Highlights
-
•
161 lower limb reconstruction surgeries for pathological or impending pathological fractures were included in the study.
-
•
46.6% of patients did not receive radiation within 12 weeks of surgery.
-
•
Among patients not receiving post-operative radiation, 6.7% required a second operation to the index surgical site.
-
•
Among patients not receiving post-operative radiation, 16.0% later received radiation to the index surgical site."?>
Keywords: Bone cancer, Bone metastases, Surgical complications, Lower extremity, Radiation therapy, Bone reconstruction
Abstract
Purpose
Pathological metastatic fractures in lower-extremity weight bearing bones often require surgical reconstruction. Post-operative radiation is routinely recommended following surgical reconstruction. This study evaluated the clinical outcomes of patients that undergo surgical fixation of an established or an impending pathologic lower extremity fracture without post-operative radiation.
Materials and methods
A retrospective chart review of patients at Sunnybrook Health Sciences Center between 2007 and 2019 was performed. Descriptive statistical analyses were performed.
Results
A total of 161 surgical reconstruction procedures were identified. Among these cases, 86/161 (53.4%) received post-operative radiation, 75/161 (47%) did not receive post-operative radiation within 12 weeks of their index surgery. Of the 75 patients not receiving post-operative radiation, 40 patients had prior radiation to the surgical site and 35 patients were radiation naïve. 5 patients (6.7%) required a second operation to the index surgical site, with 4 patients (5.3%) requiring a second fixation surgery to stabilize hardware at a median of 6.0 months post-surgery. Post-surgical radiation to the surgical site (at >12 weeks) was administered to 12 patients (16.0%) at a median of 9.1 months post-surgery.
Conclusions
The surgical revision rate was low despite absence of immediate post-operative radiation therapy and was similar to prior reports in patients receiving post-operative radiation.
1. Background
For patients with metastatic cancer, bone represents the location of 70% of all metastases [1], [2], [3]. Approximately 5–10% of all patients with bone metastases develop pathological fractures [1,4,5], and lytic lesions in weight-bearing long bones are of particular concern for fracture risk [6,7]. Following the radiological detection of an established pathological fracture(s), surgical reconstruction through either fracture fixation and/or joint reconstruction (i.e. arthroplasty) can be helpful to stabilize the bone, and alleviate pain [8]. If the local disease progresses post-operatively, prosthetic failure and/or pain may follow, and subsequent surgery may become necessary [4].
To reduce the likelihood of disease progression, immediate post-operative radiation (within 12 weeks of the surgery) is commonly performed. If successful, post-operative radiation should reduce hardware failure and decrease the need for subsequent orthopedic surgeries to the same site [4,5,9]. However, the efficacy of post-operative radiation has only been evaluated in a limited number of studies. A retrospective study by Townsend et al. was published in 1995 in which 60 patients with 64 surgical stabilizations for pathological fractures resulting from bone metastases were reviewed [5]. Of the 64 evaluated sites, 35 received radiation post-operatively (S+RT) and 29 received surgery alone (SA). Second orthopedic procedures to the same site were more common in the SA group (4 patients out of 29 sites/26 patients, 15%) and occurred at a mean time of 12.5 months (range 1.3–40 months) following the primary surgery. Alternatively, second orthopedic procedures to the same site in the S+RT group were significantly lower with only one additional surgery (1 out of 35 sites/34 patients, 2.9%) (p = 0.035) which occurred at 21 months following the initial surgery. In the multivariate analysis, only post-operative radiation was significantly associated with painless use or normal use with pain of the treated extremity (p = 0.02), improved functioning in the first year (53% vs. 11.5%) (p<0.01) and overall survival (12.4 months vs. 3.3 months, p = 0.025) when compared to SA. The author's conclusions were in support of post-operative radiation.
However, conflicting results were reported in a 1997 study by Van Geffen et al. involving 116 patients with 152 impending fractures of the extremities, where post-operative radiation was administered to only 28% of patients. For the entire cohort, 79% regained walking ability and 60% required no or occasional use of analgesic drugs. Regarding complications, 21% of patients in the SA group reported complications such as implant failure or disease progression compared to 14% in the S+RT groups, however, this was not statistically significant (p = 0.3). Unlike the study by Townsend et al., the Van Geffen study did not report on the incidence of second surgeries. The authors concluded that there was no significant difference in pain relief or use of analgesic drugs regardless of the administration of post-operative radiation treatment [4].
A recent systematic review challenged the practice of routine post-op radiation, reporting that sufficient evidence is lacking to allow for firm conclusions on its efficacy and adoption as standard care for patients undergoing surgical fixation for bony metastases [1]. To study this further, the investigators at Odette Cancer Centre, Sunnybrook Health Science Centre, Toronto, Canada conducted a retrospective review of patients who underwent surgical reconstruction (fixation or arthroplasty) of a pathologic fracture and were subsequently treated with immediate post-operative radiation to the lower extremities between January 2009 and January 2017 [9]. Of the 74 fracture sites assessed in 65 patients, only 2 (2.7%) required additional surgical intervention (at 9 and 10 months following radiation respectively) and 7 (9.5%) required re-irradiation (median 9.3 months following radiation). Additionally, of the 47 patients with available follow-up imaging, only 8 (17.0%) demonstrated local progression (median 2.1 months following radiation). This result is consistent with a study conducted by Epstein-Peterson et al. on patients with post-operative radiation for bone metastases (n = 82) which found that 14 patients (17.0%) showed local progression in imaging [10]. The study by Epstein-Peterson et al. also found that increasing coverage of the surgical prosthesis in the radiation fields was significantly associated with a reduced risk of local failure upon multivariate analysis (p = 0.03). Conversely, it was found that increased time between surgery and radiation was associated with an increased risk of local failure (p = 0.01).
