Abstract
Reactive oxygen species (ROS) have been shown or at least suggested to play an essential role for cellular signaling as second messengers. NADPH oxidases represent a source of controlled ROS formation. Accordingly, understanding the role of individual NADPH oxidases bears potential to interfere with intracellular signaling cascades without disturbing the signaling itself. Many tools have been developed to study or inhibit the functions and roles of the NADPH oxidases. This short review summarizes diseases, potentially associated with NADPH oxidases, genetically modified animals, and inhibitors.
Graphical abstract
Main
The family of NADPH oxidases consists of 7 members. Those are Nox1 through 5 and Duox1 and 2. All NADPH oxidases are able to transfer electrons across biological membranes. Those electrons are provided by NADPH. While passing the membrane through the Nox subunit, electrons are transferred onto molecular oxygen to generate superoxide anions (•O2ˉ). •O2ˉ can be released unmodified or protonated and reduced to form H2O2. Despite this, NADPH oxidases differ in their mode of activity. Both together allow for a systematic classification of the individual members of the family into three groups (Fig. 1).
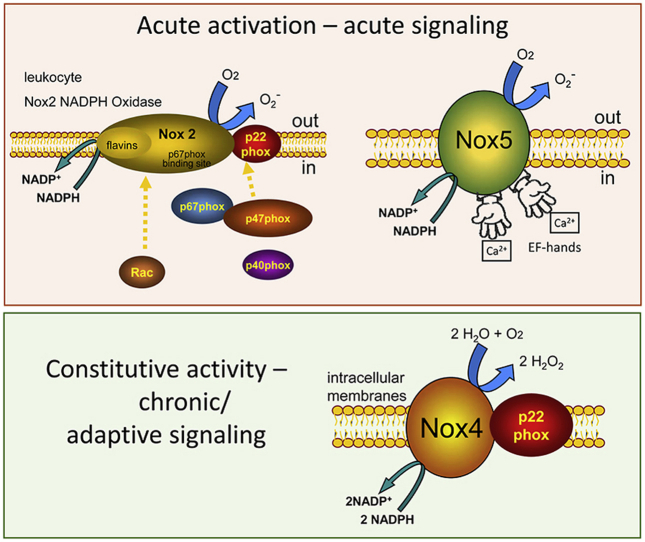
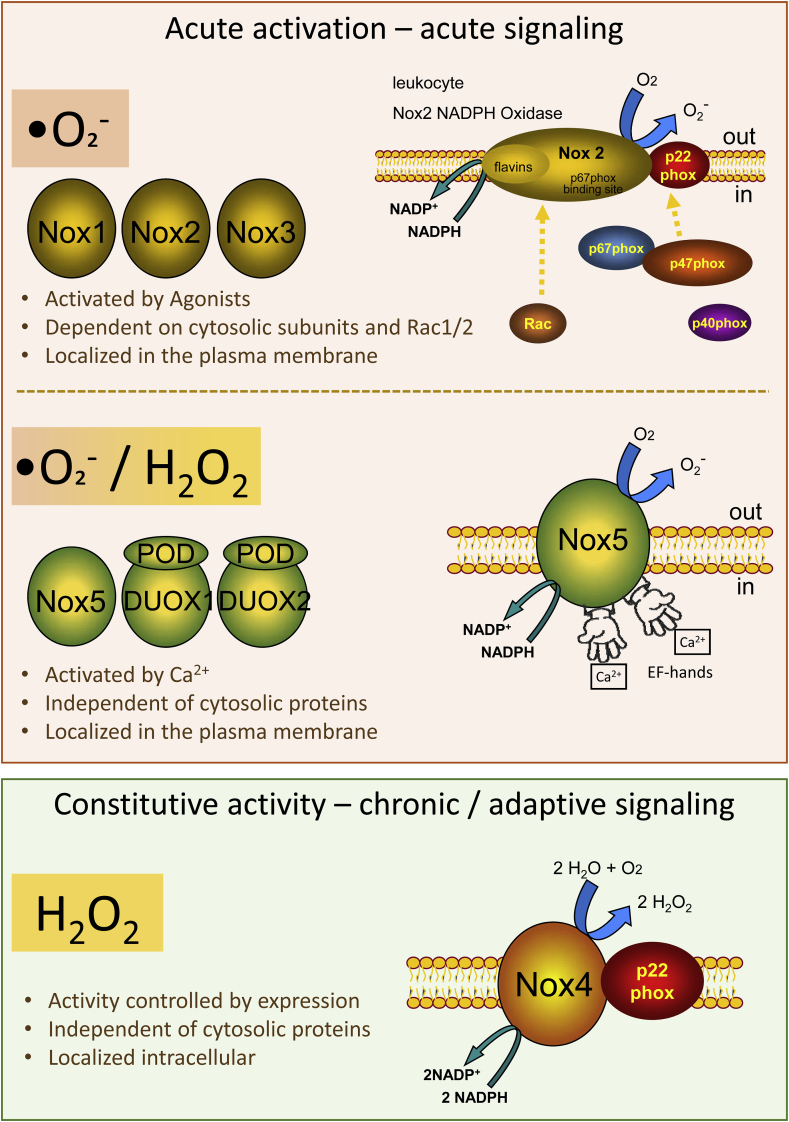
Fig. 1.
Scheme and classification of the members of the NADPH oxidase family.
NADPH oxidases can be classified into three groups according to their mode of activation. Nox1-3 are activatable via the assembly of cytosolic subunits and produce •O2ˉ. Nox5 and the Duoxes can be activated by Ca2+ and produce •O2ˉ or H2O2. The single member of group three is Nox4, which produces H2O2 in a direct manner independent from cytosolic subunits. Further explanations can be found in the text.
