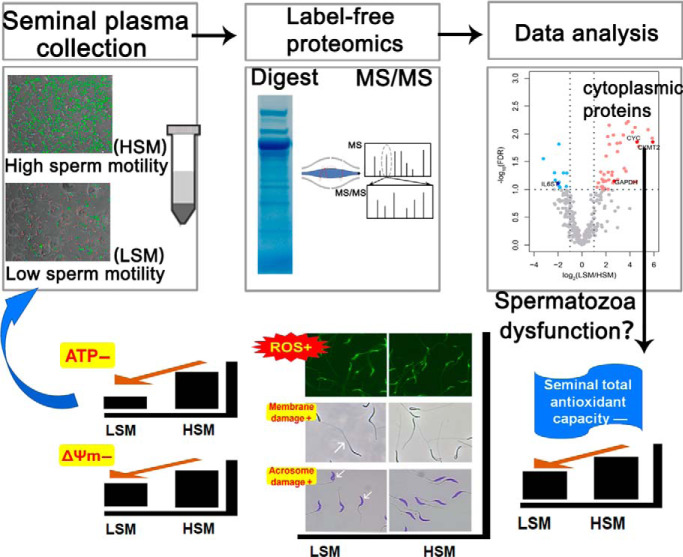
Seventy proteins, including both secretory and sperm part proteins, were identified differentially abundant in seminal plasma of chickens differ in sperm motility using a label-free mass spectrometry-based method. Higher abundance of sperm acrosome, mitochondrial and cytoskeleton proteins were noted in seminal plasma of low sperm motility (LSM). Higher spermatozoa ROS level and lower seminal plasma total antioxidant capacity in LSM suggested the occurrent oxidative stress. ROS-induced spermatozoa degradation and mitochondrial dysfunction could be causal factors for LSM.
Keywords: Label-free quantification, mass spectrometry, plasma or serum analysis, bioinformatics, protein identification, chicken sperm motility, plasma membrane integrity, spermatozoa acrosome integrity
Graphical Abstract
Highlights
Quantitative proteomes of chicken seminal plasma associated with sperm motility.
High abundant acrosome and mitochondrial proteins were noted in LSM seminal plasma.
Decreased total antioxidant capacity was highlighted in seminal plasma of LSM.
Lack of membrane, acrosome and mitochondrial integrity and high ROS may induce LSM.
Abstract
Molecular mechanisms underlying sperm motility have not been fully explained, particularly in chickens. The objective was to identify seminal plasma proteins associated with chicken sperm motility by comparing the seminal plasma proteomic profile of roosters with low sperm motility (LSM, n = 4) and high sperm motility (HSM, n = 4). Using a label-free MS-based method, a total of 522 seminal plasma proteins were identified, including 386 (∼74%) previously reported and 136 novel ones. A total of 70 differentially abundant proteins were defined, including 48 more-abundant, 15 less-abundant, and seven proteins unique to the LSM group (specific proteins). Key secretory proteins like less-abundant adhesion G-protein coupled receptor G2 (ADGRG2) and more-abundant serine peptidase inhibitor Kazal-type 2 (SPINK2) in the LSM suggested that the corresponding secretory tissues played a crucial role in maintaining sperm motility. Majority (80%) of the more-abundant and five specific proteins were annotated to the cytoplasmic domain which might be a result of higher plasma membrane damage and acrosome dysfunction in LSM. Additionally, more-abundant mitochondrial proteins were detected in LSM seminal plasma associated with lower spermatozoa mitochondrial membrane potential (ΔΨm) and ATP concentrations. Further studies showed that the spermatozoa might be suffering from oxidative stress, as the amount of spermatozoa reactive oxygen species (ROS) were largely enhanced, seminal malondialdehyde (MDA) concentrations were increased, and the seminal plasma total antioxidant capacity (T-AOC) were decreased. Our study provides an additional catalogue of chicken seminal plasma proteome and supports the idea that seminal plasma could be as an indicator of spermatozoa physiology. More-abundant of acrosome, mitochondria and sperm cytoskeleton proteins in the seminal plasma could be a marker of sperm dysfunction and loss of motility. The degeneration of spermatozoa caused by the reduced seminal T-AOC and enhanced oxidative stress might be potential determinants of low sperm motility. These results could extend our understanding of sperm motility and sperm physiology regulation.
Seminal plasma constitutes 80–90% of the ejaculate, containing diverse molecular components secreted by the testis, accessory sex glands, and genital tract (1). There are over 2000 seminal plasma proteins detected in humans (2, 3), 1159 in bulls (4), 727 in rams (5), and 607 in chickens (6). Increasing evidence indicates that seminal plasma proteins are associated with a wide range of semen quality traits, including sperm motility (7, 8), sperm morphology (9), sperm concentration (10), and sperm freezing endurance (11). Seminal plasma proteins, like zinc-alpha-2-glycoprotein, could bind to the surface of spermatozoa and stimulate sperm motility through the cAMP pathway (12). Sharma et al. (13) suggested that proteins involved in the process of oxidative stress reduction, like Albumin (ALB), could protect sperm from damage and serve as biomarkers of asthenozoospermia. Secreted by testis, epididymis, prostate, and genital tract, seminal plasma proteomic profile may be indicative of testicular and genital tract functions. Men with varicocele were identified to have altered seminal plasma proteins, including peroxiredoxin 2, fatty acid synthase, and fibronectin 1 (14, 15). Seminal transglutaminase 4, which is predominantly expressed in the prostate gland, was found to be associated with prostate cancer (16). Spermatozoa structure components may also present in the seminal plasma and are therefore regarded as signals of spermatozoa morphology and physiological state (9). Intasqui et al. (17) have demonstrated that alterations in the concentration of two intracellular proteins, inositol monophosphatase 1 and acrosin-binding protein, were responsible for sperm mitochondrial activity and acrosome damage, respectively.
With the extensive use of artificial insemination (AI) technology in poultry industry, there is an increasing demand of high-quality semen (18). Sperm motility is a primary determinant of fertility in males (19). A high proportion (>15%) of roosters with low sperm motility was noted in indigenous chicken breeds, including Beijing-You (BJY) used here (20). Avian reproductive physiological characteristics differ from humans and other mammals, mainly in the aspects of lacking the prostate and storing spermatozoa in the genital tract instead of the epididymis (21), and spermatozoa turn motile immediately after ejaculation without capacitation (22). The seminal plasma proteins may act differently in the regulation of spermatozoa functions in avian and therefore worthy of exploring.
Seminal plasma presents a wide range of protein concentrations that render low-abundance proteins difficult to be identified and quantified. A total of 80–120 proteins were identified for an individual sample in a previous study (6), which stand for merely around 10% of the predicted 1000 proteins (23). This impeded a full understanding of the roles of such low-abundance proteins for a time (24). The Orbitrap mass spectrometer enables the analysis of high-complexity samples like seminal plasma. In the present study, we performed label-free proteomics analysis based on SDS-PAGE fractionation of seminal plasma proteins obtained by Orbitrap instruments, aiming to reveal the seminal plasma proteomic profile of BJY roosters and identify the proteins associated with sperm motility. Through functional enrichment analyses, we have identified the biological functions of the seminal plasma proteins and suggested the altered proteins as potential candidate molecular regulators for sperm functions. Moreover, altered proteins might be linked to damage of the acrosome and plasma membrane. Results from this study deepen our understanding of sperm motility and sperm physiology regulation.
EXPERIMENTAL PROCEDURES
Experimental Design and Statistical Rationale
The current study was designed to identify the difference in chicken seminal plasma proteomes between low and high sperm motility semen samples. Eight roosters with similar semen volume, pH value, sperm concentration, and viability but differ in sperm motility, were selected from 210 BJY chickens. Seminal plasma proteins were purified by centrifugation and separated by SDS-PAGE electrophoresis. The gel line of each sample was cut into four fractions resulting in total of 32 samples for MS/MS analysis. An unpaired, two-tailed Student's t test was performed for identification of differentially abundant proteins (DAP) with false discovery rate (FDR)-corrected p value < 0.1 and fold-change > 1.5. Western blot analysis of four DAPs were performed to validate the MS/MS analysis. Based on the results of proteomic analysis, examinations of spermatozoa ATP level, ΔΨm, spermatozoa plasma membrane integrity, spermatozoa acrosome integrity, spermatozoa ROS, seminal plasma MDA concentrations, and seminal plasma T-AOC were performed to deepen the mechanistic insights. During the tests, the samples were numbered by the cage ID without group information. The phenotypic trait was blind to the technician until the final comparative analysis. The data were statistically analyzed using two-tailed Student's t test.
Animals
The present study was approved by the animal care and use committee of Institute of Animal Science, Chinese Academy of Agricultural Sciences (IAS-CAAS). All the animals used in this study were from a grandparent line of BJY chickens kept on the experimental farm of IAS-CAAS, Beijing, China. All chickens were individually caged and maintained under a light regime of 16L:8D (16 h light: 8 h darkness, 20 lx), with lights off at 22:00–06:00. Feed and water were available ad libitum. To collect cocks differing significantly in sperm motility for sequencing, sperm motility and semen volume were estimated five times for each male at a 2-day interval in 210 BJY male breeders from 38 to 40 weeks of age. Roosters were weighed at 38 weeks and trained for semen collection by abdominal massage technique. Semen collection during the experiment were performed by the same skilled technician. Semen volume was measured by the weighing method (assuming 1 g of semen equals 1 ml) (25). Sperm motility was estimated following a previously reported protocol (20) with slight modifications. Briefly, 10 μl of diluted semen (1:100 in 1× PBS) was transferred to a 37 °C pre-heated slide and covered with a coverslip. About 300 spermatozoa were examined under a phase-contrast microscope (DMi1, Lecia, Wetzlar, Germany) equipped with a micro heating stage setting at 37 °C. Roosters with watery semen, blood or feces polluted semen, large (> mean + standard deviation) or small (< mean - standard deviation) amount of semen volume were excluded. Average semen volume and sperm motility were calculated for each bird for a preliminary selection. The bottom four roosters with LSM and the top four roosters with HSM were selected for the following studies.
