Abstract
The discovery of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and the outbreak of coronavirus disease 2019 (COVID‐19) are causing public health emergencies. A handful pieces of literature have summarized its clinical and radiologic features, whereas therapies for COVID‐19 are rather limited. To evaluate the efficacy of convalescent plasma therapy in COVID‐19 patients, we did this timely descriptive study. Six laboratory‐confirmed COVID‐19 patients were enrolled and received the transfusion of ABO‐compatible convalescent plasma. The efficacy of this intervention was determined by the alleviation of symptoms, changes in radiologic abnormalities and laboratory tests. No obvious adverse effect observed during the treatment. Transfusion of convalescent plasma led to a resolution of ground‐glass opacities and consolidation in patients #1, #2, #3, #4, and #6. In patients #1 and #5 who presented with SARS‐CoV‐2 in throat swab, convalescent plasma therapy elicited an elimination of the virus. Serologic analysis indicated an immediate increase in anti‐SARS‐CoV‐2 antibody titers in patients #2 and #3, but not in patient #1. This study indicates that convalescent plasma therapy is effective and specific for COVID‐19. This intervention has a special significance for eliminating SARS‐CoV‐2 and is believed to be a promising state‐of‐the‐art therapy during COVID‐19 pandemic crisis.
Keywords: convalescent plasma therapy, COVID‐19, SARS‐CoV‐2
Highlights
Convalescent plasma is a specific therapy for COVID‐19.
Convalescent plasma therapy is safe and effective in COVID‐19 patients.
The SARS‐CoV‐2 neutralizing antibody in the convalescent plasma may directly lead to virus clearance.
Convalescent plasma therapy improves both clinical symptoms and radiologic abnormalities.
1. INTRODUCTION
The global outbreak of a novel human coronavirus, newly named as severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) by the international committee on taxonomy of viruses, has attracted increasing attention and public emergency. 1 , 2 This virus was initially detected in Wuhan, China, in December 2019. A cluster of pneumonia patients manifesting as fever, cough, and dyspnea with unknown etiology emerged at that time. 3 , 4 , 5 The virus was presumed to be zoonotic because preliminary investigation demonstrated that the first generation patients in Wuhan geographically linked to Hunan seafood wholesale market where live animals were sold. While patients outside of Wuhan usually had traveled to the city or had contact with city residents. 6 These epidemiologic findings strongly suggest that SARS‐CoV‐2 transmits from human‐to‐human, and causes the disease now named coronavirus disease 2019 (COVID‐19). 7 By the end of March, 2020, COVID‐19 has spread up to 199 countries and causing more than 27 000 deaths. 8
SARS‐CoV‐2 belongs to the β‐coronavirus family. Its genome is a single‐stranded RNA composed of about 30 kb nucleotides, which encodes four major structural proteins: spike protein (S), membrane protein (M), an envelope protein (E), and nucleocapsid protein (N). Among these proteins, the S protein is of special interest because this clubshaped glycoprotein spikes give the virus a crownlike appearance. 9 Translational studies have demonstrated that the interaction between the receptor‐binding motif of S protein and the angiotensin‐converting enzyme 2 (ACE2) mediates the recognition and entry of SARS‐CoV‐2 into the host cells, and ACE2 is defined as a putative receptor for SARS‐CoV‐2. 10 , 11 The homogeneity in the receptor‐binding domain between SARS‐CoV‐2 and SARS‐CoV underlies their overlapping pathogenicity and biological properties. Indeed, the clinical manifestations and radiologic features of COVID‐19 and those of SARS are quite similar. 12 , 13 For example, both diseases are highly infectious, and the incubation period ranges from several days to 2 weeks. Common symptoms at the onset of the disease include fever, cough, myalgia, and shortness of breath. Laboratory test may indicate white blood cell count below the normal range, lymphopenia, hypoxemia, deranged liver, and renal function. 3 , 5 The typical radiologic abnormalities include multifocal ground‐glass opacities (GGOs) and subsegmental areas of consolidation. 14 , 15 , 16
At the moment, the therapeutic strategy for COVID‐19 is largely supportive. 17 Several off‐label anti‐viral and anti‐HIV agents seem to be clinically beneficial, but their efficacy is far from satisfactory. 18 To this end, there are urgent needs to develop COVID‐19‐specific treatment to alleviate the symptoms and reduce the mortality. Previous experience with SARS suggested that convalescent plasma exhibits a neutralizing antibody response directed against the viral S protein. This antibody blocks SARS‐CoV‐ACE2 entry and can be detected even 24 months after infection. 19 A retrospective study by Soo et al 20 compared the clinical outcome of convalescent plasma therapy vs high‐dose steroids pulse therapy in SARS patients with the deteriorated disease. They found that patients in the plasma group had a shorter hospital stay and lower mortality than the comparator group, and no immediate adverse effect noted after plasma infusion. 20 A systemic meta‐analysis involving 1703 influenza pneumonia patients who received influenza‐convalescent human blood products, showed reduced virus load and pooled absolute reduction of 21% in mortality. 21 Since the number of COVID‐19 cases and disease‐related death is increasing at an incredible speed, an urgent question that needs to be addressed promptly is whether it is also effective to use convalescent plasma therapy in the COVID‐19 setting. One going clinical trial is recruiting patients for anti‐SARS‐CoV‐2 convalescent plasma therapy in Shanghai, China, but no relevant data has been announced yet (NCT04292340). The outcomes of this trial are definitely essential for formulating the principles of therapeutic strategy.
