Abstract
Since the outbreak of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection in humans in late 2019, it has rapidly spread worldwide. To identify the biological characteristics of SARS‐CoV‐2 in a normal laboratory environment (biosafety level 2 [BSL‐2]), a lentiviral‐based nucleocapsid was used to carry the spike protein of SARS‐CoV‐2 onto the surface of pseudoviral particles as a surrogate model to evaluate the infective characterization of SARS‐CoV‐2. This study indicated that SARS‐CoV‐2 has extensive tissue tropism for humans and may infect monkeys and tree shrews but not rodents. More importantly, the use of pseudoviral particles in this study allows rapid assessment of neutralizing antibodies in serum in a BSL‐2 laboratory. This study will provide a quick and easy tool for evaluating neutralizing antibodies in the serum of recovering patients and assessing the potency of candidate vaccines.
Keywords: infectivity, neutralization, pseudoviral particles, SARS‐CoV‐2
Highlights
Lentiviral‐based pseudo‐particles with SARS‐CoV‐2 spike proteins (SaCaov2pp) were produced to rapid evaluate SARS‐CoV‐2 infectivity and neutralizing antibodies titer in a normal laboratory environment.
SaCoV2pp has extensive tissue tropism for primates but not for human diploid embryo lung cell strain or monkey liver cell line.
The rhesus monkey neutralizing serums for SaCoV2pp has little cross‐reactivity with SaCoV1pp.
1. INTRODUCTION
In December 2019, a novel pulmonary cluster infection was found in Wuhan, China. 1 The infected person developed fever, dry cough, dyspnea, gastrointestinal upset, and other severe respiratory syndromes. 2 , 3 It was caused by a novel coronavirus infection, and the pathogen was named severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). 4 SARS‐CoV‐2 can be transmitted through saliva, droplets or close contact, and many of its biological characteristics are currently unknown. 2 , 5 Until March 17, 2020, globally, the World Health Organization announced that there were over 179 112 cases of infection including 7426 deaths. 6
Both SARS‐CoV‐2 and severe acute respiratory syndrome coronavirus 1 (SARS‐CoV‐1) can cause severe respiratory syndrome. The SARS‐CoV‐1 epidemic infection occurred in 2003, and then quickly spread to many regions around the world, causing global public health crisis with strong infectiousness and high mortality. 7 , 8 SARS‐CoV‐2 and SARS‐CoV‐1 have similarities in structure and bioinformatics, but their homology at the genome level is less than 80%. 9 Some studies have shown that intermediate hosts may be wild animals such as pangolins, 10 , 11 and then humans can be infected. The spike protein on the surface of the coronavirus plays a key role in the process of invading cells. Compared with SARS‐CoV‐1, an important variation of SARS‐CoV‐2 is the introduction of a furin protease site in the spike protein. 12 , 13 It greatly increases the infection efficiency of SARS‐CoV‐2. With the analysis of the spike protein structure of SARS‐CoV‐2 by cryoelectron microscopy, it was found that SARS‐CoV‐2 had stronger binding ability to ACE2 than SARS‐CoV‐1, 14 and the serum cross‐reactivity between SARS‐CoV‐1 and SARS‐CoV‐2 is still unknown. Since there are currently no drugs and vaccines that inhibit these viral infections, it is necessary to establish effective systems to assess their effectiveness.
Both SARS‐CoV‐1 and SARS‐CoV‐2 induce highly pathogenic infectious diseases, which need to be operated in a biosafety level 3 (BSL‐3) laboratory for biosafety consideration. In this study, SARS‐CoV spike protein coated pseudoviral particles (saCoV2pp) were established, which have similar infection characteristics to the virus but no amplification ability. saCoV2pp were used to infect cells derived from different tissues and animal species to evaluate its infective characterization and was successfully used to evaluate neutralizing antibodies in serum.
