To the Editor,
Coronavirus disease 2019 (COVID‐19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), is a disease characterized by pneumonia. The main clinical presentations are fever, dry cough, and fatigue, but in addition to respiratory symptoms, a minority of patients may present only with muscle soreness, gastrointestinal symptoms, or dispiritedness in the early stages. 1 According to limited pathological autopsy results, in addition to lung involvement, the heart, liver, kidneys, spleen, hilar lymph nodes, bone marrow, and even brain tissues are also affected in patients with COVID‐19. 2 Herein, we report a case of a patient diagnosed with COVID‐19 who manifested with neurological symptoms.
This is a case of a 64‐year‐old male patient of Han Chinese ethnicity and who lived in Wuhan City. He did not have a history of hypertension, diabetes, or cerebrovascular and cardiovascular diseases. The patient developed fever on 28 January 2020, with a maximum body temperature of 38.5°C, accompanied by mild cough. Symptomatic treatment alleviated the cough, while his body temperature fluctuated between 37.5°C and 38°C and returned to normal on 30 January. On 30 January, he experienced insomnia and muscle soreness of the whole body without significant tenderness. He was quarantined after a medical consultation on 9 February, during which his consciousness was clear, and he was able to answer questions correctly. On 10 February, the patient was found to be lethargic and unresponsive. Emergency blood tests revealed the following: white blood cell count, 3.3 × 109/L, lymphocyte percentage, 24.4%; neutrophil percentage, 62.8%; and C‐reactive protein level, 10.74 mg/L. Head computed tomography (CT) did not reveal significant abnormalities, whereas chest CT revealed multiple ground‐glass opacities with multiple fibrous cord‐like shadows in both lungs (Figure 1). Additionally, a throat swab test performed on 11 February indicated that the patient was positive for 2019‐nCoV and was later admitted to a hospital. Upon admission, the patient's vital signs were stable, with finger pulse oximetry without oxygenation, and finger pulse oximetry with oxygenation through the nasal cannula (5 L/min) of 88% and 98%, respectively. Neurological examination indicated that the patient was in a poor mental state, with his consciousness alternating between lethargy and irritability. His responses to questions were incorrect, with some indications of contextually dissociated speech. Both lower limbs showed positive ankle clonus, which was more pronounced on the left limb. The lower left limb was positive for the Babinski sign and Chaddock sign; the lower right limb was suspected positive for the Chaddock sign. The patient's neck showed slight stiffness at three fingers below the jaw and was positive for the Brudzinski sign and the straight leg raise test. After admission, the patient received oxygen inhalation, arbidol, ribavirin antiviral therapy, traditional Chinese medicine, and symptomatic and supportive care. On 16 February, he underwent lumbar puncture: intracranial pressure, 200 cmH2O (colorless and clear cerebrospinal fluid); cell count, 1 × 106/L; cerebrospinal protein level, 275.5 mg/L, glucose (Glu) level, 3.14 mmol/L; chloride level, 123 mmol/L; and instant blood Glu level, 5.1 mmol/L. Moreover, the cerebrospinal fluid tested negative for the 2019‐nCoV nucleic acid test. His 2019‐nCoV nucleic acid test (throat swab) results were negative on 18 February and positive on 21 February. On 25 February, chest CT revealed significant improvement in pulmonary infection. Based on his neurological examination that same day, he had a clear consciousness, provided correct responses to questions, and showed an essentially normal intelligence. Limb reflexes were relatively active, ankle clonus was already not observed in both lower limbs, his left lower limb was positive for pathological signs, and pathological signs could not be elicited in the right lower limb. The neck was soft and unresisting. The patient tested negative in two consecutive 2019‐nCoV nucleic acid tests (throat swab) on 25 and 27 February, and his finger pulse oximetry without oxygenation was 99%. The patient was discharged to a quarantine facility and quarantined for a further 14 days.
Figure 1.
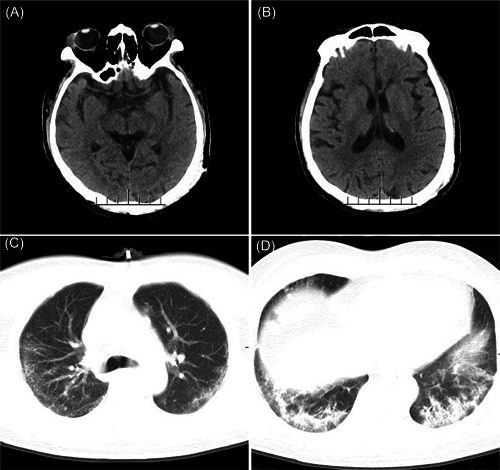
Head CT and chest CT of the patient. A,B, No obvious abnormalities were found on brain CT. C,D, Diminished bilateral lung translucence, with multiple ground‐glass opacities distributed in both in the middle and lateral fields of the lung. Multiple cord‐like shadows can be observed in the bilateral lower lungs, with interlobular septal thickening and bronchiectasis. CT, computerized tomography
SARS‐CoV‐2 is a betacoronavirus, and its genetic characteristics differ significantly from those of SARS‐CoV and Middle East respiratory syndrome coronavirus. 3 It is the main clinical presentations are fever and respiratory symptoms. However, some patients may also present with neurologic signs, such as headache, nausea, and vomiting 4 or loss of smell and taste, 5 even encephalitis. 6
Current understanding on the pathogenesis of COVID‐19 is still unclear. It is now believed that angiotensin‐converting enzyme 2 (ACE2) may be a receptor of 2019‐nCoV. 4 , 7 , 8 ACE2 has been identified as a functional receptor of SARS‐CoV. Hamming et al. 9 studied the localization of the ACE2 protein in different human organs and found that the ACE2 protein is abundantly expressed in the alveolar epithelial cells and small intestinal epithelial cells. Furthermore, ACE2 is also observed in the arterial and venous endothelial cells and arterial smooth muscle cells in all organs. Hence, respiratory and gastrointestinal symptoms are the most commonly observed symptoms among patients diagnosed with COVID‐19, but the central nervous system may also be affected through the blood vessel‐rich meninges after the blood‐brain barrier is damaged.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
RY, WF, and TW were involved in writing. GC, TW, and DC collected the data. RY, TL, and DX were involved in Data analysis.
ACKNOWLEDGEMENTS
We would like to thank the webshop of Elsevier (https://webshop.elsevier.com) for its linguistic assistance during the preparation of this manuscript.
Rong Yin, Wei Feng, and Tonghui Wang contributed equally to this work.
Contributor Information
Rong Yin, Email: yin_rong_@hotmail.com.
Tangfeng Lv, Email: bairoushui@163.com.
Dawei Xiang, Email: xdwhss@163.com.
REFERENCES
- 1. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID‐19) outbreak. J Autoimmun. 2020;109:102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS‐CoV2 may play a role in the respiratory failure of COVID‐19 patients. J Med Virol. 2020;92(6):552‐555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li YBW, Hashikawa T. Response to commentary on: “the neuroinvasive potential of SARS‐CoV‐2 may play a role in the respiratory failure of COVID‐19 patients”. J Med Virol. 2020;92(7):707‐709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of the SARS‐CoV‐2 by full‐length human ACE2. Science. 2020;367(6485):1444‐1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nataf S. An alteration of the dopamine synthetic pathway is possibly involved in the pathophysiology of COVID‐19. J Med Virol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]