In this study, we provided preliminary data showing the efficacy of convalescent plasma therapy in COVID‐19 patients. We found this intervention was effective in improving patient's symptoms and ameliorating radiologic abnormalities. More timely multi‐center randomized clinical trials are warranted to determine the safety and efficacy of convalescent plasma therapy for COVID‐19.
2. METHODS
2.1. Patients and ethics
During the outbreak of SARS‐CoV‐2 infection in Wuhan, the number of COVID‐19 patients far exceeded the capacity of the local hospitals. Therefore, the government built two designated hospital in Wuhan, one of which was named Huoshenshan. We did this study in six COVID‐19 patients admitted to Wuhan Huoshenshan Hospital from 11th February to 12th March 2020. All the patients were laboratory‐confirmed COVID‐19 cases by using throat swab SARS‐CoV‐2 real‐time polymerase chain reaction (PCR). Upon admission, all the COVID‐19 patients have been empirically treated with anti‐viral drug arbidol, which is also recommended by the New Coronavirus Pneumonia Diagnosis and Treatment Program (6th edition) published by the National Health Commission of China.* Our inclusion criteria were (a) laboratory‐confirmed cases; (b) patients with abnormalities in chest computed tomography (CT) (Case #5 was an exception); (c) patients with deteriorated symptoms after standard treatment; (d) patients with a persistent positive result of throat swab; (e) critically ill patients. Exclusion criteria were (a) patients allergic to plasma contents; (b) patients positive for HBV, HCV and HIV; (c) patients with uncontrolled bacterial mixed infection; (d) patients with malignant tumors; (e) patients who developed multiple organ dysfunction syndromes. Eligible patients' baseline characteristics are listed in Table 1. This study was reviewed and approved by the Medical Ethical Committee of Wuhan Huoshenshan Hospital. Written informed consent was obtained from each participant.
Table 1.
Characteristics of the six participants
| Patient #1 | Patient #2 | Patient #3 | Patient #4 | Patient #5 | Patient #6 | |
|---|---|---|---|---|---|---|
| Sex | Male | Female | Male | Female | Female | Male |
| Age | 69 | 75 | 56 | 63 | 28 | 57 |
| Comorbidity | No | No | Bronchitis | Sjögren syndrome | No | No |
| Fever | Tmax 39°C | No | Tmax 37.8°C | Tmax 39°C | No | Tmax 37.5 °C |
| Cough | No | No | Yes | Yes | No | Yes |
| Fatigue | No | Yes | No | Yes | No | No |
| Myalgia | Yes | No | No | No | No | Yes |
| Dyspnea | Yes | Yes | No | Yes | No | Yes |
| Diarrhea | No | No | No | No | No | No |
| Date of disease onset | Feb 7 | Feb 2 | Feb 2 | Jan 31 | Feb 10 | Jan 19 |
| Date of admission | Feb 25 | Feb 12 | Feb 12 | Feb 11 | Mar 5 | Mar 12 |
| Low white blood cell count | No | No | No | No | No | No |
| Lymphopenia | No | No | No | No | No | No |
| Requirement on oxygen supplement | No | Yes | Yes | Yes | No | Yes |
| Radiologic presentation | Patchy areas of GGOs | Multiple consolidation | Multiple GGOs, reticular opacities and fibrosis streak | Multiple GGOs with consolidation and fibrosis streak | Mostly normal | Extensive bilateral GGOs |
| Date of convalescent plasma therapy | Mar 10,13,16 | Mar 5,9 | Mar 5,6,9 | Mar 10 | Mar 13 | Mar 18 |
| Cycle of convalescent plasma therapy | 3 | 2 | 3 | 1 | 1 | 1 |
| Symptom improvement | Yes | Yes | Yes | Yes | Not applicable | Yes |
| Radiologic improvement | Yes | Yes | Yes | Yes | Not applicable | Yes |
Abbreviation: GGO, ground glass opacity.
2.2. Donors and convalescent plasma transfusion
Convalescent plasma was collected from patients who had recovered from COVID‐19. Recovery was defined as an afebrile status for at least 3 days, alleviation of respiratory symptoms, negative for SARS‐CoV‐2 nucleic acid for consecutive two RT‐PCR tests, and at least 3 weeks following disease onset. The donors need to be seronegative for anti‐HBV, HCV, and HIV, and seropositive for anti‐SARS‐CoV‐2. As a routine check with plasma donation, the convalescent plasma was also confirmed free of residual SARS‐CoV‐2 by real time PCR.
Eligible patients received the transfusion of ABO‐compatible convalescent plasma as soon as the plasma was available to the institution. In accordance with the New Coronavirus Pneumonia Convalescent Plasma Therapy Guidance of China (2nd edition),** patients received at least one cycle of ABO‐compatible convalescent plasma transfusion (200 mL for each cycle). Each transfusion was administered over a 30‐minute period.
2.3. Throat swabs analysis
Throat swabs were taken and immediately put into a transport tube. All the tested samples were processed under airborne precaution. The SARS‐CoV‐2 nucleic acid was detected by reverse transcription and real‐time PCR assays using a commercial detection kit (Changsha Sansure Biotech). Two independent primers that match the open reading frame1ab (ORF1ab) and the nucleocapsid protein (N) fragments were used. RNase P was used as an equal loading control. Reverse transcription and real‐time PCR were performed according to the manufacturer's recommendations. Each transcript provided a cycle threshold value (Ct value), which is the number of cycles required for the fluorescent signal. A Ct value of less than 40 was defined as a positive result, and a Ct value exceeds 40 was defined as a negative test.