The acutely activatable NADPH oxidases Nox1, Nox2, and Nox3 can be pooled into group 1. The appropriate complex consists of the name-giving Nox subunit and the smaller p22phox, which stabilizes the Nox protein. Nox1-3 depend on the association of the membrane bound subunits with cytosolic proteins. The interested reader is referred to Ref. [1] for detailed information concerning the cytosolic subunits of Nox1-3. Shortly: The cytosolic components are organizers (p47pox or NoxO1) and activators (p67phox or NoxA1). The organizer proteins p47phox or NoxO1, facilitate the assembling of the other cytosolic components into the full NADPH oxidase complex. P47phox contains an autoinhibitory region (AIR). Upon phosphorylation, this AIR gets inactivated and p47phox translocates to the membrane and binds p22phox. In contrast to p47phox, its homologue NoxO1 has no AIR and shows constitutive activity, which can be modified by phosphorylation. Accordingly, phosphorylation of the organizers facilitates acute cytokine-induced ROS formation by Nox1-3. Importantly, although in overexpressing systems the cytosolic subunits can substitute for each other, this does not occur in vivo, as their expression is cell specific [2,3]. Therefore, the absence of p47phox is not counterbalanced by an elevated expression of NoxO1 and vice versa. In leucocytes, an additional subunit, p40phox, is needed for the full complex to be associated. Additionally the non-NADPH oxidase specific G-protein Rac binds to the NADPH oxidase complex in order to activate the formation of superoxide radical anions (•O2¯) by the members of group 1.
The second group of NADPH oxidases consists of the Ca2+ activated Nox5, DUOX1 and DUOX2. These NADPH oxidases are independent of cytosolic factors but instead have EF-hands that facilitate the Ca2+ sensing. While Nox5 produces mainly •O2¯, DUOX1 and 2 produce both, •O2¯, as well as H2O2 probably with the aid of their peroxidase domain (POD). Both Duoxes require the maturation factors DuoxA1 and 2 for their activity.
The sole member of the third group of NADPH oxidases is Nox4. Like Nox1-3, Nox4 is stabilized by and associated with p22phox. Despite from that Nox4 does not require any further cytosolic subunit and therefore is constitutively active. Due to a special loop in its structure Nox4 is capable to restrain single reduced •O2¯ and reduce it further to H2O2 [4].
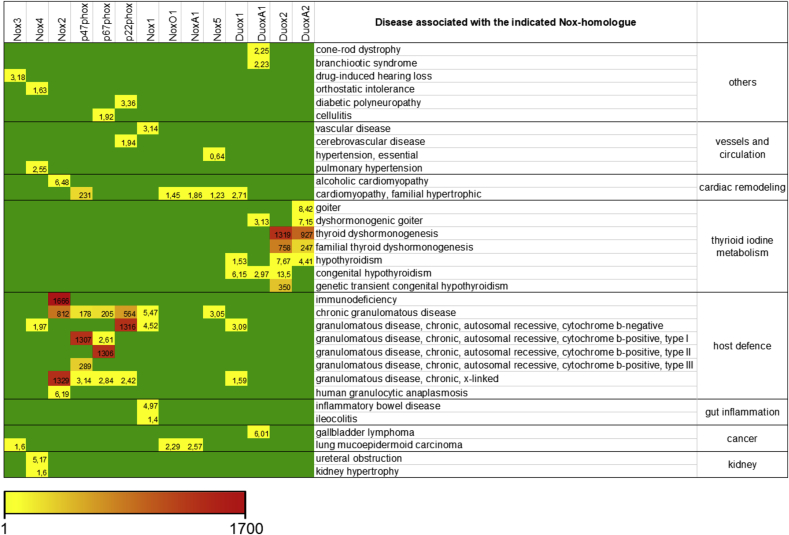
Over and above their different mode of action, NADPH oxidases also have individual intracellular localization and tissue specific expression patterns [5]. Expression and activity of NADPH oxidases are tightly controlled which enables the individual members of the family to interfere with numerous paths of signal transduction. Those include oxidation of phosphatases or kinases [6,7]. According to their complex role in regulation of cellular signaling, individual members of the family have been assigned for a number of diverse diseases in humans. Some of those are summarized in Table 1.
Table 1.
List of some diseases associated with NADPH oxidases.
All diseases listed were obtained through www.gencards.com. The analysis shows the results in the disease section of each gene in GeneCards, which is based on the MalaCard website and score. The MalaCards score ranks diseases by how closely they are associated with the gene, factoring in the relative reliability of the sources that associate them. Green indicates no relevant entry. Relevant entries are represented by numbers and the color scale indicated.
Table 1 shows basically two clusters of diseases associated with NADPH oxidases: chronic granulomatous disease and thyroid hormone production. This reflects the so far identified physiological role of the parties thereto. Nox2 and its associates p47phox and p67phox are needed for a proper fist line host defense, known as “the oxidative burst”. Accordingly, CGD (chronic granulomatous disease), a disease with inproper function or absence of one of the components of the Nox2 complex, represents with frequent infections by fungi and bacteria [8]. Duox2s physiological function is to oxidize iodine for its incorporation into the thyroid hormone. In case Duox2 or its maturation partner DuoxA2 is not present, the formation of the hormone is reduced and all kind of subsequent diseases phenotypes such as goiter and hypothyroidism occur [9]. Besides those clear cases of a physiological function of NADPH oxidases, that lead to a more or less defined and namable disease, many functions of the members of the family are unclear. Accordingly, no diseases have been identified with are solely based on the function or dysfunction of Nox1, Nox3, Nox5 or Duox1. Nevertheless, they appear to play a role in prevention or as contributors to several diseases, where their exact role often remains to be defined. In conclusion, NADPH oxidases obviously rather modulate (the development of) than cause a disease. Most literature indicates a detrimental role of NADPH oxidases in human diseases. However, beneficial roles of NADPH oxidases and ROS formation become more and more clear. As an example, Nox4 plays a role in angiogenesis, prevents bone loss upon estrogen depletion and protects from atherosclerosis [[10], [11], [12]].
