Abstract
Recently, the gut microbiome has become an important field of interest. Indeed, the microbiome has been associated to numerous drug interactions and it is thought to influence the efficacy of pharmacologic treatments. Although statins are widely prescribed medications, there remains considerable variability in its therapeutic response. In this context, we aimed to investigate how statins modulate the gut microbiome and, reversely, how can the microbiome influence the course of anti‐hypercholesterolemic treatment. We conducted a systematic review by searching four online databases, in accordance with PRISMA guidelines. Studies addressing gut microbiome changes following statin treatment and those assessing statins’ response and associating it with patients’ microbiome were included. Due to the limited number of results, we decided to include studies enrolling both humans and animals. We summarized information from three human and seven animal studies and aimed to assess the influence of gut microbiome composition on statin response (Outcome 1) and to evaluate the impact of statin treatment on the gut microbiome (Outcome 2). An association between a certain microbiome composition that promoted the lipid‐lowering effect of statins was found. However, what kind of microorganisms and how they can exert this effect remains uncertain. Furthermore, statins might have a role in the modulation of the gut microbiome, but then again, it is still unknown whether this change is directly caused by the drug or another metabolic mechanism. Even though gut microbiota may have several potential therapeutic implications, its use as a personalized predictive biomarker requires further studies.
Keywords: Gut microbiome, lipid‐lowering effect, statins, therapy related outcomes
Abbreviations
- CAMP
cathelicidin antimicrobial peptide
- IL6
interleukin‐6,
- OATP1B1
organic anion transporting polypeptide 1B1
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses
- TNF‐α
tumour necrosis factor‐alpha
1. INTRODUCTION
More than 100 trillion symbiotic microorganisms live on and within the human body, playing an important role in health and disease. These consist of a diverse consortium of archaea, bacteria, fungi, protozoa and viruses (along with their genome), forming the microbiome. 1 Unlike the host genome, the microbiome is dynamic and changes with early development and environmental factors such as diet, antibiotic exposure and, especially, in response to disease. 2 The majority of the microorganisms that inhabit the human body are in the gut, corresponding to the gut microbiota. The terms microbiome and microbiota are mostly used interchangeably. However, microbiota refers purely to the actual microorganisms that are present in a specific site, regardless of their genome or the environment in which they inhabit. In the last decades, several studies explored the interaction between the microbiome and the host as well as its influence on vital physiologic processes among which were energy and metabolism, immunity, neurobehavioral development and gut epithelial health. 3
More recently, following its impact on energy regulation and immune system homeostasis, 4 gut microbiota is becoming an attractive field with potential clinical significance. Current data suggest that the modulation of gut microbiome improves the outcome of various diseases, including inflammatory and cardiovascular, besides influencing the response to pharmacotherapy. 5 , 6 , 7 , 8
Statins are one of the most prescribed drugs in the world, 9 , 10 with a strong amount of evidence demonstrating a significant reduction in morbidity and mortality associated to cardiovascular diseases. 11 Essentially, statins can reduce blood cholesterol levels by two ways: (a) as competitive inhibitors of 3‐hydroxy‐3‐methyl‐gutaryl‐CoA (HMG‐CoA) reductase, limiting cholesterol synthesis, 12 and (b) enhancing cholesterol uptake in the liver, through upregulation of Low‐Density Lipoprotein Cholesterol (LDLc) receptor. 13 In addition, while statins are designed to reduce extracellular cholesterol in blood in the form of LDLc, they can also reduce cholesterol in the plasma membranes. 14 Notwithstanding, according to the literature, the response to statins is not always linear, with great variability in terms of LDLc levels. 15 Conversely, beyond the well‐known cardiovascular benefits, 16 other potential advantages of statins have been reported, including antioxidant, antiinflammatory, antithrombotic and antitumor activities. 17 , 18 , 19 , 20 Furthermore, several studies explored statin behavior in microbial infections, as an influencer of virulence and growth of bacterial pathogens. 21 , 22 Therefore, it is hypothesized that their use might affect the human microbiome, particularly of the gut. On the contrary, it has also been observed that antibiotics could interfere with lovastatin metabolism and, consequently, with its pharmacokinetics and pharmacodynamics; suggesting that gut microbiome could itself influence statins’ therapeutic effect. 23 In addition, Kaddurah‐Daouk et al demonstrated that higher levels of secondary bile acids derived from enteric microbes were correlated with increasing plasma concentrations of simvastatin. 24 In spite of this, still very little is known about the association of statin intake and bacterial gut composition and how the latter affects the response to statin treatment in hyperlipidemic patients.
The study of the interactions between microbiome (and its variation) and pharmaceutical agents is denominated pharmacomicrobiomics. This emerging research field allows exploring the influence of microbiome, particularly gut microbiome, in modulating drugs’ absorption, distribution, metabolism and excretion and in influencing their efficacy and toxicity, thereby influencing therapeutic outcomes. In reverse, various studies in mice and humans have emphasized the gut microbiome modulation induced by drugs. 25 , 26
In this context, we aimed to perform a systematic review of the literature in order to explore two major aspects: i) the influence of gut bacterial communities on the response to statin treatment in hyperlipidemic patients, thereby exploring whether there is enough data to support the use of a particular statin depending on gut microbiome characteristics; ii) to analyze the effect of statin intake in the gut microbiota composition. In this context, we aim to contribute to decode the interindividual variability in drug response, which cannot be totally explained by genetic factors, and still poses an important clinical and financial burden.
2. MATERIALS AND METHODS
2.1. Search strategy
This study was performed following the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses [PRISMA] Guidelines 27 and the Cochrane Collaboration Guidelines for Systemic Reviews. 28 Since there is scarce literature concerning solely humans, the authors decided to include studies performed in animals, in order to maximize the data integrated in the review. From our perspective and taking into account that this is a novel topic, besides analyzing differences between the populations enrolled it is essential to establish comparisons and correlations between the various studies. The literature search was carried out during February of 2019. Human and animal randomized controlled trials, quasi‐experimental, cohort, case‐control and review studies, were retrieved after a literature search including four electronic databases: PubMed, Web of Knowledge, ScienceDirect and Cochrane Central Register of Controlled Trials. In the first database, PubMed, the following words or medical subject heading terms were used: “Microbiome [MeSH] AND (statins or atorvastatin or cerivastatin or fluvastatin or lovastatin or mevastatin or pitavastatin or pravastatin or rosuvastatin or simvastatin)”. For the remaining databases shorter queries had to be used in order to retrieve the maximum number of possible eligible entries; for such, the search terms used were: “microbiome AND statins”. To ensure that all pertinent articles were included the reference lists of the studies selected from the databases were manually reviewed.
2.2. Eligibility and inclusion/exclusion criteria
Studies considered for inclusion met, at least, one of the following criteria: (a) articles concerning the influence of the gut microbiome on statins therapeutic effect; and (b) articles relating statin intake and its effect on the gut microbiome modulation.. No restrictions in terms of publication dates or language were applied. Studies were excluded according to the following criteria: (a) articles that were reviews, guidelines, journal indexes, book chapters or editorials; (b) articles where full text was not available; (c) articles in which patients did not receive statins; (d) articles that did not mention the gut microbiome; and (e) articles that did not evaluate, at least, the enteric bacterial composition. Note that, all studies where the gut microbiome was evaluated, emphasized the bacterial community. This may be due, at least in part, to the fact that most of the sequencing methods used to examine microbial communities were developed to study bacteria, regardless of the existence of other types of microorganisms inhabiting the gut.