2.4. Serum anti‐SARS‐CoV‐2 IgM and IgG array
Serum anti‐SARS‐CoV‐2 IgM and IgG were measured by chemiluminescence using commercially available kits (Shenzhen YHLO Biotech). Briefly, the blood sample was centrifuged at room temperature and the supernatant was removed and incubated with the SARS‐CoV‐2 antigen‐coated magnetic beads. The antigen‐antibody complex captured by the beads slurry was gently precipitated by a magnetic separation rack. The beads were then incubated with acridinium ester‐labeled mouse anti‐human IgM or IgG antibody and reacted with hydrogen peroxide in excitation buffer. Relative luminescence intensity was recorded in an iFlash‐3000 chemiluminescence system.
2.5. Safety and therapeutic outcome evaluation
Adverse events and serious adverse events associated with convalescent plasma transfusion were assessed by the treating clinician. During the transfusion, patients were under continuous supervision, with vital signs checked every 15 minutes and at 4 hours after the end of the intervention.
The primary outcome was the improvement in symptoms and chest CT in the following days after the indicated intervention. Blood and swab samples were obtained to measure serum anti‐SARS‐CoV‐2 IgM and IgG titers and throat SARS‐CoV‐2 nucleic acid, respectively.
3. RESULTS
A total number of six patients were assessed during the study period. This study was initially designed in the middle of February, however, the ABO‐compatible convalescent plasma was not available until the beginning of March. We actually recruited the first participant on 5th March and the last participant on 18th March, respectively. The other issue that needed to be addressed was that Wuhan Huoshenshan Hospital did not receive COVID‐19 patients directly from the community, instead, our patients were transferred from other nondesignated hospitals. Therefore, our patients had been treated elsewhere and were at a relatively late course of the disease when admitted to the institution. Most patients manifested as fever, shortness of breath and nonproductive cough at the onset of the disease. Other common clinical manifestations included fatigue and myalgia (Table 1). As the patients have been treated previously, blood cell count, coagulation test and biochemical analysis upon admission were mostly normal. In terms of inflammation indicators, patient #3 had a slight increase in C‐reactive protein (6.73 mg/L) and procalcitonin (0.17 μg/L). No adverse reactions were observed in the six patients during plasma transfusion and in the following 3 days.
3.1. COVID‐19 patient with persistent SARS‐CoV‐2 detection
A 69‐year‐old man complained of recurrent fever (Tmax 39°C), shortness of breath and myalgia since February7th. He admitted to a local hospital and chest CT showed diffused GGOs in bilateral lungs. He was treated with levofloxacin for 3 days and his temperature became normal on 10th February. The patient was tested for SARS‐CoV‐2 and the throat swab result was positive. He was sent to our institution on 25th February. At admission, his vital signs were stable and his saturation was 96% on ambient air. The chest CT examination on 29th February indicated patchy areas of GGOs in the right lung (Figure 1). The serum anti‐SARS‐CoV‐2 antibody titers on 3rd March were 104 and 180 for IgM and IgG, respectively. While throat swab test on 29th February, 3rd March and 8th March led to a persistently positive result. He received ABO‐compatible convalescent plasma therapy on 10th March, but the throat test on 12th March was still positive for SARS‐CoV‐2. Repeated transfusion of convalescent plasma was given on 13th and 16th March, respectively. Chest CT on 14th March indicated that the GGOs were resolved (Figure 1). Unfortunately, throat swab test on 18th March still led a positive result. However, the result turned to negative in the following two consecutive tests. Moreover, serum anti‐SARS‐CoV‐2 antibody titers on 11th and 14th March were comparable to those before plasma therapy. After careful evaluation, the patient was considered as cured and was ready to discharge from the hospital.
Figure 1.
