Abstract
An epidemic of an acute respiratory syndrome caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) in Wuhan, China, now known as coronavirus disease 2019 (COVID‐19), beginning in December 2019, has attracted an intense amount of attention worldwide. As the natural history and variety of clinical presentations of this disease unfolds, extrapulmonary symptoms of COVID‐19 have emerged, especially in the digestive system. While the respiratory mode of transmission is well known and is probably the principal mode of transmission of this disease, a possibility of the fecal‐oral route of transmission has also emerged in various case series and clinical scenarios. In this review article, we summarize four different aspects in published studies to date: (a) gastrointestinal manifestations of COVID‐19; (b) microbiological and virological investigations; (c) the role of fecal‐oral transmission; and (d) prevention and control of SARS‐CoV‐2 infection in the digestive endoscopy room. A timely understanding of the relationship between the disease and the digestive system and implementing effective preventive measures are of great importance for a favorable outcome of the disease and can help climnicians to mitigate further transmission by taking appropriate measures.
Keywords: COVID‐19, digestive system, prevention and control, severe acute respiratory syndrome coronavirus 2
1. INTRODUCTION
The novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), or coronavirus disease 2019 (COVID‐19), is a highly infectious respiratory virus that emerged in Wuhan, China and has posed a serious threat to human health worldwide since December 2019. As of March 31, 2020, a total of 697 244 confirmed cases including 33 257 deaths have been reported for COVID‐19 in a growing number of international locations (available from https://www.who.int/emergencies/diseases/novel-coronavirus-2019).
The most common presentations of COVID‐19 include fever and respiratory symptoms, such as cough, shortness of breath and dyspnea. However, concurrent gastrointestinal symptoms, mainly diarrhea and vomiting, have also been reported in some studies of the recent outbreak. 1 SARS‐CoV‐2 is an RNA virus and the nucleic acid has been detected in the feces of some patients with pneumonia who are diagnosed with novel coronavirus infection, which probably indicates the presence of live viruses in the feces as well as the possibility of a fecal‐oral route of viral transmission. This observation presents a new challenge to the diagnosis and control of COVID‐19. In this article, we review the enteric manifestations in patients with COVID‐19 based on current literature reports and aim to provide a diagnostic and preventive strategy from the gastroenterological perspective.
2. GASTROINTESTINAL MANIFESTATIONS
Human coronaviruses (HCoV) comprise two of the following genera: alpha coronavirus (HCoV‐229E and HCoV‐NL63) and beta coronavirus (HCoV‐HKU1, HCoV‐OC43, Middle East respiratory syndrome [MERS] coronavirus, severe acute respiratory syndrome coronavirus [SARS‐CoV], and the newly discovered SARS‐CoV‐2). While respiratory complaints are usually the most common presenting symptoms of HCoV infection, concurrent or isolated gastrointestinal manifestations, characterized primarily by diarrhea, nausea and vomiting, and elevated liver enzymes should not be ignored after infection with HCoV. Up to 30% of patients with SARS and MERS complain of gastrointestinal symptoms, such as vomiting and diarrhea.2, 3 Coronaviruses can also cause hematochezia in severe cases in infants and neonates.
Currently, available data show that gastrointestinal involvement in COVID‐19 is relatively infrequent compared with MERS and SARS. Among the 1602 patients enrolled in the 10 case series reported, 55 had diarrhea (average 5.6%, range 2%‐33.98%), and 72 had nausea or vomiting symptoms (average 4.49%, range 1%‐10%) (Table 1).1, 4, 5, 6, 7, 8, 9, 10, 11, 12 A recent study found that almost half of 99 patients with COVID‐19 showed liver involvement to some degree, with variable degrees of elevated alanine aminotransferase and aspartate aminotransferase. Although the exact cause remains obscure, it may be related to direct liver damage caused by COVID‐19 or antiviral drugs. 6
TABLE 1.