Semen Quality Analysis with CASA
Semen samples of the above eight roosters were collected at 41 weeks of age, and their semen quality estimation was performed three times at a 2-day interval. Semen volume was measured by the weighing method. Sperm concentration and sperm motility kinematics parameters were estimated by the computer-aided semen analysis (CASA) system (ML-608JZII, Nanning Songjingtianlun Biotechnology Co., Ltd., Guangxi, China). Briefly, 10 μl of diluted semen (1:100 in 1× PBS) was transferred to the sperm concentration and counting chamber (20 μm depth) placed on a 37 °C pre-heated microscope stage. Five fields in the chamber per sample were captured by the CASA system from the microscope equipped with a negative phase-contrast lens for further analysis. Sperm viability, the live sperm percentage, was measured using eosin-nigrosine staining technique (26). Spermatozoa with red or dark pink heads were considered dead, and those with light pink or white heads were considered alive. Semen pH value was determined with a calibrated pH meter (SevenCompact S210, Mettler-Toledo instruments Co., Ltd, Schwerzenbach, Switzerland). Principal component analysis (PCA) was performed to discriminate males on the kinetics parameters including sperm motility, curvilinear velocity (VCL), straight-line velocity (VSL), average path velocity (VAP), amplitude of lateral head displacement (ALH), linearity (LIN), beat-cross frequency (BCF), straightness (STR), and wobble (WOB).
Fertility Analysis
Eighty healthy hens from the same hatch were selected for the determination of individual fertility of roosters with a sex ratio of 1:10. These hens were inseminated with 1 × 108 spermatozoa from an individual rooster. Eggs were collected throughout a 21-day period from the second day of 2-day successive AI. Eggs were identified by hen and broken open after 5 days of incubation. Fertility was assessed by examining contents for embryonic development.
Sample Collection and Preparation
For the selected eight animals, at the age of 42 weeks, seminal plasma was immediately separated after semen collection by centrifugation at 2000 × g for 5 min at 4 °C. The supernatant was centrifuged for twice more at 12,000 × g for 20 min at 4 °C. The absence of spermatozoa in the seminal plasma was confirmed by observation under a microscope. Phenylmethylsulphonylfluoride (PMSF, 0.2 mm) was added to the sample to avoid digestion by proteases. Total protein was quantified with a BCA Protein Assay Kit (PA115-01, Tiangen Biotech Co., Ltd, Beijing, China) using BSA as a standard. Seminal plasma samples were frozen in the liquid nitrogen and stored at −80 °C until further analysis.
In-gel Digestion and LC-MS/MS
A total of 30 μg protein from each sample was treated with 500 units peptide-N-glycosidase F (PNGase F, P0704S, New England Biolabs, Ipswich), and mixed with protein loading buffer and heated for 10 min at 95 °C. Separation was performed by 10% SDS-PAGE gel (M42012C, GenScript Biotech Corp, Nanjing, China). Immediately following the electrophoresis, the gel was stained using Coomassie blue G-250. In-gel digestion was performed following the “In-gel Digestion and Extraction Protocol” from Proteomics and Mass Spectrometry Facility, BRC, Cornell University. Briefly, each lane of a sample in the gel was cut into four fractions resulting in total of 32 samples for MS/MS analysis. Each slice was cut into 1 mm2 cubes. The cubes were washed with triethylammonium bicarbonate buffer and ammonium bicarbonate to remove the Coomassie blue. The cubes were incubated with dithiothreitol and iodoacetamide in darkness at room temperature for 60 min for the reduction and alkylation of cysteine residues. The samples were digested with trypsin solution (V5111, Promega, Madison) for 12 h at 37 °C in a water bath. The peptides were collected and dried in a vacuum centrifuge. The peptides were resuspended and centrifuged at 12,000 × g for 5 min at 4 °C and transferred a volume of 20 μl to a crimp vial. The MS/MS experiment was performed on an Orbitrap Fusion mass spectrometer (Thermo Fisher Scientific Inc.) coupled to a high-pressure liquid chromatographer (Ultimate®3000 RSLC Ultra, Thermo Fisher Scientific Inc.). Peptide mixtures (2 μl) were loaded onto an Acclaim™ PepMap™ 100 C18 LC column (75 μm internal diameter × 2 cm, 3 mm particle size, Thermo Fisher Scientific Inc.). Mobile phases consisted of (A) 0.1% formic acid, 2% acetonitrile, 97.9% water and (B) 0.1% formic acid, 85% acetonitrile, 14.9% water. The column flow rate was set to 8 μl/min. The peptides were separated using an Acclaim™ PepMap™ 100C18 LC column (75 μm internal diameter × 25 cm, 2 mm particle size, Thermo Fisher Scientific, Inc.). The column flow rate was set to 300 nL/min. Data were acquired with Xcalibur™ (v.4.1, Thermo Fisher Scientific Inc.). The MS was operated in data-dependent mode. Survey MS scans were acquired in the 375–1 500 mass/charge (m/z) range, with the resolution set to a value of 120 000, automatic gain control (AGC) set to a value of 4 × 105. Peptide ions were fragmented using higher energy collision-induced dissociation. MS/MS scans were acquired with the resolution of 30,000 and AGC of 5 × 104.
Protein Identification and Quantification
Raw data were used for label-free quantitation of proteins analyzation by Proteome Discoverer (v2.3.0.523, Thermo Fisher Scientific Inc.) against the Gallus gallus reference proteome (UniProt release 2019_03, 27711 proteins, https://www.uniprot.org/proteomes/UP000000539) and contaminants database (https://lotus1.gwdg.de/mpg/mmbc/maxquant_input.nsf/7994124a4298328fc125748d0048fee2/$FILE/contaminants.fasta) with Sequesr HT search engine. The processing workflow for label-free quantification was designed according to the user guide. Oxidation on methionine was set as a variable modification, and carbamidomethyl on cysteine was set as a fixed modification. Trypsin with full enzyme specificity was selected as protease, and a maximum of two missed cleavages were allowed in the database search. The precursor mass tolerance was set at 5 ppm, and fragment mass tolerance was set to 0.5 Da. The mass spectra were set in the range of 300 Da to 8 000 Da. Decoy database search was set to 0.01 FDR at the peptide level, peptides for quantification was set as unique + razor. Proteins were grouped automatically, and the master protein per group was assigned by the parsimony principle. Contaminants were removed from the protein list. The data were normalized using total intensity. Using the normalized intensity of individual protein as variables, PCA was performed on the protein abundance to discriminate the eight males. Proteins with a minimum of two peptides and detected in at least two replicates were included for differential abundance analysis. Statistical significance was determined by Student's t test. The proteins with FDR-corrected p value < 0.1 and fold-change > 1.5 were considered as more- or less- abundant. Proteins present in all the samples of one group but are absent in another group were denoted as specific proteins. The more-abundant, less-abundant, and specific proteins were combined as DAPs.
Bioinformatics Analysis
Protein IDs were converted to Ensembl gene IDs with BioMart to make it comparable with different data sets. Gene Ontology (GO), Enrichment analysis of the Kyoto Encyclopedia of Genes and Genomes (KEGG) and Reactome (REAC) of seminal plasma proteins were performed with g:Profiler (27). Reproductive disorders association of seminal plasma proteins was investigated through the human and mouse phenotype database (https://toppgene.cchmc.org/) (28).
Western Blot Analysis
Western blotting of four proteins, Creatine kinase S-type, mitochondrial (CKMT2), Cytochrome c (CYC), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and interleukin 6 signal transducer (IL6ST), were performed following the method of Thélie et al., where total protein served as a loading control (29). The seminal plasma proteins were separated on 10% SDS-PAGE and transferred to a polyvinylidene fluoride (PVDF) membrane (Merck-Millipore, Darmstadt, Germany) at 200 mA for 90 min. Ponceau S staining was performed right after the transfer to determine the total proteins contents. The membranes were rinsed in distilled water and blocked with 5% nonfat dry milk in 1× TBST. The membranes were incubated with the primary antibodies of 1: 5000 diluted anti-CKMT2 (Rabbit Polyclonal, 13207–1-AP, Proteintech, Wuhan, Hubei, China), 1: 2000 diluted anti-CYC (Rabbit Polyclonal, bs-0013R, Bioss, Beijing, China), 1:10000 diluted anti-GAPDH (Rabbit Polyclonal, 10494–1-AP, Proteintech), or 1:2000 diluted anti-IL6ST (Rabbit Polyclonal, bs-5151R, Bioss). The membranes were washed and incubated with the secondary antibody of 1:5000 diluted HRP Goat Anti-Rabbit IgG (H+L) (SA00001–2, Proteintech). Protein bands were captured using a CCD camera system (Tanon, Shanghai, China). All images were processed in ImageJ (National Institutes of Health). Results of the four target proteins were normalized by the mean relative quantitation of HSM.