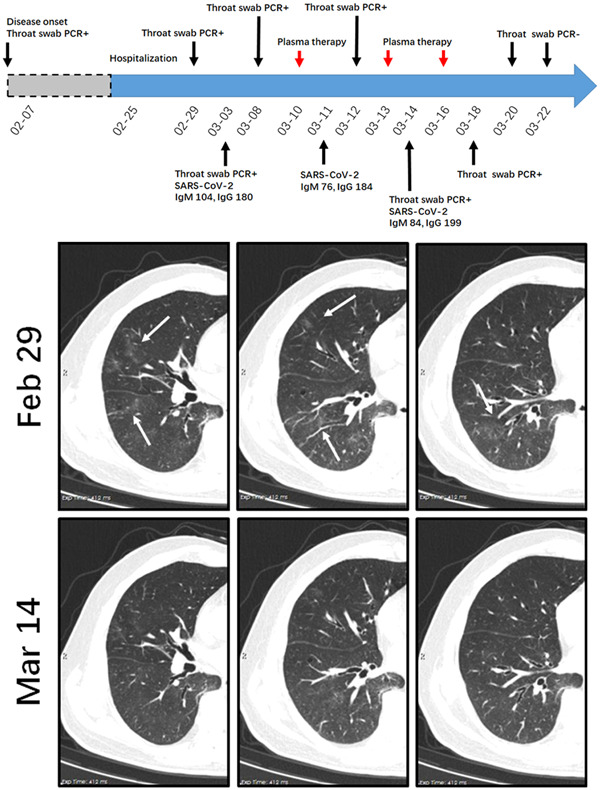
A diagram summarizes the treatment and major laboratory findings of patient #1. This patient had persistent positive results for throat tests. Transfusion of convalescent plasma was given on 10th, 13th, and 16th March, respectively. Representative chest CT images on 29th February and 4th March suggest the absorption of patchy scattered GGOs in the right lung (indicated by white arrows). Repeated throat swab test indicates clearance of residual SARS‐CoV‐2. CT, computed tomography; GGO, ground glass opacity; PCR, polymerase chain reaction
3.2. COVID‐19 patient with consolidation
A 75‐year‐old woman had fatigue and shortness of breath on 2nd February. She did not have a fever and myalgia. She was positive for the SARS‐CoV‐2 throat test and admitted to our hospital on 12th February. At admission, her saturation was 75% and she received oxygen therapy through a nasal catheter. A Chest CT scan on 22nd February showed multiple subpleural consolidations in bilateral lungs (Figure 2). Her throat swab was negative for SARS‐CoV‐2, but she still felt respiratory distress and need oxygen supplement. On 5th March, repeated chest CT examination suggested a partial resolution of the consolidation in the left down lobe, whereas the majority of lesions in the right lung were not resolved (Figure 2). Therefore, the convalescent plasma was given on 5th March. Before the treatment, serum anti‐SARS‐CoV‐2 antibodies titers for IgM and IgG were 47 and 106, respectively. The patient reported that she felt an alleviation of respiratory distress after the treatment and the second cycle of convalescent plasma was given on 9th March. Re‐evaluation of serum anti‐SARS‐CoV‐2 antibodies on 11th March suggested a twofold increase in IgM and IgG titers. Although chest CT on 11th March did not indicate a radiologic improvement, the patient did not require oxygen therapy and the saturation was 99% on ambient air. To track the dynamic changes of consolidation, a repeated chest CT scan was done on 18th March. Figure 2 showed representative images illustrating the evolution of consolidation. After two cycles of convalescent plasma intervention, the density of consolidation was gradually reduced and turned into scattered GGOs with the subpleural line. Three independent throat swab tests were all negative for SARS‐CoV‐2. The patient was considered cured and was under further clinical monitoring.
Figure 2.
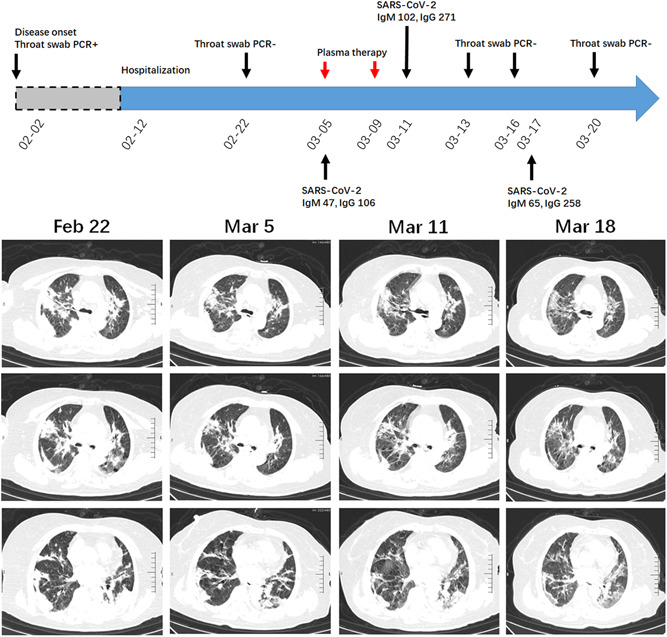
A diagram summarizes the treatment and major laboratory findings of patient #2. The patient manifested as consolidation involving multiple subsegmental lobes. The patient received convalescent plasma on 5th and 9th March. The dynamic evolution of consolidation was presented by chest CT on 22nd February, 5th, 11th, and 18th March, respectively. CT, computed tomography; PCR, polymerase chain reaction
3.3. COVID‐19 patient with extensive lung lesions
A 56‐year‐old man admitted to our hospital on February 12th. He had the fever (Tmax 37.8°C) and nonproductive cough since February 2nd. At admission, he complained of shortness of breath and his saturation was 95% on 5 L/min oxygen inhalation. Laboratory tests indicated normal blood count values and coagulation test. The patient had a slight increase in C‐reactive protein (6.73 mg/L) and procalcitonin (0.17 μg/L). Chest CT on 21st February showed consolidation in the right upper lobe and multiple GGOs in bilateral lungs. Reticular opacities with vacuole inside and fibrosis streak were evident in the right down lobe (Figure 3). Throat swab tests on 23rd and 29th February were negative. Repeated chest CT was done on March 5th and it indicated a partial resolution of the consolidation and GGOs. The serological examination showed the anti‐SARS‐CoV‐2 IgM and IgG titers were 273 and 72, respectively. However, the patient still had respiratory distress, and the arterial blood gas analysis indicated a ratio of the partial pressure of oxygen (PO2) to the fraction of inspired oxygen (FiO2) of 180 (>300 under normal condition). The patient received a transfusion of convalescent plasma on 5th March, and he reported an improvement of his symptoms. The second and third cycle of the intervention was given on 6th and 9th March, respectively. As expected, serum IgM and IgG titers increased after plasma transfusion. Repeated chest CT examination on 11th and 15th March showed a complete resolution of the consolidation and gradual resolution of GGOs around the reticular vacuole. Of noted, a septal line appeared in the left down lobe (Figure 3). Arterial blood gas analysis on 15th March indicated a PO2/FiO2 ratio of 330. The patient was cured and has discharged from the hospital.