2. MATERIALS AND METHODS
2.1. Cells
The Huh7.5 cell line was a gift from the laboratory of Charles M. Rice; RH35 was obtained from the Institute of Zoology, Chinese Academy of Sciences; immortalized tree shrew liver cells X9.0 and X9.5 and monkey liver cells RHT6.0 were isolated by our laboratory; Vero, A357, Caco‐2, KMB17, Hep2, Hacat, NIH3T3, CHO‐K1, HEK293, HEK293T, HL7702, HepG2, Hep1‐6, and Huh7 cells were preserved by our laboratory. All the cells were cultured at 37°C with 5% CO2 in flasks with Dulbecco's modified Eagle's medium (DMEM), 10% fetal bovine serum (FBS; Gibco), penicillin, and streptomycin.
2.2. Transfection and Western blot to detect spike protein expression
We transfected plasmids encoding wild‐type or codon‐optimized SARS‐CoV‐2 spike protein into HEK293T cells using jetPRIME transfection reagent. Cells were incubated for 4 hours at 37°C with transfection medium. Then, the transfection medium was replaced with DMEM containing 10% FBS, and the supernatant was removed after 48 hours. The cells were lysed with radioimmunoprecipitation assay, at 10 µg/well; samples were analyzed by 8% sodium dodecyl sulfate polyacrylamide gel electrophoresis. Proteins were transferred to polyvinylidene difluoride membranes by the Trans‐Blot TurboTM Transfer System (Bio‐Rad). The membrane was blocked with 5% nonfat milk for 1 hour at room temperature and incubated with the primary antibody (cat: 40150‐T52; Sino Biological) (1:1000 dilution) for 1 hour at room temperature and washed five times with tris‐buffered saline with Tween 20 (TBST) buffer. The goat anti‐rabbit secondary antibody (1:5000 dilution) was incubated at room temperature for 1 hour and washed five times with TBST buffer. Finally, signals were visualized using Western Detection Reagent (GE Healthcare).
2.3. Pseudoviral particle production
HEK293T cells were cotransfected with the spike protein‐encoding or spike protein knock‐out transmembrane domain (SΔTM) plasmid and the lentiviral expression vector carrying the luciferase reporter gene using Lipofectamine 3000 (Thermo Fisher Scientific). Cells were incubated for 6 hours at 37°C with transfection medium, and then the transfection medium was replaced with DMEM containing 10% FBS. The cell supernatant was harvested after 48 hours, centrifuged at 4000 rpm for 15 minutes to remove impurities such as cell debris, and then stored at −80°C.
2.4. Pseudoviral particle infection and luciferase activity measurements
The cells were plated into a 48‐well plate at a density 2 × 104 cells/well for 20 hours, the pseudovirus was removed from −80°C refrigeration, rapidly melted in a 37°C water bath, and then placed on ice, mixed with DMEM containing 2% FBS at a ratio of 1:1; then, the medium was removed from the cells, 200 µL of the pseudovirus mixture was added, and the cells were incubated at 37°C with 5% CO2 for 48 hours. The supernatant was removed, the cells were washed with 200 µL phosphate‐buffered saline (PBS), and 40 µL of lysate was lysed (Promega E1531) for 2 to 3 minutes. The lysate was removed into a tube and centrifuged at 5000 rpm for 5 minutes. The precipitate was wiped off, and the supernatant and substrate (Promega E1501) were mixed in a 1:10 ratio. Fluoroskan Ascent FL (Thermo Fisher Scientific) was used to measure the luciferase activity. The values were multiplied by a scaling factor of 106.
2.5. Neutralization antibody detection
Huh7.5 cells were plated in 96‐well plates at a density of 1 × 104 cells/well for 20 hours. The serum was inactivated at 56°C for 30 minutes, 50 µL serum was mixed with 200 µL pseudovirus (~200 ng p24/mL), and diluted twice using the pseudovirus, making the serum and pseudovirus ratios reach 1:256. Then, the cells were incubated at 37°C with 5% CO2 for 1 hour. The medium was removed from Huh7.5 cells, 50 µL of medium containing 2% FBS was added. Then, adding 50 µL neutralized the virus and serum mixture, which was mixed with gentle shaking, incubated at 37°C with 5% CO2 in an incubator for 48 hours. The supernatant was removed, the cells were washed with PBS, and 20 µL lysate was lysed (Promega E1531) for 2 to 3 minutes, then centrifuged at 12 000 rpm for 5 minutes. The supernatant and substrate (Promega E1501) were mixed at a 1:10 ratio. A Fluoroskan Ascent FL (Thermo Fisher Scientific) was used to measure the luciferase activity. The values were multiplied by a scaling factor of 106.