There is little exploration into comparative outcomes in patients who receive surgery without immediate postoperative radiotherapy other than the 2 studies published in 1995 and 1997 [4,5]. In the current paper we explored the clinical outcomes among patients receiving surgery for lower extremity bone metastasis but not immediate postoperative radiation.
2. Materials and methods
A single-arm retrospective study was conducted in cancer patients with bone metastases who received lower extremity orthopedic reconstruction at Sunnybrook Health Sciences Centre for impending or established pathological fractures between January 2007 and June 2019. Patients were included if they had received (1) surgical fixation of impending or pathologic fractures in the extremities due to bone metastases; and (2) no post-operative radiation to the surgical site for 12 weeks following initial surgery. Patients with a primary bone tumor or suspected osteoarthritis were not included in the study. Any radiotherapy to the site that occurred after 12 weeks post-surgery was considered subsequent palliative radiation, and these patients maintained eligibility for this retrospective analysis. Patient's follow-up clinical consultation notes with the treating orthopedic surgeon were also assessed to ensure that the patient did not receive postoperative radiotherapy at a different radiation center within 3 months after surgery. Pre-operative radiation was also recorded from the medical records including duration, type and dose of radiation therapy to the surgical site prior to the surgery. The reasons for not receiving post-operative radiation were recorded if available. All radiation records from the patient charts were cross referenced against the Mosaiq radiation treatment database at the Odette Cancer Center. Dates of death and/or date of last follow up were extracted from the medical records.
Patients charts were identified using various search strategies including: (1) patients referred through the Sunnybrook bone metastasis clinic between January 2007 and June 2019, (2) Ontario Health Insurance Plan (OHIP) billing codes submitted by Sunnybrook Division of Orthopedic Surgery surgeons between January 2014 and June 2019 and (3) an internal hospital record database search conducted between January 2016 to June 2019.
Demographic information, pre- and post-radiation treatment plans, and details regarding the surgical procedures were extracted from the medical records. Study approval was obtained from the Research Ethics Board. All data recorded were analyzed using descriptive statistics.
The primary objective of this study was to evaluate the need for second surgery to the initial surgical site. Operative reports from the medical records of all included patients established surgical details including date, type and site of procedure. The secondary objectives were to assess the need for re-irradiation and radiological changes at the treatment site over time following surgery. Pre-operative radiation to the surgical site was also noted. All radiation treatments were recorded with treatment details including dosage, fractionation, date, technique and site of treatment. The most recent radiological imaging of the surgical site was reviewed and analyzed to determined frequencies of tumor progression, new bony metastasis, loosening of hardware, prosthesis displacement and/or failure, and new pathological fracture at initial surgical site. All imaging studies that identified osteolytic changes were reviewed by both a staff radiologist and orthopedic surgeon. Time to specified outcome (second surgery, re-irradiation, and radiological changes) was calculated from the date of initial surgery.
3. Results
3.1. Patient inclusion
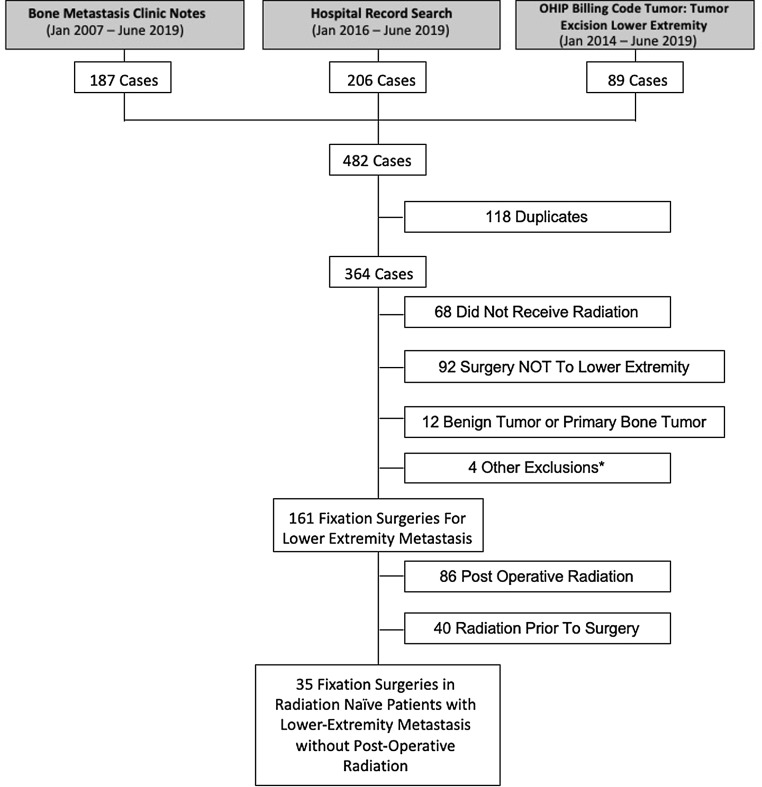
There were 482 cases screened for this study. From these 482 cases, 161 cases of reconstruction surgery for lower extremity bone metastasis were identified. Among these 161 cases, 86 (53.4%) received planned post-operative radiation within 12 weeks of their index surgery, and 75/161 (46.6%) did not receive post-operative radiation within 12 weeks of their index surgery (Fig. 1). These latter 75 patients are the subject of this report.
