Abstract
Background
Rapid spread of the severe acute respiratory syndrome‐coronavirus‐2 (SARS‐CoV‐2) and concern for viral transmission by ambulatory patients with minimal to no symptoms underline the importance of identifying early or subclinical symptoms of coronavirus disease 2019 (COVID‐19) infection. Two such candidate symptoms include anecdotally reported loss of smell and taste. Understanding the timing and association of smell/taste loss in COVID‐19 may help facilitate screening and early isolation of cases.
Methods
A single‐institution, cross‐sectional study evaluating patient‐reported symptoms with a focus on smell and taste was conducted using an internet‐based platform on adult subjects who underwent testing for COVID‐19. Logistic regression was employed to identify symptoms associated with COVID‐19 positivity.
Results
A total of 1480 patients with influenza‐like symptoms underwent COVID‐19 testing between March 3, 2020, and March 29, 2020. Our study captured 59 of 102 (58%) COVID‐19–positive patients and 203 of 1378 (15%) COVID‐19–negative patients. Smell and taste loss were reported in 68% (40/59) and 71% (42/59) of COVID‐19–positive subjects, respectively, compared to 16% (33/203) and 17% (35/203) of COVID‐19–negative patients (p < 0.001). Smell and taste impairment were independently and strongly associated with COVID‐19 positivity (anosmia: adjusted odds ratio [aOR] 10.9; 95% CI, 5.08‐23.5; ageusia: aOR 10.2; 95% CI, 4.74‐22.1), whereas sore throat was associated with COVID‐19 negativity (aOR 0.23; 95% CI, 0.11‐0.50). Of patients who reported COVID‐19–associated loss of smell, 74% (28/38) reported resolution of anosmia with clinical resolution of illness.
Conclusion
In ambulatory individuals with influenza‐like symptoms, chemosensory dysfunction was strongly associated with COVID‐19 infection and should be considered when screening symptoms. Most will recover chemosensory function within weeks, paralleling resolution of other disease‐related symptoms.
Keywords: COVID‐19, smell loss, taste loss, patient outcomes
The coronavirus disease 2019 (COVID‐19) pandemic caused by severe acute respiratory syndrome‐coronavirus‐2 (SARS‐CoV‐2) continues to spread at an exponential rate with recent concerns of significant viral transmission through asymptomatic or mildly symptomatic patients. 1 Anecdotal reports suggest smell and taste loss are potential early symptom or subclinical markers of COVID‐19 infection. A preliminary study from Iran showed a significant increase in new‐onset anosmia since the COVID‐19 outbreak. 2 An Italian report of 59 hospitalized COVID‐19 patients found that 33% reported a chemosensory disorder. 3 However, it remains unclear if these findings are unique to COVID‐19 infections requiring hospitalization, causally related to COVID‐19 infection, or simply due to more widespread recognition of postviral anosmia. Insight into the timing and association of smell/taste loss and COVID‐19 is critical because patients with acute anosmia may be otherwise asymptomatic carriers of infection who may unwittingly facilitate the spread of disease.
Patients and methods
Study design and population
A single institution, cross‐sectional study evaluating patient‐reported symptoms with a focus on smell and taste was conducted using an Internet‐based platform (Qualtrics, Provo, UT) on adult subjects who underwent polymerase chain reaction (PCR)‐confirmed testing for COVID‐19 between March 3, 2020, and March 29, 2020. An initial email invitation to a 27‐question survey was sent to 102 subjects who tested COVID‐19–positive and 1378 subjects who tested COVID‐19–negative, with a follow‐up phone call. Sense of smell at baseline, at the time of COVID‐19 testing, and at the time of survey were assessed using a subjective olfaction score. Questions were based off a continuous 10‐point slide bar (0: no sense of smell, 10: normal sense of smell), with scores from 1 to 9 indicating progressively increasing severity of hyposmia. This study was approved by the Institutional Review Board of University of California San Diego (IRB# 200485).
