Quantitative hematologic abnormalities have been described in the first patient studies of COVID‐19, the disease caused by the SARS‐CoV‐2 virus, currently responsible for a rapidly spreading pandemic, worldwide infection with high mortality rate. In severe cases, the disease progresses, through subsequent phases, from an upper respiratory tract infection with local and general flu‐like symptoms to a viral pneumonia and hyperinflammatory host reaction, which leads to acute respiratory distress and multi‐organ failure. The most common hematological findings include lymphocytopenia,1, 2, 3 neutrophilia,4, 5, 6 eosinopenia,7, 8 mild thrombocytopenia (35%) or, less frequently, thrombocytosis.2, 9 The presence of atypical, reactive lymphocytes has been only occasionally reported. 10 In one recent report from California, a mild leuco‐erythroblastic picture was observed in the peripheral blood film. 11
Italy has been, and still is severely hit by the epidemic. 12 At Fondazione Policlinico A. Gemelli of Rome, a parallel COVID‐19 hospital has been created to provide assistance to patients from the region of Lazio and central Italy. We have had up to 249 patients with COVID‐19 admitted on March 25, 2020. After microscope observation of peripheral blood films from the first 40 cases at admission, when antiviral and anti‐inflammatory treatment was not yet administered, we have noted the presence of marked morphological abnormalities of the neutrophil lineage. Platelet morphology also showed peculiar and frequent anomalies, mainly with very large, usually hyperchromatic platelets with peripheral areas of different size, not rarely protruding in pseudopodia formations (Image 1L). This occurred both in patients with thrombocytosis (four cases with a count of 503‐629 × 109/L) and in those with thrombocytopenia (66‐93 × 109/L). Quantitative cell blood count (CBC) parameters were heterogeneous and overlapped with the majority of results from the literature.1, 2, 3, 4, 5, 6, 7, 8
IMAGE 1.
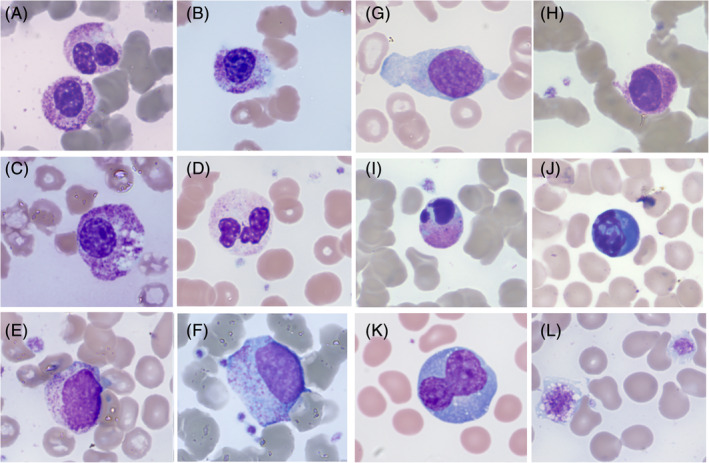
Morphological anomalies in peripheral blood films from different COVID‐19 patients. A, Neutrophil granulocytes with bilobed and unsegmented, pseudo‐Pelgerlike nuclei. B, Neutrophil granulocytes with unsegmented nucleus with coarsely clumped chromatin; cytoplasm is hypergranular with basophilic, agranular areas. C, Unsegmented, hypergranular neutrophil with multiple vacuoles. D, Neutrophil granulocyte with marked cytoplasmic hypogranularity. E, Circulating small neutrophil myelocyte. F, Circulating dysplastic promyelocyte (scattered azurophil granules, absence of paranuclear Golgi zone). G, Immature circulating cell with blasts‐like reticular chromatin and rare thin azurophilic granules. H, Unsegmented granulocyte with hyperchromic nuclear chromatin and tightly condensed cytoplasm, likely pre‐apoptotic. I, Circulating apoptotic neutrophil. J, Apoptotic cell with blue cytoplasm, of possible lymphocyte origin. K, Large polyploid reactive lymphocyte with hyperbasophilic cytoplasm. L, Giant vacuolated platelets