Spermatozoa ATP Levels
ATP concentration in spermatozoa was determined through quantifying the fluorescence generated by ATP-dependent luciferase using an Enhanced ATP Assay Kit (S0027, Beyotime, Shanghai, China) and following the manufacturer's instructions. Spermatozoa (∼2 × 107) were washed twice with 1× PBS at room temperature, collected by centrifugation at 800 × g for 5 min and disrupted in 200 μl lysis buffer at 4 °C. The suspension was collected after centrifugation at 12,000 × g for 5 min at 4 °C. Serial dilutions were made from an ATP stock provided by the kit as ATP standards. The samples and ATP standards were maintained on ice and the luminescence was measured in triplicate in 96-well black plates (Corning Costar, Corning) using a multifunctional microplate reader (Infinite 200 PRO). Each sample was measured in triplicate. Spermatozoa ATP levels were calculated based on the linearity of the relationship between fluorescence and ATP concentration.
Spermatozoa ΔΨm
Spermatozoa ΔΨm was evaluated by Mitochondrial Membrane Potential Detection Kit with JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzymidazolyl carbocyanine iodide) (M8650, Beijing Solarbio Science & Technology Co., Ltd) following the manufacturer's instructions. In brief, spermatozoa (∼1 × 106) were washed twice with 1× PBS at room temperature, collected by centrifugation at 800 × g for 5 min and incubated with 500 μl 1× JC-1 work solution for 20 min at 37 °C in darkness. The spermatozoa were washed twice with 1 000 μl 4 °C JC-1 staining wash buffer via centrifugation at 800 × g for 5 min. The precipitates were resuspended in 300 μl 4 °C JC-1 staining wash buffer, and 200 μl of the suspensions were transferred to a 96-well black plate (Corning Costar, Corning). Each sample was measured in triplicate. The fluorescent intensity of each well was measured by a multifunctional microplate reader (Infinite 200 PRO, TECAN) with excitation/emission wavelengths of 490/530 nm for green fluorescence (JC-1 monomers) and 525/590 nm for red fluorescence (JC-1 aggregates). ΔΨm was calculated as the ratio of red fluorescence intensity to green fluorescence intensity. Results were normalized by the mean fluorescence intensity of the HSM group.
Spermatozoa Plasma Membrane Integrity
Spermatozoa plasma membrane integrity was determined via the hypo-osmotic swelling test with a GENMED kit (GMS14017, GENMED Scientifics Inc.) following the method described by Santiago-Moreno et al. (30). Briefly, 10 μl fresh semen was mixed with 37 °C pre-heated 1000 μl hypo-osmotic solution and incubated for 30 min at 37 °C. A portion of 10 μl diluted semen was spread on a pre-heated slide with a coverslip. About 200 spermatozoa were examined under a phase-contrast microscope (DMi1, Lecia). Spermatozoa with swelling of the mid-piece, swelling of the tail, shortened and thickened tail were considered as intact. The percentage of intact spermatozoa was calculated.
Spermatozoa Acrosome Integrity
Spermatozoa acrosome integrity test was performed following the method of Santiago-Moreno et al. (31). Briefly, 10 μl of diluted semen (1:100 in 1× PBS) was spread onto a glass slide, air-dried, fixed with methanol, and stained with Giemsa (G1010, Beijing Solarbio Science & Technology Co., Ltd) for 30 min. Semen smear was air-dried and examined under a microscope (DMi1, Lecia). Spermatozoa with normal acrosome were evenly stained, spermatozoa with abnormal acrosome were completely not stained in acrosome. The acrosome integrity was calculated as the percentage of the intact sperm.
Spermatozoa ROS Level
Spermatozoa intracellular ROS was determined by a Reactive Oxygen Species Assay Kit (CA1410, Beijing Solarbio Science & Technology Co., Ltd) through the DCFH-DA ROS probe, following the manufacturer's instructions. In brief, spermatozoa (10 × 106 spermatozoa/ml) were incubated with the DCFH-DA (1 μm) for 15 min at 37 °C in darkness. The intracellular ROS converted the DCFH-DA to the fluorescent 2,7-dichlorofluorescein. The fluorescent intensity was visualized with an inverted fluorescent microscope (DMI 3000 B, Leica) and measured by a TECAN multifunctional microplate reader (Infinite 200 PRO, TECAN) with excitation/emission wavelengths of 488/525 nm. Results were normalized by the mean fluorescence intensity of the HSM.
Seminal Plasma MDA
The level of seminal plasma MDA were determined using a kit (A003–1, Nanjing Jiancheng Biotech Co., Ltd., Nanjing, China) according to the manufacturer's instruction. MDA can react with thiobarbituric acid and produce red compound. The MDA concentrations were measured by detection the absorbance value at 532 nm using a TECAN multifunctional microplate reader (Infinite 200 PRO).
Seminal Plasma T-AOC
The level of seminal plasma T-AOC were determined using a kit (BC1310, Beijing Solarbio Science & Technology Co., Ltd) according to the manufacturer's instruction. Fe3+-TPTZ can be reduced by antioxidants and produce blue Fe2+-TPTZ under acid condition. The antioxidant capacity of samples was calculated by detection the absorbance value at 593 nm using a TECAN multifunctional microplate reader (Infinite 200 PRO).
Statistical Analysis
Data analysis was performed using the SAS software (version 9.2; SAS Inst. Inc., Cary, NC). All percentage data were normalized with an arcsine transformation. Results are shown as non-transformed mean ± S.E. Student's t test was performed to assess the significance of differences in traits between the LSM and HSM groups. Comparisons yielding a value of p < 0.05 were regarded as statistically significant. PCA of semen quality variables was performed with JMP software (version 15.0.0, SAS Inst. Inc., Cary, NC).
RESULTS
Fertility, Semen Quality, and Sperm Kinetics Parameters
The selected animals of LSM and HSM were similar in body weight (2254.13 g versus 2236.00 g, p = 0.60). As shown in Table I, the fertility was significantly reduced in the LSM (p < 0.01), no significant difference was observed between the two groups for semen volume (p = 0.33), sperm concentration (p = 0.28), pH value (p = 0.79), or sperm viability (p = 0.45). The sperm motility tracks and the CASA analytic data revealed significant differences of sperm motility (p < 0.01) and kinetics parameters including VCL (p < 0.01), VSL (p < 0.01), VAP (p < 0.01), and ALH (p < 0.01) between groups. PCA was applied and reduced the kinetics parameters to two variables to reflect velocity and linearity, respectively (Supporting Information Fig. S1A). The samples were well-separated based on the first principal component (Supporting Information Fig. S1B).
Table I. Fertility, semen quality and sperm kinetics parameters of roosters of low and high sperm motility.
| Traits | LSM (n = 4) | HSM (n = 4) | p value |
|---|---|---|---|
| Fertility (%) | 50.52 ± 4.31 | 68.54 ± 1.40 | <0.01 |
| Sperm Motility (%) | 34.22 ± 2.09 | 82.69 ± 0.45 | <0.01 |
| Volume (μl) | 773.67 ± 87.51 | 660.00 ± 61.88 | 0.33 |
| CON (×108/ml) | 15.83 ± 0.50 | 17.05 ± 0.89 | 0.28 |
| Sperm viability (%) | 94.91 ± 1.21 | 96.32 ± 1.25 | 0.45 |
| pH | 6.95 ± 0.04 | 6.98 ± 0.10 | 0.79 |
| VCL (μm/s) | 45.26 ± 1.28 | 54.27 ± 1.27 | <0.01 |
| VSL (μm/s) | 23.18 ± 0.83 | 28.96 ± 0.62 | <0.01 |
| VAP (μm/s) | 35.79 ± 0.96 | 44.63 ± 1.41 | <0.01 |
| ALH (μm) | 3.35 ± 0.08 | 3.98 ± 0.11 | <0.01 |
| BCF (Hz) | 24.90 ± 0.87 | 23.09 ± 0.14 | 0.19 |
| LIN (%) | 51.40 ± 0.97 | 53.43 ± 1.04 | 0.20 |
| STR (%) | 64.81 ± 2.12 | 65.06 ± 2.28 | 0.94 |
| WOB (%) | 79.11 ± 1.06 | 82.22 ± 1.46 | 0.14 |
Values are specified as means ± S.E.; LSM, low sperm motility; HSM, high sperm motility; CON, Sperm concentration; VCL, curvilinear velocity; VSL, straight-line velocity; VAP, average path velocity; ALH, amplitude of lateral head displacement; BCF, beat-cross frequency; LIN, linearity; STR, straightness; WOB, wobble.
Proteomic Profile of Seminal Plasma
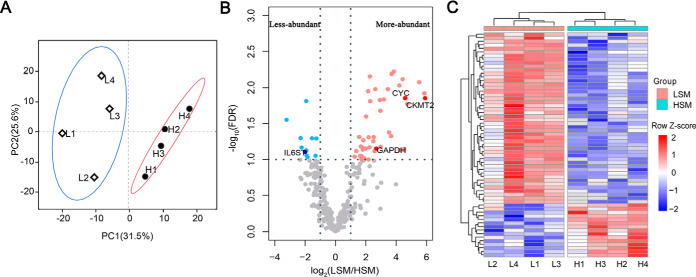
The seminal plasma protein concentration was similar between LSM and HSM (6.02 mg/ml versus 5.90 mg/ml, p = 0.73) (Table II). Strips of the SDS-PAGE gel were clear (Supporting Information Fig. S2), indicating that the gel can be used for in-gel digestion and peptide extraction. A total of 5 108 peptides (Supporting Information Table S1) belonging to 522 proteins (Supporting Information Table S2) were identified from the combined 32 LC-MS/MS runs after filtering with stated criteria. The total number of protein counts for each sample varied from 438 to 486. Of these, 511 (∼ 98%) proteins were present in at least two samples, and 339 (∼ 65%) proteins were common in all eight samples (Supporting Information Fig. S3). PCA identified seven dimensions, of which the first two explained 57.1% of the cumulative variance. PCA score plots showed that LSM and HSM samples were clustered well (Fig. 1A). Compared with the HSM, 48 proteins were more-abundant, and 15 proteins were less-abundant in the LSM (Fig. 1B, 1C), whereas seven specific proteins were uniquely identified in the LSM group (Supporting Information Table S3).
