Abstract
Objectives
Drug repositioning, that is, the use of a drug in an indication other than the one for which it was initially marketed, is a growing trend. Its origins lie mainly in the attrition experienced in recent years in the field of new drug discovery.
Key findings
Despite some regulatory and economic challenges, drug repositioning offers many advantages, and a number of recent successes have confirmed both its public health benefits and its commercial value. The first examples of successful drug repositioning mainly came about through serendipity like acetylsalicylic acid, thalidomide, sildenafil or dimethylfumarate.
Conclusion
The history of great‐repositioned drugs has given some solutions to various pathologies. Serendipity is not yet useful to find repositioning drugs. Drug repositioning is of growing interest. Nowadays, a more rational approach to the identification of drug candidates for repositioning is possible, especially using data mining.
Keywords: drug, generic, indication, repositioning
Introduction
Drug repositioning lies in repurposing an active pharmaceutical ingredient that is already on the market for a new indication. Although this strategy has a number of drawbacks and offers certain challenges, it also possesses many advantages, including helping to overcome the attrition currently experienced in the field of new drug discovery.[ 1 ] The history of medicine has been marked by several examples of repositioned drugs, including some very old drugs. Most of them came out purely through serendipity. New methods have now been developed, based in particular on data mining, to identify new candidates for drug repositioning. Many start‐ups are entirely focused on developing this concept, a journal has been devoted to it (Drug Repurposing, Rescue, and Repositioning; MA Liebert Inc., Boston, MA, USA), as well as an annual international conference (the 8th edition was held in Washington DC in 2019). This article provides a brief overview of repurposing and particularly evokes its most recent scientific basis as well as the new tools, and especially the computational ones, used to render it more efficient.
Definition, scope, limitations and scientific basis of drug repositioning
Although some historical examples exist, drug repositioning (also referred to as drug repurposing, reprofiling, redirecting, switching, etc.) is a relatively recent concept that appears to have emerged in 2004 with an article by Ashburn and Thor, who provided an initial definition.[ 2 ] They defined drug repositioning as the process of finding new uses for existing drugs, sometimes but not necessarily when they fall into the public domain and become generic drugs. The number of articles devoted to this concept grew exponentially from 2010 onwards,[ 3 ] to reach 602 in 2018 (based on a search of PubMed on November, 21th 2019 for titles or abstracts containing the terms ‘drug repositioning’ or ‘drug repurposing’ and the publication year 2018).
This original definition of drug repositioning has since been extended to include active substances that failed the clinical phase of their development on account of their toxicity or insufficient efficacy, as well as drugs withdrawn from the market because of safety concerns. It should not, however, include substances that have not yet been subjected to clinical investigation. In particular, this rules out substances held in chemical libraries by academic and industry research groups to be screened to identify those with new biological properties, different from the properties for which they were originally designed and synthesised. Such compounds usually require hit‐to‐lead chemistry, in order to optimise the new therapeutic effect sought. Similarly, selective optimisation of side activities (SOSA), as proposed by Professor Wermuth, falls outside the scope of repositioning.[ 4 ] In the SOSA approach, the biological properties deemed responsible for a drug’s adverse effects in a particular indication are isolated and amplified through chemical modification so that the modified drug can be proposed for a new indication.
The concept of drug repositioning thus excludes any structural modification of the drug. Instead, repositioning makes use in a new indication of either the biological properties for which the drug has already been approved (possibly according to a different formulation, at a new dose or via a new route of administration), or the side properties of a drug that are responsible for its adverse effects.
Drug repositioning relies on two main scientific bases: (1) the discovery, through the human genome elucidation, that some diseases share sometimes common biological targets and (2) the concept of pleiotropic drugs.
The description of the elements associated to the complex interplay between diseases, drugs and targets with in silico approaches (data mining, machine learning, ligand‐based and structure‐based approaches) is one of the key for drug repurposing.[ 5 ] It is now possible to describe diseases by their molecular profile (e.g. the genes, biomarkers, signalling pathways, environmental factors etc… involved in their pathogenicity) and to use computational methods, especially data mining, to determine the degree of similarity between diseases that share a number of these molecular features.[ 6 ] For example, Parkinson’s disease and Alzheimer’s disease share 48 genes and four signalling pathways [ 7 ]. The existence of protein targets common to several diseases suggests that a given drug might have efficacy against both conditions.