2.3. Study selection and data collection process
The identified studies were independently screened by two reviewers. Whenever the title and abstract clearly indicated that the study failed to meet the selection criteria it was immediately excluded. For the remaining studies, the full text was analyzed to decide its inclusion or exclusion. The following information was collected from the selected studies: authors’ name; publication year; country where the study was conducted; number of patients enrolled in the intervention; statin used and which dose; duration of treatment and follow‐up; the samples collected for microbiome analysis, timing and analysis’ methodology; outcomes under assessment; main results and conclusion.
2.4. Quality assessment
To ascertain the methodological quality of the included studies, we analyzed the quality of the research question and determined the adequacy of the randomization and allocation of intervention, as well as the quality of the data reported. For such, two methodological quality assessment tools were applied. The Critical Appraisal Skills Programme 29 checklist, concerning human studies, provided the sense of quality of each research and allowed us to estimate the usefulness of all of them. 30 The Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE)’s risk of bias tool which is an adaptation of Cochrane risk of bias tool, was applicable to animal studies. 31 Two authors applied the checklist independently and discrepancies were solved by discussion.
2.5. Outcomes
The two outcomes evaluated were: (a) the potential influence exerted by bacterial gut composition on the lipid‐lowering effect of statins, and (b) the role of statins in the gut bacterial environment modulation.
3. RESULTS
3.1. Bibliographic search and study selection
Figure 1 summarizes the selection strategy. The electronic database search retributed a total of 668 citations. After removing duplicates, 628 studies remained. In the screening stage, 609 studies were discarded since the analyses of the title and/or abstract revealed that those papers clearly did not meet the criteria: 400 did not study the gut microbiome and did not include statin‐treated subjects; seven did not study gut microbiome; seven did include statin‐treated subjects; 13 were review papers; 176 were book chapters; four were guidelines; one was a journal index; and one whose full text was not available. Nineteen studies were considered eligible for full‐text detailed examination. From those, nine did not meet the inclusion criteria as described: six did not evaluate the gut microbiome composition; two studied drug‐drug interaction between statin and another drug; and one was a review article. At the end, a total of 10 studies met the inclusion criteria and were included in the systematic review.
Figure 1.
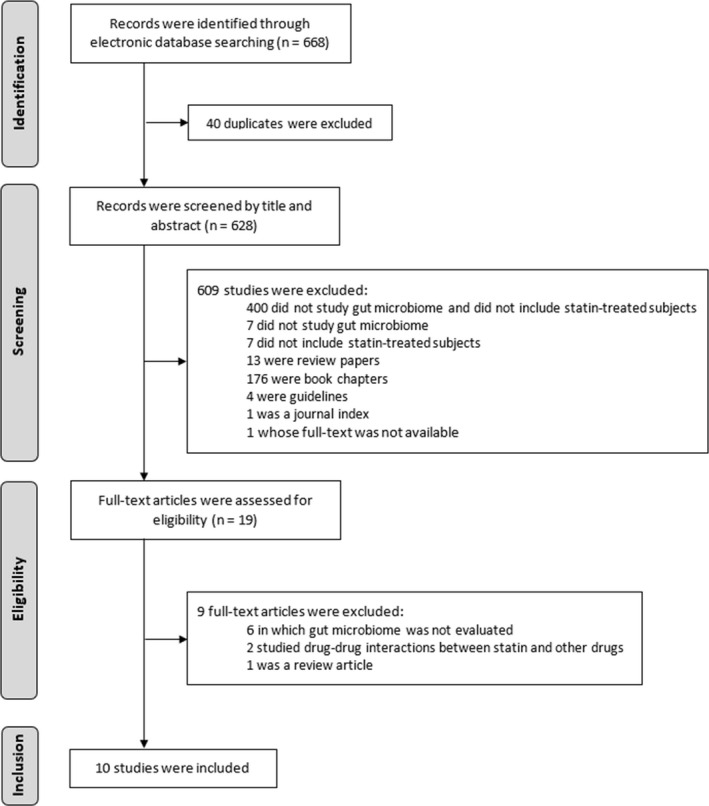
Flow diagram concerning article selection and data collection process
3.2. Studies’ characteristics
The 10 selected studies varied regarding the study design (the animal studies were all quasi‐experimental; one of the studies enrolling humans was cross‐sectional), studied population (human vs animal), country of origin and number of subjects enrolled. On the other hand, all studies are recent as they were published between 2015 and 2018. In order to simplify the article organization, primarily we present data belonging to the different outcomes separately, but also human and animal studies will be addressed apart within each outcome.
3.2.1. Human studies
From the three selected studies, two were conducted in China, with kindred study designs (quasi‐experimental; comparing only treated subjects, responders and poor responders). However, within those studies, there were peculiar differences regarding the number of subjects, statin agent and respective drug dose, and duration of treatment. The third included study was held in Saudi Arabia, a cross‐sectional study comparing three groups of subjects (healthy individuals, patients without treatment and patients treated). Additional information about these studies is illustrated in Tables 1 and 2.
Table 1.
Summary of included studies referring to the influence of gut microbiome on statins lipid‐lowering effect (Outcome 1)
| Study details (Author, year, country) |
Sample Characteristics (n) |
Treatment ‐Drug ‐dose/day ‐via of admin. ‐duration |
Study design | Sequencing method | Results | Author's conclusion | |
|---|---|---|---|---|---|---|---|
| Taxonomy | Diversity | ||||||
| Human studies | |||||||
| Sun et al, 2018, China 35 | Men, with newly diagnosed hypercholesterolemia (202) |
Atorvastatin, 20mg, Oral, 12 wk |
»Blood LDLc was measured at wk 0, 4 and 12. »Fecal samples collected at week 0. »At the end of treatment, subjects divided in two groups based on their LDLc levels: group SS < 100 mg/dL; group SR ≥ 100 mg/dL. |
Amplification by PCR of the V3‐V4 regions of 16S rRNA gene. |
Phylum »group SS: higher proportions of Firmicutes Genus »group SS: higher proportions of Lactobacillus, Eubacterium, Faecalibacterium and Bifidobacterium.»group SR: higher proportions of Clostridium |
Group SS: greater bacterial diversity than SR group. | Higher GM diversity is associated with statin sensitive response. |
| Liu et al, 2018, China 36 | Men (45) and women (19), with primary hypercholesterolemia b |
Rosuvastatin, 10mg, Oral, 4 to 8 wk |
»Blood LDLc, HDLc, TG and Tc were measured at wk 0, 4 and 8. »Fecal samples collected at wk 0. »Subjects were divided in two groups based on their BLL: group 1 if BLL dropped to normal levels from wk 4; group 2 if BLL still above normal levels after 8 wk. |
Amplification by PCR of the V3‐V4 regions of 16S rRNA gene. |
Phylum »group 1: higher proportions of Firmicutes »group 2: increased proportions of Actinobacteria Family »group 1: higher proportions of Ruminococcaceae, Lactobacillaceae and Bifidobacteriaceae »group 2: increased proportions of Bacteroidaceae, Pseudomonaceae, Streptococcaceae, Enterobacteriaceae, and other endotoxin‐producing bacteria |
Alpha diversity»group I: higher diversity than group II. Beta diversity both groups presented a much different GM composition. |
Higher diversity in GM composition was linked to RSV hypolipidemic effect. |
| Animal studies | |||||||
| He et al, 2018, China 38 | Male C57BL/6J mice (60) |
Simvastatin, 20 mg Kg‐1 BW, Oral, 4 wk |
»Random selection of control group (n = 7) and intervention group (n = 53). »Control group: fed with NCD; intervention group fed with HFD; both for 8 wk. »Then intervention group was further divided into 4 different treatment groups: HFD (n = 8); AB (n = 10), SV (n = 13), and AB_SV (n = 12). *HFD group was given high fat diet, for 4 wk *AB and AB_SV groups received imipenem/cilastatin 100 mg/Kg id, for 4 wk *AB_SV and SV groups received simvastatin, for 4 wk »Blood LDLc, HDLc, TG and Tc, and cecal samples were collected at wk 4. |
Amplification by PCR of the V3‐V4 regions of 16S rRNA gene. |
Phylum »groups AB and AB_SV: higher proportions of Proteobacteria than other 3 groups (control, HFD and SV). »groups AB and AB_SV: decreased proportions of Bacteroidetes and Firmicutes. |
