Abstract
Liver injury is common in patients with COVID-19, but little is known about its clinical presentation and severity in the context of liver transplant. We describe a case of COVID-19 in a patient who underwent transplant 3 years ago for hepatocellular carcinoma. The patient came to clinic with symptoms of respiratory disease; pharyngeal swabs for severe acute respiratory syndrome coronavirus 2 were positive. His disease progressed rapidly from mild to critical illness and was complicated by several nosocomial infections and multiorgan failure. Despite multiple invasive procedures and rescue therapies, he died from the disease. The management of COVID-19 in the posttransplant setting presents complex challenges, emphasizing the importance of strict prevention strategies.
KEYWORDS: clinical research/practice, immunosuppressant, immunosuppression/immune modulation, infection and infectious agents, infection and infectious agents - viral, liver transplantation/hepatology
Abbreviations: COVID-19, coronavirus disease 2019; RT-PCR, real-time polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
1. INTRODUCTION
The World Health Organization has labeled the spread of coronavirus disease 2019 (COVID-19) as a pandemic,1 and the death toll has already exceeded that of severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome.2 Liver injury is commonly seen in patients with COVID-19,3 but little is known regarding its clinical presentation and severity in the context of transplant. We report the case of a liver transplant recipient with COVID-19.
2. CASE REPORT
The patient was a 59-year-old man who was admitted to the isolation ward (on February 1, 2020) after 3 days of fever, cough, chills, fatigue, and diarrhea. He had a history of hepatitis B virus infection for 25 years with decompensated cirrhosis in the latter years. The patient underwent liver transplant in May 2017 for hepatocellular carcinoma. Posttransplant management included maintenance immunosuppressive therapy with tacrolimus and mycophenolate and antiviral therapy for hepatitis B with entecavir. On admission, the patient denied any history of smoking or alcohol consumption but stated that his wife had been diagnosed with COVID-19 the previous day and was in home isolation. Unfortunately, the patient did have close contact with his wife.
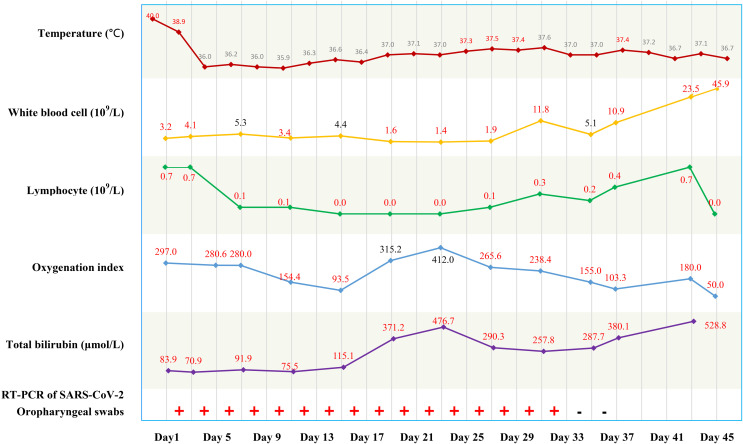
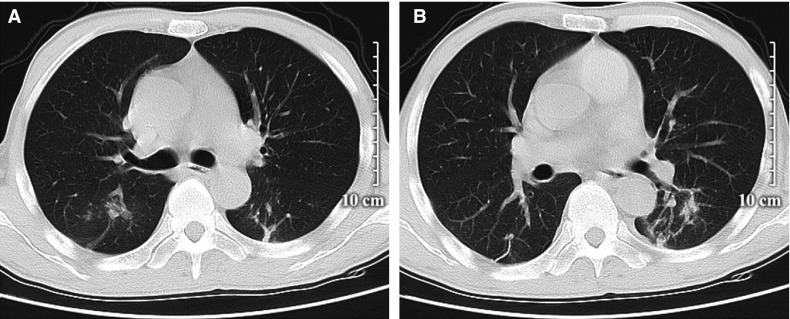
At admission (day 1), examination revealed a body temperature of 40.0°C, blood pressure of 134/86 mm Hg, heart rate of 112 beats/min, respiratory rate of 24/min, jaundice, splenomegaly, and ascites. Other laboratory findings included a white cell count of 3.2 × 109/L, lymphocyte count of 0.7 × 109/L, C-reactive protein (CRP) of 35.1 mg/L, erythrocyte sedimentation rate (ESR) of 102.0 mm/h, total bilirubin of 83.9 μmol/L, alanine aminotransferase of 60 U/L, and γ-glutamyl transpeptidase of 1087 U/L. Blood gas analysis showed a Pao 2 of 98 mm Hg and Pao 2/Fio 2 of 297 (detailed in Table S1). Real-time polymerase chain reaction (RT-PCR) assay of a pharyngeal swab for SARS-CoV-2 was positive ( Figure 1). Chest computed tomography (CT) scan showed bilateral ground-glass opacities ( Figure 2). The patient was diagnosed with mild COVID-19 pneumonia and was started on nebulized α-interferon, umifenovir, and lopinavir/ritonavir according to the Chinese COVID-19 Interim Management Guidance (fourth edition).4 Empirical intravenous piperacillin tazobactam was initiated based on the increased CRP. The dosages of tacrolimus and mycophenolate were halved because of possible drug–drug interactions with lopinavir/ritonavir.