Most drugs are now phenotypically well characterised in terms of their primary therapeutic effects and their (usually unwanted) side effects. This array of effects results from pleiotropic interactions between the drug and several (primary and secondary) biological targets. A drug can therefore have efficacy against a disease other than the one for which it was initially designed if one of its secondary targets has a role in the new disease. Note also that these pleiotropic interactions make it is possible to develop drugs with multiple effects, all intended, that act in synergy to provide greater clinical efficacy, for example, pan‐kinase inhibitors used in oncology. Like diseases, drugs can be analysed for phenotypic similarity, regardless of their therapeutic indication. If two drugs indicated in different diseases have a high similarity score, they may be effective in both indications.[ 6 ]
Advantages of drug repositioning
Drug repositioning has a number of interrelated advantages. They essentially consist of the simplification in the regulatory procedures for introducing a previously approved drug on the market, especially in certain countries such as the United States. This procedure takes into account data previously acquired, in particular on the drug’s safety and toxicity, which can make the initial phases of development for a repositioned drug considerably faster,[ 8 ] and therefore cheaper (by over 80% according to Naylor),[ 9 ] and increases the chances of introducing it on the market (by 150% compared with a novel drug, according to Thayer).[ 10 ] One important consideration, however, is that, due to the level of safety required for a drug is highly dependent on its indication, the adverse effects of a drug will be proportionately less acceptable when repositioned for a disease that is less serious or severe than its original indication.[ 11 ] Any change in formulation, dosage, or route of administration will require re‐examination of the drug’s safety profile under these new conditions, in what will be a new medicinal product.
Challenges associated with drug repositioning
The main challenges faced by repositioners lie in the relatively weak intellectual property protection afforded to such medicinal products, which can reduce their return on investment and discourage companies from developing them.[ 12 ] As the drug concerned has already been patented as a new chemical entity, subsequent medicines containing the same entity can only be protected by a new application patent, possibly backed up by a new formulation process. Patents on applications are necessarily narrower than those for a new chemical entity in terms of the therapeutic uses they cover. For example, they cannot always prevent generic products containing the same drug being prescribed off‐label for the patented application. They are also weaker, in particular in the face of a potential legal challenge on the basis that the new indication was predictable from data in the scientific literature.[ 11 ] The relative weakness of the protection provided by these patents may nevertheless be offset by certain advantages granted to companies repositioning drugs for the treatment of orphan diseases (defined in Europe as those with a prevalence no higher than 5 in 10 000), such as fee reductions and a guaranteed period of market exclusivity.[ 13 ] Some companies (such as Apteeus in France) specialised in ‘personalised’ drug repositioning, by screening candidate drugs on cells derived from patients with orphan diseases.
Examples of repositioned drugs
As mentioned above, the scientific literature abounds with studies aimed at demonstrating the value of an existing drug in another indication, yet the website http://drugrepurposing.info lists only 94 cases in which a repositioned drug made it to the market (accessed on 03 January 2018). It was estimated in 2014 that repositioned drugs generated $250 billion in sales worldwide; that is, approximately, one‐quarter of the pharmaceutical industry’s annual revenue, with five such drugs each generating over $1 billion in their new indication.[ 9 ]
Aspirin
The oldest example of drug repositioning is without doubt acetylsalicylic acid (Figure 1).
Figure 1.
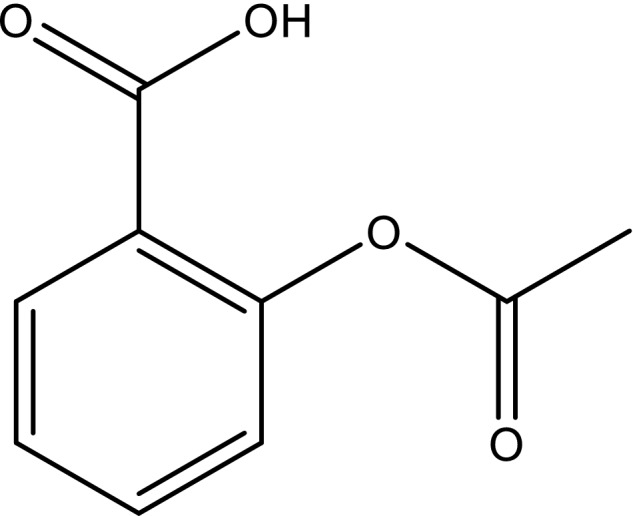
Structure of acetylsalicylic acid or aspirin.