2.6. Statistical analysis
Data were analyzed by one‐way or two‐way analysis of variance followed by Brown–Forsythe test or Bartlett's test where appropriate. At least three independent experiments were carried out, and the data from each experiment were expressed as the mean ± SD. P < .05 was considered significant.
3. RESULTS
3.1. Analysis of infectivity of SARS‐CoV‐2 pseudoviral particles on the main human vaccine‐producing cells
According to the SARS‐CoV‐1 and SARS‐CoV‐2 genome sequences, the entire wild‐type and codon‐optimized spike gene sequences were synthesized and inserted into a mammalian cell expression plasmid. After transfection and expression in HEK293T cells, the S protein expression level was measured by Western blot analysis. The S protein expression level coded by the original gene sequence was low. However, codon optimization significantly increased the expression level (Figure 1). This codon‐optimized gene with the lentiviral expression vector carrying the luciferase reporter gene was cotransfected into HEK293T cells, and the supernatant was collected to infect fresh cells. Significant luciferase reporter gene activity in infected cells than that in the control cells (Figure 2). To find suitable cell strains for the amplification of the SARS‐CoV‐2 virus for the development of inactivated vaccines. The study tested saCoV2pp on diploid cells (KMB17), green monkey kidney cells (Vero), human embryonic kidney cells (HEK293), and hamster ovary cells (CHO‐K1); mouse embryo fibroblast cells (NIH3T3) that lack transmembrane regions (SΔTM) were used as a negative control, and SARS‐CoV‐1 pseudoviral particles (saCoV1pp) were used as a positive control. The infection results suggest that saCoV2pp can effectively infect Vero and HEK293 cells, while infecting the other three cells was less efficient (Figure 2).
Figure 1.

Detection of the expression of wild‐type and codon‐optimized severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) spike proteins. The wild‐type and codon‐optimized spike protein expression plasmids were transfected into HEK293T cells. After 48 hours of transfection, Western blot analysis was performed on the transfected cell lysate to detect the protein expression levels. wt: wild‐type; cop: codon optimization; con: control, HEK293T cell lysates
Figure 2.

SARS‐CoV‐2 pseudoviral particle infectivity to vaccine‐producing cells. The saCoV2pp infection was used in vaccine‐producing cells: KMB17, NIH3T3, CHO‐K1, HEK293, and Vero. After 48 hours, the supernatant was removed to detect the luciferase activity, and the pseudovirus was prepared. SΔTM in the cell lysate was used as a negative control, and saCoV1pp was used as a positive control. n = 3, P < .05. SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
3.2. Analysis of infectivity of SARS‐CoV‐2 pseudoviral particles on human cells from different tissues
After SARS‐CoV‐2 infects the human body, in addition to typical bronchial infections and pneumonia symptoms, it can also cause various complications, including hepatitis and diarrhea. To understand the tissue tropism of SARS‐CoV‐2 in humans, we selected several human‐derived cell lines, including hepatocytes (Huh7, HepG2, and HL7702), kidney cells (HEK293 and HEK293T), epithelial colorectal cells (Caco‐2), cervical cancer cells (Hep2), skin cells (A357) and keratinocytes (Hacat) (Figure 3). SARS‐CoV‐2 efficiently infected liver, kidney, and intestinal cells but was less infectious to cervical and epithelial cells. Among them, the infectivity to hepatocytes was the highest.
Figure 3.