Figure 3.
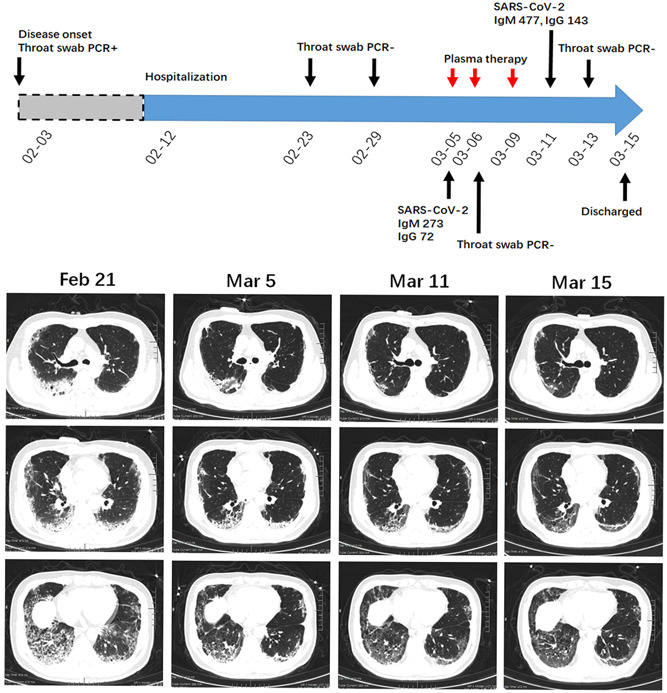
A diagram summarizes the treatment and major laboratory findings of patient #3. Chest CT on 21st February showed consolidation, multiple GGOs, reticular opacities with fibrosis streak. The patient received three cycles of convalescent plasma therapy and this intervention led to the alleviation of symptoms, as well as a gradually radiologic improvement. A septal line appeared in left down lobe after indicated treatment. CT, computed tomography; GGO, ground glass opacity; PCR, polymerase chain reaction
3.4. COVID‐19 patient with Sjögren syndrome
A 63‐year‐old woman with 10 years history of Sjögren syndrome had fever (Tmax 39°C), cough, shortness of breath and decreased exercise tolerance on 31st January. She was treated with levofloxacin, but her disease deteriorated. The patient was positive for the SARS‐CoV‐2 throat swab test and admitted to our hospital on 11th February. Chest CT examination indicated the peripheral and central distribution of multiple GGOs with partial consolidation and fibrosis streak. An air bronchogram sign could be seen inside consolidation (Figure 4). The patient received arbidol and oxygen treatment, whereas glucocorticoid was not used to avoid virus dissemination. The treatment led to a remission of the patient's symptoms, and the result of throat swab tests on 24th, 25th February 25th, and 1st March was negative. Chest CT on 5th March showed the resolution of solidified lesions, and the residual lesions presented as GGOs with partial consolidation (Figure 4). This radiologic presentation would be attributed to either SARS‐CoV‐2 infection or Sjögren syndrome‐associated interstitial lung disease. To exclude the possibility of SARS‐CoV‐2 infection, we treated the patient with a convalescent plasma transfusion on 10th March, and repeated chest CT was done on 14th March. The density of GGOs tended to reduce, while the distribution pattern was not changed (Figure 4). The serological examination showed that the patient was positive for anti‐SARS‐CoV‐2 IgM and IgG and the patient had a negative throat swab result on 11th March. The patient was discharged from our hospital on 16th March and received treatment for Sjögren syndrome.
Figure 4.