Initially marketed by Bayer in 1899 as an analgesic, aspirin was first repositioned in the 1980s, at low doses only, as an antiplatelet aggregation drug.[ 14 ] It is still widely used today in this second indication to prevent cardiovascular events, based on the work of Vane, for which he was awarded the Nobel Prize in Medicine in 1982.[ 15 ] Previously, Dr Lawrence Craven, a general practitioner at Glendale Memorial Hospital, California, had observed that aspirin, administered for analgesia for his tonsillectomy patients, had the adverse effect of increasing bleeding. Aspirin inhibits platelet aggregation by irreversibly inhibiting platelet cyclooxygenase 1 (COX‐1), an enzyme responsible for the formation of the prostaglandin precursor of thromboxane A2, a potent stimulator of platelet aggregation.
Aspirin’s analgesic and anti‐inflammatory effects stem from its inhibition of cyclooxygenase 2 (COX‐2), in particular vascular COX‐2, an enzyme involved in the synthesis of prostaglandins that generate pain and inhibit platelet aggregation. At low doses (<300 mg/day), aspirin has partial selectivity for COX‐1 and exerts its antiplatelet aggregation effect, which at higher doses is circumvented by concomitant COX‐2 inhibition. Aspirin’s inhibition of COX‐1, regardless of the dose administered, is also responsible however for its harmful effects on the gastrointestinal tract, due to this enzyme’s role in the synthesis of prostaglandins involved in its cytoprotection.
Aspirin may soon be repositioned again, this time in oncology. It has been shown that daily administration of aspirin for at least 5 years can prevent the development of many cancers, and in particular colorectal cancer[ 16 ] Aspirin’s protective effect against cancer is thought to result from COX‐2 inhibition, thus blocking the antiapoptotic effect of COX‐2 in malignant cells and promoting their apoptotic death.[ 17 ]
Although this second potential repositioning of aspirin offers great promise from a public health point of view, there is little encouragement for it from the pharmaceutical industry, probably due to the intellectual property issues outlined above.
Thalidomide
It is well known that the World Health Organization (WHO) banned thalidomide (Figure 2) in 1962 due to its teratogenicity, which affected thousands of victims worldwide, and continues to affect a second generation, mainly through its use as an antiemetic for pregnant women.
Figure 2.
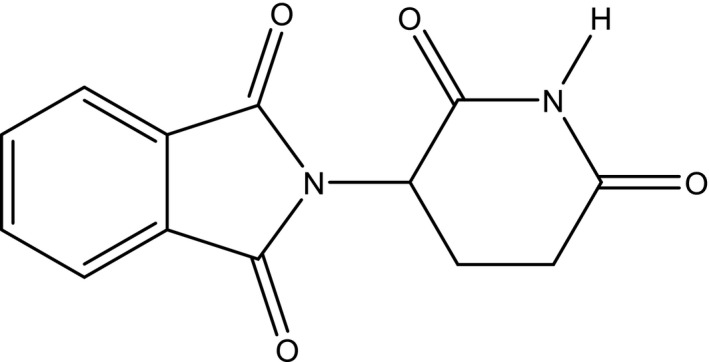
Structure of thalidomide.