Infectivity of SARS‐CoV2 pseudoviral particles on human different tissue cells.SaCoV2pp and saCoV1pp were used to infect human different tissue cells HEK293, 293T, HL7702, Huh7, Caco‐2, Hep2, Hacat, A357, and the supernatant was removed after 48 hours to detect luciferase activity, The pseudovirus particles of SΔTM as a negative control, and saCoV1pp was used as a positive control. n = 3, P < .05. SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
3.3. Analysis of the infectivity of SARS‐CoV‐2 pseudoviral particles on hepatocytes from different species
It is known that saCoV2pp has strong infectivity to human hepatocytes. Using the immortal liver cell line as the target cell for the saCoV2pp infection, the infectivity of saCoV2pp was analyzed in human (Huh7), monkey (RHT6.0), tree shrew (X9.0 and X9.5), mouse (Hep1‐6), and rat (RH35) cells, and the specificity of the infection in liver cells was determined. At the same time, hepatitis C virus pseudoviral particles (HCVpp), which have a very narrow tissue tropism, were used as controls. The results show (Figure 4) that saCoV2pp cannot effectively infect monkey hepatocytes (RHT6.0), even though RHT6.0 can be infected by HCVpp; however, saCoV2pp can infect tree shrew hepatocytes. Furthermore, the results indicated that saCoV2pp cannot infect liver cells of rodents, such as rats and mice.
Figure 4.
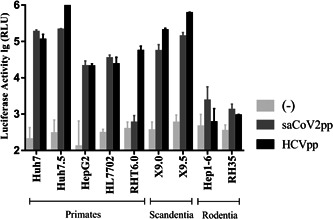
Infectivity of SARS‐CoV‐2 pseudoviral particles on hepatocytes from different species. Both saCoV2pp and HCVpp were used to infect hepatocytes from different species; tree shrew liver cells (X9.0 and X9.5), monkey liver cells (RHT6.0), mouse liver cells (Hep1‐6), and rat liver cells (RH35). and the supernatant was removed after 48 hours to detect luciferase activity. HCVpp were used as control. (‐): cell background. n = 3, P < .05. SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
3.4. SARS‐CoV‐2 pseudoviral particles for detecting neutralizing antibodies
The level of neutralizing antibodies is an important indicator for the evaluation of vaccine potency and reinfection resistance in recovering patients. For laboratories lacking BSL‐3 conditions, saCoV2pp may be a viable alternative. To test the feasibility of this, we chose three rhesus monkey serum samples with known neutralizing antibody titers (GMT: 1:4, 1:32, and 1:128) measured by SARS‐CoV‐2 virus in BSL‐3 conditions. The study selected a batch of saCoV2pp (~200 ng p24/mL) mixed with this serum in serial dilution. The results showed that the infectivity of saCoV2pp was not affected by the blank serum, but it could be neutralized by vaccine‐induced serum in a dose‐dependent manner (Figure 5). The neutralization effect was positively correlated with neutralizing antibody levels detected by the SARS‐CoV‐2 virus, suggesting that saCoV2pp can be used for the evaluation of neutralizing antibodies.
Figure 5.
Detecting the neutralizing effect of immune serum with saCoV2pp and saCoV1pp. Fifty microliters of blank serum and post‐immune serum were mixed with 200 µL of saCoV2pp or saCoV1pp, and diluted to 1:32, 1:256. After neutralization at 37°C for 1 hour, this mixture was added to Huh7.5 cells and grown for 15 hours. After 48 hours, the supernatant was removed, and the luciferase activity in the cell lysate was measured. Fetal bovine serum/Dulbecco's modified Eagle's medium (2%) was used as a negative control. Serum 1: GMT = 1:4. Serum 2: GMT = 1:32. Serum 3: GMT = 1:128. The black dotted line represents the cut‐off value. n = 2, P < .05
To measure the cross‐protection of the SARS‐CoV‐2 neutralizing serum with SARS‐CoV‐1, the same serum was used to neutralize saCoV1pp. No infection inhibition effect was observed. This result indicated that the neutralizing antibody induced by the SARS‐CoV‐2 S protein had weak cross‐protection against saCoV1pp, at least as a low‐level neutralizing antibody (Figure 5).
