Abstract
In late December 2019, China reported cases of respiratory illness in humans that involved a novel coronavirus SARS‐CoV‐2. On March 20, 2020, the first coronavirus disease 2019 (COVID‐19) in Brazil was diagnosed, and by now, we present the report on the first case of COVID among transplant recipients in our country. A liver and kidney transplant patient with SARS‐CoV‐2 pneumonia without respiratory failure was treated in a clinical multimodal strategy consisting of symptomatic support therapy, immunosuppression reduction, use of anti‐coronavirus drugs and heparin leading to a progressive improvement of patient symptoms till discharge. The authors also present a comprehensive review of published cases.
Keywords: COVID‐19, heparin, hydroxychloroquine and azithromycin, infectious diseases, kidney and liver transplantation, nonpathogen‐specifc immune parameters
1. INTRODUCTION
In late December 2019, China reported cases of respiratory illness in humans appearing first in Wuhan that involved a novel coronavirus SARS‐CoV‐2. 1 The coronavirus disease 2019 (COVID‐19) rapidly spread over countries due to the high viral contagiousness, the transmission during the asymptomatic phase, and the underestimation of the importance of the virus by the population and turned into a public health emergency of international concern in just 1 month 2 The largest case series to date of COVID‐19 is the China Center for Disease Control and Prevention's report of 44 672 people with laboratory confirmed disease. The overall case‐fatality rate (CFR) was 2.3% with poor clinical outcomes associated with older age and underlying health conditions—cardiovascular disease, diabetes, chronic respiratory disease, hypertension, and cancer. 3
The relative importance of different underlying health conditions is unclear, such as immunosuppression in solid organ transplantation. Brazil has a huge transplantation program and ranks second among all countries regarding the number of transplants performed. 4 On February 26, the virus entered Brazil and currently, after 2 months, we have 43 079 confirmed cases and 2741 deaths. 5 But so far, we do not have any COVID‐19 described case among the solid organ transplantation patient's in Brazil.
Herein, we report on the outcomes of a kidney after liver transplant recipient with COVID‐19 pneumonia admitted to the Hospital Alemão Oswaldo Cruz (São Paulo—Brazil) and review the literature.
2. CASE REPORT
A 69‐year‐old man recipient of a deceased‐donor kidney on May 2014 after deceased‐donor liver transplantation (LT) on October 2010 was admitted on March 31, 2020 due to a 24‐hour history of fever (37.8°C), fatigue, acute confusional state, diarrhea, hyporexia, and reduced urine volume.
Previous medical history included hepatitis C cirrhosis; recurrent hepatitis C after LT; post‐liver transplantation diabetes; hypertension; stroke with left hemiparesis sequelae; hepatitis C related glomerulopathy leading to end‐stage renal disease; and appendicitis 3 weeks before admission. He had been discharged after laparoscopic appendicectomy on 16 March and was at home convalescent, when he met his son just returning from Ontario the day before and asymptomatic. In Canada, there were 103 confirmed cases of COVID‐19 reported up to that day. 6 His wife presented flu‐like symptoms on 19 March and was advised to stay in self‐quarantine. Both were confirmed COVID‐19 serum‐positive afterward.
He was under maintenance immunosuppression with tacrolimus, mycophenolate sodium and prednisone and also taking omeprazole, escitalopram, lamivudine (prevention of recurrence of HBV infection with anti‐HBc positive grafts), glimepiride, and ramipril.
At first evaluation in the emergency room, the patient presented with body temperature of 37.0°C, blood pressure of 130/80 mm Hg, pulse of 66 beats per minute, respiratory rate of 16 breaths per minute, and blood oxygen saturation of 96% on room air. He was dehydrated and presented fine bilateral crackle. No murmurs, rubs, or gallops on heart examination. His abdomen was soft with diffuse tenderness, and neurologic examination revealed confusion and mild restlessness.