FIGURE 1.
Timeline of disease course according to days from hospital admission (day 1) to death (day 45) [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 2.
Chest computed tomography of the patient on admission. Both lungs had scattered ground-glass opacities (A, B)
On day 4, the patient developed respiratory failure which met the diagnostic criteria for critical illness and was placed on nasal oxygenation therapy and standard methylprednisolone based on the interim management guidance. The hypoxemia worsened rapidly with Paio 2 decreased to 65.1 mm Hg and Pao 2/Fio 2 of 100 by day 9; invasive ventilation was commenced. On day 12, he developed pneumothorax with a pleural effusion and was subject to closed chest drainage. A follow-up chest CT showed significant worsening of bilateral lung inflammation. At this stage, a blood culture was positive for candida albicans, while alveolar lavage and pleural fluid were positive for pseudomonas aeruginosa. Nosocomial infection in a transplant recipient was diagnosed. Cefperazone-sulbactam and caspofungin were given according to the pathogen drug sensitivities. The patient was given extracorporeal membrane oxygenation on day 15 due to exacerbation of respiratory failure.
As the patient’s bilirubin continued to rise to 476 μmol/L and magnetic resonance cholangiopancreatography showed significant bile duct dilatation, endoscopic retrograde cholangiopancreatography (ERCP) was performed on day 23. This drained a large amount of pus and cultures were positive for pseudomonas aeruginosa. The patient was treated over the next 10 days with antimicrobial agents including meropenem and voriconazole. The patient developed anuric acute kidney injury and was commenced on continuous renal replacement therapy (CRRT) and plasma exchange. Other treatments included infusion of albumin, immunoglobulin, blood, and plasma. Although repeat RT-PCR tests were negative on days 33 and 35 (Figure 1), the patient’s condition worsened with development of multiple organ failure and fluctuating PaO2/FiO2 levels between 76-155 mm Hg on day 37. Despite several rescue efforts, the patient’s condition rapidly deteriorated and he died on day 45 (March 16, 2020).
3. DISCUSSION
The management of posttransplant patients who develop COVID-19 is unclear. Although comorbid conditions in SARS-CoV-2 infections may increase the risk of severe disease,5 it is unknown whether transplant patients are at increased risk. In our patient, the disease progressed rapidly from mild to critical and was likely contributed to by long-term usage of immunosuppressive agents. The patient had early gastrointestinal symptoms consistent with reported presentations of SARS-CoV-2 infection. However, his presentation with icterus was uncharacteristic of COVID-19.3 The patient had several episodes of jaundice after liver transplant in 2017 and was suspected to have chronic rejection; this may have led to the observed cholestasis. It is currently unclear whether his underlying transplant status predisposed the patient to SARS-CoV-2 as an opportunistic infection. Nevertheless, the combination of liver dysfunction, multiple secondary bacterial infections, and kidney and respiratory failure caused his rapid demise. Lopinavir/ritonavir and immunosuppressive drugs are both inhibitors of CYP3A,6 and the doses of tacrolimus and mycophenolate were appropriately halved in this patient.