Alpha diversity »data not shown Beta diversity »groups AB and AB_SV: distinct GM composition than the other 3 groups. |
Hypolipidemic effect of SV might be associated with some metabolic pathways which, in turn, could be impaired by GM modulation. |
| Wang et al, 2018, China 39 | Male SPF SD rats 45 |
Rosuvastatin, 10 mg Kg−1 BW, Oral, 4 wk |
»Random selection of AB_Statin group (n = 10), Statin group (n = 10), and Water group (n = 10). »Initially AB_Statin group received ceftriaxone (2gr/Kg BW bid) for 8 days to establish a dysbiotic rat model »Then AB_Statin and Statin groups were given rosuvastatin, for 4 wk »Water group was given saline solution 2id, for 4 wk »Blood LDLc, HDLc, TG and Tc were measured at wk 0, 2 and 4. »Fecal samples collected at wk 0, 2 and 4 wks. |
Amplification by PCR of the V3‐V4 regions of 16S rRNA gene. | AB_Statin group presented diminished levels of Lactobacillus and Bifidobacterium (genus) than Statin group |
Alpha diversity»AB_Statin group: reduction in bacterial diversity at the beginning compared with Statin and Water groups. At wk 4, no differences in GM diversity between the three groups were shown. Beta diversity AB_Statin group: much different GM composition, while Statin and Water groups had similarities. |
Lipid‐lowering ability of RSV can be affected by a disruptive intestinal flora. |
Abbreviations: AB, Antibiotic; AB_SV, Antibiotic + Simvastatin; BLL, blood lipid levels; BW, Body Weight; GM, Gut Microbiome; HDLc, High Density Lipoprotein cholesterol; HFD, High Fat Diet; LDLc, Low‐Density Lipoprotein cholesterol; NCD, Normal Chow Diet; PCR, Polymerase Chain Reaction; RSV, Rosuvastatin; SR, statin resistant; SS, statin sensible; SV, Simvastatin; TC, Total cholesterol; TG, Triglycerides.
Hypercholesterolemia was considered when serum LDLc ≥ 130 mg/dl.
Hypercholesterolemia was considered when serum LDLc ≥ 3.64 mmol/L (≈140 mg/dl), HDLc ≤ 3.64 mmol/L (≈140 mg/dl), TG ≥ 1.70 mmol/L (≈150 mg/dl), and TC ≥ 5.72 mmol/L (≈220 mg/dl)
Table 2.
Summary of included studies referring to the influence of statins in gut microbiome composition (Outcome 2)
| Study details (Author, year, country) |
Sample Characteristics (n) |
Treatment ‐Drug ‐dose/day ‐via of admin. ‐duration |
Study design | Sequencing method | Results | Author's conclusion | |
|---|---|---|---|---|---|---|---|
| Taxonomy | Diversity | ||||||
| Human studies | |||||||
| Khan et al, 2018, Saudi Arabia 37 | Men and women (42), with hypercholesterolemia |
Atorvastatin, 20 mg, Oral, n/a |
»the 42 subjects enrolled were divided into three groups: *HP (n = 15) statin‐naive *At‐HP (n = 27) treated with atorvastatin for the last 2 y *HS (n = 19) as the control group »Fecal samples collected at the evaluation point. |
Amplification by PCR of the V3‐V4 regions of 16S rRNA gene. |
Phylum »HP group: higher proportions of Proteobacteria »At‐HP: increased proportions of Firmicutes Family » HP group: increased proportions of Enterobacteriaceae and Prevotellaceae »At‐HP group: higher proportions of Ruminococcaceae and Verrucomicrobiaceae |
Alpha diversity »HS group: was the most diverse, followed by HP group, being the At‐HP the less diverse from the three groups. Beta diversity »HP group: presented a separate cluster, with the other two groups, HS and At‐HP, exhibiting similar taxa. |
Although less diverse, the treated group was able to shift the bacterial composition towards a more anti‐inflammatory GM environment. |
| Animal studies | |||||||
| Khan et al, 2018, Saudi Arabia 40 | Wistar rats, pathogen‐free (42) |
Atorvastatin, 5, 10, 15 e 20 mg Kg‐1 BW, Oral, 4 wk |
»Random division in two groups: *NCD (n = 12) and HFD (n = 30) for 5 wk »NCD group was further divided into two groups: *NCD control (n = 6) and NCD‐T (n = 6) with atorvastatin »HFD was also divided into five groups: *HFD control (n = 6), HFD‐T 5mg/Kg/d; HFD‐T 10mg/Kg/d, HFD‐T 15mg/Kg/d, or HFD‐T 20 mg/Kg/d, for 4 weeks »Blood LDLc, HDLc and Tc were measured at wk 0 and 4 »Cecal samples collected at wk 4. |
Amplification by PCR of the V3‐V4 regions of 16S rRNA gene | All groups were dominated by the phyla Firmicutes, Bacteroidetes and Proteobacteria. Atorvastatin promoted significantly the relative abundance of Proteobacteria and reduced the abundance of Firmicutes. |
Alpha diversity Statin‐treated HFD groups presented higher GM diversity. Beta diversity Statin‐treated HFD groups clustered together, showing very little differences between them; in addition, their composition tends to be closer to the NCD group. The untreated HFD group presented a more distinct diversity. |
A more careful analysis should be taken, regarding GM modifying effects, when prescribing a cholesterol lowering drug, as there might be implications for host health. |
| Caparrós‐Martín et al, 2017, Australia 41 | Female C57BL/6J wild‐type mice (30‐36) |
Atorvastatin, 10 mg Kg−1 BW, or Pravastatin 10 mg Kg−1 BW, Oral, 12 wk |
»Random division in two groups: *NCD and HFD »Then each group was divided into three minor groups: *one remained as a control with no intervention, other was given ATV, another PV, for 12 wk »mice were weighed, and blood collected, weekly »at the end of treatment blood and fecal samples were collected |
Amplification by PCR of the V3‐V4 regions of 16S rRNA gene | Predominant phyla in the NCD control group were Firmicutes and Bacteroidetes. Statin treatment triggered a large enrichment within the phylum Bacteroidetes and a marked reduction in the abundance of the phylum Firmicutes. |
Alpha diversity Statin‐treated NCD groups showed a lower diversity than the NCD, with greater difference after ATV treatment. Beta diversity the three groups (NCD, ATV treated NCD and PV treated NCD) presented distinctive GM patterns. No significant differences were spotted between HFD, ATV treated HFD and PV treated HFD, regarding alpha and beta diversities. |
It is possible, through distinct metabolic pathways, that statin therapy could be a driving force for some GM alterations. |
| Ryan et al, 2017, Ireland 42 | Male ApoEtm1Unc/J mice (35) |
Atorvastatin, 1‐5 mg Kg‐1 BW, Oral, 24 wk |
»Random division into three groups of interest: *NCD (n = 7) *HFD (n = 14) *HFD_At (n = 14) was given atorvastatin 1‐5 mg/Kg BW |
Amplification by PCR of the V3‐V4 regions of 16S rRNA gene | HFD_At treated group displayed an increase in Ruminococcus relative abundance (of ~ 50%) and revealed a significant decrease in Verrucomicrobia levels. | HFD_At had reduced diversity for phylogenetic whole tree. | Statin had not a major impact in GM composition and was not the most important intervention seeking a better cardiovascular and metabolic health. |
| Nolan et al, 2017, Ireland 43 | Female C57BL/6J mice (20) |
Rosuvastatin, 9,3 mg Kg−1 BW, Oral, 4 wk |
»Mice were fed an HFD for 2 wk prior to statin intake »a group of 10 mice was given sterile water with dissolved RSV 9,3 mg kg‐1 BW/d »other group of 10 mice were only administered untreated sterile water, for 4 wk. »blood, cecal and fecal samples at wk 4. |