Laboratory tests revealed moderate acute kidney injury with serum creatinine of 3.44 mg/dL (estimated glomerular filtration rate by the MDRD Equation 22.9 mL/min/1.73 m2) with new proteinuria. His previous serum creatinine was 1.68 mg/dL 18 days before. He had severe metabolic acidosis with excess base of −9.9, hyponatremia (Na 127 mEq/L), and hyperkalemia (K 5.8 mEq/L). Abnormally increased biochemistry included lactate dehydrogenase (LDH) of 1855 U/L, aspartate aminotransferase of 106 U/L, and alanine aminotransferase of 87 U/L. He was anemic (Hemoglobin 11.4 d/dL). White blood cells were 9.24 × 103/mm3; total lymphocyte count was 1060/mm3, C‐reactive protein 14.63 mg/dL, and d‐dimer >10 000 ng/mL. A nasopharyngeal swab specimen was performed, and a direct immunofluorescence test for influenza A and B, adenovirus, respiratory syncytial virus, parainfluenza virus 1, 2, and 3 was reported back as negative. A and B Clostridium difficile toxins were negative, and fecal leukocytes were rare. Blood cultures and urine culture were negative. Immunoglobulin G (1761 mg/dL) and M (132 mg/dL), and complement C3 (94 mg/dL) and C4 (40 mg/dL) were in the normal range. Lymphocytes subpopulation were abnormally distributed with reduced counts on B lymphocytes (79/mm3), T lymphocytes (774/mm3), T CD8 lymphocytes (270/mm3), normal CD4 lymphocytes (517/mm3), NK lymphocytes (184/mm3), and CD4/CD8 relation (1.9).
Lung CT scanning at admission showed lesions in 40% of lungs’ volume, characterized as bilateral ground‐glass opacities and foci of condensation, typical radiological findings of COVID‐19 pneumonia. Baseline ECG showed a 441 ms QTc interval, and therefore, specific treatment was started, even before arrival of nasopharyngeal swab secretion RT‐PCR confirming SARS‐CoV‐2 infection (on day 2 after admission) with hydroxychloroquine 400 mg BID, nitazoxanide 500 mg BID, empirical antibiotic scheme with ceftriaxone 1 g BID and azithromycin 500 mg OD for 10 days, and unfractionated heparin 5000 IU TID. Tacrolimus was decreased to half dose for levels 3‐5 ng/mL, mycophenolate sodium was stopped, and prednisone was increased to 10 mg.
The patient did not need supplemental oxygen, and O2 saturation stayed greater than 94% during his hospitalization. Acidosis, hyponatremia, and hyperkalemia were corrected in the next 4 days with bicarbonate solution, and the patient recovered consciousness. Tacrolimus through level on D + 4 was 4.2 ng/mL. Total lymphocytes count kept above 1.0 × 103/mm3. D‐dimer reduced to 3834 ng/mL on the fourth day (D + 4) and was 1382 ng/mL on D + 21; C‐reactive protein reduced to 3.96 mg/dL after 7 days and 0.76 mf/dL on D + 21. Serum creatinine returned to baseline levels (1.7 mg/dL). CT scan repeated every 4 days is shown in Figure 1, with progressive changing patterns from ground‐glass opacities to irregular alveolar consolidation and at D + 21 as outpatient in a resolution stage the consolidation were partially absorbed, and residual laminar atelectasis and small parenchymal bands persisted. SARS‐CoV‐2 at D + 12 returned negative and patient was discharged.
FIGURE 1.