4. DISCUSSION
Because SARS‐CoV‐2 is transmitted through the respiratory tract and needs to be operated under BSL‐3 conditions, some research on the infectivity of this novel pathogen will be obviously restricted. Pseudoviral particles are defective viruses that do not have the ability to replicate, have a low biosafety concern, and are suitable for conducting research in ordinary laboratories. Currently, SARS‐CoV‐2 has evolved into a global pandemic infection. Effective vaccines are still needed to control the epidemic and eliminate pathogens from the crowd as soon as possible. Both inactivated and live attenuated vaccines require suitable cell lines for virus production. This study first tested several human vaccine‐producing cells in January. The results showed that rodent CHO‐K1 and NIH3T3 cells were not sensitive to saCoV2pp infection. The lung is known to be the primary infected tissue for SARS‐CoV‐2. The diploid human embryo lung cell KMB17 has been successfully used in the production of polio vaccine, 15 hepatitis A vaccine, 16 and EV71 vaccine, 17 and it is expected that saCoV2pp has a low infectivity to KMB17. By comparing with the most infectious hepatocyte, Huh7, and analyzing the existing transcriptome results, after normalizing and comparing, the expression of ACE2 on Huh7 cells is approximately 30 times that of KMB17 cells (unpublished data), suggesting that the abundance of the SARS‐CoV‐2 receptor ACE2 on lung cells may not be high during the embryonic development period. Clinical data showed that infants and young children are not susceptible to SARS‐CoV‐2, 18 and the expression abundance of ACE2 in lung tissue at different ages needs to be further confirmed. In addition, saCoV2pp can efficiently infect Vero and HEK293 cells. 19 Although saCoV2pp is more infectious to HEK293 cells, considering the existence of the adenovirus genome residue in HEK293 cells, it is mainly used for the production of recombinant adenoviruses and adeno‐associated viruses. Vero cells are still a better choice and are currently used for the adaptation of strains and the production of inactivated vaccines.
SARS‐CoV‐2 is a new pathogen. Some biological characteristics are still not clear. It is known that the virus can form viremia after infecting the human body through the respiratory system. Therefore, it is possible to further infect other organs. Using immortalized cell line to analyze the infectivity of saCoV2pp, it can be easily observed that saCoV2pp does not easily infect cervical and epithelial cells but can infect liver, kidney, and colon cells. This result is consistent with recent clinical studies that found that SARS‐CoV‐2 can cause hepatitis and nephritis in patients, 2 , 19 and SARS‐CoV‐2 nucleic acid and viruses have been detected in patients' stool and urine. 20 , 21
Animal models are necessary tools for studying the pathogenic mechanism of viruses. Based on the high sensitivity of SARS‐CoV‐2 to liver cells, we tested the sensitivity of commonly used animal model liver cells to saCoV2pp. The results also show that mouse and rat liver cells are not sensitive to saCoV2pp. Rhesus monkey liver cells are also less sensitive to saCoV2pp, even though RHT6 is a cell line that retains hepatocyte characteristics and can be infected by a narrow host range of HCVpp. Rhesus monkeys are an animal model, SARS‐CoV‐2 infection in the lungs of Rhesus monkeys have been established; however, compared with humans, the susceptibility is reduced, and rhesus monkeys infected with SARS‐CoV‐2 may not be prone to the symptoms of hepatitis similar to humans. Tree shrews are an ancient primate, and tree shrew liver cells can be infected by both HCVpp and saCoV2pp, suggesting the potential advantages of establishing a small animal model of SARS‐CoV‐2 infection.
In clinical tests, a method capable of detecting neutralizing antibodies in patients' serum is still lacking. In addition, the level of neutralizing antibodies in serum is also an important indicator for evaluating the vaccine protection mechanism. In this study, our results showed that the SARS‐CoV‐2 pseudovirus particle system was a successful surrogate model to evaluate the level of neutralizing antibodies in serum. Furthermore, the induced neutralizing antibodies did not have significant cross‐reactivity to saCoV1pp formed by the SARS‐CoV‐1 spike protein. Due to the slow cytopathic time after a SARS‐CoV‐2 infection when viruses are used for neutralization experiments under BSL‐3 conditions, the pseudoviral system can significantly reduce the detection time to increase the detection speed.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
TP, CD, and YWB performed the research. JMD and YDL provided the plasmid. XJL, HZ, and HWL analyzed the data. WC designed the research. TP and WC wrote the manuscript.