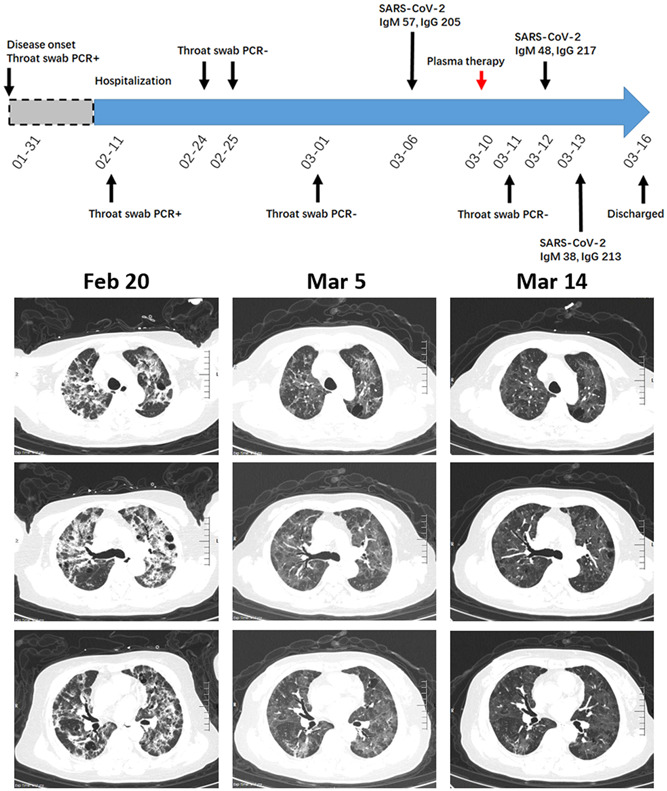
A diagram summarizes the treatment and major laboratory findings of patient #4. The 63‐year‐old female patient concurrent with Sjögren syndrome had multiple GGOs with partial consolidation and fibrosis streak at admission. After indicated treatment, she presented as GGOs with partial consolidation. Transfusion of convalescent plasma was done on 10th March, and repeated chest CT showed a slight decrease in the density of GGOs. CT, computed tomography; GGO, ground glass opacity; PCR, polymerase chain reaction
3.5. Postdischarge SARS‐CoV‐2‐positive COVID‐19 patient
A 28‐year‐old woman experienced fatigue and myalgia on 10th February. Her throat swab was positive for SARS‐CoV‐2 but her chest CT was otherwise normal. Before discharge from a local hospital, two consecutive throat swab tests were negative. She was asymptomatic and sent to our hospital for further clinical evaluation on 5th March. At admission, the serologic anti‐SARS‐CoV‐2 antibody test was positive. The first throat swab test in our institution was negative, whereas it turned positive on the following days. Chest CT examination on 8th March did not show lung lesions (Figure 5). This asymptomatic patient without radiologic abnormalities was recognized as a post‐discharge SARS‐CoV‐2‐positive COVID‐19 patient and could be a potential source of infection. Thus, convalescent plasma therapy was used on 13th March. Several consecutive SARS‐CoV‐2 throat swab tests after the intervention were negative. The patient was discharged from the hospital on 19th March.
Figure 5.
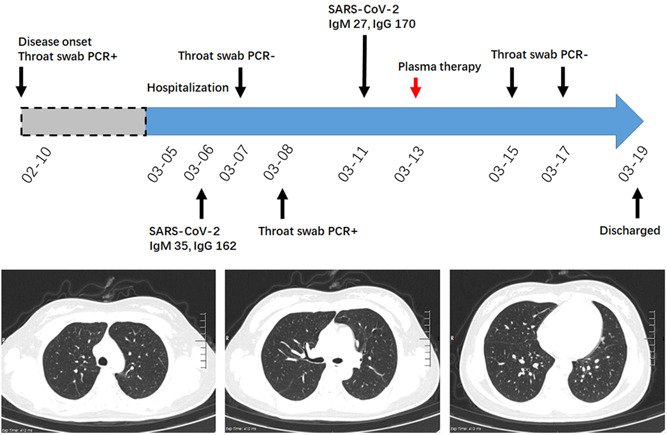
A diagram summarizes the treatment and major laboratory findings of patient #5. The patient was defined as postdischarge SARS‐CoV‐2‐positive COVID‐19, and treated with convalescent plasma on 13th March. Consecutive SARS‐CoV‐2 throat swab tests indicated an elimination of residual SARS‐CoV‐2.CT, computed tomography; GGO, ground glass opacity; PCR, polymerase chain reaction
3.6. COVID‐19 patient with GGOs
A 57‐year‐old man had fever, cough, shortness of breath and myalgia since 29th January. He was tested for throat swab SARS‐CoV‐2, and the result was positive. He was sent to our hospital on 12th March. Laboratory blood tests indicated titers of anti‐SARS‐CoV‐2 IgM and IgG were 16 and 217, respectively. Chest CT examination on 14th March showed GGOs without clear boundary in the peripheral region and down lobe of bilateral lungs (Figure 6). Although repeated throat swab test after admission was negative, the patient complained that he still had respiratory distress. The convalescent plasma was given on 18th March, and the patient reported a marked relief of symptom. Figure 6 showed the radiologic changes 3 days after the indicated intervention, in which GGOs were generally resolved after convalescent plasma transfusion. The patient was discharged from hospital on 22nd March.
Figure 6.
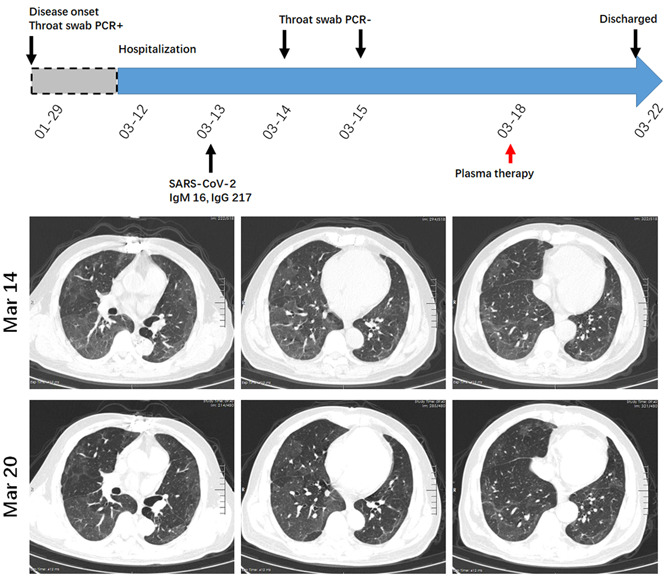
A diagram summarizes the treatment and major laboratory findings of patient #6. The radiologic feature of this patient is extensive GGOs. The convalescent plasma was given on 18th March, and repeated chest CT showed a remarkable resolution of GGOs after the intervention. CT, computed tomography; GGO, ground glass opacity; PCR, polymerase chain reaction
4. DISCUSSION
This descriptive study highlights convalescent plasma therapy as an effective and specific treatment for COVID‐19. According to the experience of SARS and severe influenza, convalescent plasma is recommended to use as early as possible because the production of endogenous IgM and IgG antibodies peaks at 2 and 4 weeks after infection, respectively. 20 , 21 However, patients admitted to Wuhan Huoshenshan Hospital have already been treated elsewhere and the duration from the onset of disease to admission usually exceeds 4 weeks. Fortunately, convalescent plasma therapy is still functional in the six patients, and all patients did not admit to ICU during treatment. To the best of our knowledge, this is a timely study evaluating the efficacy of convalescent plasma therapy in COVID‐19 patients with distinct radiologic, laboratory and clinical features.