Amplification by PCR of the V4‐V5 regions of 16S rRNA gene | In both groups, Firmicutes and Bacteroidetes were dominant. |
Alpha diversity Significant lower diversity in the RSV treated group in comparison with the untreated group. No significant differences between groups, in fecal samples analysis. Beta diversity Distinct clustering between groups was noted in GM of cecal samples, conversely to the fecal samples. |
RSV altered the GM in mice, but it is uncertain how statins influence the GM or if alterations are secondary to host responses. |
| Catry et al, 2015, Belgium 44 | Male C57BL/6J mice (29‐32) |
Simvastatin, 0,1% w/w or Ezetimibe 0,021% w/w, Oral, 1 wk |
»Mice were divided in four groups: *one was offered a control diet (DT); *another was offered the same diet supplemented with SV 0.1% w/w; *other the same diet with EZT 0.021% w/w; *and the last one with the same diet and a combination of SV and EZT. »Experiment last one week. »blood and fecal samples at wk 1. |
Amplification by PCR of the V3‐V4 regions of 16S rRNA gene |
Bacteroides‐Prevotella spp. and Roseburia spp., measured in the caecal content did not differ between groups. Also, Bifidobacteria level was not modified by the statin treatment |
Abundance of the total bacteria measured in cecal content was not affected by any treatment. | EZT alone affected GM composition in favor of Lactobacillus spp.. |
Abbreviations: At‐HP, atorvastatin‐treated hypercholesterolemic patients; ATV, Atorvastatin; HFD, High Fat Diet; HFD‐At, High Fat Diet + Atorvastatin; HFD‐T, High Fat Diet treated patient; HP, hypercholesterolemic patients; HS, healthy subjects; n/a, nonavailable; NCD, Normal Chow Diet; NCD‐T, Normal Chow Diet‐treated patient; PV, Pravastatin; RSV, Rosuvastatin; w/w, weight for weight.
3.2.2. Animal studies
From the seven studies selected, two were performed in rats and five in mice. As for the region, three took place in Asia (two in China and one in Saudi Arabia), three were carried out in Europe (two in Ireland and one in Belgium) and the last one took place in Australia. The number of animals studied widened between 20 and 60, per study. Also, four different type of statin were used, namely simvastatin, rosuvastatin, atorvastatin or pravastatin. Furthermore, the duration of treatment had significant discrepancies among studies, since it varied between 28 days to 24 weeks. Description of the details can be found in Tables 1 and 2.
3.3. Assessment of the risk of bias
Regarding the studies enrolling humans, all of them failed at reporting a good methodology in what concerns the recruitment strategy. The inclusion criteria were well defined in the three studies, but no description was provided on how the contact with the subjects was made, which may lead to selection bias. However, the study conducted by Liu et al consecutively enrolled subjects, thus minimizing selection bias. In this type of sampling, every eligible case for a time period is enrolled, ie, during enrolment period, all potential participants had 100% chance of being sampled. Therefore, there is a higher probability that the resulting sample better represents the target population. In Saudi Arabian study (Khan et al) there could be a memory bias, as the enrolled subjects had to fill a questionnaire reminding their own disease status and lifestyle factors. Only one study (Sun et al) failed to address ethical considerations. Apparently, the data obtained in each study are presented in a rigorous manner yet, globally, less information is available on the Discussion regarding the limitations of each study (all studies only display arguments favouring findings, and none against). Altogether, even though these studies point out interesting information, some methodological faults hamper the translation of such data into other settings. Regarding animal studies, in each study, the comparisons were apparently made between animals that shared the same characteristics. Indeed, the authors performed proper allocation concealment and randomization before grouping the animals in the different arms of comparison. The animal housing conditions were addressed in all studies but one (Wang et al 2018). Nevertheless, performance bias may be present, as none of the studies describe blindness of the caregivers and researchers, from knowing which intervention each animal received. Concerning outcome assessment, all animals were studied, but, again, these studies failed in blinding the assessors, so detection bias could be on the verge here. No incomplete outcome data were reported. From the seven animal studies selected, there were two where funding information was not provided (Khan et al, and Catry et al).
3.4. Summary of results
As we pointed before, due to the limited evidence concerning the topic under analysis, we had to include studies enrolling humans but also those performed in murine models. This aspect hampered direct comparisons, as mouse models cannot fully reproduce human systems, different mouse models could diverge in gut microbiome composition, and the gut microbiome and host interplay is host specific. In addition, multiple human background factors, such as diet or medical history, contribute to shape human gut microbiome, therefore their absence in mouse models cannot mimic a “real‐life” gut environment). In spite of that, there are several positive features of studies including animals that must be taken into account. Indeed, animal studies are normally faster, easier and cheaper to conduct. 32 Also, murine models allow experimental manipulations of the intestinal microbiome, thus allowing to evaluate different types of interactions established by gut microbes (for example: host, xenobiotics, diet, etc), thereby providing more insights about pathological mechanisms of certain diseases as well as pathways of drug metabolism. In this regard, one of the possibilities is the modulation of the intestinal bacterial flora of mouse models through antibiotics. This unbalanced microbiological environment known as dysbiosis, 33 is widely used in the preclinical investigation of numerous pathologies, both intestinal and extraintestinal, such as inflammatory bowel disease, celiac disease, metabolic diseases, central nervous system‐related disorders, among others. 34 Therefore, the use of these models that mimic the disrupted intestinal flora of the diseases previously mentioned, represents a fundamental step also in the search for suitable therapies.
Like the majority of investigations on the taxonomic field, all studies but two (Sun et al and Catry et al) addressed the alpha‐ and beta‐diversity concepts. Alpha‐diversity refers to the diversity within an ecosystem and is usually expressed by the number of species (ie, species richness) in that ecosystem. On the other hand, beta‐diversity consists in the examination of differences in species diversity between two or more ecosystems. Simplifying, is when we measure the total number of species that are unique to each of the ecosystems under comparison. The results of the diversity analysis are depicted in Tables 1 and 2.