Gastrointestinal manifestation in patients with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection
| Author | Country | Patients, n (total/male) | Age, y (mean, range) | Case, n (% if total case number ≥5) | |||
|---|---|---|---|---|---|---|---|
| Diarrhea | Abdominal pain | Nausea and vomiting | Fecal samples for SARS‐CoV‐2 (n) | ||||
| Pan et al1 | China | 204/107 | 52.91 | 35 (33.98) | 2 (1.94) | 4 (3.8) | NM |
| Huang et al 4 | China | 41/30 | 49 (41‐58) a | 1 (3) | NM | NM | NM |
| Chan et al 5 | China | 5/2 | NM (36‐66) | 2 (25) | NM | NM | 2/2 negative |
| Chen et al 6 | China | 99/67 | 55.5 (21‐82) | 2 (2) | NM | 1 (1) | NM |
| Chang et al 7 | China | 13 (10) | 34 (34‐48) a | 1 (7.7) | NM | NM | NM |
| Holshue et al 8 | USA | 1/1 | 35 | 1 | NM | 1 | 1/1 positive |
| Kim et al 9 | South Korea | 1/0 | 35 | 1 | NM | NS | NM |
| Phan et al 10 | Vietnam | 1/1 | 65 | 1 | NM | 1 | NM |
| Wang et al 11 | China | 138/75 | 56 (42‐68) a | 14 (10.1) | 3 (2.2) | Nausea: 14 (10.1) Vomiting: 5 (3.6) | NM |
| Guan et al 12 | China | 1099/640 | 47 (35‐58) b | 41 (3.7) | NM | 55 (5.0) | 4/62 positive fecal samples; 4 positive rectal swabs |
Abbreviation: NM, not mentioned.
Median (interquartile range).
Median (range).
The involvement of the digestive system in COVID‐19 may be underestimated as most patients initially develop respiratory symptoms. Gastrointestinal symptoms were not recognized or were neglected at the early stage of this epidemic outbreak until the first case was reported in the USA, when coronavirus RNA was identified in the patient's stool sample. 8 During the widespread outbreak, due to sudden acute surge in the number of affected patients medical resources and available medical expertise in this area was quickly outstripped by the huge demand for medical attention. With the rapidly rising medical need, available staff with medical experience were trained to focus mainly on obvious respiratory symptoms and life‐threatening complications in order to isolate these cases quickly. Gastrointestinal symptoms may be overlooked, especially in critically ill patients. Among the cases reported outside China in the countries such as the USA, South Korea, and Vietnam, diarrhea was reported as one of the main symptoms. This might be attributed to some extent to the adequate numbers of medical personnel for giving detailed care and consultation in COVID‐19 cases. Even though gastrointestinal symptoms seem to not be prominent, it would still be helpful for clinicians to identify these symptoms in the spectrum of this disease and be alert to identifying and preventing the spread of this disease at an early stage. With the emerging evidence of the viral RNA detected in the feces of patients with diarrhea, increased requirements are needed to protect and treat patients with underlying digestive diseases, such as inflammatory bowel disease (IBD). Moreover, it is necessary to find ways to prevent transmission through the sewage system.