In compliance with the New Coronavirus Pneumonia Diagnosis and Treatment Program (6th edition), the principle of discharge was based on a relief of symptoms, obvious absorption of abnormalities in chest CT, abatement of fever, and viral clearance with throat swab for two consecutive tests. In our study, we found detectable SARS‐CoV‐2 in patient #5 who has already discharged from a local hospital. This is in agreement with a previous report that the virus RNA persists for a median of 20 days in survivors. 3 Strikingly, patient #5 represents a group of post‐discharge asymptomatic “walking COVID‐19” cases that might serve as a possible source to propagate the outbreak. 22 Indeed, a recent autopsy study of COVID‐19 patient's lung indicates the presence of SARS‐CoV‐2 particles in bronchial mucosal epithelial and type II alveolar epithelial cells. 23 This has important implications for reconsidering the period of patient isolation, the principle of discharge, and warrants highly efficient that can eliminate SARS‐CoV‐2. Yeh and colleagues tested the efficacy of convalescent plasma therapy in three SARS patients, and they found viral load dropped from 495 × 103, 76 × 103, or 650 × 103 copies/mL to 0 or 1 copy/mL 1 day after transfusion. Anti‐SARS‐CoV IgM and IgG also increased in a time‐dependent manner following transfusion. 24 This treatment is also effective for influenza A (H5N1) infection, in which convalescent plasma therapy led to a 12‐fold decrease in blood virus load during the first 8 hours after transfusion, and the virus was undetectable within 32 hours. 25 These findings recommend a specific and effective strategy to eliminate residual virus. In agreement with this hypothesis, we found a clearance of SARS‐CoV‐2 in throat swab test in patient #5 who received the transfusion of convalescent plasma. Of special interest, patient #1, who has persistent positive results for a throat swab test, is free of SARS‐CoV‐2 after the same intervention. It is not surprising to notice that his serum anti‐SARS‐CoV‐2 antibody titers after three cycles of convalescent plasma therapy are not increased, probably because the residual virus consumes the protective anti‐SARS‐CoV‐2 IgM and IgG, and the antibody titer starts to increase after virus clearance (Figure 1 and Figure S1). These findings strongly indicate that convalescent plasma transfusion is a specific and effective therapy for COVID‐19.
Another important finding in our study is the dynamic change of radiologic abnormalities. We found a rapid and dramatic radiologic improvement in patient #6, who manifested as extensive pure GGOs. This effect could be recaptured in patient #2 and patient #3, while the absorption of consolidation takes a relatively longer time. Intriguingly, our initial chest CT evaluation of patient #3 showed the patient's lung was in bad condition and respiratory distress did not relieve after standard supportive treatment. Although he was negative for throat swab test and the anti‐SARS‐CoV‐2 IgM and IgG were detectable, it is reasonable to believe a clearance of virus in the upper respiratory tract, while SARS‐CoV‐2 may still exist in the lower respiratory tract and lung. Bronchoalveolar lavage fluid SARS‐CoV‐2 test would be informative, but bronchoscopy examination was not applicable in case of airborne droplets transmission. We treated patient #3 with the transfusion of convalescent plasma. As a consequence, the patient had a significant improvement in his symptom, as well as a gradually resolution of consolidation.
Our study is different from previous SARS and influenza cases because convalescent plasma were used in a relatively late course of disease. We found it is still clinically beneficial in all the six cases. The mechanism of action in this setting was not fully understood. We speculated that the anti‐SARS‐CoV‐2 IgM and IgG directly neutralizes the virus, and the anti‐inflammatory contents may prevent cytokine storms. For the latter hypothesis, there is great debate of using corticosteroids. Evidence in SARS suggests that corticosteroids do not reduce mortality, but rather delayed viral clearance. 26 Therefore, corticosteroids should not be routinely given, like what we have done in patient #4 with Sjögren syndrome.
This study is limited by the small sample size. However, by including representative patients with distinct radiologic, laboratory and clinical features, we believe our study population is representative of COVID‐19 patients in Wuhan. Since more and more patients have recovered from the infection of SARS‐CoV‐2, voluntary donation of convalescent plasma would be definitely encouraged and appreciated. Taken together, COVID‐19 is becoming a global health threat, reliable treatment is crucial for reducing mortality and preventing disease outbreak. SARS‐CoV‐2‐specific therapies, including convalescent plasma from recovery patients, would be highly effective weapons to win the war against COVID‐19.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
MY, XX, and TL conceived and designed the study. MY, DF, YR, and DW took care of the patient and contributed to the acquisition of data. XX contributed to the development of methodology. MY, XX, and TL contributed to analysis and interpretation of data. FW, FZ, and TL contributed to study supervision. MY and TL wrote this paper.