Fig. 1.
Patient Inclusion Schema. *Other reasons for exclusion included 1) surgery for soft tissue infection, 2) gunshot wound, 3) motor vehicle accident (MVA) and 4) knee arthroscopy.
3.2. Demographics
The median age was 64.8 years old (range 41–90) (Table 1). The patient population was predominantly female with 46 women (61.3%) and 29 men (38.7%) (Table 1). The most common primary cancer sites were breast (29.3%), lung (16.0%) and prostate (16.0%) (Table 1).
Table 1.
Patient demographics.
| Demographics | Combined | Radiation naïve | Pre-operative radiation |
|---|---|---|---|
| n (%) N = 75 | n (%) N = 35 | n (%) N = 40 | |
| Age (years) | |||
| 40–49 | 11 (14.7%) | 5 (14.3%) | 6 (15.0%) |
| 50–59 | 14 (18.7%) | 3 (8.6%) | 11 (27.5%) |
| 60–69 | 25 (33.3%) | 13 (37.1%) | 12 (30.0%) |
| 70–79 | 17 (22.7%) | 9 (25.7%) | 8 (20.0%) |
| 80–89 | 7 (9.3%) | 5 (14.3%) | 2 (5.0%) |
| 90–99 | 1(1.3%) | 0 (0.0%) | 1 (2.5%) |
| Gender | |||
| Female | 46 (61.3%) | 18 (51.4%) | 28 (70.0%) |
| Male | 29 (38.7%) | 17 (48.6%) | 12 (30.0%) |
| Primary cancer site | |||
| Breast | 22 (29.3%) | 8 (22.9%) | 14 (35.0%) |
| Lung | 12 (16.0%) | 5 (14.3%) | 7 (17.5%) |
| Prostate | 12 (16.0%) | 7 (20.0%) | 5 (12.5%) |
| Renal cell | 10 (13.3%) | 5 (14.3%) | 5 (12.5%) |
| Multiple myeloma | 5 (6.7%) | 2 (5.7%) | 3 (7.5%) |
| Others | 13 (17.3%) | 7 (20.0%) | 6 (15.0%) |
| Unknown | 1 (1.3%) | 1 (2.9%) | 0 (0.0%) |
3.3. Surgical characteristics
The majority of the surgeries included in this study were femur fixation surgeries (58.7%) (Table 2). The remaining surgeries were either hip arthroplasty (37.3%) or tibia fixation (4.0%) (Table 2). The surgeries were predominately for established pathologic fractures (58.7%) presenting in the emergency room or by urgent referral from the Odette Cancer Centre Bone Metastasis Clinic (Table 2). 38.7% of the surgeries were done to stabilize impending fractures (Table 2).
Table 2.
Surgical treatment information.
| Surgical treatment info | Combined | Radiation naïve | Pre-operative radiation |
|---|---|---|---|
| N (75) | N (35) | N (40) | |
| Site of surgery | |||
| Femur | 44 (58.7%) | 23 (65.7%) | 21 (52.5%) |
| Hip (Femur) | 21 (28.0%) | 9 (25.7%) | 12 (30.0%) |
| Hip (Acetabulum) | 7 (9.3%) | 2 (5.7%) | 5 (12.5%) |
| Tibia | 3 (4.0%) | 1 (2.9%) | 2 (5.0%) |
| Acute or prophylactic surgery | |||
| Established fracture | 44 (58.7%) | 21 (60.0%) | 23 (57.5%) |
| Prophylactic Stabilization | 29 (38.7%) | 13 (37.1%) | 16 (40.0%) |
| Unknown | 2 (2.7%) | 1 (2.9%) | 1 (2.5%) |
3.4. Radiation prior to surgery
Of the total cohort, 40 (53.3%) of patients received radiation treatment to the surgical site prior to surgery (Table 3). Radiation treatment occurred a median of 5.4 months prior to surgical intervention. Of the 40 patients receiving pre-surgery radiation, 28 (70.0%) of these patients had one course of radiation to the surgical site and 12 (30.0%) had multiple courses of radiation prior to surgery (Table 3). Among those receiving only one course of pre-surgery radiation a dose of 20 Gy in 5 fractions was the most common (10 patients, 40.0%) (Table 3). In those receiving multiple doses of presurgical radiation, a dosing schedule of 20 Gy in 5 fractions was the most common (10 patients, 52.6%) (Table 3).
