Abstract
During the last few months, the whole humanity is experiencing largest and most severe sudden influx of COVID‐19 outbreak caused by the novel coronavirus (CoV) originated from Wuhan, China. According to the WHO reports, total 3 862 676 positive cases and 265 961 deaths have been recorded worldwide due to COVID‐19 infection as of May 9, 2020. CoVs are a large family of viruses (enveloped, single‐stranded RNA viruses), which includes severe acute respiratory syndrome coronavirus (SARS‐CoV) and Middle East respiratory syndrome‐related coronavirus (MERS‐CoV). New SARS CoV2 is the members of Betacoronavirus genus. These viruses cause infections in bats, camels and humans, and a few other associated species. Despite many neurologic complications associated with SARS‐CoV‐2 infection, it is still unclear whether these symptoms results from direct neural injury or due to some other reason. Currently, it appears that most of the neurological symptoms of COVID‐19 are nonspecific and secondary to the systemic illness. A single case of acute hemorrhagic necrotizing encephalopathy has been reported. SARS‐CoV‐2 associated Guillain‐Barré syndrome is an atypical case. Till today, no convincing evidence is available to confirm that the SARS‐CoV‐2 virus directly affects nerves system in humans. However, postinfection surveillance will be necessary to identify the possible post‐COVID‐19 neurologic syndromes.
Keywords: coronavirus, COVID‐19, neurodegenerative disorders, viral outbreak
1. INTRODUCTION
During the last few months, the whole humanity is experiencing the largest and most severe sudden influx of COVID‐19 outbreak caused by the novel coronavirus (CoV) originated from Wuhan city. Coronavirus was officially announced as the causative pathogen of COVID‐19 by the Chinese Center for Disease Control and Prevention on January 8, 2020. The World Health Organization announced coronavirus outbreak as a public health emergency of international concern. It started to expect disturbing extents over the globe as forewarned by the World Health Organization (WHO) reports (form‐2019‐nCoV). According to the WHO reports, total 3 557 235 positive cases and 245 150 deaths have been recorded worldwide due to the COVID‐19 infection as of May 6, 2020. A total of 215 countries, areas, or territories have been affected with COVID‐19 cases. 1 CoVs are a large family of viruses (enveloped, single‐stranded RNA viruses), which includes severe acute respiratory syndrome coronavirus (SARS‐CoV) and Middle East respiratory syndrome‐related coronavirus (MERS‐CoV). Based on genetic analysis, CoVs are divided into four genera namely, Betacoronavirus, Alphacoronavirus, Gammacoronavirus, and Deltacoronavirus. The new SARS CoV2 is the members of Betacoronavirus genus. These viruses cause infections in bats, camels and humans, and a few other associated species. 2
The coronaviral genome composed of four basic structural proteins: the envelope (CoV E) protein, the spike (CoV S) protein, membrane (CoV M) protein, and nucleocapsid (CoV N) protein. The CoV E protein is a membrane protein responsible for envelope formation, budding, and pathogenesis. It functions as ion‐channeling viroporin and interacts with both host cell proteins and other CoV proteins. The CoV S protein helps the virus in binding to the host cell surface receptors. This also led to the fusion between viral and host cells and alleviates entry of virus into the host cell. 3 , 4 CoV M protein is a type III transmembrane glycoprotein which maintains the bioactive conformation and antigenic character of the CoV. 5 CoV N protein helps in stabilizing envelope assembly complexes during forming virus‐like particles, most likely through interactions with CoV M. 6
The CoV S protein of SARS‐CoV and MERS‐CoV bind to the host proteins via receptor‐binding domains (RBDs). The SARS‐CoV uses angiotensin‐converting enzyme 2 (ACE2) as main RBD. The MERS‐CoV utilizes dipeptidyl peptidase‐4 (DPP4) as their vital protein‐restricting site. COVID‐19 virus has a close relationship with SARS. 7 , 8 From a pharmacological perspective, enough proofs are available to accept that the aerosolized bead transmission of COVID‐19 viral particles in the lungs includes a few key pathological based molecular mechanisms. It is very well established that the receptor‐interceded endocytosis is the most common mechanisms of viral entrance into the host cells.