Supporting information
Supplementary information
Supplementary information
ACKNOWLEDGMENTS
All authors are voluntary front‐line clinicians in Wuhan Huoshenshan Hospital. The authors express gratitude to all the participants and their families for the invaluable support to the study. This study was partially sponsored by grants National Natural Science Foundation of China (#81802301 to Mingxiang Ye, #81772500 to Tangfeng Lv), and Jiangsu Provincial Key Research and Development Program (BE2018713 to Xinyi Xia).
Ye M, Fu D, Ren Y, et al. Treatment with convalescent plasma for COVID‐19 patients in Wuhan, China. J Med Virol. 2020;92:1890–1901. 10.1002/jmv.25882
Footnotes
Contributor Information
Xinyi Xia, Email: xiaxynju@163.com.
Tangfeng Lv, Email: bairoushui@163.com.
REFERENCES
- 1. Gillespie TR, Leendertz FH. COVID‐19: protect great apes during human pandemics. Nature. 2020;579(7800):497. [DOI] [PubMed] [Google Scholar]
- 2. Jacobsen KH. Will COVID‐19 generate global preparedness? Lancet. 2020;395:1013‐1014. 10.1016/S0140-6736(20)30559-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lian J, Jin X, Hao S, et al. Analysis of epidemiological and clinical features in older patients with corona virus disease 2019 (COVID‐19) out of Wuhan [published online ahead of print March 25, 2020]. Clin Infect Dis 2020. 10.1093/cid/ciaa242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chan JFW, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person‐to‐person transmission: a study of a family cluster. Lancet. 2020;395(10223):514‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tuite AR, Ng V, Rees E, Fisman D. Estimation of COVID‐19 outbreak size in Italy. Lancet Infect Dis. 2020;20(5):537. 10.1016/S1473-3099(20)30227-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zheng M, Song L. Novel antibody epitopes dominate the antigenicity of spike glycoprotein in SARS‐CoV‐2 compared to SARS‐CoV. Cell Mol Immunol. 2020;17(5):536–538. 10.1038/s41423-020-0385-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of the SARS‐CoV‐2 by full‐length human ACE2. Science. 2020;367:1444‐1448. 10.1126/science.abb2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Webster P. Canada and COVID‐19: learning from SARS. Lancet. 2020;395(10228):936‐937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Drosten C, Günther S, Preiser W, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348(20):1967‐1976. [DOI] [PubMed] [Google Scholar]
- 14. Guan W, Liu J, Yu CCT. Findings of coronavirus disease (COVID‐19) severe pneumonia. AJR Am J Roentgenol. 2020;214(5):W85‐W86. 10.2214/AJR.20.23035 [DOI] [PubMed] [Google Scholar]
- 15. Li M, Lei P, Zeng B, et al. Coronavirus disease (COVID‐19): spectrum of CT findings and temporal progression of the disease. Acad Radiol. 2020;27(5):603–608. 10.1016/j.acra.2020.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang Y, Dong C, Hu Y, et al. Temporal changes of CT findings in 90 patients with COVID‐19 pneumonia: a longitudinal study. Radiology. 2020:200843. 10.1148/radiol.2020200843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Matthay MA, Aldrich JM, Gotts JE. Treatment for severe acute respiratory distress syndrome from COVID‐19. Lancet Respir Med. 2020;8(5):433–434. 10.1016/S2213-2600(20)30127-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kalil AC. Treating COVID‐19‐off‐label drug use, compassionate use, and randomized clinical trials during pandemics. JAMA. 2020. 10.1001/jama.2020.4742 [DOI] [PubMed] [Google Scholar]
- 19. Liu W, Fontanet A, Zhang PH, et al. Two‐year prospective study of the humoral immune response of patients with severe acute respiratory syndrome. J Infect Dis. 2006;193(6):792‐795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Soo YOY, Cheng Y, Wong R, et al. Retrospective comparison of convalescent plasma with continuing high‐dose methylprednisolone treatment in SARS patients. Clin Microbiol Infect. 2004;10(7):676‐678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta‐analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med. 2006;145(8):599‐609. [DOI] [PubMed] [Google Scholar]
- 22. Zhang JF, Yan K, Ye HH, Lin J, Zheng JJ, Cai T. SARS‐CoV‐2 turned positive in a discharged patient with COVID‐19 arouses concern regarding the present standard for discharge. Int J Infect Dis. 2020. 10.1016/j.ijid.2020.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yao XH, Li TY, He ZC, et al. A pathological report of three COVID‐19 cases by minimally invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49:009. 10.3760/cma.j.cn112151-20200312-00193 [DOI] [PubMed] [Google Scholar]
- 24. Yeh KM, Chiueh TS, Siu LK, et al. Experience of using convalescent plasma for severe acute respiratory syndrome among healthcare workers in a Taiwan hospital. J Antimicrob Chemother. 2005;56(5):919‐922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhou B, Zhong N, Guan Y. Treatment with convalescent plasma for influenza A (H5N1) infection. N Engl J Med. 2007;357(14):1450‐1451. [DOI] [PubMed] [Google Scholar]
- 26. Hui DS. Systemic corticosteroid therapy may delay viral clearance in patients with Middle East respiratory syndrome coronavirus infection. Am J Respir Crit Care Med. 2018;197(6):700‐701. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information
Supplementary information


