional proteins and that some secreted proteins were then coopted in danger-signaling circuits. The identification of more LLSPs that exploit unconventional pathways might enable identification of the signals (e.g. protein sequence, conformation, or posttranslational modifications) and molecular machineries that permit their selection and secretion. Often, the characterization of a given mechanism helps to answer wider questions and spur important practical applications. Further dissection of the unconventional secretory pathways may shed light on their evolution and could help to identify key therapeutic avenues to manage many pathological conditions, such as dysregulated inflammation.
Acknowledgments
We are grateful to Simone Cenci and Giulio Cavalli for critically reading the manuscript and for valuable comments. We also thank Claudia Semino and Sonia Carta for helpful discussion, Gianmario Sambuceti for suggestions and support, and Marco Dalla Torre for help in editing the manuscript. We apologize to all of the colleagues whose excellent studies could not be cited because of space limitations.
Funding and additional information—This work was supported in part by grants from AIRC (2019–IG 23285), CARIPLO, and MIUR-PRIN (2017XA5J5N) (to R. S.) and the Italian Ministry of Health (2017–Cinque per mille) (to A. R.).
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
- ER
- endoplasmic reticulum
- CMA
- chaperone-mediated autophagy
- CAPS
- cryopyrin-associated periodic syndrome
- CUPS
- compartment for unconventional protein secretion
- DAMP
- damage-associated molecular pattern
- FGF
- fibroblast growth factor
- GSDMD
- gasdermin D
- IL
- interleukin
- LLSP
- leaderless secretory proteins
- MAPS
- misfolding-associated protein secretion
- MIF
- macrophage migration–inhibitory factor
- ROS
- reactive oxygen species
- SAMP
- stress-associated molecular pattern
- SOD-1
- superoxide dismutase-1
- TLR
- Toll-like receptor
- TNF
- tumor necrosis factor
- Trx
- thioredoxin
- UPS
- unconventional protein secretion
- LPS
- lipopolysaccharide
- PI(4,5)P2
- phosphatidylinositol 4,5-bisphosphate
- ICE
- IL-1–converting enzyme
- CNTF
- ciliary neurotropic factor.
References
- 1. Palade G. E. (1966) Structure and function at the cellular level. JAMA 198, 815–825 10.1001/jama.1966.03110210065024 [DOI] [PubMed] [Google Scholar]
- 2. Milstein C., Brownlee G. G., Harrison T. M., and Mathews M. B. (1972) A possible precursor of immunoglobulin light chains. Nat. New Biol. 239, 117–120 10.1038/newbio239117a0 [DOI] [PubMed] [Google Scholar]
- 3. Blobel G., and Dobberstein B. (1975) Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J. Cell Biol. 67, 835–851 10.1083/jcb.67.3.835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Auron P. E., Webb A. C., Rosenwasser L. J., Mucci S. F., Rich A., Wolff S. M., and Dinarello C. A. (1984) Nucleotide sequence of human monocyte interleukin 1 precursor cDNA. Proc. Natl. Acad. Sci. U.S.A. 81, 7907–7911 10.1073/pnas.81.24.7907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rubartelli A., Cozzolino F., Talio M., and Sitia R. (1990) A novel secretory pathway for interleukin-1β, a protein lacking a signal sequence. EMBO J. 9, 1503–1510 10.1002/j.1460-2075.1990.tb08268.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baldari C., Murray J. A., Ghiara P., Cesareni G., and Galeotti C. L. (1987) A novel leader peptide which allows efficient secretion of a fragment of human interleukin 1β in Saccharomyces cerevisiae. EMBO J. 6, 229–234 10.1002/j.1460-2075.1987.tb04743.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cooper D. N., and Barondes S. H. (1990) Evidence for export of a muscle lectin from cytosol to extracellular matrix and for a novel secretory mechanism. J. Cell Biol. 110, 1681–1691 10.1083/jcb.110.5.1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kuchler K., Sterne R. E., and Thorner J. (1989) Saccharomyces cerevisiae STE6 gene product: a novel pathway for protein export in eukaryotic cells. EMBO J. 8, 3973–3984 10.1002/j.1460-2075.1989.tb08580.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rubartelli A., Bajetto A., Allavena G., Wollman E., and Sitia R. (1992) Secretion of Trx by normal and neoplastic cells through a leaderless secretory pathway. J. Biol. Chem. 267, 24161–24164 [PubMed] [Google Scholar]
- 10. Mignatti P., Morimoto T., and Rifkin D. B. (1992) Basic fibroblast growth factor, a protein devoid of secretory signal sequence, is released by cells via a pathway independent of the endoplasmic reticulum-Golgi complex. J. Cell. Physiol. 151, 81–93 10.1002/jcp.1041510113 [DOI] [PubMed] [Google Scholar]
- 11. Nickel W., and Rabouille C. (eds) (2018) Semin. Cell Dev. Biol. 83, 1–140 [DOI] [PubMed] [Google Scholar]
- 12. Bendtsen J. D., Jensen L. J., Blom N., Von Heijne G., and Brunak S. (2004) Feature-based prediction of non-classical and leaderless protein secretion. Protein Eng. Des. Sel. 17, 349–356 10.1093/protein/gzh037 [DOI] [PubMed] [Google Scholar]