3.4.1. Influence of bacterial gut composition on the efficacy of statins
Human studies
The two analyzed studies, conducted by Sun et al and Liu et al were relatively similar and obtained comparable results: essentially, the individuals who had a more satisfactory response presented higher levels of microorganisms belonging to the Firmicutes phylum and increased proportions of Lactobacillus and Bifidobacterium genera. In both studies, the higher gut microbiome diversity was associated to a better response to statin treatment, thereby suggesting a potential role of intestinal flora in the statin action.
Animal studies
The study performed by He et al compared statin response in a dysbiotic mouse‐model with that of a nondysbiotic model. Initially, authors confirmed that the statins’ hypolipidemic effect was diminished in the dysbiosis environment. Then they observed that statins alone exert several beneficial actions among which: stimulating expression of hepatic proteins that function as regulators in the bile acid synthesis (Cholesterol 7 alpha‐hydroxylase‐Cyp7a1; 25‐hydroxycholesterol 7‐alpha‐hydroxylase‐Cyp7b1; and farnesoid X receptor‐FXR); doubling the concentration of lithocholic acid, or decreasing the levels of phospholipids. Notwithstanding, when statin was administered concomitantly with an antibiotic all those actions were impaired or even lost, meaning that intestinal bacterial composition could have a major relevance in the statin pro‐bono activity.
In a similar way, Wang et al, tried to establish a dysbiotic environment but prior to statin intake and not concomitantly. In this work it was also shown that antibiotic treatment dramatically decreased bacterial gut diversity. Because of the study design, it was possible to compare, in three different timings, how statins influence the cholesterol levels, simultaneously in a dysbiotic rat model and in a nondysbiotic rat. With no surprise, the cholesterol levels at week zero were higher, in the statin group previously treated with antibiotic (AB+Statin), compared with statin alone and water control groups. At week two, the cholesterol levels were still elevated in the AB+Statin group in comparison with the other two groups, but at this point the water group also had higher cholesterol levels than statin alone group. However, at week 4, the cholesterol levels from the AB+Statin and statin monotherapy groups were evaluated, being lower than the water group. It was demonstrated that in the presence of a decreased gut microbial diversity the cholesterol‐lowering effect of statin was reduced. However, as time passed, the communities were replenished, and statin hypolipidemic ability reappeared.
3.4.2. Role of statins in the gut bacterial composition modulation
Human studies
Only one study, from Khan et al, intended to investigate how statins could impact gut microbiome composition. It was observed that the hypercholesterolemic‐treated group inclined to an antiinflammatory profile, with increased proportions of Akkermansia muciniphila and Faecalibacterium prausnitzii. In contrast with the untreated hypercholesterolemic group that presented higher relative abundance of proinflammatory bacteria, such as Proteobacteria, Enterobacteriaceae and Desulfovibrio spp. Notwithstanding the fact that statin‐treated group showed a less diverse bacterial composition, they managed to restore a healthier profile, even comparable to the group of healthy subjects, demonstrating that statins could, in fact, be a driving force to gut microbiome modulation.
Animal studies
Five studies aimed to evaluate how statins could influence the gut bacterial microbiome composition. Khan et al obtained some contrasting results. In the Normal Chow Diet (NCD)‐treated group there was an increased level of Desulfovibrio, an endotoxin‐producing bacterium, and increased levels of Lactobacillus. The treated High Fat Diet (HFD) group presented higher relative abundance of Bacteroides and Parabacteroides genera. Also, in the HFD group, there was a rise in Oscillospira and a drop in Clostridium levels, with the curiosity of both being butyrate‐producing genera. In this case, drug‐specific effects could be produced over certain bacteria populations, without a clear predictable response on the host health. In parallel, Caparrós‐Martín et al, found that cholesterol levels were not modified by statin treatment. In addition, it was found to be an enrichment of Bacteroidetes over Firmicutes. Curiously, both pravastatin and atorvastatin, showed a reduced hepatic expression of an enzyme involved in the bile acid synthesis cascade (Cholesterol 7 alpha‐hydroxylase – Cyp7a1), but only atorvastatin induced an increase of sterol 27‐hydroxylase – Cyp27a1. The possibility of gut microbiome composition being altered, after, statin intake, is not clear. In the study performed by Nolan et al, there were distinct patterns, of cecal and faecal microbial populations, between the rosuvastatin‐treated and the untreated groups. Nevertheless, it was unclear after the conclusion of the study, if changes in gut microbial communities were due to direct action of statins or to other types of host response. Another study carried by Catry et al, showed abundance of Bacteroides‐Prevotella spp and Roseburia spp, which did not differ between groups. Also, Bifidobacterium level was not modified by the statin treatment, but a significant drop was observed in ezetimibe‐treated mice. Another work, conducted by Ryan et al, showed that statin intake had little effect in gut microbiome composition, except for the increased proportion of Ruminococcus, when compared with HFD animals. Despite this fact, change was not significant compared with other dietary interventions.
4. DISCUSSION
Statins are widely used as lipid‐lowering drugs in the context of cardiovascular disease, either in primary or in secondary prevention. From a clinical point of view, there is strong evidence that this family of drugs has many advantages with only a slightly percentage of adverse events. 46 In recent years, investigation around gut microbiome and cardiovascular disease has been growing. Therefore, this review aimed to systematize the evidence available on the literature concerning the potential influence of the bacterial intestinal flora on statin hypolipidemic effect as well as whether there was any statin‐induced modulation of gut microbiome composition.
4.1. Influence of gut microbiome on statins lipid‐lowering effect
Four studies, two including animals and two enrolling humans, tried to establish an association between gut microbiome and the therapeutic effect of statins. Within human studies, Sun et al analyzed fecal (collected before treatment) and blood (after 3 months of treatment) specimens, and showed that patients with good response to statins (LDLc ≤ 100mg/dL) presented higher bacterial diversity, with increased levels of known gut commensals (Lactobacillus and Bifidobacterium) and genera recognized to decrease cholesterol levels (Faecalibacterium and Eubacterium). The cholesterol lowering advantageous effects of these bacterial genera are well known. There are many potential mechanisms by which gut commensals contribute to the hypolipidemic activity of this class of drugs. 47 Similarly, Liu et al, found that “good responders” (LDLc drop of 58.5%) presented increased diversity with higher proportions of Firmicutes phylum and other commensal bacteria. Bacteria belonging to the Firmicutes phylum have the ability to participate in metabolic process of compounds with antidiabetic and antiobesity properties. 48 Although the beneficial effects reported in these two studies, it is unclear how does this directly influence statins’ treatment. Probably, it may be due to a broader interaction of bacterial microbiome together with host cells properties in the way of changing statin‐metabolizing enzymes and modulation of the drug receptors. The study by Liu et al excluded patients that had received antibiotics within three months; while Sun et al did not take that aspect into consideration. None of these studies provided information regarding the use of concomitant medication and/or supplements that may have influenced gut microbiome composition or function, as well as regarding patients’ dietary, exercise or smoking habits, consumption of probiotics or prebiotics.