Accordingly, research of the role of specific NADPH oxidases is needed to obtain a deeper understanding of their physiological roles. Tools such as knock out models or specific inhibitors have been developed. An overview of currently available animals with knock out, knock in and loss of function mutants of NADPH oxidases is provided in Table 2. This table however, might be incomplete and just provides an overview. Besides full animal approaches, the CrispR/Cas9 method opens a variety of possibilities to study the role of individual subunits of the NADPH oxidase complexes on cellular level. This approach has been successfully used, for example in Hek293 [13] and human HCT116 colon cancer cells [14].
Table 2.
List of some animal models for NADPH oxidase research.
| Target | Tool | ||
|---|---|---|---|
| knock out animal | knock in animal | Loss of function mutants | |
| Nox1 | Mouse [15] Mouse floxed [16] Zebrafish [17] |
||
| Nox2 | Mouse full ko [18] Mouse floxed [19] Zebrafish [17] |
||
| Nox3 | Mouse [20,21] Mouse [22] |
||
| Nox4 | Mouse full and floxed [23] | ||
| Nox5 | Rabbit [24,25] Zebrafish [17] |
Mouse [25,26] | |
| DUOX1 | Zebrafish [27] Mouse [28] |
||
| DUOX2 | Mouse [29] | ||
| DuoxA1&2 | Mouse floxed [30] | ||
| p22phox | Mouse floxed [31] | Mouse [32] mouse [33,34] + tyrosinase(−/−) rat [35] |
|
| p47phox | Mouse [36] | Mouse [37] | Rat [38] Mouse [39] |
| NoxO1 | Mouse [40] | ||
| p67phox | Mouse [41] | Rat [42] | |
| NoxA1 | Mouse floxed [43] | Mouse [44] | |
| p40phox | Mouse [45] | Mouse [46] |
Additionally, a brief collection of available inhibitors was added here (Table 3). For a detailed overview on NADPH oxidase inhibitors, the reader is referred to Ref. [47]. In that specific publication, the authors highlight the evolution as well as the limitations of Nox-inhibitors, antioxidants and other related compounds.
Table 3.
Inhibitor peptides and small molecules that act as NADPH oxidase inhibitors.
| Target | Inhibitor peptide | Pharmacological inhibitor |
|---|---|---|
| Nox1 | NoxA1ds (mimics a putative activation domain of NoxA1 and p67phox amino acids 199–210 in the FAD with substitution of Y199 by alanine 196 EPVDALGKAKV-CONH2 [48] | ML171 [49] GKT136901 and GKT137831 [50,51] |
| Nox2 | Endogenous PR-39 (RRR PRP PYL PRP RPP PFF PPR LPP RIP PGF PPR FPP RFP) [52] several peptides (peptide walking) [53] B-loop peptide of Nox2 that binds to p47phox: C85SRVRRQL93 [54] → Nox2ds-tat [55] works in vitro and in vivo (specifically inhibits the interaction of Nox2 and p47phox [56]) |
GSK2795039 [57] CYR5099 [58] Bridged tetrahydroisoquinolines: CPP11G and CPP11H [59] Perhexiline and Suramin (cell impermeable) [60] |
| Nox4 | GLX7013114 [61] GKT137831 [50] GKT137928 [62] ACD084 [63] Rosmarinic acid [64] |
|
| Nox5 | peptides pep1 and pep3 containing a KDSIT sequence at the c-terminus (D637−G661 + Y and R621−T660) [65] | |
| Duox1 and Duox2 | S–P-Re-J-L, wherein Re is a reactive electrophile and J is G or P [66] | Acrolein [67] |
Besides specific inhibitors, many global inhibitors for NADPH oxidases (or flavoproteins in general) and antioxidants are used. Those include diphenylene iodonium (DPI), apocynin, diapocynin and ebselen [68]. Some derivatives of the antioxidant ebselen, such as JM-77b, had a selectivity for Nox2 over Nox1, Nox4 and Nox5 [69]. This however does not mean, ebselen derivatives are specific Nox2 inhibitors. Especially in the light of the fact that ebselens are reported to display glutathione peroxidase-like activity [70].
In contrast, potential specific inhibitors often have been proved to be not specific or display off-target effects. The best investigated NADPH oxidase, Nox2, may serve as an example: Formerly known Nox2 inhibitors such as VAS2870 [71,72] and VAS3947 [73] did not fulfill their assigned roles as specific inhibitors. Both have been identified to exhibit off-target effects through thiol alkylation and inhibition of mitochondrial respiration and cytotoxicity [74,75]. Substances like celastrol inhibit Nox1, Nox2, Nox4 and Nox5, as it interferes with the binding of the proline rich region of p22phox to the tandem SH3 domain of p47phox and NoxO1 [76]. Alike, the PR-39 peptide binds other SH3-containing proteins, such as p130Cas and PI3Kp85α [77,78]. Recently, it was documented that also the Nox1/Nox4 inhibitors GKT136901 and 137831 are in fact non-specific [68,79]. Additionally, the Nox1 inhibitor ML-171 was also shown to be unspecific [80]. It appears that specificity of the inhibitors targeting a common domain in NADPH oxidases can be impeached. For further reading on how inhibitors work and fail the reader is referred to the work of Vincent Jaquet (Geneva) and Harald Schmidt (Maastricht).