3. MICROBIOLOGICAL AND VIROLOGICAL INVESTIGATIONS
The first confirmed COVID‐19 patient in the USA on January 20, 2020 reported nausea and vomiting that had lasted for 2 days. On the 2nd day after hospitalization, the patient had abdominal discomfort with two bowel movements. Both the stool and respiratory specimens were tested and found positive for SARS‐CoV‐2 by a real‐time reverse transcriptase polymerase chain reaction. 8 This case indicates that the gastrointestinal tract might be involved at least in the colonization of SARS‐CoV‐2. Like SARS‐CoV, with the assistance of transmembrane serine protease (TMPRSS) expressed on the host cell membrane, SARS‐CoV‐2 uses angiotensin‐converting enzyme 2 (ACE2) to mediate entry into cells. 13 A study published online in bioRxiv on January 31, 2020 analyzed four datasets with single cell transcriptomes of the lung, esophagus, stomach, ileum and colon. 14 The results indicate that ACE2 is highly expressed in absorptive intestinal epithelial cells in the ileum and colon as well as in lung cells and esophageal upper and stratified epithelial cells. Interestingly, compared with healthy adults, the expression of ACE2 is higher in the epithelial cells of the colon of patients with adenomas or colorectal cancer, which may predict a higher probability of being infected with SARS‐CoV‐2. 15 One group 16 also found ACE2 played a dual role in mediating SARS‐CoV‐2 infection in the colon after analyzing single cell‐RNA sequencing data from patients with colitis or IBD. The results show that ACE2 expression is positively associated with a viral attack, which is consistent with the abovementioned study. Regarding the abnormal liver function in patients with SARS‐CoV‐2 mentioned above, by adopting single‐cell RNA‐sequencing technology from two cohort samples, a recent study has shown that ACE2 is highly expressed in cholangiocytes rather than the hepatocytes or other interstitial cells. Therefore, during the diagnosis and treatment of patients with SARS‐CoV‐2, clinicians should pay special attention to their liver function. 17 However, another study pointed out that ACE2 also participated in the prevention of inflammation of the intestine by regulating innate immunity, cellular cytotoxicity, and energy metabolism (Figure 1). Nevertheless, it is necessary for clinicians to note that the digestive system may be invaded by SARS‐CoV‐2 and evolve into an alternative source of infection.
FIGURE 1.
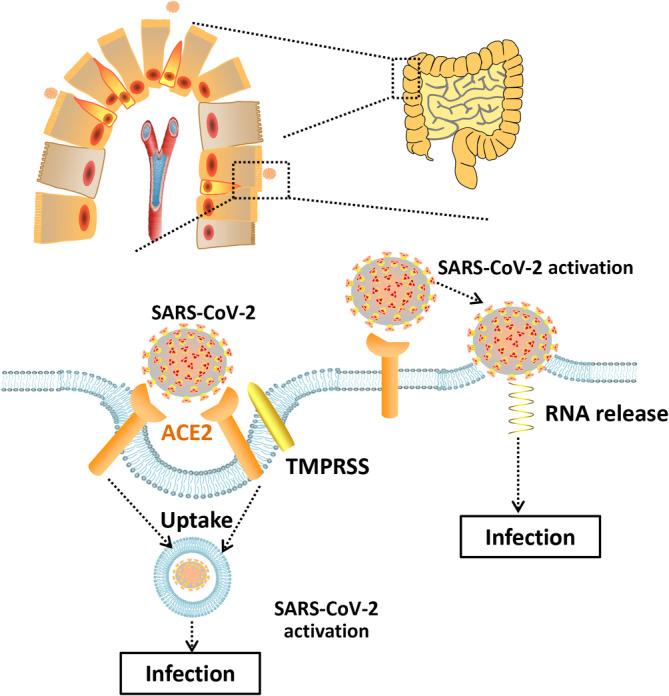
Angiotensin‐converting enzyme 2 (ACE2) is positively associated with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection of the digestive tract. Abbreviation: TMPRSS, transmembrane serine protease
4. FECAL‐ORAL TRANSMISSION
Although there been no solid evidence to confirm that SARS‐CoV‐2 can be transmitted through the fecal‐oral route, this possibility exists as the virus has been successfully isolated in a stool or anal swab of patients with COVID‐19, which is almost as accurate as a pharyngeal swab.18, 19 Moreover, SARS‐CoV‐2 was also detected in the stool sample of patients in rehabilitation, which stays positive longer than in the swab. 20 Fecal‐oral transmission occurs mainly in areas where there is limited awareness of hygiene or sanitation facilities. Nevertheless, the lesson from the transmission of SARS in Amoy Gardens in Hong Kong in 2003 resulting from the problem of aging sewage systems should warn us not to ignore biosafety in the disposal of feces from COVID‐19 patients. 21 The five “F” factors summarizing the main fecal‐oral route are fingers, flies, fields, fluids, and food. Frequent hand cleansing with sanitizers is the first and most effective step to prevent transmission. Raising population‐wide awareness of hygiene is needed, especially in rural areas, such as forbidding open defecation and swallowing water from public swimming pools or open areas that are not properly disinfected. Safe food preparation is also another vital aspect of prevention.