- 13. Garg A., and Raghava G. P. (2008) A machine learning based method for the prediction of secretory proteins using amino acid composition, their order and similarity-search. In Silico Biol. 8, 129–140 [PubMed] [Google Scholar]
- 14. Kandaswamy K. K., Pugalenthi G., Hartmann E., Kalies K. U., Möller S., Suganthan P. N., and Martinetz T. (2010) SPRED: a machine learning approach for the identification of classical and non-classical secretory proteins in mammalian genomes. Biochem. Biophys. Res. Commun. 391, 1306–1311 10.1016/j.bbrc.2009.12.019 [DOI] [PubMed] [Google Scholar]
- 15. Huang W.-L. (2012) Ranking gene ontology terms for predicting non-classical secretory proteins in eukaryotes and prokaryotes. J. Theor. Biol. 312, 105–113 10.1016/j.jtbi.2012.07.027 [DOI] [PubMed] [Google Scholar]
- 16. Graziani I., Bagalá C., Duarte M., Soldi R., Kolev V., Tarantini F., Kumar T. K., Doyle A., Neivandt D., Yu C., Maciag T., and Prudovsky I. (2006) Release of FGF1 and p40 synaptotagmin 1 correlates with their membrane destabilizing ability. Biochem. Biophys. Res. Commun. 349, 192–199 10.1016/j.bbrc.2006.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zehe C., Engling A., Wegehingel S., Schäfer T., and Nickel W. (2006) Cell-surface heparan sulfate proteoglycans are essential components of the unconventional export machinery of FGF-2. Proc. Natl. Acad. Sci. U.S.A. 103, 15479–15484 10.1073/pnas.0605997103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tanudji M., Hevi S., and Chuck S. L. (2003) The nonclassic secretion of Trx is not sensitive to redox state. Am. J. Physiol. Cell. Physiol. 284, C1272–C1279 10.1152/ajpcell.00521.2002 [DOI] [PubMed] [Google Scholar]
- 19. Zhang M., Liu L., Lin X., Wang Y., Li Y., Guo Q., Li S., Sun Y., Tao X., Zhang D., Lv X., Zheng L., and Ge L. (2020) A translocation pathway for vesicle-mediated unconventional protein secretion. Cell 181, 637–652.e15 10.1016/j.cell.2020.03.031 [DOI] [PubMed] [Google Scholar]
- 20. Rubartelli A., and Sitia R. (1997) Secretion of mammalian proteins that lack a signal sequence. in: Unusual Secretory Pathways: From Bacteria to Man (Kuchler K., Rubartelli A., and Holland B., eds) pp. 87–114, Springer, Berlin [Google Scholar]
- 21. Duran J. M., Anjard C., Stefan C., Loomis W. F., and Malhotra V. (2010) Unconventional secretion of Acb1 is mediated by autophagosomes. J. Cell Biol. 188, 527–536 10.1083/jcb.200911154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dupont N., Jiang S., Pilli M., Ornatowski W., Bhattacharya D., and Deretic V. (2011) Autophagy-based unconventional secretory pathway for extracellular delivery of IL-1β. EMBO J. 30, 4701–4711 10.1038/emboj.2011.398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Saitoh T., Fujita N., Jang M. H., Uematsu S., Yang B. G., Satoh T., Omori H., Noda T., Yamamoto N., Komatsu M., Tanaka K., Kawai T., Tsujimura T., Takeuchi O., Yoshimori T., and Akira S. (2008) Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1β production. Nature 456, 264–268 10.1038/nature07383 [DOI] [PubMed] [Google Scholar]
- 24. Harris J., Hartman M., Roche C., Zeng S. G., O'Shea A., Sharp F. A., Lambe E. M., Creagh E. M., Golenbock D. T., Tschopp J., Kornfeld H., Fitzgerald K. A., and Lavelle E. C. (2011) Autophagy controls IL-1β secretion by targeting pro-IL-1β for degradation. J. Biol. Chem. 286, 9587–9597 10.1074/jbc.M110.202911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhou R., Yazdi A. S., Menu P., and Tschopp J. (2011) A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225 10.1038/nature09663 [DOI] [PubMed] [Google Scholar]
- 26. Lee H.-J., Cho E.-D., Lee K. W., Kim J.-H., Cho S.-G., and Lee S.-J. (2013) Autophagic failure promotes the exocytosis and intercellular transfer of α-synuclein. Exp. Mol. Med. 45, e22 10.1038/emm.2013.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cruz-Garcia D., Curwin A. J., Popoff J. F., Bruns C., Duran J. M., and Malhotra V. (2014) Remodeling of secretory compartments creates CUPS during nutrient starvation. J. Cell Biol. 207, 695–703 10.1083/jcb.201407119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Andrei C., Dazzi C., Lotti L., Torrisi M. R., Chimini G., and Rubartelli A. (1999) The secretory route of the leaderless protein interleukin 1beta involves exocytosis of endolysosome-related vesicles. Mol. Biol. Cell 10, 1463–1475 10.1091/mbc.10.5.1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gardella S., Andrei C., Lotti L. V., Poggi A., Torrisi M. R., Zocchi M. R., and Rubartelli A. (2001) CD8+ T lymphocytes induce polarized exocytosis of secretory lysosomes by dendritic cells with release of interleukin-1β and cathepsin D. Blood 98, 2152–2159 10.1182/blood.V98.7.2152 [DOI] [PubMed] [Google Scholar]