Table 3.
Radiation prior to surgery.
| N (75) | % | Median time prior to surgery (months) | |
|---|---|---|---|
| No radiation to site prior to surgery | 35 | 46.7% | |
| Radiation prior to surgery | 40 | 53.3% | 5.4 |
| Single radiation Tx prior to surgery | 28 | 37.0% | 3.4 |
| Dosage of radiation | |||
| 20 Gy/5 | 10 (40.0%) | ||
| 8 Gy/1 | 6 (24.0%) | ||
| 30 Gy/10 | 6 (24.0%) | ||
| Other | 6 (24.0%) | ||
| Multiple radiation Tx prior to surgery | 12 | 16.0% | 6.7 |
| Total radiation therapy cycles | 27 | ||
| Dosage of radiation | 10 (52.6%) | ||
| 20 Gy/5 | |||
| 8 Gy/1 | 7 (36.8%) | ||
| 30 Gy/10 | 3 (15.8%) | ||
| Other | 7 (36.8%) | ||
3.5. Need for second surgery
Out of the 75 patients who received surgery, 5 (6.7%) received secondary surgery to the initial surgical site (Table 4). One of these 5 surgeries was an irrigation and debridement of infected tissue at 24 days following the initial surgery. A 62 year old female with metastatic renal cell carcinoma who underwent right hip bipolar cemented hemiarthroplasty for femoral head and neck osteolytic lesions developed right hip pain, swelling, wound erythema and drainage. Irrigation and debridement including bipolar head and neck exchange was performed with Methicillin-sensitive Staphylococcus Aureus (MSSA) isolated from wound and blood cultures. This patient developed pneumonia, episodes of hypotension, a bleeding duodenal ulcer that was treated, and expired 2 weeks after revision surgery. The remaining 4 surgeries included 2 cases (2.7%) of failed hardware and repeated fixation of initial pathological fracture, and 2 case (2.7%) of repeated fixation of fracture without hardware failure and removal (Table 4). Repeated fixation surgeries, including those with intact hardware, were performed at 2.5, 3.9, 8.1 and 8.1 months post initial surgery respectively. Among radiation naïve patients (N = 35) there was only 1 s surgery (2.9% of patients) to the initial surgical site (Table 4). This surgery was a fixation surgery with hardware failure and occurred at 3.9 months following the index surgery. Among patients with preoperative radiation (N = 40) there were 4 s surgeries (10%) including 3 fixation surgeries (7.5%) at a median of 8.1 months and 1 irrigation and debridement (2.5%) at 0.8 months (Table 4). Reasons for surgery were predominantly for emergent repair of broken hardware. For the patient who received fixation surgery without hardware failure, the patient had experienced a mechanical fall while getting out of bed and presented to emergency with pain and associated fracture. Surgery was felt to be the best option to control pain and stabilize the fracture.
Table 4.
Treatment sites requiring subsequent interventions.
| Subsequent intervention | Combined |
Radiation naïve |
Pre-operative radiation |
|||
|---|---|---|---|---|---|---|
| N (%) N = 75 | Median time (months) | N (%) N = 35 | Median time (months) | N (%) N = 40 | Median time (months) | |
| Second surgery to same site | 5 (6.7%) | 1 (2.9%) | 3.9 | 4 (10%) | 8.1 | |
| Irrigation and debridement | 1 (1.3%) | 0.8 | 1 (2.5%) | 0.8 | ||
| Second fixation | 4 (5.3%) | 6.0 | 3 (7.5) | 8.1 | ||
| with hardware failure | 2 (2.7%) | 6.0 | 1 (2.9%) | 3.9 | 2 (5.0%) | 5.3 |
| without hardware failure | 2 (2.7%) | 5.2 | 1 (2.5%) | 8.1 | ||
| Re-irradiation of postoperative site | 12 (16.0%) | 9.9 | 6 (17.1%) | 7.4 | 6 (15.0%) | 11.3 |
3.6. Need for radiation
Of the 75 patients receiving surgery alone, 12 (16.0%) patients eventually underwent delayed (beyond 3 months from the surgery date) radiation of the tumor site following surgery. Radiation occurred at a median of 9.9 months following initial surgery in these patients. The most common reason for receiving post-operative radiation was to control pain at the surgical site (7/12). In another 4 patients (4/12) radiation was given to control progression at the surgical site in the absence of patient symptoms. In one case (1/12) the purpose of the radiation was not noted in the clinical notes. Among radiation naïve patients, 6 of 35 (17.1%) required radiation at the tumor site at a median of 7.4 months post-operatively. In patients who had received pre-operative radiation, 6 of 40 (15.0%) required a re-irradiation of the tumor site at a median of 11.3 months post-operatively.