Regarding animal studies, He et al, described that simvastatin, when taken alone (without antibiotic), possesses a lipid‐lowering effect. In gut dysbiosis scenario, secondary to antibiotic treatment, the hypolipidemic effect of simvastatin was altered. This might happen for various reasons: (a) simvastatin is metabolized by anaerobic bacteria, whose composition could be altered in the presence of antibiotic, 49 (b) simvastatin stimulates expression of proteins that are responsible for bile acid synthesis, which in turn are impaired in the presence of gut dysbiosis, 50 (c) Clostridium derived lithocholic acid, which is considered as a marker of good response to statin treatment, was almost doubled in the simvastatin‐treated group but with significantly lower concentration in the antibiotic treated groups. 51 However, to date, there is not enough evidence relating the action of these bile acid homeostatic proteins and its relationship with the hypolipidemic effect of statins and how these are influenced by bacterial intestinal microbiome. In the same direction, Wang et al, demonstrated that when the mice were submitted to antibiotic treatment prior to statin start, they presented a higher lipid profile in comparison to mice receiving statin in monotherapy, after two weeks of therapy. However, four weeks after, there were no differences in the cholesterol levels between the two groups. Explaining these discrepancies was the bacterial composition found in the two groups, that were in favor of Lactobacillus and Bifidobacterium genera in the statin alone group, but antibiotic imbalance, over time diminished and the previous composition was reestablished. In the literature, Lactobacillus and Bifidobacterium, are listed as potential regulators of some cytokines (tumour necrosis factor‐alpha [TNF‐α] and interleukin‐6 [IL6]), 52 which in turn are known to be also regulators of one specific rosuvastatin transporter, the organic anion transporting polypeptide 1B1 (OATP1B1). 53 Impaired proportion of these bacteria could lead to transportation defects of rosuvastatin.
4.2. Influence of statins in gut microbiome composition
The only human‐based work that targeted the above outcome, Khan et al, tried to establish association between statin intake and bacterial gut microbiome modulation. Not surprisingly, it is difficult, if not impossible, to infer any kind of cause‐effect in a cross‐sectional study. We do not know how the bacterial composition before treatment was. More even, they relied subjects’ responses to questionnaires, about lifestyle habits and other risk factors, which could easily be a cofounding factor to the results. They found that atorvastatin‐treated subjects presented an antiinflammatory gut bacteria profile, mainly increased proportions of A muciniphila and F prausnitzii, while in the hypercholesterolemic group, an opposite trend developed, essentially, more in favor of proinflammatory bacteria, including Proteobacteria, Enterobacteriaceae and Desulfovibrio sp. 54 It has been reported that A muciniphila, in mice with HFD‐induced obesity, was not correlated to inflammatory markers, lipid synthesis and adiposity. 55 Concomitantly, F prausnitzii have the ability to decrease TNF‐α and IL12 (proinflammatory) and increase IL10 (antiinflammatory) cytokines. 56
Concerning animal studies, Khan et al, discovered a differential modulation in gut bacterial community, either in HFD and NCD, under the influence of atorvastatin. The hyperlipidic diet decreased diversity in intestinal bacterial populations, but during statin treatment the gut microbiome shifted towards a similar NCD environment. We emphasize the higher relative abundances of Bacteroides and Parabacteroides genera in atorvastatin HFD groups. Bacteroides is linked with bile acid metabolism promoting deconjugation of taurine‐conjugated bile acids in the serum. 57 Parabacteroides genus, which contains antiinflammatory bacteria, showed consistent increase in abundance with different doses of atorvastatin. 58 In contrast with all other studies, Caparrós‐Martín et al, reported that cholesterol levels were not modified after statin treatment. They also reported a lower diversity after statin treatment, with major taxa being Bacteroidales S24.7 and with reduced levels of Firmicutes phylum. Additionally, butyric acid production was impaired, probably indicating a functional dysbiosis in the gut. Bile acid pool was amplified, suggesting also, a deregulation in the synthesis and/or transport of these metabolites. Behind these alterations could be the pregnane x receptor, which is a nuclear receptor, mostly found in the liver and intestines, responsible for the metabolism of a multitude of substances from xenobiotics to bile acids. 59 , 60 Nolan et al, underline that rosuvastatin treatment also have diminished levels of several inflammation markers as well increased expression of genes responsible for regulating locally the gut bacterial composition, such as cathelicidin antimicrobial peptide (CAMP), nitric oxide synthase 2‐inducible (iNOS2), and mucin 2 (Muc2), thus potentially affecting bacterial gut composition. 61 It is still unclear though, how statins, direct or indirectly, influence the intestinal bacterial communities. One last study conducted by Catry et al, stated that ezetimibe alone, rather in combination with simvastatin, does significantly affect the composition in favor of Lactobacillus species. Which is in favor, as it was already demonstrated, of plasma cholesterol reduction levels, mainly by three mechanisms: (i) by assimilation, thus impairing intestinal absorption, (ii) by bacterial surface binding, and (iii) by incorporation to bacterial membranes. 62 , 63
Ryan et al, were also not able to detect any significant influential role of statins over gut microbiome composition. The authors compared several cardiovascular disease nutritional interventions available on the market with atorvastatin intake regarding host metabolomic and gut microbiome composition modulation. After a thorough analysis, we confirmed similar methodologic approach concerning DNA sequencing. The major differences concern the duration of the intervention, lasting 24 weeks, 2‐fold higher than the second longest study selected. Still about this second outcome, a recent review paper (Ko et al, 2017) analyzed the antimicrobial activity of statins against gram‐positive and gram‐negative agents. In this study, in general, simvastatin presented the most potent antimicrobial activity (lowest minimum inhibitory concentration), followed by atorvastatin, then rosuvastatin and finally fluvastatin. But even more interesting, based exclusively in in vitro experiments, is the idea that statins exert their modulation on bacterial communities, not only by inhibition of HMG‐CoA reductase and alteration in the bile acid pool, but also directly, by disrupting architecture of the bacterial membrane, through some specific features of their chemical structure. 64
Before we initiated the submission process, we made one last search, to verify if there were new works in the field. Interestingly, we were confronted by a review article (Ashrafizadeh and Ahmadi, 2019) with the objective of reviewing the interactions between statins and gut microbiota. After detailed analysis we realized that it was not a systematic review, they only focused on the influence of statins over gut microbiome, and no real discussion was made on the possible reasons for the inferences assumed by them. 65
4.3. Limitations
The evidence available regarding the possible correlation between statins and gut microbiome is very scarce and only 10 studies fulfilled our search criteria. Few quantitative data were available limiting the use of meta‐analysis. Among the included studies, three were conducted in human subjects and seven in mice models. This is an important limitation, because comparisons are difficult to perform and the number of studies is too small to allow broader generalizations. Also, all studies presented, at least, one type of bias, thus weakening potential inferences. Differences existed also regarding the study design. The animal studies were conducted in a laboratory‐controlled environment, but still there were discrepancies, in duration of treatment, type and dose of statin used, number of subjects enrolled, arms of treatment and controls, and timing of collected samples, making analogies impossible. Regarding the human‐based studies, one (Khan et al) was an observational cross‐sectional, conducted in Saudi Arabia, while the remaining two were quasi‐experimental Chinese studies, relatively similar despite some differences in number of subjects, statin used (type and dose) and duration of treatment.
