Abstract
During COVID‐19 outbreak there are discordant opinions toward the impact of biologics in psoriatic (PsO) patients. Thus we performed a single‐center case‐control study in Lombardia, the Italian region with the higher number of COVID‐19 confirmed cases. We enrolled 1193 PsO patients treated with biologics and small molecules and we used the entire Lombardia population as controls. Notably, 17 PsO patients COVID‐19 confirmed were quarantined at home and five hospitalized, no PsO patients were admitted to intensive care unit (ICU) or died. With respect to the general population of Lombardy, patients on biologics were at higher risk to test positive for COVID‐19 (odds ratio [OR] 3.43 [95% confidence interval (CI) 2.25‐5.73], P < .0001), to be self‐quarantined at home (OR 9.05 [95% CI 5.61‐14.61], P < .0001) and hospitalized (OR 3.59 [95% CI 1.49‐8.63], P = .0044), however, not increased risk of ICU admission or death were found. PsO patients on biologics should be carefully monitored with telemedicine during COVID‐19 outbreak and early treated at home to limit hospital overwhelm.
Keywords: bilateral interstitial pneumonia, biologics, COVID‐19, pandemic, psoriasis, psoriatic arthritis, SARS‐CoV‐2
1. INTRODUCTION
Since March 8, 2020, Lombardia region experienced COVID‐19 lockdown and only after 2 days the entire Italy became red‐zone. Nowadays, Lombardia is the Italian region with more confirmed, hospitalized, and dead COVID‐19 patients.
Despite the higher risk for COVID‐19 displayed by obese, active smokers, and COPD patients, 1 almost no data are present toward psoriatic (PsO) patients and biologics.
Moderate to severe psoriasis benefit from systemic treatment as biologics and small molecules; at the same time these drugs, capable to clear psoriasis, are related also to an increased risk of airway infections. 2 PsO patients also display a baseline airway inflammation that triggers the constellation of chronic respiratory comorbidities, such as asthma and chronic obstructive pulmonary disease (COPD). 3 Furthermore, PsO patients are frequently smokers and cigarettes increased flares as well as psoriasis severity, predisposing to COPD and exacerbating asthma crisis. 4
Thus, we performed this study aiming to understand the effect of biologics in PsO patients during COVID‐19 outbreak focusing on symptomatic patients quarantined at home, hospitalized, and their prognosis.
2. METHODS
2.1. Study design
This case‐control study was performed using the adult PsO patients under biologics and small molecules that attended PsoCare ambulatory in San Donato Hospital in Milan, Italy, compared with the Lombardia population from February 21, 2020 (first case of Coronavirus in Italy) to April 9, 2020. Since during red‐zone declaration period routine dermatologic visits were reprioritized and in‐person visits were limited, we had assessed patients every 2 weeks with video teledermatology and we delivered at home the chronic anti‐PsO systemic therapies. Teledermatology softwares allowing a videocall were WhatsApp, FaceTime, Skype, and Zoom.
This cohort of PsO patients is the largest in the whole region and healthcare records were informatized. In fact, clinical, pharmacological, and demographic data were collected in a weekly updated database. Furthermore, Lombardia was the first Italian region to be entirely quarantined in Italy during COVID‐19 outbreak and still have the highest number of cases. Thus, these characteristics made it ideal to perform our study that aimed to quantify the COVID‐19 impact on PsO patients treated with biologics and small molecules.
Controls data were collected from the EPICENTRO ISS, and Regione Lombardia public available websites (http://www.epicentro.iss.it/coronavirus/, http://www.regione.lombardia.it). Lombardy is an Italian Region consisting of 10 060 574 residents.
2.2. Inclusion and exclusion criteria
Inclusion criteria for PsO patients were: (a) adult patients (>18 years); (b) moderate to severe (PASI >10 before treatment) plaque psoriasis for more than 1 year; (c) approved anti‐PsO monotherapy (biologics or small molecules); (d) being in the maintaining phase; (e) signed consent form for participation to epidemiological studies, (f) phone number and/or contacts for teledermatology; and (g) residence in Lombardia.
Exclusion criteria for PsO patients were: (a) pediatric patients (<18 years); (b) different psoriasis type (guttate psoriasis, erythroderma, pustular psoriasis); (c) plaque psoriasis with duration <1 year; (d) being in the induction phase or autonomously discontinued the therapy; (e) not signed consent form for participation to epidemiological studies; and (f) lack of phone number and/or contacts for teledermatology.