3.7. Assessment of radiological changes
A total of 55 patients (73%) had radiological imaging post-surgery (Table 5). These 55 patients were followed with radiological imaging for a median of 3.6 months post-surgery (Range - 0.13–88.7 months) (Table 5). There were 16 (29.1%) patients that showed evidence of osteolysis at the index surgical site. Only 1 of 16 patients with osteolysis was determined to have progressive osteolysis, and these progressive osteolytic changes were first observed at 106 days following the initial operation. Another 5 patients (9.1%) showed new bone metastasis, 2 (3.6%) showed hardware loosening, 8 (14.5%) had migration of implanted hardware or a new pathologic fracture, and 2 patients (3.6%) had hardware failure at the surgical site at 4.1 and 7.8 months respectively (Table 5). Among radiation naïve patients, no patients had evidence of osteolysis at the surgical site, 1(3.3%) had new bone metastasis at 18.3 months post-operatively. 1 (3.3%) had loosening of hardware at 32.3 months post-operatively, and 2 (6.7%) had migration of hardware at a median of 12.5 months. Only 1 (3.3%) patient experienced hardware failure at 4.1 months post-surgery.
Table 5.
Radiological changes to surgical site.
| Radiological changes | Combined | Radiation naïve |
Pre-operative radiation |
|||
|---|---|---|---|---|---|---|
| N (55) | Median time to event (months) | N (30) | Median time to event (months) | N (25) | Median time to event (months) | |
| Osteolysis at surgical site | 16 (29%) | |||||
| Progressive Osteolysis | 1 (2%) | 2.1 | 0 (0%) | 1 (4%) | 2.1 | |
| Stable Osteolysis | 15 (27%) | 6 (20%) | 9 (36%) | |||
| New Bone Metastasis | 5 (9%) | 9.2 | 1 (3%) | 18.3 | 4 (16%) | 8.2 |
| Loosening of Hardware | 2 (4%) | 20.1 | 1 (3%) | 32.3 | 1 (4%) | 7.8 |
| Migration of Hardware or New Pathologic Fracture | 8 (15%) | 2.1 | 2 (7%) | 12.5 | 6 (24%) | 0.7 |
| Failure of Hardware | 2 (4%) | 11.3 | 1 (3%) | 4.1 | 1 (4%) | 18.5 |
| Radiological Follow Up | N (75) | |||||
| No Radiological Follow-Up | 20 (26.7%) | |||||
| Radiological Follow-up | 55 (73.3%) | |||||
| Months | ||||||
| Median Radiological Follow Up | 3.6 | |||||
| IQR | 15.1 (Q1:1.0, Q3:16.1) | |||||
| Min, Max | 0.13, 88.7 | |||||
| Mean Radiological Follow Up | 9.9 | |||||
3.8. Post-surgical survival and follow-up
Patients were followed for a median of 4.7 months following their initial surgery (Table 6). As of July 2019, 27 patients (36.0%) were deceased among the study cohort with a mean time of 5.1 months between surgical stabilization and death. Among the 35 radiation naïve patients, the median follow up was 4.7 months, and 17 (48.6%) were decreased with a median time to death of 6.6 months. Most post-surgical deaths were related to complications and/or decreased functional status following the initial surgery.
Table 6.
Post-surgical survival and follow-up.
| Combined (N = 75) | Radiation naïve (N = 35) | Pre-operative radiation (N = 40) | |
|---|---|---|---|
| Follow up | Months | ||
| Median follow up post surgery | 4.7 | 4.7 | 4.0 |
| Min, Max | 0.0, 133.9 | 0.0, 120.7 | 0.2, 133.9 |
| IQR | 16.7 | 19.47 | 13.9 |
| Survival | N (75) | N (35) | N (40) |
| Alive or lost to follow-up | 48 (62%) | 18 (51.4%) | 30 (72.5%) |
| Deceased | 27 (36%) | 17 (48.6%) | 10 (25%) |
| Patients surviving <3 Months | 18 (24.0%) | 11 (31.4%) | 7 (17.5%) |
| Median time to death | 1.3 months | 1.2 months | 1.7 months |
3.9. Reasons for not receiving radiation treatment
The reasons for not receiving post-operative radiation were recorded if available (Table 7). The most common reasons for not receiving treatment were lack of proper referral to radiation oncology (28/75, 37.3%) and complications post-surgery resulting in prolonged hospitalization (26/75, 34.7%).
Table 7.
Reasons for not receiving post-operative radiation.
| Reasons for not receiving post-operative radiation | Number (N = 75) |
|---|---|
| Not seen or referred to radiation oncology | 28 |
| Managed in emergency department | 3 |
| Managed as outpatient/inpatient surgical referral | 25 |
| Complicated post-surgery course | 26 |
| Not discharged from hospital, patient deceased | 18 |
| Discharged from hospital with recovery | 8 |
| Radiation to surgical site not within post-operative window | 5 |
| Radiation therapy considered unnecessary with pain improvement post-surgery | 4 |
| Receiving chemotherapy and/or other cancer treatment post-operatively | 3 |
| radiation to the surgical site prior to surgery, radiation deemed unnecessary | 2 |
| tumor previously did not respond to radiation treatment | 1 |
| Concerns about functional consequences of radiation treatment to surgical site | 1 |
| Received radio-ablation to surgical site during surgery | 1 |
| Other/Unknown | 1 |
| Total | 75 |
4. Discussion
Pathological and impending fractures in advanced cancer patients with bone metastasis often can have profound implications on patient quality of life and mobility and often require surgical stabilization [13]. Early postoperative radiation is routinely used in clinical practice and is thought to decrease the incidence of tumor progression and/or recurrence, which would then theoretically reduce the incidence of post-surgical complications such as hardware failure, pain, and the need for a second surgery [4,5,9]. In this study, we report on a total of 161 patients who had surgery for pathologic fractures or impending fractures, focusing on the 75 patients (46.6%) who did not receive immediate post-operative radiation. While none of the patients included in the current study received immediate post-operative radiation, a majority of them did receive radiation to the surgical site in the months leading up to their operation. As stated in Table 3, 53.3% (40/75) of the cohort had radiation to the site at a median of 5.4 months prior to their operation.