Neutrophil absolute counts were frequently increased in the first days after admission (>5 x109/L in 14/40 cases), before or just after the beginning of treatment, with a trend to decrease 1 week thereafter (1.48‐3.23 x109/Lin seven followed‐up cases). Their morphological abnormalities concerned both nuclear and cytoplasmic granulation (Image 1A‐D). In particular, we underline the presence of many, crowded, dark granulations in the cytoplasm (similar to “toxic” granules) and of peripheral light blue agranular areas. In a minority of cases, cytoplasmic hypogranularity was very frequent. Abnormalities of nuclear shape were striking, with increased frequency of band forms but also of dysmorphic cells with total absence of nuclear segmentation, consistent with pseudo‐Pelger morphology. Apoptotic cells were easily found in many peripheral blood films (Image 1H‐J). They appeared with liquefied nuclear chromatin and granulated or deep blue cytoplasm, suggesting possible derivation from different types of cells (ie, neutrophils and lymphocytes, respectively). Immature granulocytes, especially small myelocytes and metamyelocytes, were also frequently present in early phase cases (Image 1E), as recently described. 11 More immature cells, sometimes showing immature nuclei and small azurophilic granules, were occasionally observed (Image 1F,G). Using the automated cytochemical reaction for myeloperoxidase, provided by the blood cell counter ADVIA 2120 (Siemens, Milan, Italy), in two of the 40 cases at diagnosis the neutrophil population showed a marked decrease of the peroxidase activity (Image 2), with myeloperoxidase index of −10.4 and −14.4, respectively (manufacturer’s normal range ± 10).
IMAGE 2.
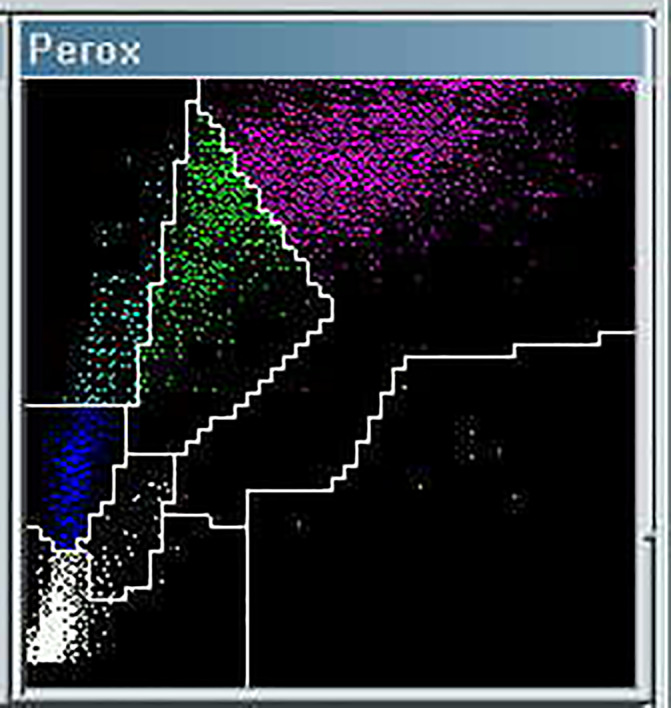
Partial myeloperoxidase (MPO) deficiency in a patient with dysmorphic neutrophils (same as Image 1A). The neutrophil population (purple signals) on the ADVIA 2120 scattergram is shifted to the left, partially invading the monocyte area (green signals), which determines a false increase of the monocyte percentage. Quantitative data: WBC 2.78 × 109/L, neutrophils 1.29 × 109/L, monocytes 0.89 × 109/L (falsely including a fraction of MPO‐deficient neutrophils), lymphocytes 0.41 × 109/L, large unstained cells (LUC) 0.19 × 109/L
In seven patients the peripheral blood film was re‐observed after 5 to 7 days of antiviral and anti‐inflammatory treatment. The above described neutrophil morphological changes had almost completely disappeared and the lymphocyte population showed wide morphological heterogeneity with large atypical lymphocytes (Image 1K), lymphoplasmacytoid cells and increased proportion of large granular lymphocytes. Treatment comprised antiviral, anti‐inflammatory and anti‐interleukin 6 products.