Table II. Seminal plasma protein concentration of roosters of low and high sperm motility.
| Group | Sample | Protein concentration (mg/ml) |
|---|---|---|
| Low sperm motility | L1 | 6.44 |
| L2 | 6.51 | |
| L3 | 5.76 | |
| L4 | 5.35 | |
| High sperm motility | H1 | 5.98 |
| H2 | 5.88 | |
| H3 | 6.17 | |
| H4 | 5.58 |
Fig. 1.
Principal component analysis (A) and differential abundance analysis (B, C) for seminal plasma proteins. A, Distribution of individuals according to PC1 and PC2, individual points are grouped in ellipses; PC1, principal component 1; PC2, principal component 2; L1-L4, samples of low sperm motility group; H1-H4, samples of high sperm motility group. B, Volcano diagram of sperm motility related differentially abundant proteins; The light/dark blue and light/dark red dots represent less-abundant and more-abundant proteins in LSM, respectively; The dotted lines represent the cutoffs that determine significance of protein abundance; Four proteins validated by Western blotting are named. C, Hierarchical clustering of differentially abundant proteins, the abundance values were log2-transformed and normalized by Z-score transformation; LSM, low sperm motility; HSM, high sperm motility.
Comprehensive Functional Annotation of Seminal Plasma Proteins
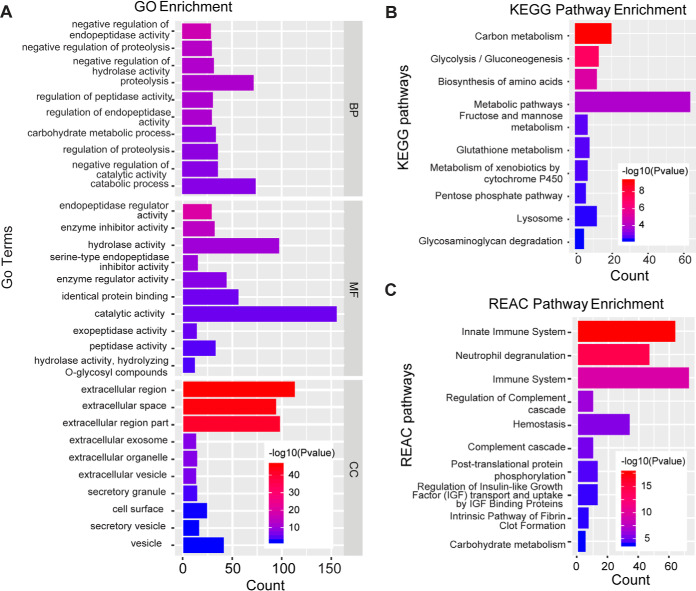
The 339 common proteins were submitted to g:Profiler to obtain an overview of the biological associations of the seminal plasma proteins. As a result, 290 proteins were annotated to the GO database and classified into 151 functional groups. The top 10 significant terms of biological processes (BP), cellular components (CC), and molecular functions (MF) were presented in Fig. 2A. Over 40% of the proteins were annotated to be localized in the extracellular milieu. The top-ranked BP terms were negative regulators of endopeptidase activity, proteolysis, and carbohydrate metabolic process, followed by protein processing, protein maturation, zymogen activation, glycolytic process, immune response, and removal of superoxide radicals. The seminal plasma proteins were mainly involved in catalytic activity, and binding process, including endopeptidase activity, enzyme inhibitor activity, and identical protein binding. This finding is consistent with the enriched KEGG and REAC pathways, such as energy production, glutathione metabolism, innate immune system, and post-translational protein phosphorylation pathways (Fig. 2B, 2C).
Fig. 2.
Gene Ontology (GO) analysis (A), Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway classification (B), and Reactome (REAC) pathway classification (C) of common seminal plasma proteins in eight Beijing-You roosters.
Bioinformatics Analysis of the DAPs
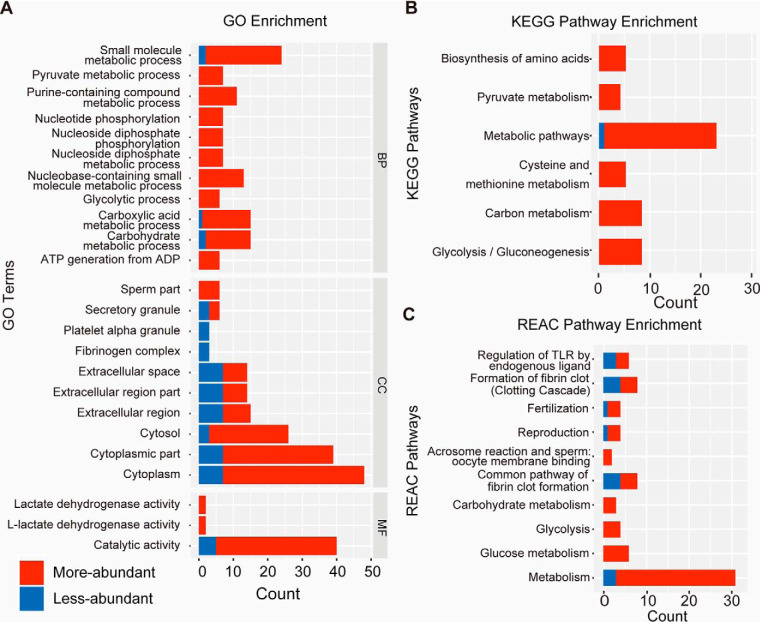
As shown in Fig. 3A, DAPs were related to energy-producing processes such as carbohydrate metabolic process and glycolytic process. For CC analysis, 68.57%, 21.43%, and 8.57% of the proteins were annotated to the cytoplasm, extracellular region, and sperm part, respectively. These proteins were enriched in energy production pathways, including metabolic pathway, glycolysis and gluconeogenesis pathway, acrosome reaction, formation of fibrin clot, and fertilization (Fig. 3B, 3C).
Fig. 3.
Gene ontology (GO) analysis (A), Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway classification (B), and Reactome (REAC) pathway classification (C) of differentially abundant seminal plasma proteins in the low and high sperm motility groups. Blue and red color indicate less-abundant and more-abundant proteins in the low motility semen samples, respectively.
Association Analysis of DAPs and Male Fertility-Related Disorders
As shown in Fig. 4, 11 DAPs were annotated to be associated with male infertility traits, including abnormal spermatogenesis, abnormal sperm morphology, and abnormal sperm physiology. Acrosin (ACR), ADGRG2, Annexin A1 (ANXA1), and Sperm Associated Antigen 16 (SPAG16) were annotated to be associated with asthenozoospermia.
Fig. 4.
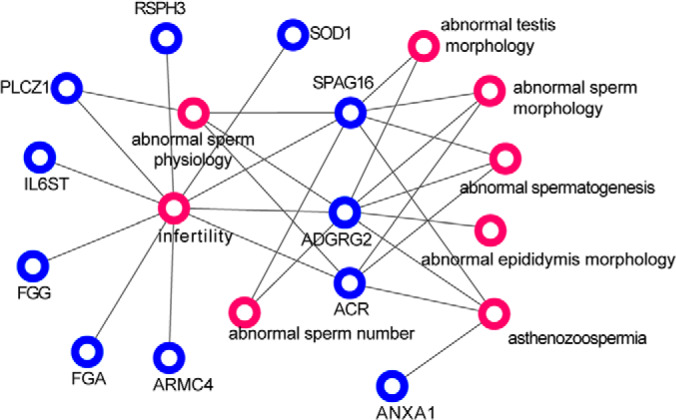
Relation network of differentially abundant proteins (blue circles) and infertility related disorders (red circles).
Western Blot Analysis
To further confirm the DAPs, the abundance of CKMT2, CYC, IL6ST, and GAPDH were analyzed by Western blotting. CKMT2, CYC, and GAPDH were more-abundant, and IL6ST was less-abundant in the LSM group (Fig. 5). These were consistent with the results of the proteomic analysis as presented in Fig. 1B.
Fig. 5.