Concluding remarks
Understanding the role of individual NADPH oxidases bears potential to interfere on a modulatory basis with intracellular signaling cascades. Within the last years, the collection of tools to analyze and target NADPH oxidases increased constantly. Therefore, it is important to provide an overview from time to time. This short review summarizes diseases potentially associated with NADPH oxidases, genetically modified animals, and inhibitors for some members of the family. Most references either point to a location, where to get the animals or to the first description of the animal or inhibitor. This should enable the reader to find a way to his/her tool of interest.
Funding
This work was supported by grants from the Deutsche Forschungsgemeinschaft (DFG) (to KS SCHR1241/1-1, SFB815/TP1, SFB834/TPA2).
Declaration of competing interest
The author declares no conflict of interest.
References
- 1.Schröder K., Weissmann N., Brandes R.P. Organizers and activators: cytosolic Nox proteins impacting on vascular function. Free Radic. Biol. Med. 2017;109:22–32. doi: 10.1016/j.freeradbiomed.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 2.Moll F., Walter M., Rezende F., Helfinger V., Vasconez E., de Oliveira T., Greten F.R., Olesch C., Weigert A., Radeke H.H., Schröder K. NoxO1 controls proliferation of colon epithelial cells. Front. Immunol. 2018;9:973. doi: 10.3389/fimmu.2018.00973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rezende F., Moll F., Walter M., Helfinger V., Hahner F., Janetzko P., Ringel C., Weigert A., Fleming I., Weissmann N., Kuenne C., Looso M., Rieger M.A., Nawroth P., Fleming T., Brandes R.P., Schröder K. The NADPH organizers NoxO1 and p47phox are both mediators of diabetes-induced vascular dysfunction in mice. Redox Biol. 2018;15:12–21. doi: 10.1016/j.redox.2017.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takac I., Schröder K., Zhang L., Lardy B., Anilkumar N., Lambeth J.D., Shah A.M., Morel F., Brandes R.P. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J. Biol. Chem. 2011;286:13304–13313. doi: 10.1074/jbc.M110.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sirokmány G., Donkó Á., Geiszt M. Nox/Duox family of NADPH oxidases: lessons from knockout mouse models. Trends Pharmacol. Sci. 2016;37:318–327. doi: 10.1016/j.tips.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Schröder K., Kohnen A., Aicher A., Liehn E.A., Büchse T., Stein S., Weber C., Dimmeler S., Brandes R.P. NADPH oxidase Nox2 is required for hypoxia-induced mobilization of endothelial progenitor cells. Circ. Res. 2009;105:537–544. doi: 10.1161/CIRCRESAHA.109.205138. [DOI] [PubMed] [Google Scholar]
- 7.Helfinger V., von Gall F.F., Henke N., Kunze M.M., Schmid T., Heidler J., Wittig I., Radeke H.H., Marschall V., Anderson K., Shah A.M., Fulda S., Brüne B., Brandes R.P., Schröder K. 2017. Hydrogen Peroxide Formation by Nox4 Limits Malignant Transformation. [Google Scholar]
- 8.Roos D. Chronic granulomatous disease. Methods Mol. Biol. 1982:531–542. doi: 10.1007/978-1-4939-9424-3_32. 2019. [DOI] [PubMed] [Google Scholar]
- 9.de Deken X., Miot F. DUOX defects and their roles in congenital hypothyroidism. Methods Mol. Biol. 1982:667–693. doi: 10.1007/978-1-4939-9424-3_37. 2019. [DOI] [PubMed] [Google Scholar]
- 10.Vogel J., Kruse C., Zhang M., Schröder K. Nox4 supports proper capillary growth in exercise and retina neo-vascularization. J. Physiol. (Lond ) 2015;593:2145–2154. doi: 10.1113/jphysiol.2014.284901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schürmann C., Rezende F., Kruse C., Yasar Y., Löwe O., Fork C., van de Sluis B., Bremer R., Weissmann N., Shah A.M., Jo H., Brandes R.P., Schröder K. The NADPH oxidase Nox4 has anti-atherosclerotic functions. Eur. Heart J. 2015;36:3447–3456. doi: 10.1093/eurheartj/ehv460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goettsch C., Babelova A., Trummer O., Erben R.G., Rauner M., Rammelt S., Weissmann N., Weinberger V., Benkhoff S., Kampschulte M., Obermayer-Pietsch B., Hofbauer L.C., Brandes R.P., Schröder K. NADPH oxidase 4 limits bone mass by promoting osteoclastogenesis. J. Clin. Invest. 2013;123:4731–4738. doi: 10.1172/JCI67603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prior K.-K., Leisegang M.S., Josipovic I., Löwe O., Shah A.M., Weissmann N., Schröder K., Brandes R.P. CRISPR/Cas9-mediated knockout of p22phox leads to loss of Nox1 and Nox4, but not Nox5 activity. Redox Biol. 2016;9:287–295. doi: 10.1016/j.redox.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joo J.H., Oh H., Kim M., An E.J., Kim R.-K., Lee S.-Y., Kang D.H., Kang S.W., Keun Park C., Kim H., Lee S.-J., Lee D., Seol J.H., Bae Y.S. NADPH oxidase 1 activity and ROS generation are regulated by grb2/cbl-mediated proteasomal degradation of NoxO1 in colon cancer cells. Canc. Res. 2016;76:855–865. doi: 10.1158/0008-5472.CAN-15-1512. [DOI] [PubMed] [Google Scholar]
- 15.The Jackson Laboratory https://www.jax.org/strain/018787 018787 - B6.129X1-Nox1<tm1Kkr>/J. accessed.