The whole Lombardia population records were extracted and included.
2.3. Classification of COVID‐19 disease states
COVID‐19 progression was recently divided in three stages (Figure 1): (a) early infection clinically characterized by fever dry cough, diarrhea, and headache and lymphopenia but no dyspnea; (b) pulmonary phase clinically characterized by a viral pneumonia (IIA without hypoxia, IIB with hypoxia [PaO2/FiO2 of <300 mmHg]) with lymphopenia and hyper‐transaminasemia; and (c) hyperinflammation phase with extrapulmonary manifestations driven by the cytokines storm. 5
FIGURE 1.
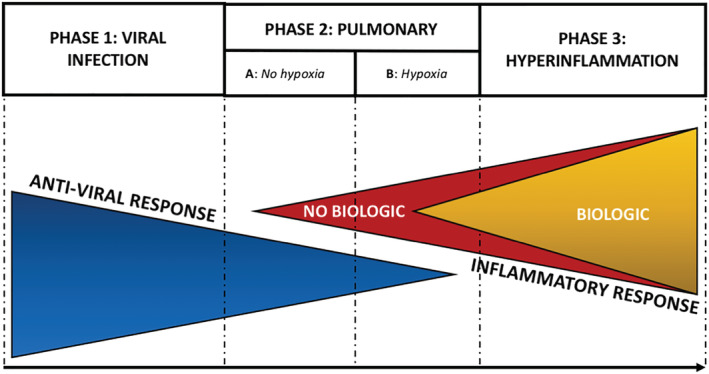
COVID‐19 disease states and biologics impact
2.4. Statistical analysis
Descriptive statistics was performed computing mean and SD. Odds ratio (OR) was computed to shed light on the risk of patients under biologics with respect to the general population of Lombardy. Figures with P‐value equal to or less than .05 were considered statistically significant. All statistical analyses were performed by means of the commercial software “Statistical Package for Social Sciences” (SPSS for Windows, version 24.0, IBM Corp., Armonk, New York).
3. RESULTS
3.1. Sample characteristics
We enrolled 1193 PsO patients (mean age 55 years, males 68%) and 10 060 574 controls (mean age 65 years, males 48.9%). Smokers were 28% and 24% in PsO patients and controls.
From a therapeutic point of view, 262 (22%) PsO patients underwent tumor necrosis factor‐alpha (TNFα) inhibitors, 238 (19.9%) interleukin (IL) 12/23 inhibitors, 542 (45.4%) IL‐17 inhibitors, 62 (5.2%) IL‐23 inhibitors, and 89 (7.5%) small molecules. Demographic and clinical characteristics for PsO patients are detailed in Table 1.
TABLE 1.
Characteristics of the San Donato Hospital cohort
| San Donato Hospital cohort (N = 1193) | |
|---|---|
| Male, N (%) | 811 (68) |
| Age, mean ± SD, years | 55 ± 12.7 |
| BMI, mean ± SD, kg/m2 | 28.7 ± 2.5 |
| PsO duration, mean ± SD, years | 12.3 ± 9.1 |
| Smoking, N (%) | 350 (29.3) |
| Comorbidities | |
| Obesity, N (%) | 215 (18) |
| Cardiovascular disease, N (%) | 167 (15) |
| Hypertension, N (%) | 346 (29) |
| Diabetes mellitus, N (%) | 143 (12) |
| COPD, N (%) | 197 (16.5) |
| OSA, N (%) | 53 (4.4) |
| PsA, N (%) | 298 (25) |
| Systemic therapies | |
| TNF‐inhibitors, N (%) | 262 (22) |
| Etanercept and its biosimilars | 86 (33) |
| Adalimumab and its biosimilars | 176 (67) |
| IL‐12/23‐inhibitors, N (%) | 238 (19.9) |
| Ustekinumab | 238 (100) |
| IL‐17‐inhibitors, N (%) | 542 (45.4) |
| Secukinumab | 287 (53) |
| Ixekizumab | 201 (37) |
| Brodalumab | 54 (10) |
| IL‐23 inhibitors, N (%) | 62 (5.2) |
| Guselkumab | 53 (85.5) |
| Risankizumab | 8 (12.9) |
| Tidrakizumab | 1 (1.6) |
| Small molecules, N (%) | 89 (7.5) |
| Apremilast | 77 (6.5) |
| Dimetil‐fumarate | 12 (1) |
| COVID‐19 | |
| Quarantined at home | 17 (1.4) |
| Hospitalized | 5 (0.4) |
| Death | 0 (0) |
| Healthy | 1171 (98.2) |
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease;, IL, interleukin; OSA, obstructive sleep apnea; PsA, psoriatic arthritis; PsO, psoriasis; TNF, tumor necrosis factor.