In summary, it is our aim to point out that different cell morphological changes can be seen in the subsequent phases of COVID‐19. In particular, in the early phase of symptom aggravation, usually coinciding with hospital admission, a pronounced granulocytic reaction with immaturity, dysmorphism and apoptotic‐degenerative morphology was evident in peripheral blood. After several days of treatment, the hematologic picture tended to shift toward impressive lymphocyte activation, often with numerical increase, and heterogeneous morphological expression.
These abnormalities mainly highlight the severe, transitory and reversible perturbation of myelopoiesis, especially in the form of accelerated and disordered granulopoiesis, in patients with COVID‐19 in severe symptomatic phase. According to recent updates, such quantitative and qualitative abnormalities can be related to the cytokine storm and hyperinflammation, which is a fundamental pathogenic factor in the evolution of COVID‐19 pneumonia. Possibly this is in the form of secondary hemophagocytic lymphohistiocytosis, leading to an often fatal multi‐organ failure.13, 14, 15 Our preliminary observation could therefore call for further studies of the involvement of myelopoiesis in the pathogenesis and evolution of COVID‐19.
Zini G, Bellesi S, Ramundo F, d'Onofrio G. Morphological anomalies of circulating blood cells in COVID‐19. Am J Hematol. 2020;95:870–872. 10.1002/ajh.25824
REFERENCES
- 1. Guan WJ, Ni ZY, Hu Y, Liang WH, et al; China Medical Treatment Expert Group for Covid‐19.Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan. China Intensive Care Med. 2020;46:846‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang F, Nie J, Wang H, Zhao Q, et al. Characteristics of peripheral lymphocyte subset alteration in COVID‐19 pneumonia. J Infect Dis. 2020;221(11):1762‐1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. 10.1016/S0140-6736(20)30211-30217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Qian GQ, Yang NB, Ding F, et al. Epidemiologic and clinical characteristics of 91 hospitalized patients with COVID‐19 in Zhejiang, China: a retrospective, multi‐centre case series. QJM. 2020. 10.1093/qjmed/hcaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mo P, Xing Y, Xiao Y, et al. Clinical characteristics of refractory COVID‐19 pneumonia in Wuhan, China. Clin Infect Dis. 2020. 10.1093/cid/ciaa270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. 2020. 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 8. Liu F, Xu A, Zhang Y, et al. Patients of COVID‐19 may benefit from sustained lopinavir‐combined regimen and the increase of eosinophil may predict the outcome of COVID‐19 progression. Int J Inf Dis. 2020;95:183‐191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID‐19) infections: a meta‐analysis. Clin Chim Acta. 2020;506:145‐148. 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fan BE, Chong VCL, Chan SSW, et al. Hematologic parameters in patients with COVID‐19 infection. Am J Hematol. 2020;95(6):E131‐E132. [DOI] [PubMed] [Google Scholar]
- 11. Mitra A, Dwyre DM, Schivo M, et al. Leukoerythroblastic reaction in a patient with COVID‐19 infection. Am J Hematol. 2020. 10.1002/ajh.25793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Buoro S, Di Marco F, Rizzi M, et al. Papa Giovanni XXIII Bergamo Hospital at the time of the COVID‐19 outbreak: letter from the warfront. Int J Lab Hematol. 2020. 10.1111/ijlh.13207. [DOI] [PubMed] [Google Scholar]
- 13. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID‐19 in Wuhan, China. Clin Infect Dis 2020. 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mehta P, McAuley DF, Brown M, et al. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033‐1034. 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Siddiqi HK, Mehra MR. COVID‐19 Illness in Native and Immunosuppressed States: A Clinical‐Therapeutic Staging Proposal. J Heart Lung Transplant. 2020;39(5):405‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]