- 30. Gardella S., Andrei C., Ferrera D., Lotti L. V., Torrisi M. R., Bianchi M. E., and Rubartelli A. (2002) The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 3, 995–1001 10.1093/embo-reports/kvf198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee J. G., Takahama S., Zhang G., Tomarev S. I., and Ye Y. (2016) Unconventional secretion of misfolded proteins promotes adaptation to proteasome dysfunction in mammalian cells. Nat. Cell Biol. 18, 765–776 10.1038/ncb3372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fontaine S. N., Zheng D., Sabbagh J. J., Martin M. D., Chaput D., Darling A., Trotter J. H., Stothert A. R., Nordhues B. A., Lussier A., Baker J., Shelton L., Kahn M., Blair L. J., Stevens S. M. Jr., and Dickey C. A. (2016) DnaJ/Hsc70 chaperone complexes control the extracellular release of neurodegenerative-associated proteins. EMBO J. 35, 1537–1549 10.15252/embj.201593489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pernègre C., Duquette A., and Leclerc N. (2019) Tau secretion: good and bad for neurons. Front. Neurosci. 13, 649 10.3389/fnins.2019.00649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Merezhko M., Brunello C. A., Yan X., Vihinen H., Jokitalo E., Uronen R. L., and Huttunen H. J. (2018) Secretion of Tau via an unconventional non-vesicular mechanism. Cell Rep. 25, 2027–2035.e4 10.1016/j.celrep.2018.10.078 [DOI] [PubMed] [Google Scholar]
- 35. Katsinelos T., Zeitler M., Dimou E., Karakatsani A., Muller H. M., Nachman E., Steringer J. P., Ruiz de Almodovar C., Nickel W., and Jahn T. R. (2018) Unconventional secretion mediates the trans-cellular spreading of tau. Cell Rep. 23, 2039–2055 10.1016/j.celrep.2018.04.056 [DOI] [PubMed] [Google Scholar]
- 36. Scott R. E., Perkins R. G., Zschunke M. A., Hoerl B. J., and Maercklein P. B. (1979) Plasma membrane vesiculation in 3T3 and SV3T3 cells. I. Morphological and biochemical characterization. J. Cell Sci. 35, 229–243 [DOI] [PubMed] [Google Scholar]
- 37. Benedikter B. J., Volgers C., van Eijck P. H., Wouters E. F. M., Savelkoul P. H. M., Reynaert N. L., Haenen G. R. M. M., Rohde G. G., Weseler A. R., and Stassen F. R. M. (2017) Cigarette smoke extract induced exosome release is mediated by depletion of exofacial thiols and can be inhibited by thiol-antioxidants. Free Radic. Biol. Med. 108, 334–344 10.1016/j.freeradbiomed.2017.03.026 [DOI] [PubMed] [Google Scholar]
- 38. Li X., Gulbins E., and Zhang Y. (2012) Oxidative stress triggers Ca-dependent lysosome trafficking and activation of acid sphingomyelinase. Cell. Physiol. Biochem. 30, 815–826 10.1159/000341460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Semino C., Carta S., Gattorno M., Sitia R., and Rubartelli A. (2018) Progressive waves of IL-1β release by primary human monocytes via sequential activation of vesicular and gasdermin D-mediated secretory pathways. Cell. Death. Dis. 9, 1088 10.1038/s41419-018-1121-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rayne F., Debaisieux S., Yezid H., Lin Y. L., Mettling C., Konate K., Chazal N., Arold S. T., Pugnière M., Sanchez F., Bonhoure A., Briant L., Loret E., Roy C., and Beaumelle B. (2010) Phosphatidylinositol-(4,5)-bisphosphate enables efficient secretion of HIV-1 Tat by infected T-cells. EMBO J. 29, 1348–1362 10.1038/emboj.2010.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Steringer J. P., Bleicken S., Andreas H., Zacherl S., Laussmann M., Temmerman K., Contreras F. X., Bharat T. A., Lechner J., Müller H. M., Briggs J. A., García-Sáez A. J., and Nickel W. (2012) Phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2)-dependent oligomerization of fibroblast growth factor 2 (FGF2) triggers the formation of a lipidic membrane pore implicated in unconventional secretion. J. Biol. Chem. 287, 27659–27669 10.1074/jbc.M112.381939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kayagaki N., Stowe I. B., Lee B. L., O'Rourke K., Anderson K., Warming S., Cuellar T., Haley B., Roose-Girma M., Phung Q. T., Liu P. S., Lill J. R., Li H., Wu J., Kummerfeld S., et al. (2015) Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671 10.1038/nature15541 [DOI] [PubMed] [Google Scholar]
- 43. Shi J., Zhao Y., Wang K., Shi X., Wang Y., Huang H., Zhuang Y., Cai T., Wang F., and Shao F. (2015) Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 10.1038/nature15514 [DOI] [PubMed] [Google Scholar]
- 44. He W. T., Wan H., Hu L., Chen P., Wang X., Huang Z., Yang Z. H., Zhong C. Q., and Han J. (2015) Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 25, 1285–1298 10.1038/cr.2015.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mulvihill E., Sborgi L., Mari S. A., Pfreundschuh M., Hiller S., and Müller D. J. (2018) Mechanism of membrane pore formation by human gasdermin-D. EMBO J. 37, e983231 10.15252/embj.201798321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Russo H. M., Rathkey J., Boyd-Tressler A., Katsnelson M. A., Abbott D. W., and Dubyak G. R. (2016) Active caspase-1 induces plasma membrane pores that precede pyroptotic lysis and are blocked by lanthanides. J. Immunol. 197, 1353–1367 10.4049/jimmunol.1600699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hamon Y., Luciani M. F., Becq F., Verrier B., Rubartelli A., and Chimini G. (1997) Interleukin-1β secretion is impaired by inhibitors of the Atp binding cassette transporter, ABC1. Blood 90, 2911–2915 10.1182/blood.V90.8.2911 [DOI] [PubMed] [Google Scholar]