Statistical analysis
Categorical variables were evaluated by chi‐square (χ2) test. Unadjusted odds ratios (ORs) with 95% confidence intervals (CIs) were calculated by univariable logistic regression, with COVID‐19 test result considered as the independent variable. Adjusted ORs (aORs) were calculated using multivariable logistic regression analysis, a priori criteria for variable inclusion in the multivariable model included: (1) maximum of 6 variables to minimize potential model overfitting; (2) magnitude of association with COVID‐19 positivity of at least 2‐fold (ie, OR ≥ 2.0 or OR ≤ 0.5); (3) statistically significant associations (p < 0.05); and (4) we prioritized “any symptoms” (eg, any symptoms reported during the entire disease period) over “presenting symptoms” (eg, initial symptoms of presentation) because we had no a priori hypothesis for the natural history of anosmia and thus believed that inputting “any symptoms” for our multivariate correlation analyses would be more sensitivity for establishing associations between anosmia/ageusia and COVID‐19 status. Given the known colinear relationship between anosmia and ageusia, each variable was adjusted separately as a dependent variable. Goodness‐of‐fit was performed for both final multivariable models (anosmia and ageusia). All permutations of models tested showed p values >0.05, demonstrating that goodness‐of‐fit of both final models as evaluated by both Pearson's method (anosmia p = 0.64, ageusia p = 0.44), as well as Hosmel‐Lemeshow's method (anosmia p = 0.36, ageusia p = 0.11), demonstrated that our models fit the data well. Analysis was performed using Stata 15.1 software (StataCorp, College Station, TX).
Results
A total of 59 COVID‐19–positive (58% response rate) and 203 COVID‐19–negative subjects completed the survey (15% response rate) between March 31, 2020, and April 3, 2020. Demographics and clinical characteristics of the 2 cohorts are summarized in Table 1. There were no differences in regard to age (grouped by decade) or rate of illness improvement between the 2 groups. Hospital admission rates were low and comparable in both groups (4/58 [7%] of COVID‐19–positive patients and 14/200 [7%] COVID‐19–negative patients, χ2 test p = 0.98), with predominantly ambulatory cases responding to the survey. The patients who were later found to be COVID‐19–negative were admitted for evaluation of fever and/or dyspnea. Sex distribution in COVID‐19–positive patients was balanced, but was skewed toward females (65%) in COVID‐19–negative patients.
TABLE 1.
Baseline characteristics*
|
COVID‐19–positive (n = 59) n (%) |
COVID‐19–negative (n = 203) n (%) |
p | |
|---|---|---|---|
| Age group a | 0.19 | ||
| 18–29 years | 10 (17.0) | 26 (12.9) | |
| 30–39 years | 11 (18.6) | 67 (33.2) | |
| 40–49 years | 17 (28.8) | 39 (19.3) | |
| 50–59 years | 9 (15.3) | 36 (17.8) | |
| 60–69 years | 7 (11.9) | 19 (9.4) | |
| 70–79 years | 5 (8.5) | 10 (5.0) | |
| ≥80 years | 0 | 5 (2.5) | |
| Gender b | 0.033 | ||
| Male | 29 (49.2) | 69 (34.0) | |
| Female | 29 (49.2) | 132 (65.0) | |
| Gender diverse | 1 (1.7) | 0 | |
| Improvement of illness c | 0.66 | ||
| No | 8 (13.6) | 38 (18.7) | |
| Yes | 50 (84.8) | 162 (79.8) | |
| Hospital admission d | 0.98 | ||
| No | 54 (93.1) | 186 (93.0) | |
| Yes | 4 (6.9) | 14 (7.0) | |
| Any symptoms | |||
| Fatigue | 48 (81.4) | 116 (57.1) | 0.001 |
| Ageusia | 42 (71.2) | 35 (17.2) | <0.001 |
| Fever | 41 (69.5) | 87 (42.9) | <0.001 |
| Anosmia | 40 (67.8) | 33 (16.3) | <0.001 |
| Cough | 39 (66.1) | 156 (76.9) | 0.096 |
| Headache | 39 (66.1) | 99 (48.8) | 0.019 |
| Myalgia/arthralgia | 37 (62.7) | 65 (32.0) | <0.001 |
| Dyspnea | 32 (54.2) | 88 (43.4) | 0.14 |
| Diarrhea | 28 (47.5) | 50 (24.6) | 0.001 |