5. PREVENTION AND CONTROL OF INFECTION IN THE DIGESTIVE ENDOSCOPY ROOM
Due to digestive symptoms of SARS‐CoV‐2 infection, preoperative epidemiological screening in high‐risk individuals (including their travel history and the presence of suspicious symptoms) and strict protective measures are particularly important in the prevention of cross‐infection during endoscopic examination in epidemic areas.22, 23
5.1. Epidemiological investigations
At registration and during a medical appointment, an epidemiological investigation should be conducted and the following information needs to be obtained for both the patient and their companions: (a) travel history in epidemic areas in the previous 2 weeks; (b) any exposure to patients confirmed or suspected of having an SARS‐CoV‐2 infection in the past 2 weeks; (c) any symptoms of SARS‐CoV‐2 infection, including but not limited to fever, fatigue, cough, dyspnea, and gastrointestinal symptoms; and (d) the travel history and health of their family members. The temperature of the examinee should be measured before the endoscopic examination. Patients with an unexplained fever or suspected SARS‐CoV‐2 infection should be recommended for isolation for further evaluation. It is preferable to reschedule elective endoscopic procedures to after the outbreak of COVID‐19. For emergency endoscopic procedures, an algorithm for managing patients with suspected COVID‐19 in the epidemic region is shown in Figure 2.
FIGURE 2.
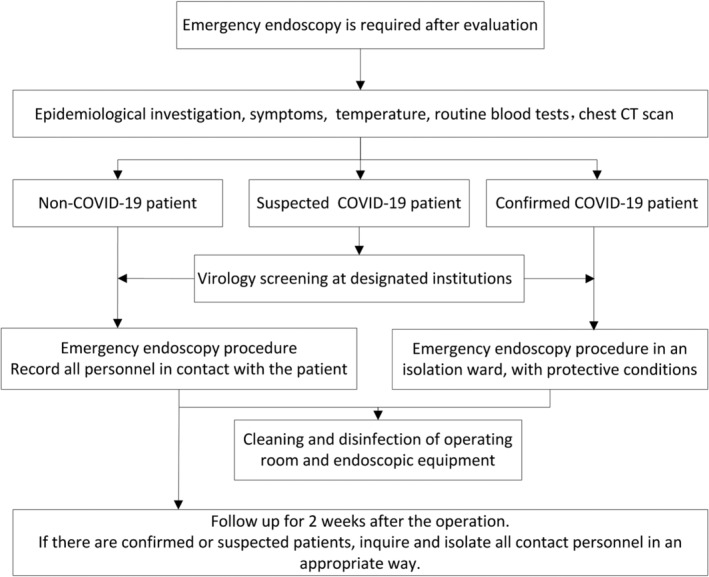
Flowchart of evaluations for emergency endoscopy in an epidemic region for coronavirus disease 19 (COVID‐19) outbreak. Abbreviation: CT, computed tomography
5.2. Personal protective measures and equipment
To reduce the risk of infecting medical staff in the digestive endoscopy unit, appropriate protective measures should be taken during the endoscopic procedures. Protective measures and equipment needed are divided into the following categories: the non‐epidemic region, epidemic region, and wards with confirmed patients. The corresponding protection methods and equipment requirements are shown in Table 2 and Figure 3.
TABLE 2.
Personal protective measures and equipment for medical staff in the digestive endoscopy in different epidemic areas
| Non‐epidemic region | Epidemic region | Wards for confirmed patients | |
|---|---|---|---|
| Hand hygiene | Yes | Yes | Yes |
| Gloves | Yes | Yes | Double gloves |
| Impervious gowns | Yes | Yes | Yes |
| Face or eye shields or masks | Yes | Yes | Yes |
| Hair and shoe cover | Yes | Yes | Yes |
| Protective suits | Not necessary | Yes | Yes |
| Fresh air respirator system supplied | Not necessary | Not necessary | Yes |
FIGURE 3.