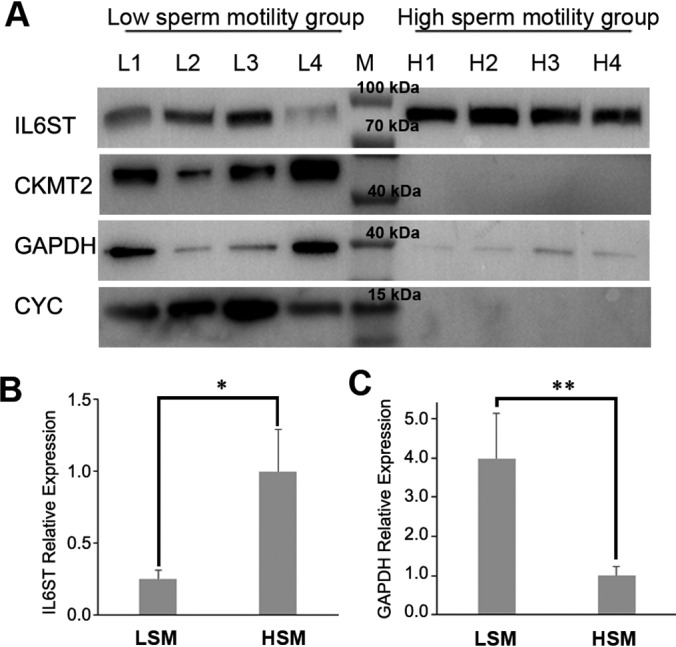
Western blot analysis of seminal IL6ST, CKMT2, GAPDH and CYC abundance in low and high motility semen samples. A, Western blotting showing protein abundances of IL6ST, CKMT2, GAPDH and CYC in seminal plasma of LSM and HSM; M, pre-stained protein ladder; L1-L4, samples of low sperm motility group; H1-H4, samples of high sperm motility group. B, Bars represent relative protein quantification of IL6ST; C, Bars represent relative protein quantification of GAPDH; Protein quantification was normalized to the mean of HSM; Values are specified as mean ± S.E.; LSM, low sperm motility; HSM, high sperm motility; * p < 0.05, ** p < 0.01.
ATP Level and ΔΨm in Spermatozoa
As shown in Fig. 6, compared with the HSM group, both the spermatozoa ATP and ΔΨm were significantly reduced in the LSM group (p < 0.05).
Fig. 6.
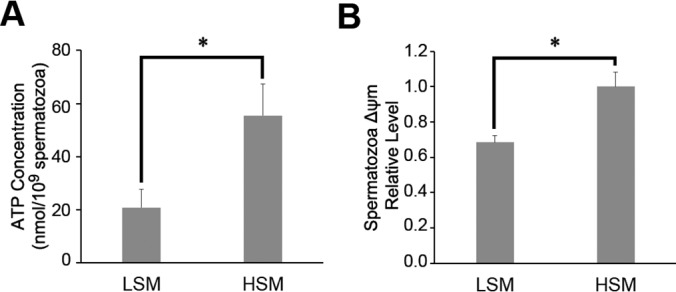
ATP concentration (A) and mitochondrial membrane potentials levels (B) in low and high motility sperm samples. ΔΨm, Mitochondrial membrane potentials; LSM, low sperm motility; HSM, high sperm motility; * p < 0.05.
Plasma Membrane Integrity in Spermatozoa
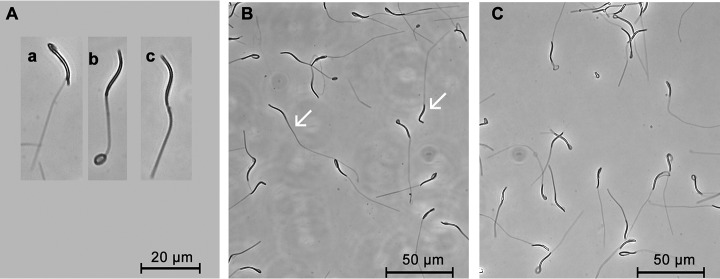
Spermatozoa with intact plasma membrane showed swelling or thickened tails (Fig. 7). The HSM samples showed a high percentage (93.81%±0.85%) of intact spermatozoa, whereas the percentage was significantly reduced in the LSM samples (86.23%±1.26%) (p = 0.02).
Fig. 7.
Evaluation of plasma membrane integrity in low and high sperm motility samples. A, Morphological changes in spermatozoa with integrity membrane: swelling of the mid-piece (a), swelling of the tail (b), shortened and thickened tail (c); B, Low sperm motility sample with degraded plasma membrane as denoted by white arrows; C, High sperm motility sample with intact plasma membrane.
Acrosome Integrity in Spermatozoa
As shown in Fig. 8, Giemsa staining of spermatozoa with intact acrosome exhibit dark purple acrosome on the head. Significant differences were observed in spermatozoa acrosome integrity between LSM and HSM (82.20%±4.24% versus 96.43%±0.68%, p = 0.04).
Fig. 8.
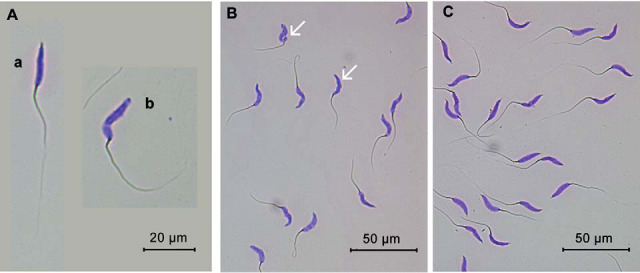
Evaluation of spermatozoa acrosome integrity in low and high sperm motility samples. A, Spermatozoa stained with Giemsa with an intact acrosome (a) or a damaged acrosome (b); B, Low sperm motility sample with degraded acrosomes as denoted by white arrows; C, High sperm motility sample with intact acrosomes.
ROS Level in Spermatozoa
Under a fluorescence microscope, spermatozoa with low motility showed strong green fluorescence on the head and neck (Fig. 9A), whereas those with high motility showed strong green fluorescence around the neck (Fig. 9B). Determined by a microplate reader, spermatozoa intracellular ROS of LSM was 15-times higher than that of the HSM (p = 0.01) (Fig. 9C).
Fig. 9.
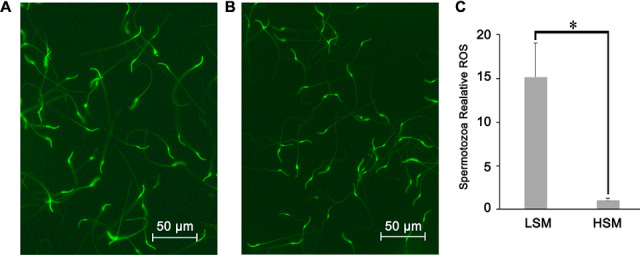
Evaluation of spermatozoa reactive oxygen species (ROS) level in low and high sperm motility samples. A, Fluorescent microscopic images of LSM sample; B, Fluorescent microscopic images of HSM sample; C, Bars represent spermatozoa ROS normalized by mean fluorescence intensity of the HSM samples, values are specified as mean ± S.E.; LSM, low sperm motility; HSM, high sperm motility; * p < 0.05.
MDA and T-AOC in Seminal Plasma
As shown in Table III, compared with the HSM group, MDA concentration was higher whereas the T-AOC was lower in the LSM group at a level of p < 0.10.
Table III. MDA and T-AOC concentrations in seminal plasma of roosters of low and high sperm motility groups.
| Traits | LSM (n = 4) | HSM (n = 4) | p value |
|---|---|---|---|
| MDA (nmol/ml) | 1.35 ± 0.50 | 0.70 ± 0.34 | 0.05 |
| T-AOC (U/ml) | 1.51 ± 0.16 | 1.71 ± 0.08 | 0.09 |
Values are specified as means ± S.E.; LSM, low sperm motility; HSM, high sperm motility; MDA, malondialdehyde; T-AOC, total antioxidant capacity.
DISCUSSION
With the application of MS-based label-free quantitative proteomics, we were able to identify 522 proteins in the seminal plasma of BJY chickens. Seventy proteins were found to be differentially-abundant between the LSM and HSM groups. Bioinformatics analyses and verification studies further extended the understanding of their roles in sperm motility.
Protein concentration in seminal plasma of roosters reported in previous studies (3–10 mg/ml) is consistent with 6 mg/ml for BJY chickens presented here (32, 33). Factors like breed, technical features, and protein databases may affect the efficiency of protein identification. SDS-PAGE fractionation followed by LC-MS/MS is one of the most efficient methods for the proteomic analysis of body fluid like seminal plasma with extensive concentrations of different proteins (34, 35). In the present study, 438 to 486 proteins were identified in an individual sample with four fractionations in BJY chickens. Borziak et al. (23) identified 454–803 proteins with 12 fractionations in Red jungle fowls, whereas Labas et al. (6) reported 95–128 proteins without fractionation and 607 proteins with 40 fractionations in meat-type chickens. Of the 522 proteins identified in this study, 386 (∼74%) were reported previously, and the remaining 136 novel proteins may be attributed to the detection methods and chicken breeds. These contribute to the seminal plasma proteome database of avian species.
GO analysis revealed that these seminal plasma proteins were related to metabolic process, proteolysis, ion homeostasis, and immune system processes, which correspond to the seminal plasma functions of nutrition, regulation, and protection. Similar findings were reported in other species like humans (3) and turkeys (36). A total of 70 proteins were denoted as candidate molecules for sperm motility because they were differentially abundant between LSM and HSM samples.