Despite this, Dr Jacob Sheskin from Hadassah University, Jerusalem, demonstrated in 1964 its dramatic efficacy against erythema nodosum leprosum, an autoimmune complication of leprosy. Thalidomide, which achieves this effect by inhibiting the synthesis of the proinflammatory cytokine tumour necrosis factor‐alpha (TNF‐α), was consequently repositioned by Celgene in 1998 as an orphan drug for complications of leprosy.[ 18 ] Its use must obviously be accompanied by drastic measures to prevent exposure to the drug during pregnancy, including stringent contraceptive measures. This example illustrates how even drugs with an exceptionally poor toxicity profile can be repositioned if the new indication is a rare disease (the estimated incidence of leprosy is 250 000 cases per year according to http://www.orpha.net, accessed November, 21th 2019).
Like aspirin, thalidomide was repositioned for a second time in the field of oncology. Investigation of the mechanism underlying thalidomide’s teratogenicity demonstrated its antiangiogenic activity, responsible for the arrested limb development (phocomelia) that occurs after in utero exposure. This activity led to research into its potential use to block or destroy blood vessels supplying malignant tumours, culminating in 2006 in its second repositioning as a first‐line treatment for multiple myeloma. The same precautions apply, and in Europe, the prescribing and dispensing of thalidomide is subject to specific monitoring.
Sildenafil
Sildenafil (Figure 3) is an example of a pharmaceutical substance that was repurposed before it reached the market.
Figure 3.
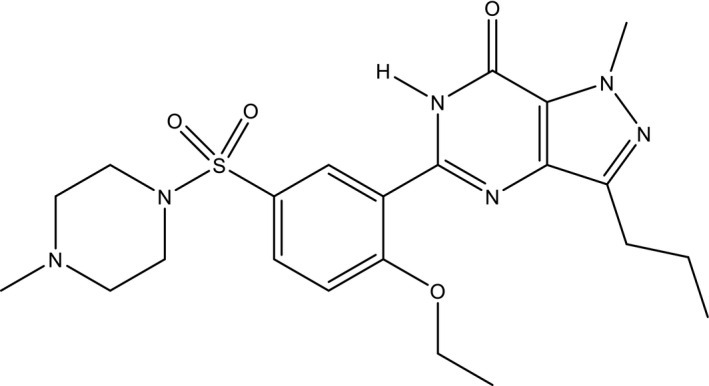
Structure of sildenafil.
Sildenafil was initially investigated by Pfizer in 1985 as a potential antihypertensive drug. It was shown to produce vasodilation and to inhibit platelet aggregation by inhibiting phosphodiesterase type‐5 (PDE5), the enzyme that degrades cGMP. In light of these properties, the focus was shifted onto its potential in the treatment for angina. An unexpected side effect emerged during clinical trials conducted in the United Kingdom, in the form of penile erections. This effect is the consequence of vasodilation, by inhibiting PDE5 and consequently blocking cGMP degradation. Sildenafil only produces an erection however in the presence of sexual stimulation, leading to the release of nitric oxide (NO), which in turn leads to cGMP production. It differs in this way from amyl nitrite, contained for example in ‘poppers’, which provokes NO release and therefore, unlike sildenafil, can be termed an aphrodisiac. This physiological effect led Pfizer to market sildenafil in 1998 for erectile dysfunction, under the brand name Viagra®, generating peak annual sales in this indication in excess of $2 billion.[ 9 ]
Sildenafil was subsequently repositioned for a second time. Pfizer continued to study the potentially therapeutic effects of inhibiting PDE5 and proposed exploiting the vasodilation it produces to treat pulmonary arterial hypertension, at one‐fifth of the dose used in erectile dysfunction. The idiopathic form of pulmonary hypertension is considered a rare disease (2–3 million cases per year) and is potentially fatal, which explains why it was relatively easy for Pfizer to obtain approval for a second sildenafil product (Revatio®) in 2005 in this new indication.[ 11 ]
Unlike aspirin and thalidomide, which act on different biological targets in the various indications for which they have been approved, and can therefore be described as pleiotropic, sildenafil acts on the same target (PDE5) to treat both erectile dysfunction and pulmonary arterial hypertension.
Dimethyl fumarate
Dimethyl fumarate (Figure 4) was first synthesised in 1819. For 150 years, it was only known as a mould inhibitor to protect leather and a cause of allergies, which led to a ban on its use in Europe in 2009.
Figure 4.