| Nasal obstruction | 28 (47.5) | 91 (44.8) | 0.72 |
| Sore throat | 19 (32.2) | 122 (60.1) | <0.001 |
| Rhinorrhea | 18 (30.5) | 83 (40.9) | 0.15 |
| Nausea | 16 (27.1) | 23 (11.3) | 0.004 |
| Presenting symptoms | |||
| Fatigue | 25 (42.4) | 62 (30.5) | 0.089 |
| Ageusia | 12 (20.3) | 10 (4.9) | <0.001 |
| Fever | 32 (54.2) | 53 (26.1) | <0.001 |
| Anosmia | 13 (22.0) | 9 (4.4) | <0.001 |
| Cough | 21 (35.6) | 104 (51.2) | 0.034 |
| Headache | 25 (42.4) | 40 (19.7) | <0.001 |
| Myalgia/arthralgia | 20 (33.9) | 39 (19.2) | 0.017 |
| Dyspnea | 7 (11.9) | 47 (23.2) | 0.059 |
| Diarrhea | 5 (8.5) | 16 (7.9) | 0.88 |
| Nasal obstruction | 11 (18.6) | 43 (21.2) | 0.67 |
| Sore throat | 10 (17.0) | 92 (45.3) | <0.001 |
| Rhinorrhea | 6 (10.2) | 40 (19.7) | 0.09 |
| Nausea | 3 (5.1) | 8 (3.9) | 0.7 |
| Comorbidities | |||
| Allergic rhinitis | 20 (33.9) | 77 (37.9) | 0.57 |
| Other immunosuppressed state | 9 (15.3) | 32 (15.8) | 0.92 |
| Hypertension | 8 (13.6) | 30 (14.8) | 0.82 |
| Diabetes | 5 (8.5) | 15 (7.4) | 0.78 |
| Cardiac disease | 3 (5.1) | 13 (6.4) | 0.71 |
| Chronic lung disease | 3 (5.1) | 31 (15.3) | 0.04 |
| Cancer | 2 (3.4) | 10 (4.9) | 0.62 |
| Sinus disease | 2 (3.4) | 20 (9.9) | 0.12 |
| History of head trauma | 1 (1.7) | 13 (6.4) | 0.16 |
| Neurologic disease | 0 | 6 (3.0) | 0.18 |
*Differences in self‐reported clinical feature distributions across COVID‐19–positive and COVID‐19–negative patients were evaluated by chi‐square test with p values reported in the right column.
aOne patient did not answer question on age group. All other reported results based on 100% response rates.
bTwo patients did not answer question on gender. All other reported results based on 100% response rates.
cFour patients did not answer question on improvement of illness. All other reported results based on 100% response rates.
dFour patients did not answer question on hospital admission. All other reported results based on 100% response rates.
Olfactory and gustatory impairment was reported in (40/59) 68% and (42/59) 71% of COVID‐19–positive patients, respectively, compared to 16% and 17% of COVID‐19–negative patients (χ2 test p < 0.001). Two patients reported acute ageusia without acute anosmia due to a preexisting history of baseline chronic rhinosinusitis–associated anosmia. Self‐reported symptoms associated with COVID‐19 positivity in order of descending frequency included fatigue (81%), ageusia (71%), fever (70%), anosmia (68%), myalgia or arthralgia (63%), diarrhea (48%), and nausea (27%). Sore throat was associated with COVID‐19 negativity (60% vs 32% in COVID‐19–positive patients). Fever was the most common presenting symptom (54%), whereas 22% reported anosmia at initial presentation of COVID‐19–positive illness.
Compared to other symptoms of COVID‐19 infection, loss of smell and taste showed the largest magnitudes of association with COVID‐19 positivity (anosmia: OR 10.9; 95% CI, 5.6‐21.0; ageusia: OR 11.9; 95% CI, 6.1‐23.2, Table 2, left column). Multivariable logistic regression adjusting for myalgia/arthralgia, fatigue, fever, nausea, and sore throat demonstrated that both smell and taste impairment independently associated with COVID‐19 positivity (anosmia: aOR 10.9; 95% CI, 5.08‐23.5; taste: aOR 10.2; 95% CI, 4.74‐22.1, Table 2, middle and right columns). Conversely, sore throat was independently associated with COVID‐19 negativity, with COVID‐19–negative patients being 4‐fold to 5‐fold more likely to report sore throat as a symptom (aOR 0.23; 95% CI, 0.11‐0.50). Beyond smell loss, taste loss, and sore throat, only nausea was found to be consistently and independently associated with COVID‐19 positivity. None of the evaluated comorbidities listed in Table 1 associated with COVID‐19 status.
TABLE 2.