- 48. Flieger O., Engling A., Bucala R., Lue H., Nickel W., and Bernhagen J. (2003) Regulated secretion of macrophage migration inhibitory factor is mediated by a non-classical pathway involving an ABC transporter. FEBS Lett. 551, 78–86 10.1016/S0014-5793(03)00900-1 [DOI] [PubMed] [Google Scholar]
- 49. Lee Y. R., Joo H. K., Lee E. O., Cho H. S., Choi S., Kim C. S., and Jeon B. H. (2019) ATP binding cassette transporter A1 is involved in extracellular secretion of acetylated APE1/Ref-1. Int. J. Mol. Sci. 20, E3178 10.3390/ijms20133178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dean M., Hamon Y., and Chimini G. (2001) The human ATP-binding cassette (ABC) transporter superfamily. J. Lipid Res. 42, 1007–1017 [PubMed] [Google Scholar]
- 51. Zhang M., Kenny S. J., Ge L., Xu K., and Schekman R. (2015) Translocation of interleukin-1β into a vesicle intermediate in autophagy-mediated secretion. eLife 4, e11205 10.7554/eLife.11205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dice J. F., Chiang H. L., Spencer E. P., and Backer J. M. (1986) Regulation of catabolism of microinjected ribonuclease A. Identification of residues 7–11 as the essential pentapeptide. J. Biol. Chem. 261, 6853–6859 [PubMed] [Google Scholar]
- 53. Cuervo A. M., and Dice J. F. (1996) A receptor for the selective uptake and degradation of proteins by lysosomes. Science 273, 501–503 10.1126/science.273.5274.501 [DOI] [PubMed] [Google Scholar]
- 54. Zhou D., Li P., Lin Y., Lott J. M., Hislop A. D., Canaday D. H., Brutkiewicz R. R., and Blum J. S. (2005) Lamp-2a facilitates MHC class II presentation of cytoplasmic antigens. Immunity 22, 571–581 10.1016/j.immuni.2005.03.009 [DOI] [PubMed] [Google Scholar]
- 55. Lee J., Xu Y., Zhang T., Cui L., Saidi L., and Ye Y. (2018) Secretion of misfolded cytosolic proteins from mammalian cells is independent of chaperone-mediated autophagy. J. Biol. Chem. 293, 14359–14370 10.1074/jbc.RA118.003660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sloan I. S., Horowitz P. M., and Chirgwin J. M. (1994) Rapid secretion by a non classical pathway of overexpressed mammalian mitochondrial rhodanese. J. Biol. Chem. 269, 27625–27630 [PubMed] [Google Scholar]
- 57. Cleves A. E., Cooper D. N., Barondes S. H., and Kelly R. B. (1996) A new pathway for protein export in Saccharomyces cerevisiae. J. Cell Biol. 133, 1017–1026 10.1083/jcb.133.5.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tanudji M., Hevi S., and Chuck S. L. (2002) Improperly folded green fluorescent protein is secreted via a non-classical pathway. J. Cell Sci. 115, 3849–3857 10.1242/jcs.00047 [DOI] [PubMed] [Google Scholar]
- 59. Wiedłocha A., and Sørensen V. (2004) Signaling, internalization, and intracellular activity of fibroblast growth factor. Curr. Top. Microbiol. Immunol. 286, 45–79 10.1007/978-3-540-69494-6_3 [DOI] [PubMed] [Google Scholar]
- 60. Maizel A., Tassetto M., Filhol O., Cochet C., Prochiantz A., and Joliot A. (2002) Engrailed homeoprotein secretion is a regulated process. Development 129, 3545–3553 [DOI] [PubMed] [Google Scholar]
- 61. Schwertassek U., Balmer Y., Gutscher M., Weingarten L., Preuss M., Engelhard J., Winkler M., and Dick T. P. (2007) Selective redox regulation of cytokine receptor signaling by extracellular Trx-1. EMBO J. 26, 3086–3097 10.1038/sj.emboj.7601746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Debaisieux S., Rayne F., Yezid H., and Beaumelle B. (2012) The ins and outs of HIV-1 Tat. Traffic 13, 355–363 10.1111/j.1600-0854.2011.01286.x [DOI] [PubMed] [Google Scholar]
- 63. Livi G. P., Lillquist J. S., Miles L. M., Ferrara A., Sathe G. M., Simon P. L., Meyers C. A., Gorman J. A., and Young P. R. (1991) Secretion of N-glycosylated interleukin-1β in Saccharomyces cerevisiae using a leader peptide from Candida albicans: effect of N-linked glycosylation on biological activity. J. Biol. Chem. 266, 15348–15355 [PubMed] [Google Scholar]
- 64. Yayon A., and Klagsbrun M. (1990) Autocrine transformation by chimeric signal peptide-basic fibroblast growth factor: reversal by suramin. Proc. Natl. Acad. Sci. U.S.A. 87, 5346–5350 10.1073/pnas.87.14.5346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lotze M. T., Zeh H. J., Rubartelli A., Sparvero L. J., Amoscato A. A., Washburn N. R., Devera M. E., Liang X., Tör M., and Billiar T. (2007) The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol. Rev. 220, 60–81 10.1111/j.1600-065X.2007.00579.x [DOI] [PubMed] [Google Scholar]
- 66. Carta S., Lavieri R., and Rubartelli A. (2013) Different members of the IL-1 family come out in different ways: DAMPs vs. cytokines? Front. Immunol. 4, 123–132 10.3389/fimmu.2013.00123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Scaffidi P., Misteli T., and Bianchi M. E. (2002) Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418, 191–195 10.1038/nature00858 [DOI] [PubMed] [Google Scholar]
- 68. Gross O., Yazdi A. S., Thomas C. J., Masin M., Heinz L. X., Guarda G., Quadroni M., Drexler S. K., and Tschopp J. (2012) Inflammasome activators induce interleukin-1α secretion via distinct pathways with differential requirement for the protease function of caspase-1. Immunity 36, 388–400 10.1016/j.immuni.2012.01.018 [DOI] [PubMed] [Google Scholar]