3.2. COVID‐19‐related data
In April 9, 2020, among controls 54 801 subjects were COVID‐19 positive, 16 042 were self‐isolated (quarantined) at home, 11796 were hospitalized, 1236 were admitted to intensive care unit (ICU), and 10 222 died. Conversely, 17 PsO patients were quarantined at home, five hospitalized, and none dead. Age of hospitalized PsO patients was 62 ± 11.3 years, whereas age of those quarantined was 56 ± 12.9 years. Males were 76.5% and 60.0% among the quarantined and hospitalized groups, respectively. PsO patients COVID‐19 confirmed quarantined at home display a stage 1 of COVID‐19 disease, among the hospitalized two had stage IIA and three had stage IIB. Remarkably, none displayed a progression on stage III (Table 2).
TABLE 2.
Clinical characteristics and therapies of COVID‐19 confirmed psoriatic patients
| COVID‐19 positive quarantined at home (N = 17) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Age | Gender | PsA | PsO therapy | Hospitalization, days | COVID‐19 diagnosis | COVID‐19 therapy | Comorbidities | |
| 1 | 44 | F | _ | Ustekinumab 45 mg | 0 | Fever, anosmia, ageusia, astenia, cough, no hypoxia, no dyspnea | Paracetamol 1000 mg every 8‐12 h × 10 days + azitromicin 500 mg/die × 7 days | _ |
| 2 | 40 | F | _ | Ustekinumab 90 mg | 0 | Fever, anosmia, ageusia, astenia, cough, no hypoxia, no dyspnea | Paracetamol 1000 mg every 8‐12 h × 12 days | _ |
| 3 | 67 | M | _ | Ustekinumab 90 mg | 0 | Fever, anosmia, ageusia, astenia, cough, no hypoxia, no dyspnea | Paracetamol 1000 mg every 8–12 h × 10 days + azitromicin 500 mg/die × 6 days | Hypertension, myocardial infarction |
| 4 | 68 | M | _ | Ustekinumab 90 mg | 0 | Fever, anosmia, ageusia, astenia, cough, no hypoxia, no dyspnea | Paracetamol 1000 mg every 8–12 h × 10 days | Hypertension |
| 5 | 51 | M | _ | Ustekinumab 45 mg | 0 | Fever, anosmia, ageusia, astenia, cough, no hypoxia, no dyspnea | Paracetamol 1000 mg twice/day × 10 days | _ |
| 6 | 63 | M | _ | Adalimumab biosimilar | 0 | Fever, anosmia, ageusia, astenia, cough, no hypoxia, no dyspnea | Paracetamol 1000 mg twice/day × 10 days | Obesity and diabetes |
| 7 | 72 | M | X | Adalimumab biosimilar | 0 | Fever, anosmia, ageusia, astenia, cough, no hypoxia, no dyspnea | Paracetamol 1000 mg twice/day × 10 days | Hypertension |
| 8 | 42 | F | X | Adalimumab | 0 | Fever, anosmia, ageusia, astenia, cough, no hypoxia, no dyspnea | Paracetamol 1000 mg twice/day × 10 days | _ |
| 9 | 67 | M | _ | Etanercept biosimilar | 0 | Fever, anosmia, ageusia, astenia, cough, no hypoxia, no dyspnea | Amoxicillin 1000 mg twice per day × 7 days, paracetamol 1000 mg twice/day × 10 days | _ |
| 10 | 56 | M | _ | Etanercept biosimilar | 0 | Fever, anosmia, ageusia, astenia, cough, no hypoxia, no dyspnea | Paracetamol 1000 mg twice/day × 10 days | _ |
| 11 | 43 | F | _ | Etanercept biosimilar | 0 | Fever, anosmia, ageusia, astenia, cough, no hypoxia, no dyspnea | Paracetamol 1000 mg twice/day × 10 days | _ |