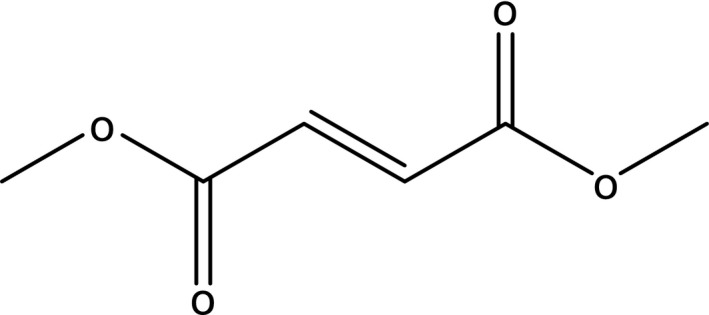
Structure of dimethylfumarate.
Nevertheless, dimethyl fumarate has also been marketed as a drug since 1994. It is commonly used in Germany to treat psoriasis, under the brand name Fumaderm®. Having discovered that dimethyl fumarate’s anti‐inflammatory activity was mediated by increased expression of NRF2‐dependent antioxidative genes, Biogen proposed its use in another autoimmune disease, multiple sclerosis (MS), at higher doses. It was marketed for this indication in 2013, under the brand name Tecfidera®. It constituted a major advance in the treatment of MS, because it could be taken orally and is less cardiotoxic and hepatotoxic than the other drugs used in this condition, although it does expose patients to the risk of developing progressive multifocal leukoencephalopathy. In 2015, this product generated almost $3 billion in annual revenue.[ 8 ] Finally, it should be noted that unlike the previous examples, dimethyl fumarate was not repositioned to make use of a side effect considered undesirable in the treatment of psoriasis, but on the basis of the similarity between the molecular profiles of psoriasis and MS.
The drug pipeline for Alzheimer’s disease
If there is one field in which attrition in the introduction of new drugs is particularly evident, it is Alzheimer’s disease, for which no new drugs have been marketed since galantamine in 2000. It is undoubtedly for this reason that so many drug repositioning trials have been and continue to be conducted in this field, albeit unsuccessfully to date. In 2017, over 100 drugs were undergoing clinical trials for this disease.[ 19 ] One‐quarter (27) of these drugs have already been marketed and are therefore being investigated for potential repositioning (Table 1). Methylthioninium chloride (methylene blue) can also be added to this list, as it has been used to treat malaria and methaemoglobinaemia and is always used as an antiseptic to treat urinary tract infections and non‐infectious conjunctivitis. Many mechanisms of action that have already been exploited in other therapeutic fields have been reinvestigated in Alzheimer’s disease for their potential to offer symptomatic treatments (e.g. levetiracetam, cannabinoids, lithium and neuroleptics) or disease‐modifying therapies (e.g. insulins, dihydropyridines, nicotine and sartans).
Table 1.
Previously approved drugs undergoing clinical trials in Alzheimer’s disease in 2017
| ICD | Mechanism of action | Original indication | Stage |
|---|---|---|---|
| Aripiprazole | Dopaminergic Agonist | Atypical Antipsychotic | Stage 1 |
| Brexpiprazole | Dopaminergic Agonist | Atypical Antipsychotic | |
| Insulin | Insulin Receptor Ligand | Types 1 and 2 Diabetes | |
| Methylphenidate | Dopamin Reuptake Inhibitor | ADHD | |
| Nabilone | Cannabinoid | Nausea, Vomiting | |
| Nilvadipine | Calcic Antagonist, Dihydropyridines | Hypertension | |
| Pioglitazone | PPARγ Agonist receptor | Type 2 Diabetes | |
| Atomoxetine | Adrenaline Reuptake inhibitor | ADHD | Stage 2 |
| Candesartan | Angiotensin Agonist Receptor | Hypertension | |
| Cilostazol | PDE3 Inhibitor | Intermittent Claudication | |
| Dronabinol | Cannabinoid | Nausea, Vomiting | |
| Formoterol | β‐2 adrenergic receptor Agonist | Asthma | |
| Detemir Insulin | Insulin Receptor Ligand | Types 1 and 2 Diabetes | |
| Glulisine Insulin | Insulin Receptor Ligand | Types 1 and 2 Diabetes | |
| Levetiracetam | (SV2A) Protein Ligand | Epilepsy | |
| Liraglutide | Glucagon Receptor Binding | Type 2 Diabetes | |