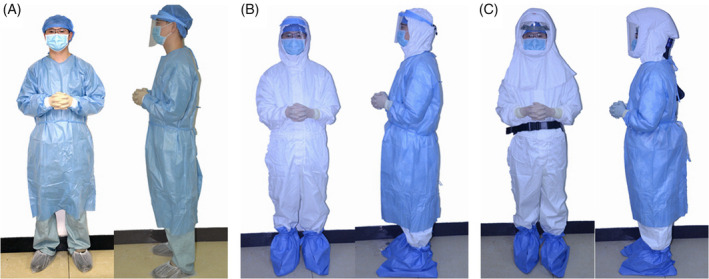
Personal protective measures and equipment for medical staff in the digestive endoscopy center in: A, a non‐epidemic region; B, an epidemic region; C, wards with confirmed patients
6. CONCLUSIONS
SARS‐CoV‐2 is a serious threat to human health worldwide due to its strong ability for human‐to‐human transmission. In the early days of the disease outbreak, medical staff focused on screening for respiratory symptoms. However, as the disease progresses and understanding unfolds, digestive symptoms related to COVID‐19 have also been identified. A literature review has shown that the SARS‐CoV‐2 and the SARS that broke out in 2003 belong to the same β‐coronavirus family and possess highly similar genomes. 24 In addition, the receptors for both SARS and SARS‐CoV‐2 is ACE2. 25 Therefore, it is not difficult to understand why SARS‐CoV‐2 may give rise to digestive system symptoms similar to those induced by SARS‐CoV. A recent case series in China has confirmed that SARS‐CoV‐2 nucleic acid which is negative in throat swabs can still be detected in the feces of three COVID‐19 patients, two of whom manifested diarrhea before treatment. 26 Nevertheless, as it remains unknown whether the virus in the digestive system is derived from cellular fragments from the respiratory system or consists of replicates in the digestive tract, it is sensible to take early steps to prevent fecal‐oral transmission both in the hospital and in the community. Additional clinical case reports and laboratory studies are needed to confirm the existence of this transmission route. More importantly, efforts should be made to formulate the clinical protocols and develop antiviral drugs targeting the digestive system in the future.
CONFLICTS OF INTEREST
All authors have no conflicts of interest or financial ties to disclose.
FUNDING INFORMATION
None.
Li LY, Wu W, Chen S, et al. Digestive system involvement of novel coronavirus infection: Prevention and control infection from a gastroenterology perspective. J Dig Dis. 2020;21:199–204. 10.1111/1751-2980.12862
Contributor Information
He Ming Yang, Email: yhming306@163.com.
Qiang Cai, Email: qcai@emory.edu.
REFERENCES
- 1. Pan L, Mu M, Yang P, et al. Clinical characteristics of COVID‐19 patients with digestive symptoms in Hubei, China: a descriptive, cross‐sectional, multicenter study [Epub ahead of print]. Am J Gastroenterol. 2020. 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Leung WK, To KF , Chan PKS, et al. Enteric involvement of severe acute respiratory syndrome‐associated coronavirus infection. Gastroenterology. 2003;125(4):1011‐1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hui DS, Memish ZA, Zumla A. Severe acute respiratory syndrome vs. the Middle East respiratory syndrome. Curr Opin Pulm Med. 2014;20(3):233‐241. [DOI] [PubMed] [Google Scholar]
- 4. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person‐to‐person transmission: a study of a family cluster. Lancet. 2020;395(10223):514‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chang D, Lin M, Wei L, et al. Epidemiologic and clinical characteristics of novel coronavirus infections involving 13 patients outside Wuhan, China. JAMA. 2020;323(11):1092‐1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Holshue ML, DeBolt C, Lindquist S, et al; Washington State 2019‐nCoV Case Investigation Team . First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382(10):929‐936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim JY, Choe PG, Oh Y, et al. The first case of 2019 novel coronavirus pneumonia imported into Korea from Wuhan, China: implication for infection prevention and control measures. J Korean Med Sci. 2020;35(5):e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Phan LT, Nguyen TV, Luong QC, et al. Importation and human‐to‐human transmission of a novel coronavirus in Vietnam. N Engl J Med. 2020;382(9):872‐874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of 2019 novel coronavirus infection in China [Epub ahead of print February 28, 2020]. N Engl J Med 10.1056/NEJMoa2002032. [DOI]
- 13. Chen Y, Guo Y, Pan Y, Zhao ZZJ. Structure analysis of the receptor binding of 2019‐nCoV. Biochem Biophys Res Commun. 2020;525(1):135‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang H, Kang Z, Gong H, et al. The digestive system is a potential route of 2019‐nCov infection: a bioinformatics analysis based on single‐cell transcriptomes. https://www.biorxiv.org/content/10.1101/2020.01.30.927806v1.full.pdf. Accessed April 12, 2020.