Other limitations that must be taken into consideration are that the differences in microbial relative abundances may be an artifact of a corresponding increase/decrease in another organism and different practices of fecal samples collection and processing may explain differences observed amongst studies. In addition, the effect of confounding variables (patient characteristics and lifestyle factors) in the gut microbiome cannot be neglected.
Therefore, in order to better understand the reciprocal relationship between gut microbiome and statins, further studies with more homogenous designs and methodologies must be developed. Particular attention must be paid to the accuracy in defining the dysbiotic environment (overall diversity or relative proportion of microbial communities?) in studies enrolling humans and even more in those conducted in animals, since several dysbiosis models exist and the manipulation of the gut microbiome is undeniably easier in this setting. Few studies were conducted in humans, making it difficult to understand the possible cofounding influence of diet, lifestyle, concomitant medications, diseases, among others. Last but not the least, the comparison of different types of statins, in different doses and treatment durations is also a topic to be investigated, in order to determine which changes are common to the class and those that are drug specific.
5. CONCLUSION
Taken together, in almost all studies there were variations in gut microbiome diversity after statin intake, and at the same time, the lipid‐lowering effect of statins were compromised in the presence of intestinal bacterial dysbiosis. Despite that, to date, there is not enough information to personalize the choice of hyperlipidaemia's treatment based on gut microbiome composition.
This is the first systematic revision on this topic, confirming the lack of evidence available. Therefore, further research is needed to understand the correlation between statins and the bacterial gut microbiome. Indeed, with modern technologies and increasing knowledge in distinct areas of science, from genomics to metabolomics, and constant pressure to develop new targets for therapies, excellent discoveries could blossom in a short‐medium term, allowing a more personalized use of the current therapeutic options.
6. Funding Informatiom
The authors did not receive external funding for this review article.
DISCLOSURES
All authors have no conflict of interest to disclose.
AUTHORS' CONTRIBUTIONS
GC and AMD were involved in the acquisition, analysis, interpretation of data and drafting of the manuscript; MME, RV, LF, and MRC helped with data interpretation, manuscript drafting and medical writing assistance; and FM was involved in the study concept and design, data interpretation, and manuscript drafting. All authors read and approved the final manuscript.
Dias AM, Cordeiro G, Estevinho MM, et al; on behalf of the Clinical Pharmacology Unit, São João Hospital University Centre . Gut bacterial microbiome composition and statin intake ‐ A systematic review. Pharmacol Res Perspect. 2020;8:e00601 10.1002/prp2.601
REFERENCES
- 1. Young VB. The role of the microbiome in human health and disease: an introduction for clinicians. BMJ. 2017;356:j831. [DOI] [PubMed] [Google Scholar]
- 2. Amon P, Sanderson I. What is the microbiome? Archives of disease in childhood ‐ Education & practice edition. 2017;102(5):257‐260. [DOI] [PubMed] [Google Scholar]
- 3. Barko PC, McMichael MA, Swanson KS, et al. The gastrointestinal microbiome: a Review. J Vet Intern Med. 2018;32(1):9‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu HJ, Wu E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes. 2012;3(1):4‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fu J, Bonder MJ, Cenit MC, et al. The gut microbiome contributes to a substantial proportion of the variation in blood lipids. Circ Res. 2015;117(9):817‐824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ianiro G, Bibbo S, Gasbarrini A, et al. Therapeutic modulation of gut microbiota: current clinical applications and future perspectives. Curr Drug Targets. 2014;15(8):762‐770. [DOI] [PubMed] [Google Scholar]
- 7. Wilson ID, Nicholson JK. The role of gut microbiota in drug response. Curr Pharm Des. 2009;15(13):1519‐1523. [DOI] [PubMed] [Google Scholar]
- 8. Swanson HI. Drug metabolism by the host and gut microbiota: a partnership or Rivalry? Drug Metab Dispos. 2015;43(10):1499‐1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS guidelines for the management of Dyslipidaemias. Eur Heart J. 2016;37(39):2999‐3058. [DOI] [PubMed] [Google Scholar]
- 10. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082‐e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davies JT, Delfino SF, Feinberg CE, et al. Current and emerging uses of statins in clinical therapeutics: a review. Lipid Insights. 2016;9:13‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Costa S, Reina‐Couto M, Albino‐Teixeira A, Sousa T. António Albino‐Teixeira, Teresa Sousa, Estatinas e stresse oxidativo na insuficiência cardíaca crónica . Revista Portuguesa de Cardiologia (Portuguese Journal of Cardiology). 2016;35(1):41‐57. [DOI] [PubMed] [Google Scholar]
- 13. Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2005;45:89‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abdelmaksoud AA, Girerd PH, Garcia EM, et al. Association between statin use, the vaginal microbiome, and Gardnerella vaginalis vaginolysin‐mediated cytotoxicity. PLoS ONE. 2017;12(8):e0183765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boekholdt SM, Hovingh GK, Mora S, et al. Very low levels of atherogenic lipoproteins and the risk for cardiovascular events: a meta‐analysis of statin trials. J Am Coll Cardiol. 2014;64(5):485‐494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oesterle A, Laufs U, Liao JK. Pleiotropic effects of statins on the cardiovascular system. Circ Res. 2017;120(1):229‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lahera V, et al. Endothelial dysfunction, oxidative stress and inflammation in atherosclerosis: beneficial effects of statins. Curr Med Chem. 2007;14(2):243‐248. [DOI] [PubMed] [Google Scholar]
- 18. Blum A, Shamburek R. The pleiotropic effects of statins on endothelial function, vascular inflammation, immunomodulation and thrombogenesis. Atherosclerosis. 2009;203(2):325‐330. [DOI] [PubMed] [Google Scholar]
- 19. Thurnher M, Nussbaumer O, Gruenbacher G. Novel aspects of mevalonate pathway inhibitors as antitumor agents. Clin Cancer Res. 2012;18(13):3524‐3531. [DOI] [PubMed] [Google Scholar]
- 20. Greenwood J, Steinman L, Zamvil SS. Statin therapy and autoimmune disease: from protein prenylation to immunomodulation. Nat Rev Immunol. 2006;6(5):358‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hennessy E, Adams C, Reen FJ, O'Gara F. Is there potential for repurposing statins as novel antimicrobials? Antimicrob Agents Chemother. 2016;60(9):5111‐5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smit J. Community‐acquired Staphylococcus aureus bacteremia: Studies of risk and prognosis with special attention to diabetes mellitus and chronic heart failure. Dan Med J. 2017;64(5). [PubMed] [Google Scholar]
- 23. Yoo D‐H, Kim IS, Van Le TK, et al. Gut microbiota‐mediated drug interactions between lovastatin and antibiotics. Drug Metab Dispos. 2014;42(9):1508‐1513. [DOI] [PubMed] [Google Scholar]
- 24. Kaddurah‐Daouk R, Baillie RA, Zhu H, et al. Enteric microbiome metabolites correlate with response to simvastatin treatment. PLoS ONE. 2011;6(10):e25482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sharma A, Buschmann MM, Gilbert JA. Pharmacomicrobiomics: the holy grail to variability in drug response? Clin Pharmacol Ther. 2019;106(2):317‐328. [DOI] [PubMed] [Google Scholar]
- 26. Doestzada M, Vila AV, Zhernakova A,, et al. Pharmacomicrobiomics: a novel route towards personalized medicine? Protein Cell. 2018;9(5):432‐445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Int J Surg. 2010;8(5):336‐341. [DOI] [PubMed] [Google Scholar]
- 28. Higgins JPT, GSe. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, 2011, [updated March 2011].