- 69. Kakkar R., Hei H., Dobner S., and Lee R. T. (2012) Interleukin 33 as a mechanically responsive cytokine secreted by living cells. J. Biol. Chem. 287, 6941–6948 10.1074/jbc.M111.298703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bulau A. M., Nold M. F., Li S., Nold-Petry C. A., Fink M., Mansell A., Schwerd T., Hong J., Rubartelli A., Dinarello C. A., and Bufler P. (2014) Role of caspase-1 in nuclear translocation of IL-37, release of the cytokine, and IL-37 inhibition of innate immune responses. Proc. Natl. Acad. Sci. U.S.A. 111, 2650–2655 10.1073/pnas.1324140111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang H., Yang H., and Tracey K. J. (2004) Extracellular role of HMGB1 in inflammation and sepsis. J. Intern. Med. 255, 320–331 10.1111/j.1365-2796.2003.01302.x [DOI] [PubMed] [Google Scholar]
- 72. Jones S. A., and Jenkins B. J. (2018) Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat. Rev. Immunol. 18, 773–789 10.1038/s41577-018-0066-7 [DOI] [PubMed] [Google Scholar]
- 73. Park M. S., Choi S., Lee Y. R., Joo H. K., Kang G., Kim C. S., Kim S. J., Lee S. D., and Jeon B. H. (2016) Secreted APE1/Ref-1 inhibits TNF-α-stimulated endothelial inflammation via thiol-disulfide exchange in TNF receptor. Sci. Rep. 6, 23015 10.1038/srep23015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Goedert M. (2015) Neurodegeneration. Alzheimer's and Parkinson's diseases: the prion concept in relation to assembled Aβ, tau, and α-synuclein. Science 349, 1255555 10.1126/science.1255555 [DOI] [PubMed] [Google Scholar]
- 75. Münch C., O'Brien J., and Bertolotti A. (2011) Prion-like propagation of mutant superoxide dismutase-1 misfolding in neuronal cells. Proc. Natl. Acad. Sci. U.S.A. 108, 3548–3553 10.1073/pnas.1017275108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Xu Y., Cui L., Dibello A., Wang L., Lee J., Saidi L., Lee J.G., and Ye Y. (2018) DNAJC5 facilitates USP19-dependent unconventional secretion of misfolded cytosolic proteins. Cell Discov. 4, 11 10.1038/s41421-018-0012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhang Q. S., Heng Y., Yuan Y. H., and Chen N. H. (2017) Pathological α-synuclein exacerbates the progression of Parkinson's disease through microglial activation. Toxicol. Lett. 265, 30–37 10.1016/j.toxlet.2016.11.002 [DOI] [PubMed] [Google Scholar]
- 78. Perea J. R., Ávila J., and Bolós M. (2018) Dephosphorylated rather than hyperphosphorylated Tau triggers a pro-inflammatory profile in microglia through the p38 MAPK pathway. Exp. Neurol. 310, 14–21 10.1016/j.expneurol.2018.08.007 [DOI] [PubMed] [Google Scholar]
- 79. Naviaux R. K. (2012) Oxidative shielding or oxidative stress? J. Pharmacol. Exp. Ther. 342, 608–618 10.1124/jpet.112.192120 [DOI] [PubMed] [Google Scholar]
- 80. Lorenzen I., Mullen L., Bekeschus S., and Hanschmann E. M. (2017) Redox regulation of inflammatory processes is enzymatically controlled. Oxid. Med. Cell Longev. 2017, 8459402 10.1155/2017/8459402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Castellani P., Balza E., and Rubartelli A. (2014) Inflammation, DAMPs, tumor development, and progression: a vicious circle orchestrated by redox signaling. Antioxid. Redox Signal. 20, 1086–1097 10.1089/ars.2012.5164 [DOI] [PubMed] [Google Scholar]
- 82. Yi M. C., and Khosla C. (2016) Thiol-disulfide exchange reactions in the mammalian extracellular environment. Annu. Rev. Chem. Biomol. Eng. 7, 197–222 10.1146/annurev-chembioeng-080615-033553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sharda A., and Furie B. (2018) Regulatory role of thiol isomerases in thrombus formation. Expert Rev. Hematol. 11, 437–448 10.1080/17474086.2018.1452612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Matthias L. J., Yam P. T., Jiang X. M., Vandegraaff N., Li P., Poumbourios P., Donoghue N., and Hogg P. J. (2002) Disulfide exchange in domain 2 of CD4 is required for entry of HIV-1. Nat. Immunol. 3, 727–732 10.1038/ni815 [DOI] [PubMed] [Google Scholar]
- 85. Checconi P., Salzano S., Bowler L., Mullen L., Mengozzi M., Hanschmann E. M., Lillig C. H., Sgarbanti R., Panella S., Nencioni L., Palamara A. T., and Ghezzi P. (2015) Redox proteomics of the inflammatory secretome identifies a common set of redoxins and other glutathionylated proteins released in inflammation, influenza virus infection and oxidative stress. PLoS ONE 10, e0127086 10.1371/journal.pone.0127086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Rubartelli A., and Sitia R. (2009) Stress as an intercellular signal: the emergence of stress-associated molecular patterns (SAMP). Antioxid. Redox Signal. 11, 2621–2629 10.1089/ars.2009.2377 [DOI] [PubMed] [Google Scholar]