Secreted by the testis, epididymis, and genital tract, seminal plasma proteomic profile may be an indicator of testicular and genital tract functions (37). In humans, ADGRG2 is X-linked and secreted in the efferent ducts and epididymis where spermatozoa acquire motility (38). It is involved in the reabsorption process of the testicular fluid, which carries immature spermatozoa (39). ADGRG2 mutant men displayed azoospermia (40, 41) or oligozoospermia (42). The abundance of ADGRG2 was significantly reduced in the LSM group, which may suggest the dysfunction of epididymis. Family members of annexin are involved in calcium ion binding and signaling receptor binding, and have active roles in the organization of cell membrane (43). ANXA1, ANXA2, and ANXA5 were less-abundant in the seminal plasma of asthenospermia (44–46) and might be caused by sperm DNA fragmentation (17). Our data showed that ANXA1 was 2.5-fold decreased in seminal plasma of LSM, suggesting the possible involvement of ANXA1 in avian sperm motility. Seminal SPINK2, also named chicken liver trypsin inhibitor 1 (ClTI-1) (29), originates from testis, epididymis, and genital tract. It functions as an acrosin/trypsin inhibitor protecting the spermatozoa against damage by binding to the sperm membrane (29). Its more-abundance in the seminal plasma of LSM roosters make us speculate that failure of SPINK2 to bind to the membrane and acrosome of the damaged and disfunction spermatozoa. In a previous study, SPINK2 was reported to be more-abundant in the seminal plasma of roosters with high fertility (6, 29). This inconstancy might be caused by the different causal factors between studies. A previous study in turkey pointed out that excess of ALB may reduce sperm motility (47). No significant difference in ALB abundance was found between LSM and HSM groups in this study, indicating that the poor motility in BJY might not be caused by the variation of this protein.
Function analysis revealed that more-abundant proteins, including GAPDH and PKM, were involved in glycolysis. It is a fact that sperm motility is directly dependent upon the energy obtained through ATP hydrolysis. Glycolysis is the top energy-generating pathway for sperm motility in mammals, whereas mitochondrial ATP synthesis plays a preponderant role rather than glycolysis in avian (48). We found a lower ATP level in spermatozoa of LSM group with no difference of fuel substrate (including fructose, glucose, sorbitol, lactate, and pyruvate) in seminal plasma (unpublished data). The release and significant more-abundance of mitochondrial membrane proteins in seminal plasma of LSM, including CKMT2, CYC, and mitochondria-eating protein (SPATA18), gave rise to dysfunction in mitochondria (49). This speculation was further verified by the detection of reduced ΔΨm of LSM samples.
The intracellular proteins including GAPDH and CKMT2 present in seminal plasma may originate from spermatozoa (50). These proteins are an inseparable part of seminal plasma and may reflect the physiological and morphological characteristics of spermatozoa. Majority (74.54%) of the more-abundant proteins in LSM were annotated to the cytoplasmic and sperm part. The higher concentration of intracellular proteins in the seminal plasma might be related to higher degradation of spermatozoa. It is worthy of note that five of the seven LSM-specific proteins, viz. ARMC4, CFAP52, RSPH3, RSPH14, and SPAG16, were critical structural components of motile cilia (51) and involved in ciliary assembly (52, 53). The abundance of these proteins in seminal plasma may indicate the structural damage of sperm flagella. An intact sperm membrane was reported as a necessary criterion for motility (54, 55). We found that 13.77% of the spermatozoa in LSM group have damaged cell membranes, which was twice of that in the HSM group. This observation suggests that high frequency of cell membrane damage might be a causing factor for immobile spermatozoa.
ACR is one of the most abundant proteins in the LSM group. It is involved in the digestion of the innermost glycoprotein layer of the oocyte during the gamete fusion of both mammals and birds (56, 57). ACR is typically stored in the acrosome of mature spermatozoa, and its abundance in the LSM seminal plasma probably caused by the degradation of the acrosome cap of sperm in the ejaculate (6). Previous studies have demonstrated that altered abundance of seminal plasma proteins, including ACR, Pyruvate kinase, and Superoxide Dismutase 1 (SOD1), may be related to acrosome dysfunction in humans (17, 58), as suggested by our findings. This speculation was confirmed by the spermatozoa acrosome integrity test, as 17.80% of the spermatozoa in the LSM were acrosome damaged. Similar to the previous report in humans (59), the results here suggest that acrosome degradation might be one of the factors leading to poor sperm motility.
These more-abundant proteins, like GAPDH, CKMT2 and ACR, give us a clue that the LSM might be related to the dysfunction of sperm membrane, mitochondria and acrosome. Oxidative stress by ROS is a major cause of sperm dysfunction and reduced sperm motility (60). In this study, ROS level of the LSM spermatozoa was found to be 15-times higher than that of HSM ones. Additionally, seminal plasma MDA, a marker of lipid peroxidation in cells (61), was higher in LSM. Spermatozoa have limited antioxidative defense mechanisms because of the small amount of cytoplasm (62, 63), especially for chickens. Seminal plasma is enriched with both enzymatic and nonenzymatic antioxidants, such as SOD, Glutathione peroxidase (GPXs) and vitamins (63). Here, seven enzymatic antioxidants were identified in seminal plasma and the seminal plasma proteins were enriched in removal of superoxide radicals. SOD1, an antioxidant enzyme localized in both the mitochondria intermembrane space and cytosol, was more-abundant in seminal plasma with low motility and fertility, which might be caused by the dysfunction of spermatozoa plasma and mitochondrial as discussed above. Further, the more-abundant of SOD1 could potentially increase production of hydrogen peroxide leading to increased cellular injury(64, 65). Lower T-AOC in LSM group might be a result of enzymatic and nonenzymatic antioxidants. The excess generation of ROS by the spermatozoa and lower level of T-AOC in seminal plasma results in the occurrence of oxidative stress, thus, lead to sperm dysfunction, lower ATP production, and poor sperm motility.
CONCLUSIONS
The present study provides a comprehensive proteomic profile of chicken seminal plasma. Detection of a more-abundance of cytosol, mitochondria and sperm cytoskeleton proteins in the seminal plasma, together with the membrane and acrosome integrity test, suggesting that ROS-induced spermatozoa degradation and mitochondria dysfunction might be potential determinants of low sperm motility. In addition, quantity alteration of some secretory proteins like ADGRG2 shed light on their potential roles in maintaining sperm motility, which is worthy of further experimental confirmation.
DATA AVAILABILITY
Raw mass spectrometry data and Proteome Discoverer output files were deposited to the ProteomeXchange Consortium via PRIDE (https://www.ebi.ac.uk/pride/archive/) with the dataset identifier PXD016220.
Supplementary Material
Acknowledgments
We thank Xingping Zheng (Institute of Animal Science, Chinese Academy of Agricultural Sciences, Beijing, China) for his assistance with raising animals. We thank Sheng Zhang (Biotechnology Resource Center, Cornell University) for technical recommendation.
Footnotes
This article contains supplemental data.
Funding and additional information—This work was supported by the National Natural Science Foundation of China (31672406), Beijing Municipal Science and Technology Project (D171100007817005), China Agriculture Research Systems (CARS-40), the Fundamental Research Funds for Central Non-profit Scientific Institution (2017ywf-2d-7), Key Laboratory of Animal (Poultry) Genetics Breeding and Reproduction, Ministry of Agriculture (poultrylab2018-5), and Agricultural Science and Technology Innovation Program (ASTIP-IAS04).
Conflict of interest—Authors declare no competing interests.
Abbreviations—The abbreviations used are:
- ACR
- Acrosin
- ADGRG2
- adhesion G-protein coupled receptor G2
- AGC
- automatic gain control
- AI
- artificial insemination
- ALB
- albumin
- ALH
- amplitude of lateral head displacement
- ANXA1
- annexin A1
- BCF
- beat-cross frequency
- BJY
- Beijing-You
- BP
- biological processes
- CASA
- computer-aided semen analysis
- CC
- cellular components
- CKMT2
- creatine kinase S-type mitochondrial
- CYC
- cytochrome C
- DAPs
- differential abundant proteins
- FDR
- false discovery rate
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- GO
- Gene Ontology
- GPXs
- glutathione peroxidase
- HSM
- high sperm motility
- IL6ST
- interleukin 6 signal transducer
- KEGG
- Kyoto Encyclopedia of Genes And Genomes
- LIN
- linearity
- LSM
- low sperm motility
- MDA
- malondialdehyde
- MF
- molecular functions
- PCA
- principal component analysis
- PMSF
- phenylmethylsulphonylfluoride
- REAC
- reactome
- ROS
- reactive oxygen species
- SOD1
- superoxide dismutase 1
- SPAG16
- sperm associated antigen 16
- SPATA18
- mitochondria-eating protein
- SPINK2
- serine peptidase inhibitor Kazal-type 2
- STR
- straightness
- T-AOC
- total antioxidant capacity
- VAP
- average path velocity
- VCL
- curvilinear velocity
- VSL
- straight-line velocity
- WOB
- wobble
- ΔΨm
- mitochondrial membrane potential.