- 16.Leoni G., Alam A., Neumann P.-A., Lambeth J.D., Cheng G., McCoy J., Hilgarth R.S., Kundu K., Murthy N., Kusters D., Reutelingsperger C., Perretti M., Parkos C.A., Neish A.S., Nusrat A. Annexin A1, formyl peptide receptor, and NOX1 orchestrate epithelial repair. J. Clin. Invest. 2013;123:443–454. doi: 10.1172/JCI65831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weaver C.J., Terzi A., Roeder H., Gurol T., Deng Q., Leung Y.F., Suter D.M. nox2/cybb deficiency affects zebrafish retinotectal connectivity. J. Neurosci. 2018;38:5854–5871. doi: 10.1523/JNEUROSCI.1483-16.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Jackson Laboratory https://www.jax.org/strain/002365 002365 - B6.129S-Cybb<tm1Din>/J. accessed.
- 19.The Jackson Laboratory, 031777 - B6(Cg)-Cybb<tm1.1Abk>/J: NOX2-Flox. NOX2-FL. https://www.jax.org/strain/031777 (accessed 4 December 2019).
- 20.Paffenholz R., Bergstrom R.A., Pasutto F., Wabnitz P., Munroe R.J., Jagla W., Heinzmann U., Marquardt A., Bareiss A., Laufs J., Russ A., Stumm G., Schimenti J.C., Bergstrom D.E. Vestibular defects in head-tilt mice result from mutations in Nox3, encoding an NADPH oxidase. Genes Dev. 2004;18:486–491. doi: 10.1101/gad.1172504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson Laboratory The. B6.129S1-Nox3het-3J/GrsrJ: Head Tilt 3 Jackson. https://www.jax.org/strain/006228
- 22.The Jackson Laboratory, C57BL/6J-Nox3het-4J/J: C57BL/6J-Nox3nmf250/J, head tilt 4 Jackson.
- 23.The jackson laboratory. https://www.jax.org/strain/022996 022996 - B6.129-Nox4<tm1Kkr>/J. accessed.
- 24.Jha J., Dai A., Kennedy C., Ekinci E., Cooper M., Jandeleit-Dahm K. SUN-302 the relative roles of pro-oxidant ezymes Nox4 versus Nox5 in diabetic kidney disease. Kidney International Reports. 2019;4:S285. doi: 10.1016/j.ekir.2019.05.708. [DOI] [Google Scholar]
- 25.Funding Drives New Directions in Oxidative Stress Research for Diabetes and Heart Disease. 2018. https://www.monash.edu/medicine/news/latest/2018-articles/funding-drives-new-directions-in-oxidative-stress-research-for-diabetes-and-heart-disease accessed. [Google Scholar]
- 26.Kleikers P.W.M., Dao V.T., Göb E., Hooijmans C., Debets J., van Essen H., Kleinschnitz C., Schmidt H.H.H.W. SFRR-E Young Investigator AwardeeNOXing out stroke: identification of NOX4 and 5as targets in blood-brain-barrier stabilisation and neuroprotection. Free Radic. Biol. Med. 2014;75(Suppl 1):S16. doi: 10.1016/j.freeradbiomed.2014.10.593. [DOI] [PubMed] [Google Scholar]
- 27.Park J.-S., Choi T.-I., Kim O.-H., Hong T.I., Kim W.-K., Lee W.-J., Kim C.-H. Targeted knockout of duox causes defects in zebrafish growth, thyroid development, and social interaction. Journal of Genetics and Genomics. 2019;46:101–104. doi: 10.1016/j.jgg.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Donkó Á., Ruisanchez É., Orient A., Enyedi B., Kapui R., Péterfi Z., de Deken X., Benyó Z., Geiszt M. Urothelial cells produce hydrogen peroxide through the activation of Duox1. Free Radic. Biol. Med. 2010;49:2040–2048. doi: 10.1016/j.freeradbiomed.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 29.The Jackson Laboratory, 005543 - B6(129)-Duox2<thyd>/J. https://www.jax.org/strain/005543 accessed.
- 30.Grasberger H., de Deken X., Mayo O.B., Raad H., Weiss M., Liao X.-H., Refetoff S. Mice deficient in dual oxidase maturation factors are severely hypothyroid. Mol. Endocrinol. 2012;26:481–492. doi: 10.1210/me.2011-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lob H.E., Schultz D., Marvar P.J., Davisson R.L., Harrison D.G. Role of the NADPH oxidases in the subfornical organ in angiotensin II-induced hypertension. Hypertension. 2013;61:382–387. doi: 10.1161/HYPERTENSIONAHA.111.00546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.International Mouse Strain Resource Cyba<nmf333> Chemically Induced Allele Detail MGI Mouse. http://www.informatics.jax.org/allele/key/35281 (MGI:3526726), accessed.
- 33.Nakano Y., Longo-Guess C.M., Bergstrom D.E., Nauseef W.M., Jones S.M., Bánfi B. Mutation of the Cyba gene encoding p22phox causes vestibular and immune defects in mice. J. Clin. Invest. 2008;118:1176–1185. doi: 10.1172/JCI33835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.The Jackson Laboratory, 005445 - A.B6 Tyr<+>-Cyba<nmf333>/J. https://www.jax.org/strain/005445 (accessed 29 December 2019).
- 35.Mori M., Li G., Hashimoto M., Nishio A., Tomozawa H., Suzuki N., Usami S.-i., Higuchi K., Matsumoto K. Pivotal Advance: eosinophilia in the MES rat strain is caused by a loss-of-function mutation in the gene for cytochrome b(-245), alpha polypeptide (Cyba) J. Leukoc. Biol. 2009;86:473–478. doi: 10.1189/jlb.1108715. [DOI] [PubMed] [Google Scholar]
- 36.The Jackson Laboratory https://www.jax.org/strain/027331 027331 - B6N.129S2-Ncf1<tm1Shl>/J. accessed.