The primary endpoint in this study was second surgery to index surgical site, and the frequency of repeat fixation second surgery in our patient cohort was 5.3% (4 patients). These surgeries occurred at 2.5, 3.9, 8.1 and 8.1 months after the index surgery. Looking specifically at the subgroup of patients in our study who were radiation naïve preoperatively, only 1 patient (2.9% of this subgroup) required a second surgery, which occurred at 3.9 months following the index operation.
In 2017, Drost et al. [9] reported on radiation naïve patients receiving fixation surgery with planned post-operative radiation, finding that 2 of 74 patients (2.7%) required second surgery, occurring at 9 and 10 months after the original surgery. Comparing this study to our results, the findings suggest similar rates of second surgery in radiation naïve patients receiving early post-operative radiation and those not receiving early post-operative radiation. Interestingly, in our study the rates of second surgery were slightly elevated among patients with pre-operative radiation exposure. In this group, 3/40 patients (7.5%) had a repeated fixation surgery and 1/40 (2.5%) required irrigation and debridement of an infected surgical site. It is possible that these patients represent a group with worsened disease course and functional status going into surgery. It is also possible that the pre-operative radiation had a negative effect on bone and/or soft tissue healing, predisposing these patients to worse biological and biomechanical properties at the surgical site.
The results of our study seem to contradict the previous results of Townsend et al. [5], which suggest that the rates of second surgical intervention were significantly higher in patients receiving surgery alone (with no post-operative radiation) for bony metastases. Townsend et al. reported rates of second surgery of 15% (4/26 patients) in the surgery alone (SA) group, and 2.9% (1/34 patients) in the surgery plus radiation therapy (S+RT) group. Like the Drost et al. study, Townsend also reported longer times between the index surgery and the secondary surgery in the S+RT group. The second surgeries in the surgery alone arm of the Townsend study occur at a mean of 12.5 months after the index surgery, and those in the Drost study also occur at a mean of 9.5 months after the index surgery, compared to a mean of 5.7 months in our current study. These results suggest that post-surgery radiation may be prolonging the interval between initial surgery and second surgery for hardware complications.
Of importance is the single infection identified in our study. In this patient, the diagnosis of infection was made within days of the surgery, and the repeat surgery (irrigation and debridement) was performed at day 24 post-op from the index surgery. As the typical application of planned post-op radiation in these patients is 4–12 weeks post-op, the infection in this case would have presented prior to the patient having had a chance to receive the radiation, had this been planned.
This study also highlights the significant rates of mortality observed in this cohort. Among the included surgery alone patient population, 36% (27/75) patients were deceased during the study period with an average survival time of 5.1 months between surgical stabilization and death. Among these patients, 18 patients (24% of total surgery alone group) died within the initial 12-week postoperative period.
The low rate of repeat surgical fixation procedures in this surgery alone group in conjunction with the high mortality rate observed in this cohort is clinically significant. Orthopedic surgery is an invasive procedure, especially in advanced cancer patients whose survival and quality of life is often limited [1]. However, if the rates of second fixation surgery are comparable in both the surgery alone and surgery with post-operative radiation treatment groups it may be possible to spare patients from having post-operative radiation to the treatment site. Post-operative radiation comes with acute and long-term risks. In particular, post-operative radiation may put patients at risk for temporary pain flare [11,12], local soft tissue irritation [13] as well as fatigue and nausea [14,15]. Radiation therapy also carries with it a significant time and effort commitment from the patient who has to commute to and from the hospital while receiving their course of radiation treatment, typically on a daily basis for 6 weeks. Therefore, at least during the 4 to 6 weeks of therapy, radiation therapy has a significant negative effect on the quality of life for these patients. There is also a significant cost associated with post-operative radiation therapy. In Ontario, it is estimated that a course of radiation therapy costs between $5270 CAD and $14,155 CAD depending on the radiation technique, disease site, and planning complexity [16].
The costs and implications of radiation therapy on quality of life should above all be considered in the context of high mortality rates in advanced cancer patients. In a study of pathologic fractures in patients with malignant bone disease, overall mortality at 24 months was estimated to be 56.4% [17]. Our current guidelines are suggesting post-operative radiation therapy to this cohort of patients in order to reduce the risk of second fixation surgery to the index surgical site. Not only is the risk of second surgery very low at 2.8–10.0% (Table 4) depending on the radiation status pre-surgery, but the 2-year survival of this patient cohort is less than 50%. Thus, it needs to be seriously considered whether subjecting patients to post-operative radiation to limit the small risk of repeat fixation surgery is warranted in a patient population with such a high risk of mortality unrelated to the index surgery.