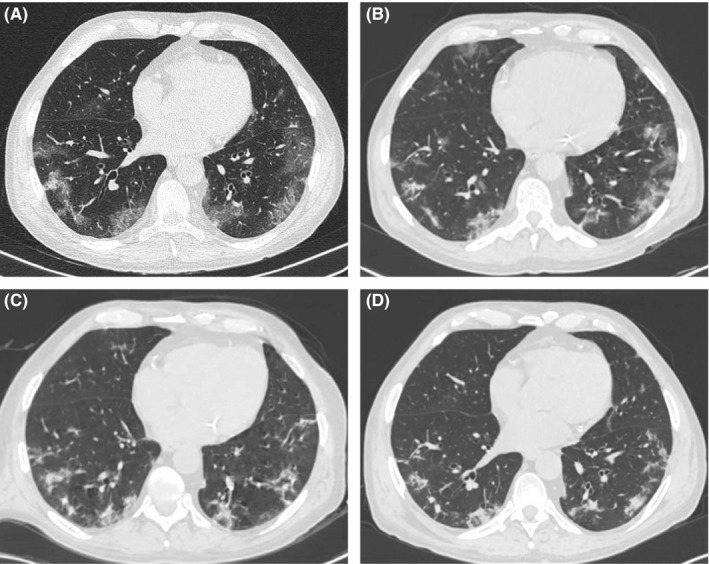
CT scan (A) at admission showing bilateral ground‐glass opacities and sparse bilateral consolidation foci, predominating in peripheral regions; CT scan (B) after 4 d with reduced extension of ground‐glass opacities and appearance of parenchymal consolidation and septal thickening; CT scan (C) after 8 d with reduced extension of ground‐glass opacities and septal thickening, more parenchymal consolidation and fibrous bands in both lungs and CT scan (D) at D + 21 in a resolution stage the consolidation were partially absorbed, and residual laminar atelectasis and small parenchymal bands persisted
3. DISCUSSION
Our case shows successful outcome of COVID‐19 in a long‐term liver‐kidney transplant patient admitted in early phase of disease to the ward. Diagnosis was promptly made in addition to stratification for complication risk. We considered our patient as having a moderate disease as he has never presented hypoxemia, lung lesions compromised <50% of lungs volume, but he was an elderly patient, with elevated d‐dimer, LDH and C‐reactive protein and under triple immunosuppression. Furthermore, our patient had normal non‐pathogen‐specific immune parameters such as immunoglobulin G level, complement C3 level, and CD4 lymphocytes count which could have rendered him this better evolution. Such immunological variables should be included in prospective studies.
As well as in other viral diseases in transplanted patients, we suppose SARS‐CoV‐2 could manifest as a flu‐like syndrome or tissue‐invasive disease with clinical symptoms and signs of end‐organ disease like colitis, pneumonia, and acute respiratory distress syndrome. Two liver recipients reported by Fernández‐Ruiz et al were managed as outpatients. 7 On the other hand, Gandolfini et al reported on an elderly male that developed abrupt worsening of his respiratory conditions due to “cytokine storm” over a period of 24‐38 hours and expired 5 days after admission before intubation. 8
By now, we identified 40 kidney transplanted patients with COVID published, together with this one, and their clinical characteristics are shown in Table 1, 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 .
TABLE 1.
Baseline clinical characteristics of 40 kidney transplant recipients with COVID‐19
| Characteristics | Value | |
|---|---|---|
| Kidney transplanted—n | 40 | |
| Gender (male:female) | 33:7 | |
| Months post‐Tx (range) | 124 | (3‐361) |
| Age (y) (range) | 58,6 | (32‐80) |
| Previous history—n (%) | ||
| Hypertension | 32 | (80) |
| Diabetes | 8 | (20) |
| Heart disease | 6 | (15) |
| Cancer& PTLD | 6 | (15) |
| HCV + | 3 | (7.5) |
| Obesity | 2 | (5) |
| Lung disease | 1 | (2.5) |
| Maintenance immunosuppression—n (%) | ||
| CNI | 36 | (90) |
| Mycophenolate | 30 | (75) |
| Steroids | 32 | (80) |
| mTORi | 6 | (15) |
| Belatacept | 1 | (2.5) |
| Admission clinical presentation—n (%) | ||
| Fever | 39 | (97.5) |
| Cough | 22 | (55) |
| Dyspnea | 11 | (27.5) |
| Gastrointestinal symptoms | 9 | (22.5) |
| Myalgias | 8 | (20) |
| Fatigue | 2 | (5) |
| Headache | 1 | (2.5) |
| Admission chest imaging—n (%) | ||
| No infiltrates | 6 | (15) |
| Unilateral infiltrates | 11 | (27.5) |
| Bilateral infiltrates | 23 | (57.5) |
| Admission Biochemistry in 32 patients | ||
| Serum creatinine (mg/dL) | 1.92 | |
| CRP elevated (n) | 32 | |
| WBC (×109/L) | 6.1 | |
| Lymphocytes (×109/L) | 1.03 | |
Gastrointestinal symptoms (vomiting, nausea, hyporexia, diarrhea).
Abbreviations: CNI, calcineurin inhibitor; CRP, C‐reactive protein; mTORi, mammalian target or rapamycin inhibitor; WBC, white blood cells.