- 87. Angelini G., Gardella S., Ardy M., Ciriolo M. R., Filomeni G., Di Trapani G., Clarke F., Sitia R., and Rubartelli A. (2002) Antigen-presenting dendritic cells provide the reducing extracellular microenvironment required for T lymphocyte activation. Proc. Natl. Acad. Sci. U.S.A. 99, 1491–1496 10.1073/pnas.022630299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Tassi S., Carta S., Vené R., Delfino L., Caorsi R., Martini A., Gattorno M., and Rubartelli A. (2010) Altered redox state of monocytes from cryopyrin-associated periodic syndromes causes accelerated IL-1β secretion. Proc. Natl. Acad. Sci. U.S.A. 107, 9789–9794 10.1073/pnas.1000779107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Vené R., Delfino L., Castellani P., Balza E., Bertolotti M., Sitia R., and Rubartelli A. (2010) Redox remodeling allows and controls B-cell activation and differentiation. Antioxid. Redox Signal. 13, 1145–1155 10.1089/ars.2009.3078 [DOI] [PubMed] [Google Scholar]
- 90. Xu S. Z., Sukumar P., Zeng F., Li J., Jairaman A., English A., Naylor J., Ciurtin C., Majeed Y., Milligan C. J., Bahnasi Y. M., Al-Shawaf E., Porter K. E., Jiang L. H., Emery P., et al. (2008) TRPC channel activation by extracellular Trx. Nature 451, 69–72 10.1038/nature06414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Rubartelli A., and Lotze M. T. (2007) Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol. 28, 429–436 10.1016/j.it.2007.08.004 [DOI] [PubMed] [Google Scholar]
- 92. Vénéreau E., Ceriotti C., and Bianchi M. E. (2015) DAMPs from cell death to new life. Front. Immunol. 6, 422 10.3389/fimmu.2015.00422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Cecchinato V., D'Agostino G., Raeli L., Nerviani A., Schiraldi M., Danelon G., Manzo A., Thelen M., Ciurea A., Bianchi M. E., Rubartelli A., Pitzalis C., and Uguccioni M. (2018) Redox-mediated mechanisms fuel monocyte responses to CXCL12/HMGB1 in active rheumatoid arthritis. Front. Immunol. 9, 2118–2130 10.3389/fimmu.2018.02118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Jin X., Stamnaes J., Klöck C., DiRaimondo T. R., Sollid L. M., and Khosla C. (2011) Activation of extracellular transglutaminase 2 by Trx. J. Biol. Chem. 286, 37866–37873 10.1074/jbc.M111.287490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Andrei C., Margiocco P., Poggi A., Lotti L. V., Torrisi M. R., and Rubartelli A. (2004) Phospholipases C and A2 control lysosome-mediated IL-1β secretion: implications for inflammatory processes. Proc. Natl. Acad. Sci. U.S.A. 101, 9745–9750 10.1073/pnas.0308558101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. MacKenzie A., Wilson H. L., Kiss-Toth E., Dower S. K., North R. A., and Surprenant A. (2001) Rapid secretion of interleukin-1β by microvesicle shedding. Immunity 15, 825–835 10.1016/S1074-7613(01)00229-1 [DOI] [PubMed] [Google Scholar]
- 97. Qu Y., Franchi L., Nunez G., and Dubyak G. R. (2007) Nonclassical IL-1β secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J. Immunol. 179, 1913–1925 10.4049/jimmunol.179.3.1913 [DOI] [PubMed] [Google Scholar]
- 98. Cerretti D. P., Kozlosky C. J., Mosley B., Nelson N., Van Ness K., Greenstreet T. A., March C. J., Kronheim S. R., Druck T., and Cannizzaro L. A. (1992) Molecular cloning of the interleukin-1 beta converting enzyme. Science 256, 97–100 10.1126/science.1373520 [DOI] [PubMed] [Google Scholar]
- 99. Martinon F., Burns K., and Tschopp J. (2002) The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell. 10, 417–426 10.1016/S1097-2765(02)00599-3 [DOI] [PubMed] [Google Scholar]
- 100. Kovacs S. B., and Miao E. A. (2017) Gasdermins: effectors of pyroptosis. Trends Cell Biol. 27, 673–684 10.1016/j.tcb.2017.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Johnson D. E., Ostrowski P., Jaumouillé V., and Grinstein S. (2016) The position of lysosomes within the cell determines their luminal pH. J. Cell Biol. 212, 677–692 10.1083/jcb.201507112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Sundler R. (1997) Lysosomal and cytosolic pH as regulators of exocytosis in mousemacrophages. Acta Physiol. Scand. 161, 553–556 10.1046/j.1365-201X.1997.00262.x [DOI] [PubMed] [Google Scholar]
- 103. Gardella S., Andrei C., Costigliolo S., Olcese L., Zocchi M. R., and Rubartelli A. (2000) Secretion of bioactive interleukin-1β by dendritic cells is modulated by interaction with antigen specific T cells. Blood 95, 3809–3815 10.1182/blood.V95.12.3809 [DOI] [PubMed] [Google Scholar]
- 104. Carta S., Tassi S., Pettinati I., Delfino L., Dinarello C. A., and Rubartelli A. (2011) The rate of interleukin-1β secretion in different myeloid cells varies with the extent of redox response to Toll-like receptor triggering. J. Biol. Chem. 286, 27069–27080 10.1074/jbc.M110.203398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Mariathasan S., Weiss D. S., Newton K., McBride J., O'Rourke K., Roose-Girma M., Lee W. P., Weinrauch Y., Monack D. M., and Dixit V. M. (2006) Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440, 228–232 10.1038/nature04515 [DOI] [PubMed] [Google Scholar]