Patients who are not scheduled for early post-op radiation therapy may ultimately undergo delayed radiotherapy for a variety of reasons including no improvement in pain, or partial relief or relapse in pain after experiencing initial relief following surgery [18,19]. Rates of post-surgery radiation occurring at >12 weeks in this study seem to be consistent or slightly elevated when compared with rates of re-irradiation in patients with bone metastases receiving surgery and post-operative radiation. In our study we report a rate of delayed (>12weeks) radiation of 16%. Drost et al. [9] reports a lower re-irradiation rate of 9.5%. A recent systematic review by Huisman et al. suggests that rates of re-irradiation differ quite significantly ranging from 8% to 42% in advanced cancer patients and is dependent on the dose of the initial treatment [18].
Most of the patients (55/75, 73%) in this study had some form of radiological follow-up post-operatively. Of particular interest were any osteolytic changes observed at the treatment site post-operatively, which were observed in 16 of 55 (29%) of the patient population. However, only 1 of these 16 cases of osteolysis was considered to be progressive. While the results seem comparable to those reported by Drost et al. [9], given the heterogenous nature of the patient population with respect to age, cancer type, and prior cancer treatment, osteolytic lesions may be more likely in certain patients and depending on the primary cancer, may appear differently on radiological imaging. As such, this endpoint is likely not as reliable as repeat surgery and radiation of the surgical site.
This study has several limitations. It was a retrospective study. We did not have a comprehensive database to check if patients in our study received radiation or surgery at another cancer treatment center. However most of the patients were followed in our orthopedic clinic and there was no documentation of receiving radiation or second surgery from other centers besides the Sunnybrook Odette Cancer Center. We did not have patient reported outcome scores, which would be needed to fully elucidate and compare the effects of early radiation on the patient outcomes. Further research is required that examines differences in outcomes in a controlled prospective randomized setting between patients with surgery alone and patients with surgery and immediate post-operative radiation. It should also be noted that this patient population is quite heterogenous with respect to the type of cancer type, surgical history, age and prognosis. In this setting there are many other variables that could potentially contribute to risk of complications and hardware failure. Particularly systemic chemotherapy and cancer type can substantially impact bone metabolism and osteoporosis, predisposing certain patients to surgical complications independent of post-operative radiation.
5. Conclusion
The surgical revision rate was low for patients treated with surgical reconstruction (fixation or arthroplasty) of pathologic fractures in the lower extremities, despite the absence of early post-operative radiation therapy. These results were comparable with those previously published involving patients who did receive early post-operative radiation. Given the low rates of revision surgery in both groups, these findings should be further explored with a randomized study in a larger patient population to evaluate the benefits (or lack thereof) of early post-operative radiation treatment following orthopedic fixation of pathologic fractures in the lower extremity.
Project contributions
| William Pidduck* | Formal analysis – Application of statistical, mathematical, computational, or other formal techniques to analyze or synthesize study data. |
| Writing – original draft – Preparation, creation and/or presentation of the published work, specifically writing the initial draft (including substantive translation). | |
| Writing – review & editing – Preparation, creation and/or presentation of the published work by those from the original research group, specifically critical review, commentary or revision – including pre- or post-publication stages. | |
| Leah Drost | Project administration – Management and coordination responsibility for the research activity planning and execution. |
| Software – Programming, software and supporting algorithms. | |
| Albert Yee | Conceptualization – Ideas; formulation or evolution of overarching research goals and aims. |
| Methodology – Development or design of methodology; creation of models. | |
| Writing – review & editing – Preparation, creation and/or presentation of the published work by those from the original research group, specifically critical review, commentary or revision – including pre- or post-publication stages. | |
| Edward Chow | Conceptualization – Ideas; formulation or evolution of overarching research goals and aims. |
| Methodology – Development or design of methodology; creation of models. | |
| Writing – review & editing – Preparation, creation and/or presentation of the published work by those from the original research group, specifically critical review, commentary or revision – including pre- or post-publication stages. | |
| Ravi Tuazon | Project administration – Management and coordination responsibility for the research activity planning and execution. |
| Patrick Henry* | Conceptualization – Ideas; formulation or evolution of overarching research goals and aims. |
| `Methodology – Development or design of methodology; creation of models. | |
| Supervision – Oversight and leadership responsibility for the research activity planning and execution, including mentorship external to the core team. | |
| Writing – review & editing – Preparation, creation and/or presentation of the published work by those from the original research group, specifically critical review, commentary or revision – including pre- or post-publication stages. |
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jbo.2020.100289.