| 12 | 69 | M | _ | Secukinumab | 0 | Fever, anosmia, ageusia, astenia, cough, no hypoxia, no dyspnea | Paracetamol 1000 mg twice/day × 10 days | _ |
| 13 | 37 | M | _ | Secukinumab | 0 | Fever, anosmia, ageusia, astenia, cough, no hypoxia, no dyspnea | Paracetamol 1000 mg twice/day × 10 days | _ |
| 14 | 39 | M | _ | Ixekizumab | 0 | Fever, anosmia, ageusia, astenia, cough, no hypoxia, no dyspnea | Paracetamol 1000 mg twice/day × 10 days | _ |
| 15 | 65 | M | _ | Ixekizumab | 0 | Fever, anosmia, ageusia, astenia, cough, no hypoxia, no dyspnea | Paracetamol 1000 mg twice/day × 10 days + azitromicin 500 mg/die × 7 days | Hypertension, obesity |
| 16 | 66 | M | _ | Ixekizumab | 0 | Fever, anosmia, ageusia, astenia, cough, no hypoxia, no dyspnea | Paracetamol 1000 mg every 8 h × 10 days + azitromicin 500 mg/die × 7 days | Hypertension, stroke |
| 17 | 69 | M | _ | Guselkumab | 0 | Fever, anosmia, ageusia, astenia, cough, no hypoxia, no dyspnea | Amoxicillin 1000 mg twice per day × 7 days, paracetamol 1000 mg twice/day × 10 days | Hypertension, diabetes |
| COVID‐19 positive hospitalized (N = 5) | ||||||||
| 1 | 55 | F | X | Adalimumab (suspended) | 5 | Bilateral interstitial pneumonia | Lopinavir‐ritonavir 400 mg twice/day × 15 days + paracetamol 1000 mg every 8 h × 10 days | Diabetes, hypertension |
| 2 | 62 | M | _ | Ustekinumab 90 mg | 7 | Bilateral interstitial pneumonia | Oxigen × 2 days, lopinavir‐ritonavir 400 mg twice/day × 15 days, hydroxychloroquine 800 mg/die × 15 days, prednisone 25 mg per 8 days and 12.5 per 8 days | _ |
| 3 | 73 | M | _ | Ixekizumab | 7 | Bilateral interstitial pneumonia | Oxigen 1 day, amoxicillin 1000 mg twice per day × 7 days, hydroxychloroquine 800 mg/die × 10 days | _ |
| 4 | 74 | M | _ | Secukinumab | 5 | Bilateral interstitial pneumonia | Oxigen × 1 day, Amoxicillin 1000 mg twice per day× 7 days | Hypertension, transient ischemic attack |
| 5 | 48 | F | _ | Ustekinumab 90 mg | 8 | Bilateral interstitial pneumonia | Lopinavir‐ritonavir 400 mg twice/day × 10 days + paracetamol 1000 mg every 8 h × 10 days | |
[Correction added on 3 July, after first online publication: The N values in Table 2 have been corrected.]
3.3. Biologics and COVID‐19 risk
With respect to the general population of Lombardy, patients on biologics were at higher risk of testing positive for COVID‐19 (unadjusted OR 3.43 [95% CI 2.25‐5.73], P < .0001). Similarly, the risk of being self‐quarantined at home (OR 9.05 [95% CI 5.61‐14.61], P < .0001) and being hospitalized (unadjusted OR 3.59 [95% CI 1.49‐8.63], P = 0.0044) was higher. The risks of being admitted to ICU (unadjusted OR 3.41 [95% CI 0.21‐54.55], P = .3861) and of dying (unadjusted OR 0.41 [95% CI 0.03‐6.59], P = .5306) were not statistically significant.
4. DISCUSSION
PsO patients on biologics displayed higher risk to be infected and to be hospitalized/self‐quarantined at home, but ICU hospitalization and death did not differ from the general population.
