Dear Editor,
Coronavirus disease (COVID‐19) is spreading across the world. 1 Many different clinical manifestations of COVID‐19 have been described in the literature; though there have not been any reported cases in the literature about skin manifestations related to COVID‐19 in pediatric patients. We have tried to analyze the appearance of the rash in pediatric patients with and/or without using hydroxychloroquine (HCQ) treatment for the treatment of COVID‐19 who have been hospitalized at our hospital.
Twenty pediatric patients with COVID‐19 were hospitalized at the hospital from 20 March to 25 April. In all of 20 pediatric patients, nine of them received HCQ treatment. The exact diagnosis of each patient was confirmed by nasopharyngeal and oropharyngeal swab PCR. Three patients (15%) developed cutaneous manifestations. Two patients (10%) developed cutaneous involvement at the onset, one patient (5%) developed cutaneous involvement after HCQ treatment during the hospitalization. Cutaneous manifestations were maculopapular rashes (two patients) and erythematosus eruptions (one patient). The youngest patient, an 8‐month‐old girl, had an erythematous skin rash and had a similar appearance to the rash of roseola. Her rash and fever lasted for 2 days, and both clinical conditions disappeared concomitantly (Figure 1A,B). The second patient, an 11‐year‐old girl, had a maculopapular and itchy rash at the time of admission to the hospital, which lasted for 5 days (Figure 1C,D). During the fourth day of hospitalization of the oldest, a 17‐year‐old patient, the patient developed a maculopapular mild itchy rash on the third day of HCQ treatment (Figure 1E,F). Therefore, we concluded that her skin rash occurred due to the usage of HCQ and her rash after the termination of HCQ treatment. Generally, all of the patients' rashes started on their faces and continued on the extremities and ended on the trunk. We did not detect any correlation between the exacerbation of the rash and the disease's severity. Three patients had normal coagulation patterns. All of the other viral markers were normal for these three patients.
FIGURE 1.
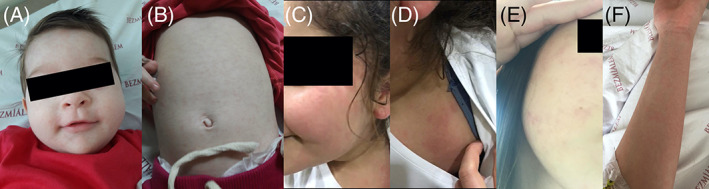
A, B Figures showing an erythematous skin rash and had a similar appearance to the rash of roseola on his face and trunk in an 8‐month‐old child. C, D Figures showing a maculopapular and itchy rash on her face and shoulder in a 11‐year‐old child. E, F Figures showing a maculopapular mild itchy rash on her face and extremities after HCQ treatment in a 17‐year‐old teenager
The first scientific report about the relationship between COVID‐19 and rashes was done in China and was about skin involvement. 2 Recalcati et al did a study on 88 patients, and 18 of them developed cutaneous manifestations. Clinical cutaneous manifestations of the patients were erythematous rash, chickenpox‐like vesicles, and widespread urticaria. Also, itching was low, and all of the lesions ameliorated last than 5 days. 3 In our case, the frequency of appearance of skin rash in our pediatric COVID‐19 patients was so much lower than Recalcati et al's study. Unlike the study, which was done by Recalcati et al, one of our patients developed skin rash secondary to HCQ treatment. The skin rashes of our two cases were itchy, similar to those in this study.
HCQ is one of the most effective drugs against COVID‐19. 4 However, there have been a few reported cases about the side effects of HCQ on the skin in dermatomyositis and lupus erythematosus patients. 4 , 5 , 6 The characteristic morphology of skin rashes was variable. Generally, the form of intensely pruritic morbilliform eruptions occurred. Each reaction ameliorated once the drug regimen was terminated. 5 The eruptions that occurred in our patients were in the form of maculopapular. Our patient developed a maculopapular mild itchy rash on the third day of HCQ treatment. The rash resolved on discontinuation of the drug regimen. In conclusion, we concluded that skin rash might occur in pediatric patients with and/or without using HCQ for treatment in COVID‐19. To the best of our knowledge, this is the first report of the appearance of the rash in pediatric patients with and/or without using HCQ for treatment in COVID‐19.
CONFLICT OF INTEREST
The author(s) declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
DECLARATION OF PATIENT CONSENT
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
AUTHORS CONTRIBUTIONS
All authors participated in creating content for the manuscript, editing and provided final approval for submission. No undisclosed authors contributed to the manuscript.
ACKNOWLEDGMENTS
This research received no specific grant from any funding agency or commercial or not‐for‐profit sectors.
REFERENCES
- 1. Morand A, Fabre A, Minodier P, et al. COVID‐19 virus and children: what do we know? Arch Pediatr. 2020;27(3):117‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Recalcati S. Cutaneous manifestations in COVID‐19: a first perspective [published online ahead of print, 2020 Mar 26]. J Eur Acad Dermatol Venereol. 2020. 10.1111/jdv.16387. [DOI] [PubMed] [Google Scholar]
- 4. Rainsford KD, Parke AL, Clifford‐Rashotte M, Kean WF. Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology. 2015;23(5):231‐269. [DOI] [PubMed] [Google Scholar]
- 5. Pelle MT, Callen JP. Adverse cutaneous reactions to hydroxychloroquine are more common in patients with dermatomyositis than in patients with cutaneous lupus erythematosus. Arch Dermatol. 2002;138(9):1231‐1233. [DOI] [PubMed] [Google Scholar]
- 6. Murphy M, Carmichael AJ. Fatal toxic epidermal necrolysis associated with hydroxychloroquine [published correction appears in Clin Exp Dermatol 2001 Sep;26(6):560]. Clin Exp Dermatol. 2001;26(5):457‐458. [DOI] [PubMed] [Google Scholar]