ACKNOWLEDGMENT
This study was supported by the CAMS Initiative for Innovative Medicine (2016‐I2M‐1‐019 and 2016‐I2M‐1‐013), National Science and Technology Major Projects (2018ZX09738003‐007, 2018ZX10101001‐002), Major Science and Technology Projects of Yunnan Province (2019ZF001), National Natural Science Foundation of China (81971947), and Talent development project (D2017003).
Pu T, Ding C, Li Y, et al. Evaluate severe acute respiratory syndrome coronavirus 2 infectivity by pseudoviral particles. J Med Virol. 2020;92:1609–1614. 10.1002/jmv.25865
Tao Pu and Chen Ding contributed equally to this study.
REFERENCES
- 1. WHO . Pneumonia of unknown cause–China. 2020/3/1. https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-china/en/
- 2. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. New Engl J Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gorbalenya AE, Baker SC, Baric RS, et al. Severe acute respiratory syndrome‐related coronavirus: The species and its viruses – a statement of the Coronavirus Study Group. bioRxiv. 2020. 10.1101/2020.02.07.937862 [DOI] [Google Scholar]
- 5. Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. WHO . Situation reports. 2020/3/18. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports
- 7. Ksiazek T. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:348‐1966. [DOI] [PubMed] [Google Scholar]
- 8. Drosten C, Günther S, Preiser W, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348(20):1967‐1976. [DOI] [PubMed] [Google Scholar]
- 9. Zhou P, Yang X, Wang X, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xiao K, Zhai J, Feng Y, et al. Isolation and characterization of 2019‐nCoV‐like coronavirus from Malayan pangolins. bioRxiv. 2020. 10.1101/2020.02.17.951335 [DOI] [Google Scholar]
- 11. Tsan‐Yuk Lam T, Shum MH, Zhu HC, et al. Identification of 2019‐nCoV related coronaviruses in Malayan pangolins in southern China. bioRxiv. 2020. 10.1101/2020.02.13.945485 [DOI] [Google Scholar]
- 12. Xin L, Guangyou D, Wei Z, et al. A furin cleavage site was discovered in the Wuhan 2019 human coronavirus S protein. Chinese J Bioinformatics. 2020. [Google Scholar]
- 13. Walls AC, Park Y, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS‐CoV‐2 spike glycoprotein. Cell. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wrapp D, Wang N, Corbett KS, et al. Cryo‐EM structure of the 2019‐nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260‐1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ning‐zhu HU, Rong‐fu W, QUSu, et al. Culture of live attenuated poliomyelitis vaccine virus in human embryonic lung diploid cells (KMB17) by suspension adsorption. Chinese J Biol. 2005;18(6):50‐51. [Google Scholar]
- 16. Huang X, Yang J, Zhou D, Bai H, Cao Y, Dong D. Virulence and nucleotide sequences of HAV (H2 strain) cultured in KMB17 cell. Chinese J Biol. 2000. [Google Scholar]
- 17. Liu Xin, Liao Yun. Analysis of Enterovirus 71 associated receptors expression on the membrane of human diploid cell line KMB17 membrane. Chinese Med Biotechnol. 2011;6(4):4‐255. [Google Scholar]
- 18. Xu Y, Li X, Zhu B, et al. Characteristics of pediatric SARS‐CoV‐2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li Z, Wu M, Guo J, et al. Caution on kidney dysfunctions of 2019‐nCoV patients. medRxiv. 2020. 10.1101/2020.02.08.20021212v2 [DOI] [Google Scholar]
- 20. Chinese Center for Disease Control and Prevention . 2020. http://www.chinacdc.cn/yw_9324/202002/t20200214_212635.html [DOI] [PMC free article] [PubMed]
- 21. The laboratory team successfully isolated the virus from the urine of COVID‐19 patients – State Key Laboratory of Aspiration Disease. 2020/3/2. http://www.sklrd.cn/show.php?id=1384