REFERENCES
- 1. Camargo M., Intasqui P., and Bertolla R. P. (2018) Understanding the seminal plasma proteome and its role in male fertility. Basic Clin. Andro. 28, 6–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Batruch I., Lecker I., Kagedan D., Smith C. R., Mullen B. J., Grober E., Lo K. C., Diamandis E. P., and Jarvi K. A. (2011) Proteomic analysis of seminal plasma from normal volunteers and post-vasectomy patients identifies over 2000 proteins and candidate biomarkers of the urogenital system. J. Proteome Res. 10, 941–953 [DOI] [PubMed] [Google Scholar]
- 3. Gilany K., Minai-Tehrani A., Savadi-Shiraz E., Rezadoost H., and Lakpour N. (2015) Exploring the human seminal plasma proteome: An unexplored gold mine of biomarker for male Infertility and male reproduction disorder. J. Reprod. Infertil. 16, 61–71 [PMC free article] [PubMed] [Google Scholar]
- 4. Viana A. G. A., Martins A. M. A., Pontes A. H., Fontes W., Castro M. S., Ricart C. A. O., Sousa M. V., Kaya A., Topper E., Memili E., and Moura A. A. (2018) Proteomic landscape of seminal plasma associated with dairy bull fertility. Sci. Rep. 8, 16323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Soleilhavoup C., Tsikis G., Labas V., Harichaux G., Kohnke P. L., Dacheux J. L., Guérin Y., Gatti J. L., de Graaf S. P., and Druart X. (2014) Ram seminal plasma proteome and its impact on liquid preservation of spermatozoa. J. Proteomics 109, 245–260 [DOI] [PubMed] [Google Scholar]
- 6. Labas V., Grasseau I., Cahier K., Gargaros A., Harichaux G., Teixeira-Gomes A. P., Alves S., Bourin M., Gérard N., and Blesbois E. (2015) Qualitative and quantitative peptidomic and proteomic approaches to phenotyping chicken semen. J. Proteomics 112, 313–335 [DOI] [PubMed] [Google Scholar]
- 7. Rodrigues M. A. M., Souza C. E. A., Martins J. A. M., Rego J. P. A., Oliveira J. T. A., Domont G., Nogueira F. C. S., and Moura A. A. (2013) Seminal plasma proteins and their relationship with sperm motility in Santa Ines rams. Small Rumin. Res. 109, 94–100 [Google Scholar]
- 8. Lin Y., Liang A., He Y., Li Z., Li Z., Wang G., and Sun F. (2019) Proteomic analysis of seminal extracellular vesicle proteins involved in asthenozoospermia by iTRAQ. Mol. Reprod. Dev. 86, 1094–1105 [DOI] [PubMed] [Google Scholar]
- 9. De Lazari F. L., Sontag E. R., Schneider A., Moura A. A. A., Vasconcelos F. R., Nagano C. S., Mattos R. C., Jobim M. I. M., and Bustamante-Filho I. C. (2019) Seminal plasma proteins and their relationship with sperm motility and morphology in boars. Andrologia 51, e13222. [DOI] [PubMed] [Google Scholar]
- 10. Zou P., Wang X., Chen Q., Yang H., Zhou N., Sun L., Chen H., Liu J., Ao L., Cui Z., and Cao J. (2019) Kisspeptin protein in seminal plasma is positively associated with semen quality: results from the MARHCS study in Chongqing, China. Biomed. Res. Int. 2019, 5129263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Al-Essawe E. M., Wallgren M., Wulf M., Aurich C., Macías-García B., Sjunnesson Y., and Morrell J. M. (2018) Seminal plasma influences the fertilizing potential of cryopreserved stallion sperm. Theriogenology 115, 99–107 [DOI] [PubMed] [Google Scholar]
- 12. Qu F., Ying X., Guo W., Guo Q., Chen G., Liu Y., and Ding Z. (2007) The role of Zn-α2 glycoprotein in sperm motility is mediated by changes in cyclic AMP. Reproduction 134, 569–576 [DOI] [PubMed] [Google Scholar]
- 13. Sharma R., Agarwal A., Mohanty G., Du Plessis S. S., Gopalan B., Willard B., Yadav S. P., and Sabanegh E. (2013) Proteomic analysis of seminal fluid from men exhibiting oxidative stress. Reprod. Biol. Endocrinol. 11, 85–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zylbersztejn D. S., Andreoni C., Del Giudice P. T., Spaine D. M., Borsari L., Souza G. H. M. F., Bertolla R. P., and Fraietta R. (2013) Proteomic analysis of seminal plasma in adolescents with and without varicocele. Fertil. Steril. 99, 92–98 [DOI] [PubMed] [Google Scholar]
- 15. Panner Selvam M., Agarwal A., and Baskaran S. (2019) Proteomic analysis of seminal plasma from bilateral varicocele patients indicates an oxidative state and increased inflammatory response. Asian J. Androl. 21, 544–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Drabovich A. P., Saraon P., Drabovich M., Karakosta T. D., Dimitromanolakis A., Eric Hyndman M., Jarvi K., and Diamandis E. P. (2019) Multi-omics biomarker pipeline reveals elevated levels of protein-glutamine gammaglutamyltransferase 4 in seminal plasma of prostate cancer patients. Mol. Cell Proteomics 18, 1807–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Intasqui P., Camargo M., Antoniassi M. P., Cedenho A. P., Carvalho V. M., Cardozo K. H. M., Zylbersztejn D. S., and Bertolla R. P. (2016) Association between the seminal plasma proteome and sperm functional traits. Fertil. Steril. 105, 617–628 [DOI] [PubMed] [Google Scholar]
- 18. Dhama K., Singh R. P., Karthik K., Chakraborty S., Tiwari R., Wani M. Y., and Mohan J. (2014) Artificial insemination in poultry and possible transmission of infectious pathogens. Asian J. Anim. Vet. Adv. 9, 211–228 [Google Scholar]
- 19. Mohan J., Sharma S. K., Kolluri G., and Dhama K. (2018) History of artificial insemination in poultry, its components and significance. Worlds Poult. Sci. J. 74, 475–488 [Google Scholar]
- 20. Hu J., Chen J. L., Wen J., Zhao G. P., Zheng M. Q., Liu R. R., Liu W. P., Zhao L. H., Liu G. F., and Wang Z. W. (2013) Estimation of the genetic parameters of semen quality in Beijing-You chickens. Poult. Sci. 92, 2606–2612 [DOI] [PubMed] [Google Scholar]
- 21. Clulow J., and Jones R. C. (1982) Production, transport, maturation, storage and survival of spermatozoa in the male Japanese quail, Coturnix coturnix. J. Reprod. Fertil. 64, 259–266 [DOI] [PubMed] [Google Scholar]
- 22. Howarth B. (1970) An examination for sperm capacitation in the fowl. Biol. Reprod. 3, 338–341 [DOI] [PubMed] [Google Scholar]
- 23. Borziak K., Álvarez Fernández -A., Karr T. L., Pizzari T., and Dorus S. (2016) The Seminal fluid proteome of the polyandrous Red junglefowl offers insights into the molecular basis of fertility, reproductive ageing and domestication. Sci. Rep. 6, 35864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Drabovich A. P., Saraon P., Jarvi K., and Diamandis E. P. (2014) Seminal plasma as a diagnostic fluid for male reproductive system disorders. Nat. Rev. Urol. 11, 278–288 [DOI] [PubMed] [Google Scholar]
- 25. Organization W. H. O. (2010) WHO laboratory manual for the examination and processing of human semen. 5th edition. [PubMed] [Google Scholar]
- 26. Chalah T., and Brillard J. P. (1998) Comparison of assessment of fowl sperm viability by eosin-nigrosin and dual fluorescence (SYBR-14/PI). Theriogenology 50, 487–493 [DOI] [PubMed] [Google Scholar]
- 27. Raudvere U., Kolberg L., Kuzmin I., Arak T., Adler P., Peterson H., and Vilo J. (2019) g:Profiler: a web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 47, 191–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen J., Bardes E. E., Aronow B. J., and Jegga A. G. (2009) ToppGene suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res. 37, 305–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thélie A., Rehault-Godbert S., Poirier J. C., Govoroun M., Fouchécourt S., and Blesbois E. (2019) The seminal acrosin-inhibitor ClTI1/SPINK2 is a fertility-associated marker in the chicken. Mol. Reprod. Dev. 86, 762–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Santiago-Moreno J., Castaño C., Coloma M. A., Gómez-Brunet A., Toledano-Díaz A., López-Sebastián A., and Campo J. L. (2009) Use of the hypo-osmotic swelling test and aniline blue staining to improve the evaluation of seasonal sperm variation in native Spanish free-range poultry. Poult. Sci. 88, 2661–2669 [DOI] [PubMed] [Google Scholar]
- 31. Santiago-Moreno J., Gil M. G., Dávila S. G., Campo J. L., Castãno C., Toledano-Díaz A., Prieto M. T., and Blesbois E. (2018) Access to pasture in an outdoor housing system affects welfare indicators and improves rooster sperm quality in two native Mediterranean breeds. Poult. Sci. 97, 4433–4441 [DOI] [PubMed] [Google Scholar]
- 32. Murugesan S., Matam N., Kulkarni R., Bhattacharya T. K., and Chatterjee R. (2013) Semen quality in white leghorn chicken selected for egg production traits. Turk. J. Vet. Anim. Sci. 37, 747–749 [Google Scholar]
- 33. AI-Aghbari A., Engel H. N., and Froman D. P. (1992) Analysis of seminal plasma from roosters carrying the Sd (Sperm Degeneration) allele1. Biol. Reprod. 47, 1059–1063 [DOI] [PubMed] [Google Scholar]
- 34. Paulo J. A. (2016) Sample preparation for proteomic analysis using a GeLC-MS/MS strategy. J. Biol. Methods 3, e45, 10.14440/jbm.2016.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fountoulakis M., Juranville J. F., Jiang L., Avila D., Röder D., Jakob P., Berndt P., Evers S., and Langen H. (2004) Depletion of the high-abundance plasma proteins. Amino Acids 27, 249–259 [DOI] [PubMed] [Google Scholar]