- 37.Sareila O., Hagert C., Kelkka T., Linja M., Xu B., Kihlberg J., Holmdahl R. Reactive oxygen species regulate both priming and established arthritis, but with different mechanisms. Antioxidants Redox Signal. 2017;27:1473–1490. doi: 10.1089/ars.2016.6981. [DOI] [PubMed] [Google Scholar]
- 38.Olofsson P., Holmberg J., Tordsson J., Lu S., Akerström B., Holmdahl R. Positional identification of Ncf1 as a gene that regulates arthritis severity in rats. Nat. Genet. 2003;33:25–32. doi: 10.1038/ng1058. [DOI] [PubMed] [Google Scholar]
- 39.Hultqvist M., Olofsson P., Holmberg J., Bäckström B.T., Tordsson J., Holmdahl R. Enhanced autoimmunity, arthritis, and encephalomyelitis in mice with a reduced oxidative burst due to a mutation in the Ncf1 gene. Proc. Natl. Acad. Sci. U. S. A. 2004;101:12646–12651. doi: 10.1073/pnas.0403831101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brandes R.P., Harenkamp S., Schürmann C., Josipovic I., Rashid B., Rezende F., Löwe O., Moll F., Epah J., Eresch J., Nayak A., Kopaliani I., Penski C., Mittelbronn M., Weissmann N., Schröder K. The cytosolic NADPH oxidase subunit NoxO1 promotes an endothelial stalk cell phenotype. Arterioscler. Thromb. Vasc. Biol. 2016;36:1558–1565. doi: 10.1161/ATVBAHA.116.307132. [DOI] [PubMed] [Google Scholar]
- 41.Jacob C.O., Yu N., Yoo D.-G., Perez-Zapata L.J., Barbu E.A., Kaplan M.J., Purmalek M., Pingel J.T., Idol R.A., Dinauer M.C. Haploinsufficiency of NADPH oxidase subunit neutrophil cytosolic factor 2 is sufficient to accelerate full-blown lupus in NZM 2328 mice. Arthritis & rheumatology (Hoboken, N.J.) 2017;69:1647–1660. doi: 10.1002/art.40141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Evans L.C., Ryan R.P., Broadway E., Skelton M.M., Kurth T., Cowley A.W. Null mutation of the nicotinamide adenine dinucleotide phosphate-oxidase subunit p67phox protects the Dahl-S rat from salt-induced reductions in medullary blood flow and glomerular filtration rate. Hypertension. 2015;65:561–568. doi: 10.1161/HYPERTENSIONAHA.114.04468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flaherty J.P., Spruce C.A., Fairfield H.E., Bergstrom D.E. Generation of a conditional null allele of NADPH oxidase activator 1 (NOXA1) Genesis. 2010;48:568–575. doi: 10.1002/dvg.20655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.The Jackson Laboratory, 014601 - B6(Cg)-Noxa1<tm1Brg>/BrgJ. https://www.jax.org/strain/014601 (accessed 19 December 2019).
- 45.Ellson C.D., Davidson K., Ferguson G.J., O'Connor R., Stephens L.R., Hawkins P.T. Neutrophils from p40phox-/- mice exhibit severe defects in NADPH oxidase regulation and oxidant-dependent bacterial killing. J. Exp. Med. 2006;203:1927–1937. doi: 10.1084/jem.20052069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Winter S., Hultqvist Hopkins M., Laulund F., Holmdahl R. A reduction in intracellular reactive oxygen species due to a mutation in NCF4 promotes autoimmune arthritis in mice. Antioxidants Redox Signal. 2016;25:983–996. doi: 10.1089/ars.2016.6675. [DOI] [PubMed] [Google Scholar]
- 47.Altenhöfer S., Radermacher K.A., Kleikers P.W.M., Wingler K., Schmidt H.H.H.W. Evolution of NADPH oxidase inhibitors: selectivity and mechanisms for target engagement. Antioxidants Redox Signal. 2015;23:406–427. doi: 10.1089/ars.2013.5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ranayhossaini D.J., Rodriguez A.I., Sahoo S., Chen B.B., Mallampalli R.K., Kelley E.E., Csanyi G., Gladwin M.T., Romero G., Pagano P.J. Selective recapitulation of conserved and nonconserved regions of putative NOXA1 protein activation domain confers isoform-specific inhibition of Nox1 oxidase and attenuation of endothelial cell migration. J. Biol. Chem. 2013;288:36437–36450. doi: 10.1074/jbc.M113.521344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gianni D., Taulet N., Zhang H., DerMardirossian C., Kister J., Martinez L., Roush W.R., Brown S.J., Bokoch G.M., Rosen H. A novel and specific NADPH oxidase-1 (Nox1) small-molecule inhibitor blocks the formation of functional invadopodia in human colon cancer cells. ACS Chem. Biol. 2010;5:981–993. doi: 10.1021/cb100219n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gorin Y., Cavaglieri R.C., Khazim K., Lee D.-Y., Bruno F., Thakur S., Fanti P., Szyndralewiez C., Barnes J.L., Block K., Abboud H.E. Targeting NADPH oxidase with a novel dual Nox1/Nox4 inhibitor attenuates renal pathology in type 1 diabetes. Am. J. Physiol. Ren. Physiol. 2015;308:F1276–F1287. doi: 10.1152/ajprenal.00396.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gaggini F., Laleu B., Orchard M., Fioraso-Cartier L., Cagnon L., Houngninou-Molango S., Gradia A., Duboux G., Merlot C., Heitz F., Szyndralewiez C., Page P. Design, synthesis and biological activity of original pyrazolo-pyrido-diazepine, -pyrazine and -oxazine dione derivatives as novel dual Nox4/Nox1 inhibitors. Bioorg. Med. Chem. 2011;19:6989–6999. doi: 10.1016/j.bmc.