Biologics and small molecules, as previously suggested by both real‐life registries and trials, increased airway infections 2 , 6 and this detrimental effect is still valid in COVID‐19. However, their inhibition of pro‐inflammatory cytokines detrimental in the viral phase, seems to be fundamentally beneficial in the hyperinflammatory phase protecting PsO patients to the progression to the extrapulmonary manifestations and death. 5
Both TNFα and IL‐17, together with IL‐6, IL‐8, and IL‐1, play a pivotal role in driving the hyperinflammatory final stage of COVID‐19 progression and their possible selective antagonism may be helpful in interrupting the cytokinic storm. Likewise, tocilizumab, an IL‐6 receptor antagonist, is obtaining encouraging results in decreasing mortality of ICU COVID‐19 patients. 7 The blood of ICU COVID‐19 patients displayed circulating CD14+CD16+IL+ monocytes and granulocyte‐macrophage colony‐stimulating factor interferon‐γ+ Th1 lymphocytes that release several pro‐inflammatory cytokines, included high levels of IL‐6, capable to determine the multi‐organ damage concretizing in acute respiratory distress syndrome. 7
The same concept is inspiring two recently approved trials on the Chinese Clinical Trials Registry (http://www.chictr.org.cn/abouten.aspx) that aimed to treat COVID‐19 hyperinflammatory phase with adalimumab alone (ChiCTR2000030089) and ixekizumab combined with conventional antiviral drugs (ChiCTR2000030703).
Notably, PsO patients display baseline airway inflammation that clears with anti‐PsO therapies, preliminary data suggest that airways inflammation is downregulated by treating skin inflammation. 8 The lung‐skin inflammatory reciprocal interactions was modelized by Nadeem et al claiming that skin inflammation via IL‐23/STAT3 signaling modulates airway inflammation and vice versa. 9 These findings offer also a rationale to continue biologics in PsO patients to prevent the lung‐skin inflammatory axis and to inhibit the progression to the hyperinflammatory phase.
Despite this study is the first to assess the impact of biologics among PsO patients during COVID‐19, it did not assess the family members, so future studies should also evaluate this aspect.
Biologics may not increase the risk of ICU hospitalization and death; however, they increase the risk of COVID‐19 mild to moderate disease. Thus, PsO patients on biologics should be carefully monitored with teledermatology and early treated at COVID‐19 symptoms early onset.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Damiani G, Pacifico A, Bragazzi NL, Malagoli P. Biologics increase the risk of SARS‐CoV‐2 infection and hospitalization, but not ICU admission and death: Real‐life data from a large cohort during red‐zone declaration. Dermatologic Therapy. 2020;33:e13475. 10.1111/dth.13475
REFERENCES
- 1. Zhang G, Hu C, Luo L, et al. Clinical features and short‐term outcomes of 221 patients with COVID‐19 in Wuhan, China. J Clin Virol. 2020;127:104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bronckers IMGJ, Seyger MMB, West DP, et al. Safety of systemic agents for the treatment of pediatric psoriasis. JAMA Dermatol. 2017;153(11):1147‐1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Santus P, Rizzi M, Radovanovic D, et al. Psoriasis and respiratory comorbidities: the added value of fraction of exhaled nitric oxide as a new method to detect, evaluate, and monitor psoriatic systemic involvement and therapeutic efficacy. Biomed Res Int. 2018;2018:3140682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ko SH, Chi CC, Yeh ML, Wang SH, Tsai YS, Hsu MY. Lifestyle changes for treating psoriasis. Cochrane Database Syst Rev. 2019;7(7):CD011972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Siddiqi HK, Mehra MR. COVID‐19 illness in native and immunosuppressed states: a clinical‐therapeutic staging proposal. J Heart Lung Transpl. 2020.39(5):405‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spelman L, Rubel D, Brnabic A, Burkhardt N, Riedl E, Foley P. A subset analysis of efficacy and safety outcomes from phase 3 clinical studies of ixekizumab for the treatment of patients with severe plaque psoriasis. J Dermatolog Treat. 2020;1‐7. [DOI] [PubMed] [Google Scholar]
- 7. Fu B, Xu X, Wei H. Why tocilizumab could be an effective treatment for severe COVID‐19? J Transl Med. 2020;18(1):164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Damiani G, Radaeli A, Olivini A, Calvara‐Pinton P, Malerba M. Increased airway inflammation in patients with psoriasis. Br J Dermatol. 2016;175(4):797‐799. [DOI] [PubMed] [Google Scholar]
- 9. Nadeem A, Al‐Harbi NO, Ansari MA, et al. Psoriatic inflammation enhances allergic airway inflammation through IL‐23/STAT3 signaling in a murine model. Biochem Pharmacol. 2017;124:69‐82. [DOI] [PubMed] [Google Scholar]