- 36. Slowińska M., Nynca J., Arnold G. J., Fröhlich T., Jankowski J., Kozłowski K., Mostek A., and Ciereszko A. (2017) Proteomic identification of Turkey (Meleagris gallopavo) seminal plasma proteins. Poult. Sci. 96, 3422–3435 [DOI] [PubMed] [Google Scholar]
- 37. Camargo M., Intasqui P., and Bertolla R. P. (2016) Proteomic profile of seminal plasma in adolescents and adults with treated and untreated varicocele. Asian J. Androl. 18, 194–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang D. L., Sun Y. J., Ma M. L., Wang Y. J., Lin H., Li R. R., Liang Z. L., Gao Y., Yang Z., He D. F., Lin A., Mo H., Lu Y. J., Li M. J., Kong W., Chung K. Y., Yi F., Li J. Y., Qin Y. Y., Li J., Thomsen A. R. B., Kahsai A. W., Chen Z. J., Xu Z. G., Liu M., Li D., Yu X., and Sun J. P. (2018) Gq activity-and β-arrestin-1 scaffolding-mediated ADGRG2/CFTR coupling are required for male fertility. eLife, 7, pii: e33432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Davies B., Baumann C., Kirchhoff C., Ivell R., Nubbemeyer R., Habenicht U. F., Theuring F., and Gottwald U. (2004) Targeted deletion of the epididymal receptor HE6 results in fluid dysregulation and male infertility. Mol. Cell Biol. 24, 8642–8648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang B., Wang J., Zhang W., Pan H., Li T., Liu B., Li H., and Wang B. (2017) Pathogenic role of ADGRG2 in CBAVD patients replicated in Chinese population. Andrology 5, 954–957 [DOI] [PubMed] [Google Scholar]
- 41. Khan M. J., Pollock N., Jiang H., Castro C., Nazli R., Ahmed J., Basit S., Rajkovic A., and Yatsenko A. N. (2018) X-linked ADGRG2 mutation and obstructive azoospermia in a large Pakistani family. Sci. Rep. 8, 16280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Oud M. S., Ramos L., O'Bryan M. K., McLachlan R. I., Okutman Ö Viville S, de Vries P. F., Smeets D. F. C. M., Lugtenberg D., Hehir-Kwa J. Y., Gilissen C., van de Vorst M., Vissers L., Hoischen A., Meijerink A. M., Fleischer K., Veltman J. A., and Noordam M. J. (2017) Validation and application of a novel integrated genetic screening method to a cohort of 1,112 men with idiopathic azoospermia or severe oligozoospermia. Hum. Mutat. 38, 1592–1605 [DOI] [PubMed] [Google Scholar]
- 43. Lizarbe M. A., Barrasa J. I., Olmo N., Gavilanes F., and Turnay J. (2013) Annexin-phospholipid interactions. Functional implications. Int. J. Mol. Sci. 14, 2652–2683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wu Y., Yuan Y., Chen L., Wang M., Yang Y., Wang Y., Quan C., Chen D., Chen Y., Huang X., and Zhou T. (2019) Quantitative proteomic analysis of human seminal plasma from normozoospermic and asthenozoospermic individuals. Biomed. Res. Int. 2019, 2735038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Munuce M. J., Marini P. E., and Teijeiro J. M. (2019) Expression profile and distribution of Annexin A1, A2 and A5 in human semen. Andrologia 51, e13224. [DOI] [PubMed] [Google Scholar]
- 46. Inokuchi J., Lau A., Tyson D. R., and Ornstein D. K. (2009) Loss of annexin A1 disrupts normal prostate glandular structure by inducing autocrine IL-6 signaling. Carcinogenesis 30, 1082–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Slowińska M., Kozłowski K., Jankowski J., and Ciereszko A. (2015) Proteomic analysis of white and yellow seminal plasma in turkeys (Meleagris gallopavo). J. Anim. Sci. 93, 2785–2795 [DOI] [PubMed] [Google Scholar]
- 48. Froman D. P., and Kirby J. D. (2005) Sperm mobility: phenotype in roosters (Gallus domesticus) determined by mitochondrial function. Biol. Reprod. 72, 562–567 [DOI] [PubMed] [Google Scholar]
- 49. Emokpae A., Chima H., and Ahmed M. (2016) Seminal plasma caspase 3, cytochrome c and total antioxidant capacity in oligospermic males and association with sperm indices. J. Exp. Integrative Med. 6, 10.5455/jeim.091116.or.164 [DOI] [Google Scholar]
- 50. Jodar M., Soler-Ventura A., and Oliva R. (2017) Semen proteomics and male infertility. J. Proteomics 162, 125–134 [DOI] [PubMed] [Google Scholar]
- 51. Coutton C., Vargas A. S., Amiri-Yekta A., Kherraf Z. E., Ben Mustapha S. F., Le Tanno P., Wambergue-Legrand C., Karaouzene T., Martinez G., Crouzy S., Daneshipour A., Hosseini S. H., Mitchell V., Halouani L., Marrakchi O., Makni M., Latrous H., Kharouf M., Deleuze J. F., Boland A., Hennebicq S., Satre V., Jouk P. S., Thierry-Mieg N., Conne B., Dacheux D., Landrein N., Schmitt A., Stouvenel L., Lores P., El Khouri E., Bottari S. P., Faure J., Wolf J. P., Pernet-Gallay K., Escoffier J., Gourabi H., Robinson D. R., Nef S., Dulioust E., Zouari R., Bonhivers M., Toure A., Arnoult C., and Ray P. F. (2018) Mutations in CFAP43 and CFAP44 cause male infertility and flagellum defects in Trypanosoma and human. Nat. Commun. 9, 686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Silva F. P., Hamamoto R., Nakamura Y., and Furukawa Y. (2005) WDRPUH, a novel WD-repeat-containing protein, is highly expressed in human hepatocellular carcinoma and involved in cell proliferation. Neoplasia 7, 348–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Onoufriadis A., Shoemark A., Munye M. M., James C. T., Schmidts M., Patel M., Rosser E. M., Bacchelli C., Beales P. L., Scambler P. J., Hart S. L., Danke-Roelse J. E., Sloper J. J., Hull S., Hogg C., Emes R. D., Pals G., Moore A. T., Chung E. M. K., and Mitchison H. M. (2014) Combined exome and whole-genome sequencing identifies mutations in ARMC4 as a cause of primary ciliary dyskinesia with defects in the outer dynein arm. J. Med. Genet. 51, 61–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ramirez J. P., Carreras A., and Mendoza C. (1992) Sperm plasma membrane integrity in fertile and infertile men. Andrologia 24, 141–144 [DOI] [PubMed] [Google Scholar]
- 55. Rajashri M., Reddy K. R., Kumari G. A., Kumari N. N., and Kesharwani S. (2017) Correlation between hypo-osmotic swelling test (host) and other seminal characteristics of Deccani Ram semen. J. Exp. Biol. Agricultural Sci. 5, 195–200 [Google Scholar]
- 56. Blesbois E. (2012) Biological features of the avian male gamete and their application to biotechnology of conservation. J. Poult. Sci. 49, 141–149 [Google Scholar]
- 57. Ito C., and Toshimori K. (2016) Acrosome markers of human sperm. Anat. Sci. Int. 91, 128–142 [DOI] [PubMed] [Google Scholar]
- 58. Intasqui P., Camargo M., Del Giudice P. T., Spaine D. M., Carvalho V. M., Cardozo K. H. M., Zylbersztejn D. S., and Bertolla R. P. (2013) Sperm nuclear DNA fragmentation rate is associated with differential protein expression and enriched functions in human seminal plasma. BJU Int. 112, 835–843 [DOI] [PubMed] [Google Scholar]
- 59. Li T., Liu W., Xie N., Yang S., Zhang C., Fu H., and Gao X. (2017) Value analysis of sperm spontaneous acrosome reaction in male fertility evaluation. Andrology 6, 10.4172/2167-0250.1000195 [DOI] [Google Scholar]
- 60. Saleh R. A., and Agarwal A. (2002) Oxidative stress and male infertility: From research bench to clinical practice. J. Androl. 23, 737–752 [PubMed] [Google Scholar]
- 61. Colagar A. H., Karimi F., and Jorsaraei S. G. A. (2013) Correlation of sperm parameters with semen lipid peroxidation and total antioxidants levels in astheno- and oligoasheno- teratospermic men. Iran. Red. Crescent. Med. J. 15, 780–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Aitken J., Buckingham D., and Krausz C. (1994) Relationships between biochemical markers for residual sperm cytoplasm, reactive oxygen species generation, and the presence of leukocytes and precursor germ cells in human sperm suspensions. Mol. Reprod. Dev. 39, 268–279 [DOI] [PubMed] [Google Scholar]
- 63. Alahmar A. (2019) Role of oxidative stress in male infertility: An updated review. J. Hum. Reprod. Sci. 12, 4–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Usui S., Oveson B. C., Iwase T., Lu L., Lee S. Y., Jo Y. J., Wu Z., Choi E. Y., Samulski R. J., and Campochiaro P. A. (2011) Overexpression of SOD in retina: Need for increase in H2O2-detoxifying enzyme in same cellular compartment. Free Radic. Biol. Med. 51, 1347–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bustamante-Filho I. C., Rosa A. P., Van der Linden L. S., Pederzolli C. D., Neves A. P., Dutra-Filho C. S., Jobim M. I. M., and Mattos R. C. (2014) Enzymatic scavengers in the epididymal fluid: Comparison between pony and miniature breed stallions. Anim. Reprod. Sci. 151, 164–168 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw mass spectrometry data and Proteome Discoverer output files were deposited to the ProteomeXchange Consortium via PRIDE (https://www.ebi.ac.uk/pride/archive/) with the dataset identifier PXD016220.