- 106. Netea M. G., Nold-Petry C. A., Nold M. F., Joosten L. A., Opitz P., van der Meer J. H., van de Veerdonk F. L., Ferwerda G., Heinhuis B., Devesa I., Funk C. J., Mason R. J., Kullberg B. J., Rubartelli A., van der Meer J. W., and Dinarello C. A. (2009) Differential requirement for the activation of the inflammasome for processing and release of IL-1β in monocytes and macrophages. Blood 113, 2324–2335 10.1182/blood-2008-03-146720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Zanoni I., Tan Y., Di Gioia M., Broggi A., Ruan J., Shi J., Donado C. A., Shao F., Wu H., Springstead J. R., Kagan J. C. (2016) An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells. Science 352, 1232–1236 10.1126/science.aaf3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Dostert C., Pétrilli V., Van Bruggen R., Steele C., Mossman B. T., and Tschopp J. (2008) Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320, 674–677 10.1126/science.1156995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Piccini A., Carta S., Tassi S., Lasiglié D., Fossati G., and Rubartelli A. (2008) ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1β and IL-18 secretion in an autocrine way. Proc. Natl. Acad. Sci. U.S.A. 105, 8067–8072 10.1073/pnas.0709684105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Carta S., Penco F., Lavieri R., Martini A., Dinarello C. A., Gattorno M., Rubartelli A. (2015) Cell stress increases ATP release in NLRP3 inflammasome-mediated autoinflammatory diseases, resulting in cytokine imbalance. Proc. Natl. Acad. Sci. U.S.A. 112, 2835–2840 10.1073/pnas.1424741112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Di Virgilio F. (2007) Liaisons dangereuses: P2X(7) and the inflammasome. Trends Pharmacol. Sci. 28, 465–472 10.1016/j.tips.2007.07.002 [DOI] [PubMed] [Google Scholar]
- 112. Dinarello C. A., Simon A., and van der Meer J. W. (2012) Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug Discov. 11, 633–652 10.1038/nrd3800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Martinon F. (2010) Signaling by ROS drives inflammasome activation. Eur. J. Immunol. 40, 616–619 10.1002/eji.200940168 [DOI] [PubMed] [Google Scholar]
- 114. Lavieri R., Piccioli P., Carta S., Delfino L., Castellani P., and Rubartelli A. (2014) TLR costimulation causes oxidative stress with unbalance of proinflammatory and anti-inflammatory cytokine production. J. Immunol. 192, 5373–5381 10.4049/jimmunol.1303480 [DOI] [PubMed] [Google Scholar]
- 115. Tassi S., Carta S., Vené R., Delfino L., Ciriolo M. R., and Rubartelli A. (2009) Pathogen-induced interleukin-1β processing and secretion is regulated by a biphasic redox response. J. Immunol. 183, 1456–1462 10.4049/jimmunol.0900578 [DOI] [PubMed] [Google Scholar]
- 116. Sitia R., and Rubartelli A. (2018) The unconventional secretion of IL-1β: handling a dangerous weapon to optimize inflammatory responses. Semin. Cell Dev. Biol. 83, 12–21 10.1016/j.semcdb.2018.03.011 [DOI] [PubMed] [Google Scholar]
- 117. Manthiram K., Zhou Q., Aksentijevich I., and Kastner D. L. (2017) The monogenic autoinflammatory diseases define new pathways in human innate immunity and inflammation. Nat. Immunol. 18, 832–842 10.1038/ni.3777 [DOI] [PubMed] [Google Scholar]
- 118. Carta S., Tassi S., Delfino L., Omenetti A., Raffa S., Torrisi M. R., Martini A., Gattorno M., and Rubartelli A. (2012) Deficient production of IL-1 receptor antagonist and IL-6 coupled to oxidative stress in cryopyrin-associated periodic syndrome monocytes. Ann. Rheum. Dis. 71, 1577–1581 10.1136/annrheumdis-2012-201340 [DOI] [PubMed] [Google Scholar]
- 119. Keller M., Rüegg A., Werner S., and Beer H. D. (2008) Active caspase-1 is a regulator of unconventional protein secretion. Cell 132, 818–831 10.1016/j.cell.2007.12.040 [DOI] [PubMed] [Google Scholar]
- 120. Gardella S., Andrei C., Poggi A., Zocchi M. R., and Rubartelli A. (2000) Control of interleukin-18 secretion by dendritic cells: role of calcium influxes. FEBS Lett. 481, 245–248 10.1016/S0014-5793(00)02015-9 [DOI] [PubMed] [Google Scholar]
- 121. Gattorno M., Tassi S., Carta S., Delfino L., Ferlito F., Pelagatti M. A., D'Osualdo A., Buoncompagni A., Alpigiani M. G., Alessio M., Martini A., and Rubartelli A. (2007) Pattern of interleukin-1β secretion in response to lipopolysaccharide and ATP before and after interleukin-1 blockade in patients with CIAS1 mutations. Arthritis Rheum. 56, 3138–3148 10.1002/art.22842 [DOI] [PubMed] [Google Scholar]
- 122. Prudovsky I., Tarantini F., Landriscina M., Neivandt D., Soldi R., Kirov A., Small D., Kathir K. M., Rajalingam D., and Kumar T. K. (2008) Secretion without Golgi. J. Cell. Biochem. 103, 1327–1343 10.1002/jcb.21513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Steringer J. P., and Nickel W. (2018) A direct gateway into the extracellular space: unconventional secretion of FGF2 through self-sustained plasma membrane pores. Semin. Cell Dev. Biol. 83, 3–7 10.1016/j.semcdb.2018.02.010 [DOI] [PubMed] [Google Scholar]
- 124. Popa S. J., Stewart S. E., and Moreau K. (2018) Unconventional secretion of annexins and galectins. Semin. Cell. Dev. Biol. 83, 42–50 10.1016/j.semcdb.2018.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Rammes A., Roth J., Goebeler M., Klempt M., Hartmann M., and Sorg C. (1997) Myeloid-related protein (MRP) 8 and MRP14, calcium-binding proteins of the S100 family, are secreted by activated monocytes via a novel, tubulin-dependent pathway. J. Biol. Chem. 272, 9496–9502 10.1074/jbc.272.14.9496 [DOI] [PubMed] [Google Scholar]