| Lithium | Ionic Modulator | Bipolar Disorders | |
| Nicotine | Cholinergic Receptors Agonist | Smoking Cessation | |
| Nilotinib | Tyrosine kinase inhibitor | Chronic myeloid leukaemia | |
| Pimavanserine | 5‐HT2A receptor reverse agonist | Psychotic disorders | |
| Probucol | LDL Promoter | Hypercholesterolaemia | |
| Rasagiline | IMAO‐B | Antiparkinsonian | |
| Riluzole | Glutamate antagonist receptor | Amyotrophic Lateral Sclerosis | |
| Sargramostim | Cell growth factor | Neutropenia | |
| Simvastatin | HMG‐CoA reductase Inhibitor | Hypercholesterolaemia | |
| Valaciclovir | DNA polymerase inhibitor | Herpes virus infection | |
| Telmisartan | Angiotensin Agonist Receptor | Hypertension | Stage 3 |
The high proportion of existing drugs in the Alzheimer’s disease pipeline is a good illustration of the fields that rely heavily on the concept of drug repositioning (Table 2).
Table 2.
Key papers and their related key findings
| Paper | Findings |
|---|---|
| [1] Pushpakom S et al. Nat Rev Drug Discov 2019; 18(1): 41–58. https://doi.org/10.1038/nrd.2018.168 | Recommended innovative ways to overcome challenges in repurposing approach |
| [5] March‐Vila E et al. Front Pharmacol 2017; 8: 298–298. https://doi.org/10.3389/fphar.2017.00298 | Computational approaches for repurposing |
| [6] Iwata H et al. J Chem Inf Model 2015; 55(2): 446–459. https://doi.org/10.1021/ci500670q | Drug repurposing for neurodegenerative diseases: the in silico tools |
| [7] Dovrolis N et al. Drug Discov Today 2017; 22(5): 805–813. https://doi.org/10.1016/j.drudis.2017.03.009 | In silico tools to establish drug‐disease association network and newly predicted drug indications |
| [20] Zhang M et al. PLoS One 2016; 11(12). https://doi.org/10.1371/journal.pone.0168812 | Omics data mining for repurposing |
| [22] Brown AS, Patel CJ. Sci Data 2017; 4. https://doi.org/10.1038/sdata.2017.29 | Computational repurposing based on a database of approved and failed drugs and their indications |
Conclusion
The repositioning of drugs for a therapeutic indication other than the one for which they were initially marketed is a growing trend. An illustrative and very recent example of useful repositioning is the study led in Marseille which showed that chloroquine and hydroxychloroquine could constitute useful weapons against COVID‐19.[ 20 ]
The first examples of repositioned drugs largely came out through serendipity. Information technology, and cheminformatics in particular, have now made it possible to facilitate and accelerate the process. Data mining is one current method for identifying candidate drugs for repositioning.[ 7 , 21 , 22 ] By defining descriptors from databases for both drugs and diseases, this technology can be used to establish drug–disease pairs and construct models to predict new pairs, which will undoubtedly yield new candidates for repositioning through a more rational process.
The main aim of drug repositioning is to combat the attrition and rising costs which, according to ‘Eroom’s law’,[ 23 ] are having a dramatic effect on the number of new drugs entering the pharmaceuticals market. However, this approach must be considered as an add‐on rather than an alternative to the search for novel drugs.
Declarations
Conflicts of interest
None.
Author’s contribution
All Authors participate to this work. All authors revised, added figures, tables and polished it in English.
References
Key papers and their related key findings are summarized in Table 2.