Mostly of them (82.5%) were male. They were 58.6 years (32‐80) and transplanted for 124 months (3‐361) under use of calcineurin inhibitors, mycophenolate, and steroids in 22 (55%). The majority has high blood pressure, and 14 (35%) were taking angiotensin‐converting enzyme inhibitors or angiotensin‐receptor blockers. Fever was the main clinical sign presenting in 97.5% of patients, associated with cough in 55% and dyspnea in 27.5%. Individual laboratory data were not available in all cases. Our patient had greater d‐dimer and lactate dehydrogenase (LDH) levels, which were also highly elevated in 7 and 4 other cases reported, respectively. The Italian case series patients presented slightly elevated LDH (<1.2‐fold of upper limit of normal) and d‐dimer (<2.6‐fold of upper limit of normal). CT scan with typical signs of viral infection was described in 85% of cases.
Regarding treatment, we proceeded as always with potential severe respiratory diseases and promptly reduced total immunosuppression. As our patient presented with pulmonary infiltrates but without hypoxemia or toxemic (moderate impairment), we did not stop but reduced tacrolimus to reach through levels of 3‐5 ng/mL, withdrawn mycophenolate and doubled prednisone dose. Carbajo‐Lozoya J et al had already shown that replication of SARS‐CoV was diminished after tacrolimus treatment, but whether SARS‐CoV‐2 is also inhibited is unknown. 15 Reviewing the 40 cases, we observed that mycophenolate was withdrawn in 90% of patients and calcineurin inhibitor in 72.2%. Steroids were maintained in 26 (81.3%) and started in seven patients. Due to immunosuppression modification, two patients presented clinical acute rejection that were rescued with methylprednisolone pulse therapy.
Considering a patient with high risk for COVID‐19 complications due to older age, cardiovascular disease, diabetes, history of hypertension, and currently using triple immunosuppressive scheme, we believed specific treatment should also be given, despite only unapproved drugs were available. We discussed with the patient and her wife about off‐label use of anti‐coronavirus drugs, and by mutual agreement, we decided to use hydroxychloroquine and nitazoxanide.
Several investigational approaches are being explored for antiviral treatment of COVID‐19, based in observational series and/or on in vitro extrapolated evidence. Despite the lack of evidence on clinical efficacy, 39 (97.5%) patients received antiviral therapy as shown in Table 2. Hydroxychloroquine was given in 32 (80%), as shown in Table 2. Hydroxychloroquine has immunomodulatory, antithrombotic, and antiviral effects. Its antiviral effect is attributed to the alkalinization of intracellular acidic organelles and to the inhibition of entry steps and viral proteins glycosylation. Recently, chloroquine was shown to inhibit the replication and spread of coronavirus in vitro and to prevent infection with hCoV in newborn mice showing promise as a potential therapy of this resistant virus. 16 In an open‐label study of 36 patients with COVID‐19, use of hydroxychloroquine was associated with a higher rate of undetectable SARS‐CoV‐2 RNA on nasopharyngeal specimens at day 6 compared with no specific treatment. 17 Hydroxychloroquine was given for 10 days in our patient with no adverse event. The US Food and Drug Administration and the Ministry of Health (Brazil) both issued an emergency use authorization to allow the use of hydroxychloroquine in hospitalized for COVID‐19 when participation in clinical trials is not feasible. We also prescribed nitazoxanide. It exhibits in vitro activity against Middle East respiratory syndrome coronavirus and other coronaviruses, inhibiting expression of the viral N protein. Nitazoxanide also suppresses production of pro‐inflammatory cytokines in peripheral blood mononuclear cells and suppresses interleukin‐6 production in mice. 18 Empirical antibiotics were given in 17 of 32 cases reporting the data (53%).
TABLE 2.