In a previously reported case of successfully recovery from COVID-19 in a 52-year-old recipient of a renal transplant 12 years earlier, the patient was treated with a regimen that included low-dose corticosteroids.7 In contrast, our patient was in a state of chronic rejection. The patient developed multiple nosocomial infections despite changes in treatment. After a retrospective review of his disease course, we suggest that an earlier and more aggressive approach with antiviral and antibacterial drug treatment might have been warranted. The primary cause of this patient’s death was multiple infections that led to septic shock, rather than COVID-19. Early blood cultures in similar patients are mandatory for guiding treatment decisions. Notably, the immunosuppressive drugs used for suspected chronic rejection in this patient likely contributed to his increased risk of infection. This suggests that either lowering the dosage or withholding some of the immunosuppressive drugs early during COVID-19 might have averted the fatal outcome. It is important to balance the use of corticosteroids to prevent septic shock with the need to avoid nosocomial infections.
In sum, we report a case of COVID-19 post liver transplant with a poor outcome despite multiple aggressive therapeutic measures. Secondary nosocomial infection should be seriously entertained in posttransplant patients with compromised immune function when treating COVID-19; using as low a dose of immunosuppressive agents as possible or temporarily withholding some of the agents should be considered.
Acknowledgments
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
AUTHOR CONTRIBUTIONS
Study concept and design: Jiao-Feng Huang and Kenneth I. Zheng; Acquisition of data: Hai-Nv Gao, Ru-Nan Wei and Hua-Dong Yan; Analysis and interpretation of data: Hua-Dong Yan and Kenneth I. Zheng; Drafting of the manuscript: Jiao-Feng Huang and Kenneth I. Zheng; Critical revision of the manuscript for important intellectual content: Jacob George; Study supervision: Ming-Hua Zheng. All authors contributed to the manuscript for important intellectual contents and approved the submission.
ETHICS
Study procedures were approved by the institutional review board (IRB) at Ningbo No. 2 Hospital, University of Chinese Academy of Sciences. The clinical activities being reported are consistent with the principles of the declaration of Istanbul as outlined in the “Declaration of Istanbul on Organ Trafficking and Transplant Tourism.”
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Funding information This work was supported by grants from the National Natural Science Foundation of China (81500665), Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (2018ZD039), High Level Creative Talents from Department of Public Health in Zhejiang Province, Zhejiang Key Research and Development Plan Emergency Project (2020C03123), and Project of New Century 551 Talent Nurturing in Wenzhou.
Footnotes
Jiao-Feng Huang and Kenneth I. Zheng are co-first authors.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
Table S1
REFERENCES
- 1.World Health Organization. WHO Characterizes COVID-19 as a Pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen. Accessed March 11, 2020.
- 2.Joo B. Strengthening China’s public health response system: from SARS to COVID-19. Am J Public Health. 2020;e1-e2. [DOI] [PMC free article] [PubMed]
- 3.Feng G, Zheng KI, Yan QQ, et al. COVID-19 and liver dysfunction: current insights and emergent therapeutic strategies. J Clin Transl Hepatol. 2020;8:18–24. doi: 10.14218/JCTH.2020.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Commission China National Health. Diagnosis and Treatment of COVID-19 in China (4th edition). In Chinese Published January 27, 2020. http://wwwnhcgovcn/yzygj/s7653p/202001/4294563ed35b43209b31739bd0785e67shtml. Accessed January 27, 2020.
- 5.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China [published online ahead of print 2020]. N Engl J Med. 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed]
- 6.Yeh RF, Gaver VE, Patterson KB, et al. Lopinavir/ritonavir induces the hepatic activity of cytochrome P450 enzymes CYP2C9, CYP2C19, and CYP1A2 but inhibits the hepatic and intestinal activity of CYP3A as measured by a phenotyping drug cocktail in healthy volunteers. J Acquir Immune Defic Syndr (1999) 2006;42(1):52–60. doi: 10.1097/01.qai.0000219774.20174.64. [DOI] [PubMed] [Google Scholar]
- 7.Zhu L, Xu X, Ma KE, et al. Successful recovery of COVID-19 pneumonia in a renal transplant recipient with long-term immunosuppression [published online ahead of print 2020]. Am J Transplant. 10.1111/ajt.15869. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.