Appendix. Supplementary materials
References
- 1.Willeumier J.J., van der Linden Y.M., Dijkstra P.D.S. Lack of clinical evidence for postoperative radiotherapy after surgical fixation of impending or actual pathologic fractures in the long bones in patients with cancer; a systematic review. Radiother. Oncol. 2016;121(1):138–142. doi: 10.1016/j.radonc.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 2.Pires A.O., Borges U.S., Lopes-Costa P V. Gebrim LH, da Silva BB. Evaluation of bone metastases from breast cancer by bone scintigraphy and positron emission tomography/computed tomography imaging. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014;180:138–141. doi: 10.1016/j.ejogrb.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 3.Tubiana-Hulin M. Incidence, prevalence and distribution of bone metastases. Bone. 1991;12:S9–10. doi: 10.1016/8756-3282(91)90059-r. [DOI] [PubMed] [Google Scholar]
- 4.Van Geffen E., Wobbes T., Veth R.P., Gelderman W.A. Operative management of impending pathological fractures: a critical analysis of therapy. J. Surg. Oncol. 1997;64(3):190–194. doi: 10.1002/(sici)1096-9098(199703)64:3<190::aid-jso3>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 5.Townsend P.W., Smalley S.R., Cozad S.C., Rosenthal H.G., Hassanein R.E.S. Role of postoperative radiation therapy after stabilization of fractures caused by metastatic disease. Int. J. Radiat. Oncol. 1995;31(1):43–49. doi: 10.1016/0360-3016(94)E0310-G. [DOI] [PubMed] [Google Scholar]
- 6.Kawabata Y., Matsuo K., Nezu Y., Kamiishi T., Inaba Y., Saito T. The risk assessment of pathological fracture in the proximal femur using a CT-based finite element method. J. Orthop. Sci. 2017;22(5):931–937. doi: 10.1016/j.jos.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 7.McGregor A.K., Greystoke B., Wood K., Bedwell C., Oakes R. Pathological fracture due to lytic lesion caused by a myeloid neoplasm with FIP1L1 – PDGFRA. Br. J. Haematol. 2016;174(5):660. doi: 10.1111/bjh.14203. [DOI] [PubMed] [Google Scholar]
- 8.Malviya A., Gerrand C. Evidence for orthopaedic surgery in the treatment of metastatic bone disease of the extremities: a review article. Palliat. Med. 2012;26(6):788–796. doi: 10.1177/0269216311419882. [DOI] [PubMed] [Google Scholar]
- 9.Drost L., Ganesh V., Wan B.A., Raman S., Chan S., Christakis M. Efficacy of postoperative radiation treatment for bone metastases in the extremities. Radiother. Oncol. 2017;124(1):45–48. doi: 10.1016/j.radonc.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Epstein-Peterson Z.D., Sullivan A., Krishnan M., Chen J.T., Ferrone M., Ready J. Postoperative radiation therapy for osseous metastasis: outcomes and predictors of local failure. Pract. Radiat. Oncol. 2015;5(5):e531–e536. doi: 10.1016/j.prro.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Hird A., Chow E., Zhang L., Wong R., Wu J., Sinclair E. Determining the Incidence of pain flare following palliative radiotherapy for symptomatic bone metastases: results from three Canadian Cancer Centers. Int. J. Radiat. Oncol. 2009;75(1):193–197. doi: 10.1016/j.ijrobp.2008.10.044. [DOI] [PubMed] [Google Scholar]
- 12.Gomez-Iturriaga A., Cacicedo J., Navarro A., Morillo V., Willisch P., Carvajal C. Incidence of pain flare following palliative radiotherapy for symptomatic bone metastases: multicenter prospective observational study. BMC Palliat. Care. 2015;14:48. doi: 10.1186/s12904-015-0045-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haubner F., Ohmann E., Pohl F., Strutz J., Gassner H. Wound healing after radiation therapy: review of the literature. Radiat. Oncol. 2012;7(1) doi: 10.1186/1748-717x-7-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsiao C., Daly B., Saligan L. The Etiology and management of radiotherapy-induced fatigue. Expert Rev. Qual. Life Cancer Care. 2016;1(4):323–328. doi: 10.1080/23809000.2016.1191948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feyer P., Jahn F., Jordan K. Radiation induced nausea and vomiting. Eur. J. Pharmacol. 2014;722:165–171. doi: 10.1016/j.ejphar.2013.09.069. [DOI] [PubMed] [Google Scholar]
- 16.Yong J., McGowan T., Redmond-Misner R. Estimating the costs of intensity-modulated and 3-dimensional conformal radiotherapy in Ontario. Curr. Oncol. 2016;23(3):228. doi: 10.3747/co.23.2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saad F., Lipton A., Cook R., Chen Y., Smith M., Coleman R. Pathologic fractures correlate with reduced survival in patients with malignant bone disease. Cancer. 2007;110(8):1860–1867. doi: 10.1002/cncr.22991. [DOI] [PubMed] [Google Scholar]
- 18.Wong E., Hoskin P., Bedard G. Re-irradiation for painful bone metastases – a systematic review. Radiother. Oncol. 2014;110:61–70. doi: 10.1016/j.radonc.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Huisman M., van den Bosch M.A.A.J., Wijlemans J.W. Effectiveness of re-irradiation for painful bone metastases: a systematic review and meta- analysis. Int. J. Radiat. Oncol. Biol. Phys. 2012;84:8–14. doi: 10.1016/j.ijrobp.2011.10.080. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.