Support therapy, immunosuppression modification, and anti‐coronavirus drugs used in 40 kidney transplant recipients with COVID‐19
| Treatment | Values | |||
|---|---|---|---|---|
| Immunosuppression drug—n | Withdrawn | Maintained | Reduced | Started |
| CNI n (%) | 26 (72.2) | 2 (5.5) | 8 (22.3) | 1 |
| Mycophenolate n (%) | 27 (90) | 2 (6.7) | 1 (3.3) | |
| mTORi n (%) | 6 (100) | |||
| Steroids n (%) | 6 (18.7) | 26 (81.3) | 7 | |
| Belatacept | 1 (100) | |||
| Specific Treatment—n | ||||
| Hydroxycloroquine | 32 | |||
| Lopinavir/ritonavir | 22 | |||
| Tocilizumab | 6 | |||
| Oseltamivir or umifenovir | 7 | |||
| Darunavir/ritonavir | 4 | |||
| IVIg | 3 | |||
| Nitazoxanide | 1 | |||
| Interferon‐beta | 1 | |||
| Interferon‐alpha | 1 | |||
| Darunavir/cobicistat | 1 | |||
| Colchicin | 1 | |||
| Empirical antibiotics (n/cases reporting) | 17/32 | |||
| Oxygen support device | ||||
| No oxygen support—n (%) | 10 (25) | |||
| Nasal catheter/Low support—n (%) | 12 (30) | |||
| Face mask/High‐flux nasal delivery/High support—n (%) | 7 (17.5) | |||
| Non‐invasive ventilation—n (%) | 8 (20) | |||
| Invasive ventilation—n (%) | 3 (7.5) | |||
Abbreviations: CNI, calcineurin inhibitor; IVIg intravenous immunoglobulin; mTORi, mammalian target or rapamycin inhibitor.
Our patient received unfractionated heparin either to prevent venous thrombosis but also to avoid COVID‐19 complications owing to diffuse endothelial lesion. He had extremely elevated fibrin D‐dimer that is one of the major fibrin degradation products released upon cleavage of crosslinked fibrin by plasmin. Elevated concentrations of plasma D‐dimer indicate recent or ongoing intravascular coagulation and fibrinolysis. 19 Based in a retrospective cohort of consecutive patients with severe COVID‐19, Tang at al proposed that anticoagulant therapy is associated with better prognosis in severe COVID‐19 patients meeting sepsis‐induced coagulopathy (SIC) score criteria or with markedly elevated D‐dimer (>6‐fold of upper limit of normal). 20 Our patient had d‐dimer >10 000 ng/mL or >20‐fold of upper limit of normal.
Three patients used mechanical ventilation, and only five were admitted to intensive care units (ICU), as presented in Table 3. However, eight patients had died at publications date. The reasons for not providing mechanical ventilation and ICU are not clear in publications, except in one already cited. 8 Importantly, renal function should be monitored because acute kidney injury is frequent (22.5%). Two patients (5%) required dialysis. Most cases reported have incomplete evolution and outcomes data due to short follow‐up time. Only nine patients had been discharged.
TABLE 3.
Clinical outcomes of 40 kidney transplant recipients with COVID‐19
| Clinical evolution | Value |
|---|---|
| Acute renal injury—n (%) | 9 (22.5) |
| Allograft rejection—n (%) | 2 (5) |
| Intensive care unit—n (%) | 5 (12.5%) |
| Outcome | |
| Discharged—n (%) | 9 (22.5) |
| Death—n (%) | 8 (20) |
| Inpatient—n (%) | 23 (57.5) |
In conclusion in this liver and kidney transplant patient with SARS‐CoV‐2 pneumonia without respiratory failure, we applied a multimodal strategy consisting of symptomatic support therapy, total immunosuppression reduction, use of anti‐coronavirus drugs, and heparin leading to a progressive improvement of the patient symptoms till discharge. Except for heparin, it has been the main used scheme. In the literature cases, management was individualized according to severity, but case‐fatality rate cannot be extrapolated because the reasons for not providing mechanical ventilation and ICU are not clear and the follow‐up time is very short. Clinical approach protocols have been frequently used but need to be improved to impact on the poor prognosis. Immunological variables should be included in the studies to better understand recipient × virus interaction. Tools like complication risk score and anti‐COVID‐19 therapies to mitigate organ/tissue injures by COVID‐19 need to be identified in future researches.
CONFLICTS OF INTEREST
The authors of this manuscript have no conflicts of interest to disclose.
AUTHORS CONTRIBUTION
DJBM and LEI contributed to the analysis of the data and the writing of the manuscript.