- 126. Lee J. P., Foote A., Fan H., Peral de Castro C., Lang T., Jones S. A., Gavrilescu N., Mills K. H., Leech M., Morand E. F., and Harris J. (2016) Loss of autophagy enhances MIF/macrophage migration inhibitory factor release by macrophages. Autophagy 12, 907–916 10.1080/15548627.2016.1164358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Wakasugi K., and Schimmel P. (1999) Two distinct cytokines released from a human aminoacyl-tRNA synthetase. Science 284, 147–151 10.1126/science.284.5411.147 [DOI] [PubMed] [Google Scholar]
- 128. Andersson U., and Tracey K. J. (2011) HMGB1 is a therapeutic target for sterile inflammation and infection. Annu. Rev. Immunol. 29, 139–162 10.1146/annurev-immunol-030409-101323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Niinaka Y., Paku S., Haga A., Watanabe H., and Raz A. (1998) Expression and secretion of neuroleukin/phosphohexose isomerase/maturation factor as autocrine motility factor by tumor cells. Cancer Res. 58, 2667–2674 [PubMed] [Google Scholar]
- 130. Söderberg A., Sahaf B., and Rosén A. (2000) Trx 2, a redox-active selenoprotein, is secreted by normal and neoplastic cells: presence in human plasma. Cancer Res. 60, 2281–2289 [PubMed] [Google Scholar]
- 131. Salzano S., Checconi P., Hanschmann E. M., Lillig C. H., Bowler L. D., Chan P., Vaudry D., Mengozzi M., Coppo L., Sacre S., Atkuri K. R., Sahaf B., Herzenberg L. A., Herzenberg L. A., Mullen L., and Ghezzi P. (2014) Linkage of inflammation and oxidative stress via release of glutathionylated peroxiredoxin-2, which acts as a danger signal. Proc. Natl. Acad. Sci. U.S.A. 111, 12157–12162 10.1073/pnas.1401712111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Mondola P., Annella T., Santillo M., and Santangelo F. (1996) Evidence for secretion of cytosolic CuZn superoxide dismutase by HEPG2 cells and human fibroblast. Int. J. Biochem. Cell Biol. 28, 677–681 10.1016/1357-2725(96)00004-0 [DOI] [PubMed] [Google Scholar]
- 133. Plugis N. M., Weng N., Zhao Q., Palanski B. A., Maecker H. T., Habtezion A., and Khosla C. (2018) Interleukin 4 is inactivated via selective disulfide-bond reduction by extracellular Trx. Proc. Natl. Acad. Sci. U.S.A. 115, 8781–8786 10.1073/pnas.1805288115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Sendtner M., Stöckli K. A., and Thoenen H. (1992) Synthesis and localization of ciliary neurotrophic factor in the sciatic nerve of the adult rat after lesion and during regeneration. J. Cell Biol. 118, 139–148 10.1083/jcb.118.1.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Pooler A. M., Phillips E. C., Lau D. H., Noble W., and Hanger D. P. (2013) Physiological release of endogenous tau is stimulated by neuronal activity. EMBO Rep. 14, 389–394 10.1038/embor.2013.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Lee H. J., Patel S., and Lee S. J. (2005) Intravesicular localization and exocytosis of α-synuclein and its aggregates. J. Neurosci. 25, 6016–6024 10.1523/JNEUROSCI.0692-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Emmanouilidou E., Melachroinou K., Roumeliotis T., Garbis S. D., Ntzouni M., Margaritis L. H., Stefanis L., and Vekrellis K. (2010) Cell-produced α-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J. Neurosci. 30, 6838–6851 10.1523/JNEUROSCI.5699-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Rodriguez L., Marano M. M., and Tandon A. (2018) Import and export of misfolded α-synuclein. Front. Neurosci. 12, 344 10.3389/fnins.2018.00344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Punia N., Primon M., Simpson G. R., Pandha H. S., and Morgan R. (2019) Membrane insertion and secretion of the Engrailed-2 (EN2) transcription factor by prostate cancer cells may induce antiviral activity in the stroma. Sci. Rep. 9, 5138 10.1038/s41598-019-41678-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Lorand L., and Graham R. M. (2003) Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat. Rev. Mol. Cell Biol. 4, 140–156 10.1038/nrm1014 [DOI] [PubMed] [Google Scholar]
- 141. Zemskov E. A., Mikhailenko I., Hsia R. C., Zaritskaya L., and Belkin A. M. (2011) Unconventional secretion of tissue transglutaminase involves phospholipid-dependent delivery into recycling endosomes. PLoS ONE 6, e19414 10.1371/journal.pone.0019414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Tamboli I. Y., Barth E., Christian L., Siepmann M., Kumar S., Singh S., Tolksdorf K., Heneka M. T., Lütjohann D., Wunderlich P., and Walter J. (2010) Statins promote the degradation of extracellular amyloid-peptide by microglia via stimulation of exosome-associated insulin-degrading enzyme (IDE) secretion. J. Biol. Chem. 285, 37405–37414 10.1074/jbc.M110.149468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Holmgren A. (1984) Enzymatic reduction-oxidation of protein disulfides by Trx. Methods Enzymol. 107, 295–300 10.1016/0076-6879(84)07019-1 [DOI] [PubMed] [Google Scholar]