- 1. Pushpakom S et al. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov 2019; 18: 41–58. [DOI] [PubMed] [Google Scholar]
- 2. Ashburn TT, Thor KB. Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discov 2004; 3: 673–683. [DOI] [PubMed] [Google Scholar]
- 3. Langedijk J et al. Drug repositioning and repurposing: terminology and definitions in literature. Drug Discov Today 2015; 20: 1027–1034. [DOI] [PubMed] [Google Scholar]
- 4. Wermuth CG. Selective optimization of side activities: the SOSA approach. Drug Discov Today 2006; 11: 160–164. [DOI] [PubMed] [Google Scholar]
- 5. March‐Vila E et al. On the integration of in silico drug design methods for drug repurposing. Front Pharmacol 2017; 8: 298–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Iwata H et al. Systematic drug repositioning for a wide range of diseases with integrative analyses of phenotypic and molecular data. J Chem Inf Model 2015; 55: 446–459. [DOI] [PubMed] [Google Scholar]
- 7. Dovrolis N et al. Laying in silico pipelines for drug repositioning: a paradigm in ensemble analysis for neurodegenerative diseases. Drug Discov Today 2017; 22: 805–813. [DOI] [PubMed] [Google Scholar]
- 8. Nosengo N. Can you teach old drugs new tricks? Nature 2016; 534: 314–316. [DOI] [PubMed] [Google Scholar]
- 9. Naylor S, Schonfeld J. Therapeutic drug repurposing: repositioning and rescue. Drug Discov World 2014: 50–63. Available at: https://www.ddw‐online.com/drug‐discovery/p274232‐therapeutic‐drug‐repurposingrepositioning‐and‐rescue‐part‐i:‐overview.html. Accessed February 12, 2020. [Google Scholar]
- 10. Thayer A. Drug Repurposing|October 1, 2012 Issue – Vol. 90 Issue 40|Chemical & Engineering News. Available at: https://cen.acs.org/articles/90/i40/Drug‐Repurposing.html. Accessed February 12, 2020.
- 11. Cavalla D. Scientific commercial value of drug repurposing. In: Dudley J, Berliocchi LE, eds. Drug Repositioning – Approaches and Applications for Neurotherapeutics. Abingdon: Taylor & Francis Group, 2017: 3–22. [Google Scholar]
- 12. Rastegar‐Mojarad M et al. Opportunities for drug repositioning from phenome‐wide association studies. Nat Biotechnol 2015; 33: 342–345. [DOI] [PubMed] [Google Scholar]
- 13. Xu K, Coté TR. Database identifies FDA‐approved drugs with potential to be repurposed for treatment of orphan diseases. Brief Bioinform 2011; 12: 341–345. [DOI] [PubMed] [Google Scholar]
- 14. Monneret C, Bohuon C. Fabuleux hasards: histoire de la découverte de médicaments. Paris: EDP, 2009. [Google Scholar]
- 15. Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin‐like drugs. Nat New Biol 1971; 231: 232–235. [DOI] [PubMed] [Google Scholar]
- 16. Rothwell PM et al. Effect of daily aspirin on long‐term risk of death due to cancer: Analysis of individual patient data from randomised trials. Lancet 2011; 377: 31–41. [DOI] [PubMed] [Google Scholar]
- 17. Rüschoff J et al. Aspirin suppresses the mutator phenotype associated with hereditary nonpolyposis colorectal cancer by genetic selection. Proc Natl Acad Sci USA 1998; 95: 11301–11306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Raje N, Anderson K. Thalidomide – a revival story. N Engl J Med 1999; 341: 1606–1609. [DOI] [PubMed] [Google Scholar]
- 19. Cummings J et al. Alzheimer’s disease drug development pipeline: 2017. Alzheimer’s Dement Transl Res Clin Interv 2017; 3: 367–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Raoult D et al. Chloroquine and hydroxychloroquine as available weapons to fight COVID‐19. Int J Antimicrob Agents 2020; 105932. 10.1016/j.ijantimicag.2020.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang M et al. Drug repositioning for Alzheimer’s disease based on systematic “omics” data mining. PLoS ONE 2016; 11: e0168812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brown AS, Patel CJ. A standard database for drug repositioning. Sci Data 2017; 4. 10.1038/sdata.2017.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Scannell JW et al. Diagnosing the decline in pharmaceutical R&D efficiency. Nat Rev Drug Discov 2012; 11: 191–200. [DOI] [PubMed] [Google Scholar]