- 29. Wood AR, Esko T, Yang J, et al. Defining the role of common variation in the genomic and biological architecture of adult human height. Nat Genet. 2014;46(11):1173‐1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Programme C.A.S . CASP Qualitative Checklist. 2018.
- 31. Hooijmans CR, Rovers MM, De Vries RB, Leenaars M, Ritskes‐Hoitinga M, Langendam MW. SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nguyen TL, Vieira‐Silva S, Liston A, Raes J. How informative is the mouse for human gut microbiota research? Dis Model Mech. 2015;8(1):1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tamboli CP, Neut C, Desreumaux P, Colombel JF. Dysbiosis in inflammatory bowel disease. Gut. 2004;53(1):1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kennedy EA, King KY, Baldridge MT. Mouse microbiota models: comparing germ‐free mice and antibiotics treatment as tools for modifying gut bacteria. Front Physiol. 2018;9:1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sun B, Li L, Zhou X. Comparative analysis of the gut microbiota in distinct statin response patients in East China. J Microbiol. 2018;56(12):886‐892. [DOI] [PubMed] [Google Scholar]
- 36. Liu Y, Song X, Zhou H, et al. Gut microbiome associates with lipid‐lowering effect of rosuvastatin in vivo. Front Microbiol. 2018;9:530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Khan TJ, Ahmed YM, Zamzami MA, et al. Atorvastatin treatment modulates the gut microbiota of the hypercholesterolemic patients. OMICS. 2018;22(2):154‐163. [DOI] [PubMed] [Google Scholar]
- 38. He X, Zheng N, He J, et al. Gut microbiota modulation attenuated the hypolipidemic effect of simvastatin in high‐fat/cholesterol‐diet fed mice. J Proteome Res. 2017;16(5):1900‐1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang L, Wang Y, Wang H, et al. The influence of the intestinal microflora to the efficacy of Rosuvastatin. Lipids Health Dis. 2018;17(1):151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Khan TJ, Ahmed YM, Zamzami MA, et al. Effect of atorvastatin on the gut microbiota of high fat diet‐induced hypercholesterolemic rats. Nature/Sci Rep. 2018; 8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Caparrós‐Martín JA, Lareu RR, Ramsay JP, et al. Statin therapy causes gut dysbiosis in mice through a PXR‐dependent mechanism. Microbiome. 2017;5(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ryan PM, London L, Bjorndah TC, et al. Microbiome and metabolome modifying effects of several cardiovascular disease interventions in apo‐E neg neg mice. Microbiome. 2017;5(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nolan JA, Skuse P, Govindarajan K, et al. The influence of rosuvastatin on the gastrointestinal microbiota and host gene expression profiles. Am J Physiol Gastrointest Liver Physiol. 2017;312(5):G488‐G497 [DOI] [PubMed] [Google Scholar]
- 44. Catry E, Pachikian BD, Salazar N, et al. Ezetimibe and simvastatin modulate gut microbiota and expression of genes related to cholesterol metabolism. Life Sci. 2015;132:77‐84. [DOI] [PubMed] [Google Scholar]
- 45. Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab‐paclitaxel in advanced triple‐negative breast cancer. N Engl J Med. 2018;379(22):2108‐2121. [DOI] [PubMed] [Google Scholar]
- 46. Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388(10059):2532‐2561. [DOI] [PubMed] [Google Scholar]
- 47. Reis SA, Conceição LL, Rosa DD, et al. Mechanisms responsible for the hypocholesterolaemic effect of regular consumption of probiotics. Nutr Res Rev. 2017;30(1):36‐49. [DOI] [PubMed] [Google Scholar]
- 48. Eid HM, et al. Significance of microbiota in obesity and metabolic diseases and the modulatory potential by medicinal plant and food ingredients. Front Pharmacol. 2017;8:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Aura A‐M, Mattila I, Hyötyläinen T, et al. Drug metabolome of the simvastatin formed by human intestinal microbiota in vitro. Mol Biosyst. 2011;7(2):437‐446. [DOI] [PubMed] [Google Scholar]
- 50. Claudel T, Staels B, Kuipers F. The Farnesoid X receptor: a molecular link between bile acid and lipid and glucose metabolism. Arterioscler Thromb Vasc Biol. 2005;25(10):2020‐2030. [DOI] [PubMed] [Google Scholar]
- 51. Houten SM, Watanabe M, Auwerx J. Endocrine functions of bile acids. EMBO J. 2006;25(7):1419‐1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Veiga P, Juste C, Lepercq P, et al. Correlation between faecal microbial community structure and cholesterol‐to‐coprostanol conversion in the human gut. FEMS Microbiol Lett. 2005;242(1):81‐86. [DOI] [PubMed] [Google Scholar]
- 53. Le Vee M, Lecureur V, Stieger B, Fardel O. Regulation of drug transporter expression in human hepatocytes exposed to the proinflammatory cytokines tumor necrosis factor‐alpha or interleukin‐6. Drug Metab Dispos. 2009;37(3):685‐693. [DOI] [PubMed] [Google Scholar]
- 54. Yin J, Liao S‐X, He Y, et al. Dysbiosis of gut microbiota with reduced Trimethylamine‐N‐Oxide level in patients with large‐artery atherosclerotic stroke or transient ischemic attack. J Am Heart Assoc. 2015;4(11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schneeberger M, Everard A, Gómez‐Valadés AG, et al. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci Rep. 2015;5:16643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sokol H, Pigneur B, Watterlot L, et al. Faecalibacterium prausnitzii is an anti‐inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA. 2008;105(43):16731‐16736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Narushima S, Itoh K, Miyamoto Y, et al. Deoxycholic acid formation in gnotobiotic mice associated with human intestinal bacteria. Lipids. 2006;41(9):835‐843. [DOI] [PubMed] [Google Scholar]
- 58. Ishioka M, Miura K, Minami S, et al. Altered gut microbiota composition and immune response in experimental steatohepatitis mouse models. Dig Dis Sci. 2017;62(2):396‐406. [DOI] [PubMed] [Google Scholar]
- 59. Lehmann JM, McKee DD, Watson MA, et al. The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J Clin Invest. 1998;102(5):1016‐1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Giguere V. Orphan nuclear receptors: from gene to function. Endocr Rev. 1999;20(5):689‐725. [DOI] [PubMed] [Google Scholar]
- 61. Jager S, Stange EF, Wehkamp J. Antimicrobial peptides in gastrointestinal inflammation. Int J Inflam. 2010;2010:910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pereira DI, Gibson GR. Cholesterol assimilation by lactic acid bacteria and bifidobacteria isolated from the human gut. Appl Environ Microbiol. 2002;68(9):4689‐4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Song M, Park S, Lee H, et al. Effect of Lactobacillus acidophilus NS1 on plasma cholesterol levels in diet‐induced obese mice. J Dairy Sci. 2015;98(3):1492‐1501. [DOI] [PubMed] [Google Scholar]
- 64. Ko HHT, Lareu RR, Dix BR, et al. Statins: antimicrobial resistance breakers or makers? PeerJ. 2017;5:e3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ashrafizadeh M, Ahmadi Z. Effects of Statins on Gut Microbiota (Microbiome). Rev. Clin Med. 2019;6(2):55‐59. [Google Scholar]