COVID‐19 may affect the central nervous system (CNS) via four possible pathways: (a) Immediate viral injury of nervous tissue. In spite of some interesting case reports, there is no clear evidence is available that how SARS‐CoV virus harms the CNS. 9 (b) Injury results from an excessive immune response as a cytokine storm. Cytokines can cross the blood‐brain barrier and can cause acute necrotizing encephalopathy. 10 , 11 (c) Unintended host immune response effects after an acute infection. Guillain‐Barré disorder (GBS) is the case of indirect CNS injury. One instance of GBS related with COVID‐19 has been reported. However, the proofs of circumstances and logical results are still unclear. 12 (d) Indirect viral injury due to systemic sickness. The COVID‐19 can enter the nervous system via the olfactory nerve, blood circulation or neuronal pathways. Binding of virus to the angiotensin‐converting enzyme 2 (ACE2) receptor allow it to get enters into the host cells and take cellular machineries under viral control and undergo replication. Minimization of lysosomal activity leads to protein aggregation in neurons and thus may cause neurodegenerative diseases (Figure 1).
FIGURE 1.
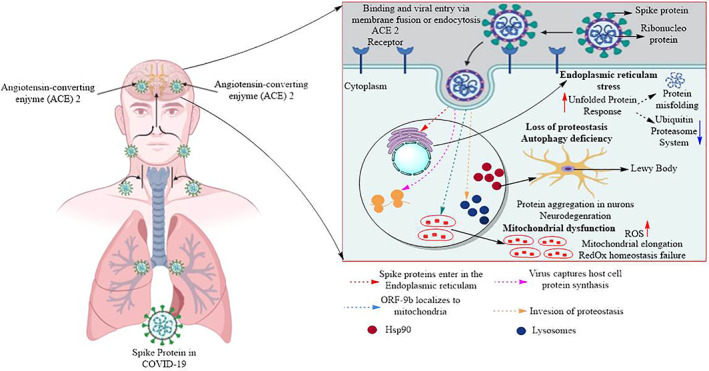
The mechanisms of COVID‐19 infections and neurological damage caused by COVID‐19
2. CASE REPORT
Neurologists are now more attentively observing the patients suffering from serious neurologic symptoms and COVID‐19 infection. Such patients may face problems due to the limitation of intensive care beds and hesitancy to treat with certain necessary medications given risk of nosocomial COVID‐19 infection. 13 , 14 Guan and coworkers reported the clinical symptoms of SARS‐CoV‐2 contamination in 1099 patients. The neurologic symptoms in patients with COVID‐19 included myalgias (14.9%) and headache (13.6%). Five percentage of the patients required intensive care beds and 2.3% patients underwent invasive mechanical ventilation. Thus, SARS‐CoV‐2 can directly or indirectly attack the nervous system. 15
A clinical study based on 214 COVID‐19 patients in Wuhan, China demonstrated a high degree of incidence of neurologic symptoms. A total of 78 (36.4%) patients had shown neurologic disorders, 19 (8.9%) peripheral nervous system (PNS), 53 (24.8%) CNS, and 23 (10.7%) skeletal muscle injuries. The two most common CNS related symptoms were headache 28 (13.1%) and dizziness 36 (16.8%), with epilepsy, acute cerebrovascular disease, impaired consciousness and ataxia additionally revealed. Seriously sick patients had neurologic symptoms, like, ischemic or hemorrhagic stroke, muscle injury and adjusted mental status. Hypogeusia 12 (5.6%) and hyposmia 11 (5.1%) were the most common PNS symptoms with vision impairment and neuropathic pain (in light of the fact that taste and smell are dependent on cranial nerves, these would all the more precisely viewed as shortages because of CNS injury). 16
It has been hypothesized that SARS‐CoV‐2 may enter into the nervous system by means of the ACE2 receptor, which is present in neurons, glial cells, skeletal muscle, and different organs. Potential passage to the CNS incorporates hematogenous spread and retrograde neuronal transmission via olfactory neurons in the cribriform plate. 17 SARS‐CoV has direct access to the brain. The direct COVID‐19 entry to the medullary cardiorespiratory center may lead to the failure of respiratory system. SARS‐CoV nucleic acids have been observed in the brain tissues and cerebro‐spinal liquid of patients infected with SARS‐CoV. Intranasally administered MERS‐COV or SARS‐CoV could enter the brain of transgenic mice, possibly via the olfactory nerves and could spread to the brainstem and thalamus. 18
Li et al conducted a retrospective, single center, observational analysis of 221 COVID‐19 patients in Wuhan, China. The study demonstrated that severe COVID‐19 patients commonly had neurologic symptoms. Thirteen COVID‐19 patients developed new onset of cerebrovascular disease. One patient was diagnosed with cerebral venous sinus thrombosis; 11 patients were diagnosed as ischemic stroke and one patient with cerebral hemorrhage. Six ischemic stroke patients received antiplatelet treatment with Clopidogrel or Aspirin and five received Clexane with anticoagulant treatment. As of February 29, 2020, seven patients remained hospitalized. Three patients who received antiplatelet treatment died. 19 Poyiadji et al reported first presumptive case of acute necrotizing hemorrhagic encephalopathy associated with COVID‐19. This could be due to the cytokine storm. This is the example of indirect viral nerve injury caused by Guillain‐Barré syndrome associated with SARS‐CoV‐2 infection. However, this was an atypical case as Guillain‐Barré syndrome occurred 1 week before the development of clinical symptoms. 11 , 12