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 52.Shi J., Ross C.R., Leto T.L., Blecha F. PR-39, a proline-rich antibacterial peptide that inhibits phagocyte NADPH oxidase activity by binding to Src homology 3 domains of p47 phox. Proc. Natl. Acad. Sci. U. S. A. 1996;93:6014–6018. doi: 10.1073/pnas.93.12.6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dahan I., Molshanski-Mor S., Pick E. Inhibition of NADPH oxidase activation by peptides mapping within the dehydrogenase region of Nox2-A "peptide walking" study. J. Leukoc. Biol. 2012;91:501–515. doi: 10.1189/jlb.1011507. [DOI] [PubMed] [Google Scholar]
- 54.DeLeo F.R., Yu L., Burritt J.B., Loetterle L.R., Bond C.W., Jesaitis A.J., Quinn M.T. Mapping sites of interaction of p47-phox and flavocytochrome b with random-sequence peptide phage display libraries. Proc. Natl. Acad. Sci. U. S. A. 1995;92:7110–7114. doi: 10.1073/pnas.92.15.7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rey F.E., Cifuentes M.E., Kiarash A., Quinn M.T., Pagano P.J. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O(2)(-) and systolic blood pressure in mice. Circ. Res. 2001;89:408–414. doi: 10.1161/hh1701.096037. [DOI] [PubMed] [Google Scholar]
- 56.Csányi G., Cifuentes-Pagano E., Al Ghouleh I., Ranayhossaini D.J., Egaña L., Lopes L.R., Jackson H.M., Kelley E.E., Pagano P.J. Nox2 B-loop peptide, Nox2ds, specifically inhibits the NADPH oxidase Nox2. Free Radic. Biol. Med. 2011;51:1116–1125. doi: 10.1016/j.freeradbiomed.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hirano K., Chen W.S., Chueng A.L.W., Dunne A.A., Seredenina T., Filippova A., Ramachandran S., Bridges A., Chaudry L., Pettman G., Allan C., Duncan S., Lee K.C., Lim J., Ma M.T., Ong A.B., Ye N.Y., Nasir S., Mulyanidewi S., Aw C.C., Oon P.P., Liao S., Li D., Johns D.G., Miller N.D., Davies C.H., Browne E.R., Matsuoka Y., Chen D.W., Jaquet V., Rutter A.R. Discovery of GSK2795039, a novel small molecule NADPH oxidase 2 inhibitor. Antioxidants Redox Signal. 2015;23:358–374. doi: 10.1089/ars.2014.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu F.-C., Yu H.-P., Chen P.-J., Yang H.-W., Chang S.-H., Tzeng C.-C., Cheng W.-J., Chen Y.-R., Chen Y.-L., Hwang T.-L. A novel NOX2 inhibitor attenuates human neutrophil oxidative stress and ameliorates inflammatory arthritis in mice. Redox Biol. 2019;26:101273. doi: 10.1016/j.redox.2019.101273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Y., Cifuentes-Pagano E., DeVallance E.R., de Jesus D.S., Sahoo S., Meijles D.N., Koes D., Camacho C.J., Ross M., St Croix C., Pagano P.J. NADPH oxidase 2 inhibitors CPP11G and CPP11H attenuate endothelial cell inflammation & vessel dysfunction and restore mouse hind-limb flow. Redox Biol. 2019;22:101143. doi: 10.1016/j.redox.2019.101143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gatto G.J., Ao Z., Kearse M.G., Zhou M., Morales C.R., Daniels E., Bradley B.T., Goserud M.T., Goodman K.B., Douglas S.A., Harpel M.R., Johns D.G. NADPH oxidase-dependent and -independent mechanisms of reported inhibitors of reactive oxygen generation. J. Enzym. Inhib. Med. Chem. 2013;28:95–104. doi: 10.3109/14756366.2011.636360. [DOI] [PubMed] [Google Scholar]
- 61.Wang X., Elksnis A., Wikström P., Walum E., Welsh N., Carlsson P.-O. The novel NADPH oxidase 4 selective inhibitor GLX7013114 counteracts human islet cell death in vitro. PloS One. 2018;13 doi: 10.1371/journal.pone.0204271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Helfinger V., Palfi K., Weigert A., Schröder K. The NADPH oxidase Nox4 controls macrophage polarization in an NFκB-dependent manner. Oxid. Med. Cell. Longev. 2019:3264858. doi: 10.1155/2019/3264858. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kofler P.A., Pircher H., von Grafenstein S., Diener T., Höll M., Liedl K.R., Siems K., Jansen-Dürr P. Characterisation of Nox4 inhibitors from edible plants. Planta Med. 2013;79:244–252. doi: 10.1055/s-0032-1328129. [DOI] [PubMed] [Google Scholar]
- 64.Revoltella S., Baraldo G., Waltenberger B., Schwaiger S., Kofler P., Moesslacher J., Huber-Seidel A., Pagitz K., Kohl R., Jansen-Duerr P., Stuppner H. Identification of the NADPH oxidase 4 inhibiting principle of lycopus europaeus. Molecules. 2018;23 doi: 10.3390/molecules23030653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tirone F., Radu L., Craescu C.T., Cox J.A. Identification of the binding site for the regulatory calcium-binding domain in the catalytic domain of NOX5. Biochemistry. 2010;49:761–771. doi: 10.1021/bi901846y. [DOI] [PubMed] [Google Scholar]