- 15. Chen H, Xuan B, Yan Y, et al. Profiling ACE2 expression in colon tissue of healthy adults and colorectal cancer patients by single‐cell transcriptome analysis. https://www.medrxiv.org/content/10.1101/2020.02.15.20023457v1.full.pdf. Accessed April 12, 2020.
- 16. Wang J, Zhao S, Liu M, et al. ACE2 expression by colonic epithelial cells is associated with viral infection, immunity and energy metabolism. https://www.medrxiv.org/content/10.1101/2020.02.05.20020545v1.full.pdf. Accessed April 12, 2020.
- 17. Chai X, Hu L, Zhang Y, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019‐nCoV infection. https://www.biorxiv.org/content/10.1101/2020.02.03.931766v1.full.pdf. Accessed April 12, 2020.
- 18. Zhang J, Wang S, Xue Y. Fecal specimen diagnosis 2019 novel coronavirus‐infected pneumonia . J Med Virol. 2019;92(6):680‐682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gao QY, Chen YX, Fang JY. Novel coronavirus infection and gastrointestinal tract. J Dig Dis. 2020;21(3):125‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ling Y, Xu SB, Lin YX, et al. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients [Epub ahead of print February 28, 2020]. Chin Med J (Engl). 10.1097/CM9.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peiris JSM, Chu CM, Cheng VCC, et al. Clinical progression and viral load in a community outbreak of coronavirus‐associated SARS pneumonia: a prospective study. Lancet. 2003;361(9371):1767‐1772. [DOI] [PMC free article] [PubMed]
- 22. The Subspecialty Group of Gastroenterology, the Society of Pediatrics, Chinese Medical Association . Prevention and control program on 2019 novel coronavirus infection in children's digestive endoscopy center [in Chinese]. Chin J Pediatr. 2020;58(3):175‐178. [DOI] [PubMed] [Google Scholar]
- 23. Working Group of Novel Coronavirus , Peking Union Medical College Hospital. Diagnosis and clinical management of 2019 novel coronavirus infection: an operational recommendation of Peking Union Medical College Hospital (V2.0) [in Chinese]. Chin J Intern Med. 2020;59(3):186‐188. [DOI] [PubMed] [Google Scholar]
- 24. Wu A, Peng Y, Huang B, et al. Genome composition and divergence of the novel coronavirus (2019‐nCoV) originating in China. Cell Host Microbe. 2020;27(3):325‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lei C, Fu W, Qian K, et al. Potent neutralization of 2019 novel coronavirus by recombinant ACE2‐Ig. https://www.biorxiv.org/content/10.1101/2020.02.01.929976v2.full.pdf. Accessed April 12, 2020.
- 26. Yang Z, Li G, Dai X, Liu G, Li G, Jie Y. Three cases of novel coronavirus pneumonia with viral nucleic acids still positive in stool after throat swab detection turned negative [Epub ahead of print February 14, 2020] [in Chinese]. Chin J Digestion. 2020. 10.3760/cma.j.issn.0254-1432.2020.0002. [DOI] [Google Scholar]