3. CONCLUSIONS
Despite many neurologic complications associated with SARS‐CoV‐2 infection, it is still unclear whether these symptoms results from direct neural injury or due to some other reason. Currently, it appears that most of the neurological symptoms of COVID‐19 are nonspecific and secondary to the systemic illness. A single case of acute hemorrhagic necrotizing encephalopathy has been reported. SARS‐CoV‐2 associated Guillain‐Barré syndrome is an atypical case. Till today, no convincing evidence is available to confirm that the SARS‐CoV‐2 virus directly affects the CNS or PNS in humans. However, postinfection surveillance will be necessary to identify the possible post‐COVID‐19 neurologic syndromes.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Singh AK, Bhushan B, Maurya A, Mishra G, Singh SK, Awasthi R. Novel coronavirus disease 2019 (COVID‐19) and neurodegenerative disorders. Dermatologic Therapy. 2020;33:e13591. 10.1111/dth.13591
REFERENCES
- 1.WHO Report on Coronavirus Disease (COVID‐19) Pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed May 10, 2020.
- 2. Martya AM, Jones MK. The novel coronavirus (SARS‐CoV‐2) is a one health issue. One Health. 2020;9:100123. 10.1016/j.onehlt.2020.100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schoeman D, Fielding BC. Coronavirus envelope protein: current knowledge. Virol J. 2019;16(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Haan CA, Smeets M, Vernooij F, Vennema H, Rottier PJ. Mapping of the coronavirus membrane protein domains involved in interaction with the spike protein. J Virol. 1999;73(9):7441‐7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alsaadi EAJ, Jones IM. Membrane binding proteins of coronaviruses. Future Virol. 2019;14(4):275‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arndt AL, Larson BJ, Hogue BG. A conserved domain in the coronavirus membrane protein tail is important for virus assembly. J Virol. 2010;84(21):11418‐11428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu A, Peng Y, Huang B, et al. Genome composition and divergence of the novel coronavirus (2019‐nCoV) originating in China. Cell Host Microbe. 2020;27(3):325‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270‐273. 10.1101/2020.01.22.914952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu Y, Xu X, Chen Z, et al. Nervous system involvement after infection with COVID‐19 and other coronaviruses. Brain Behav Immun. 2020. 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID‐19‐associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. 2020;201187. 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain‐Barré syndrome associated with SARS‐CoV‐2 infection: causality or coincidence? Lancet Neurol. 2020;19(5):383‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Needham EJ, Chou SHY, Coles AJ, Menon DK. Neurological implications of COVID‐19 infections. Neurocrit Care. 2020. 10.1007/s12028-020-00978-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Manji H, Carr AS, Brownlee WJ, Lunn MP. Neurology in the time of COVID‐19. J Neurol Neurosurg Psychiatry. 2020;91(6):568‐570. [DOI] [PubMed] [Google Scholar]
- 15. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020. 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID‐19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS Chem Nerosci. 2020;11(7):995‐998. [DOI] [PubMed] [Google Scholar]
- 18. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS‐CoV2 may play a role in the respiratory failure of COVID‐19 patients. J Med Virol. 2020;92(6):552‐555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li Y, Wang M, Zhou Y, et al. Acute Cerebrovascular Disease Following COVID‐19: A Single Center, Retrospective, Observational Study; 2020. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3550025. Accessed May 6, 2020. [DOI] [PMC free article] [PubMed]