- 66.van der, Albert V., Heppner D. Earl, Danyal Karamatullah. 2016. Covalent Inhibitors Of Dual Oxidase 1 (Duox 1)http://www.freepatentsonline.com/y2017/0128517.html accessed. [Google Scholar]
- 67.Danyal K., de Jong W., O'Brien E., Bauer R.A., Heppner D.E., Little A.C., Hristova M., Habibovic A., van der Vliet A. Acrolein and thiol-reactive electrophiles suppress allergen-induced innate airway epithelial responses by inhibition of DUOX1 and EGFR. Am. J. Physiol. Lung Cell Mol. Physiol. 2016;311:L913–L923. doi: 10.1152/ajplung.00276.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Augsburger F., Filippova A., Rasti D., Seredenina T., Lam M., Maghzal G., Mahiout Z., Jansen-Dürr P., Knaus U.G., Doroshow J., Stocker R., Krause K.-H., Jaquet V. Pharmacological characterization of the seven human NOX isoforms and their inhibitors. Redox Biol. 2019;26:101272. doi: 10.1016/j.redox.2019.101272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smith S.M.E., Min J., Ganesh T., Diebold B., Kawahara T., Zhu Y., McCoy J., Sun A., Snyder J.P., Fu H., Du Y., Lewis I., Lambeth J.D. Ebselen and congeners inhibit NADPH oxidase 2-dependent superoxide generation by interrupting the binding of regulatory subunits. Chem. Biol. 2012;19:752–763. doi: 10.1016/j.chembiol.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nakamura Y., Feng Q., Kumagai T., Torikai K., Ohigashi H., Osawa T., Noguchi N., Niki E., Uchida K. Ebselen, a glutathione peroxidase mimetic seleno-organic compound, as a multifunctional antioxidant. Implication for inflammation-associated carcinogenesis. J. Biol. Chem. 2002;277:2687–2694. doi: 10.1074/jbc.M109641200. [DOI] [PubMed] [Google Scholar]
- 71.Frank T., Walter Ulrich, Reinhard S., Kirstin W., Peter S., Harald S. https://europepmc.org/article/pat/wo2005111041
- 72.ten Freyhaus H., Huntgeburth M., Wingler K., Schnitker J., Bäumer A.T., Vantler M., Bekhite M.M., Wartenberg M., Sauer H., Rosenkranz S. Novel Nox inhibitor VAS2870 attenuates PDGF-dependent smooth muscle cell chemotaxis, but not proliferation. Cardiovasc. Res. 2006;71:331–341. doi: 10.1016/j.cardiores.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 73.Wind S., Beuerlein K., Eucker T., Müller H., Scheurer P., Armitage M.E., Ho H., Schmidt H.H.H.W., Wingler K. Comparative pharmacology of chemically distinct NADPH oxidase inhibitors. Br. J. Pharmacol. 2010;161:885–898. doi: 10.1111/j.1476-5381.2010.00920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sun Q.-A., Hess D.T., Wang B., Miyagi M., Stamler J.S. Off-target thiol alkylation by the NADPH oxidase inhibitor 3-benzyl-7-(2-benzoxazolyl)thio-1,2,3-triazolo4,5-dpyrimidine (VAS2870) Free Radic. Biol. Med. 2012;52:1897–1902. doi: 10.1016/j.freeradbiomed.2012.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zielonka J., Cheng G., Zielonka M., Ganesh T., Sun A., Joseph J., Michalski R., O'Brien W.J., Lambeth J.D., Kalyanaraman B. High-throughput assays for superoxide and hydrogen peroxide: design of a screening workflow to identify inhibitors of NADPH oxidases. J. Biol. Chem. 2014;289:16176–16189. doi: 10.1074/jbc.M114.548693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jaquet V., Marcoux J., Forest E., Leidal K.G., McCormick S., Westermaier Y., Perozzo R., Plastre O., Fioraso-Cartier L., Diebold B., Scapozza L., Nauseef W.M., Fieschi F., Krause K.-H., Bedard K. NADPH oxidase (NOX) isoforms are inhibited by celastrol with a dual mode of action. Br. J. Pharmacol. 2011;164:507–520. doi: 10.1111/j.1476-5381.2011.01439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chan Y.R., Gallo R.L. PR-39, a syndecan-inducing antimicrobial peptide, binds and affects p130(Cas) J. Biol. Chem. 1998;273:28978–28985. doi: 10.1074/jbc.273.44.28978. [DOI] [PubMed] [Google Scholar]
- 78.Tanaka K., Fujimoto Y., Suzuki M., Suzuki Y., Ohtake T., Saito H., Kohgo Y. PI3-kinase p85alpha is a target molecule of proline-rich antimicrobial peptide to suppress proliferation of ras-transformed cells. Jpn. J. Cancer Res. 2001;92:959–967. doi: 10.1111/j.1349-7006.2001.tb01187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dao V.T.-V., Elbatreek M.H., Altenhöfer S., Casas A.I., Pachado M.P., Neullens C.T., Knaus U.G., Schmidt H.H.H.W. Isoform-selective NADPH oxidase inhibitor panel for pharmacological target validation. Free Radic. Biol. Med. 2020;148:60–69. doi: 10.1016/j.freeradbiomed.2019.12.038. [DOI] [PubMed] [Google Scholar]
- 80.Seredenina T., Chiriano G., Filippova A., Nayernia Z., Mahiout Z., Fioraso-Cartier L., Plastre O., Scapozza L., Krause K.-H., Jaquet V. A subset of N-substituted phenothiazines inhibits NADPH oxidases. Free Radic. Biol. Med. 2015;86:239–249. doi: 10.1016/j.freeradbiomed.2015.05.023. [DOI] [PubMed] [Google Scholar]