Machado DJDB, Ianhez LE. COVID‐19 pneumonia in kidney transplant recipients—Where we are?. Transpl Infect Dis. 2020;22:e13306. 10.1111/tid.13306
REFERENCES
- 1. WHO . Novel Coronavirus (2019‐nCoV): Situation Report – 1, 20 January 2020. Geneva, Switzerland: World Health Organization; 2020. (https://www.who.int/docs/default‐source/coronaviruse/situation‐reports/20200121‐sitrep‐1‐2019‐ncov.pdf?sfvrsn=20a99c10_4) [Google Scholar]
- 2. WHO . Novel coronavirus (2019‐nCoV): Situation Report – 11, 31 January 2020. Geneva, Switzerland: World Health Organization; 2020. (https://www.who.int/docs/default‐source/coronaviruse/situation‐reports/20200131‐sitrep‐11‐ncov.pdf?sfvrsn=de7c0f7_4) [Google Scholar]
- 3. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239. [DOI] [PubMed] [Google Scholar]
- 4. Pêgo‐Fernandes PM, Pestana JOM, Garcia VD. Transplants in Brazil: where are we? Clinics. 2019;74: e832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. COVID19 Painel Coronavírus . Ministério da Saúde. In https://covid.saude.gov.br/. Accessed 21 April 2020.
- 6. Coronavirus disease (COVID‐19): outbreak update. https://www.canada.ca/en/public‐health/services/diseases/2019‐novel‐coronavirus‐infection.html#a1. Accessed: 08 April 2020.
- 7. Mario Fernández‐Ruiz M, Andrés A, Loinaz C, et al. COVID‐19 in solid organ transplant recipients: a single‐center case series from Spain. Am J Transplant. 2020. [Epub ahead of print] 10.1111/ajt.15929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gandolfini I, Delsante M, Fiaccadori E, et al. COVID‐19 in kidney transplant recipients. Am J Transplant. 2020;1‐3. 10.1111/ajt.15891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guillen E, Pineiro GJ, Revuelta I, et al. Case report of COVID‐19 in a kidney transplant recipient: does immunosuppression alter the clinical presentation? Am J Transplant. 2020;1‐4. 10.1111/ajt.15874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhu L, Xu Z, Ma K, et al. Successful recovery of COVID‐19 pneumonia in a renal transplant recipient with long‐term immunosuppression. Am J Transplant. 2020;1‐5. 10.1111/ajt.15869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alberici F, Delbarba E, Manent C, et al. A single center observational study of the clinical characteristics and short‐term outcome of 20 kidney transplant patients admitted for SARS‐CoV2 pneumonia. Kidney Int. 2020, in press. 10.1016/j.kint.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marx D, Moulin B, Sl F‐K, et al. First case of COVID‐19 in a kidney transplant recipient treated with belatacept. Am J Transplant. 2020. 1‐3. 10.1111/ajt.15919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bussalino E, De Maria A, Russo R, et al. Immunosuppressive therapy maintenance in a kidney transplant recipient SARS‐CoV‐2 pneumonia: a case report. Am J Transplant. 2020;1‐3. 10.1111/ajt.15920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang H, Chen Y, Yuan Q, et al. Identification of kidney transplant recipients with coronavirus disease 2019. Eur Urol. 2020, in press. 10.1016/j.eururo.2020.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carbajo‐Lozoya J, Muller MA, Kallies S, Thiel V, Drosten C, von Brunn A. Replication of human coronaviruses SARS‐CoV, HCoV‐NL63 and HCoV‐ 229E is inhibited by the drug FK506. Virus Res. 2012;165:112‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ben‐Zvi I, Kivity S, Langevitz P, et al. Hydroxychloroquine: from malaria to autoimmunity. Clinic Rev Allerg Immunol. 2012;42:145‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID19: results of an open‐label non‐randomized clinical trial. Int J Antimicrob Agents. 2020, in press. 10.1016/j.ijantimicag.2020.105949 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18. Nitazoxanide RJF. Nitazoxanide, a new drug candidate for the treatment of Middle East respiratory syndrome coronavirus. J Infect Public Health. 2016;9(3):227‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weitz JI, Fredenburgh JC, Eikelboom JW. A test in context: d‐dimer. J Am Coll Cardiol. 2017;70(19):2411. [DOI] [PubMed] [Google Scholar]
- 20. Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. 10.1111/jth.14817 [DOI] [PMC free article] [PubMed] [Google Scholar]