Self‐reported clinical feature associations with COVID‐19‐positivity*
| Parameter |
Univariable regression OR (95% CI) |
p |
Multivariable regression aOR (95% CI) a |
p |
Multivariable regression aOR (95% CI) b |
p |
|---|---|---|---|---|---|---|
| Age group (years) | 0.72 (0.33–1.60) | 0.43 | ||||
| Gender | 0.53 (0.30–0.96) | 0.036 | ||||
| Improvement of illness | 1.47 (0.64–3.35) | 0.36 | ||||
| Hospital admission | 0.98 (0.31–3.11) | 0.98 | ||||
| Any symptoms | ||||||
| Ageusia c | 11.86 (6.06–23.19) | <0.001 | — | — | 10.23 (4.74–22.09) | <0.001 |
| Anosmia c | 10.85 (5.60–21.01) | <0.001 | 10.92 (5.08–23.53) | <0.001 | — | — |
| Myalgia/arthralgia c | 3.57 (1.95–6.54) | <0.001 | 1.74 (0.79–3.84) | 0.17 | 1.53 (0.71–3.33) | 0.28 |
| Fatigue c | 3.27 (1.61–6.67) | 0.001 | 1.53 (0.61–3.84) | 0.37 | 1.23 (0.50–3.04) | 0.66 |
| Fever c | 3.03 (1.63–5.65) | <0.001 | 1.55 (0.71–3.40) | 0.27 | 1.67 (0.77–3.60) | 0.19 |
| Nausea c | 2.91 (1.42–5.98) | 0.004 | 2.86 (1.16–7.01) | <0.001 | 2.71 (1.07–6.83) | 0.035 |
| Diarrhea | 2.76 (1.51–5.05) | 0.001 | — | — | — | — |
| Headache | 2.05 (1.12–3.75) | 0.02 | — | — | — | — |
| Dyspnea | 1.55 (0.86–2.77) | 0.064 | — | — | — | — |
| Nasal obstruction | 1.11 (0.62–1.99) | 0.72 | — | — | — | — |
| Rhinorrhea | 0.63 (0.34–1.18) | 0.096 | — | — | — | — |
| Cough | 0.59 (0.31–1.10) | 0.098 | — | — | — | — |
| Sore throat c | 0.32 (0.17–0.58) | <0.001 | 0.20 (0.09‐0.44) | <0.001 | 0.23 (0.11–0.50) | <0.001 |
| Presenting symptoms | ||||||
| Fatigue | 1.67 (0.92–3.04) | 0.091 | ||||
| Ageusia | 4.93 (2.01–12.10) | <0.001 | ||||
| Fever | 3.35 (1.84–6.11) | <0.001 | ||||
| Anosmia | 6.09 (2.46–15.11) | <0.001 | ||||
| Cough | 0.53 (0.29–0.96) | 0.036 | ||||
| Headache | 3.00 (1.61–5.58) | 0.001 | ||||
| Myalgia/arthralgia | 2.16 (1.13–4.10) | 0.019 | ||||
| Dyspnea | 0.45 (0.19–1.05) | 0.064 | ||||
| Diarrhea | 1.08 (0.38–3.09) | 0.88 | ||||
| Nasal obstruction | 0.85 (0.41–1.78) | 0.67 | ||||
| Sore throat | 0.25 (0.12–0.51) | <0.001 | ||||
| Rhinorrhea | 0.46 (0.19–1.15) | 0.096 | ||||
| Nausea | 1.31 (0.34–5.09) | 0.70 |
*Associations of self‐reported clinical feature associations to COVID‐19‐status were tested using univariable (left column, reporting unadjusted ORs) and multivariable (middle and right columns, reporting aORs) logistic regression models. Separate multivariable regression models were conducted for anosmia (middle column) and ageusia (right column), given the observed collinearity of these variables.
Multivariable regression including anosmia.
Multivariable regression including ageusia.
Variables included in the multivariable regression analysis.
aOR = adjusted odds ratio; OR = odds ratio; CI = confidence interval; COVID‐19 = coronavirus 2019.
Patterns of COVID‐19–related olfactory/gustatory impairment demonstrate a profound to complete anosmia/ageusia, with a significant majority achieving spontaneous improvement (Figs. 1 and 2). Notably, the degree of COVID‐19–related anosmia and ageusia correlate closely in affected individuals. No patients in this study received treatment for olfactory or gustatory loss. Among COVID‐19–positive subjects who experienced smell loss, 29 of 40 (72.5%) reported improvement at time of survey (18% by <1 week, 37.5% by 1 to 2 weeks, 18% by 2 to 4 weeks).
FIGURE 1.
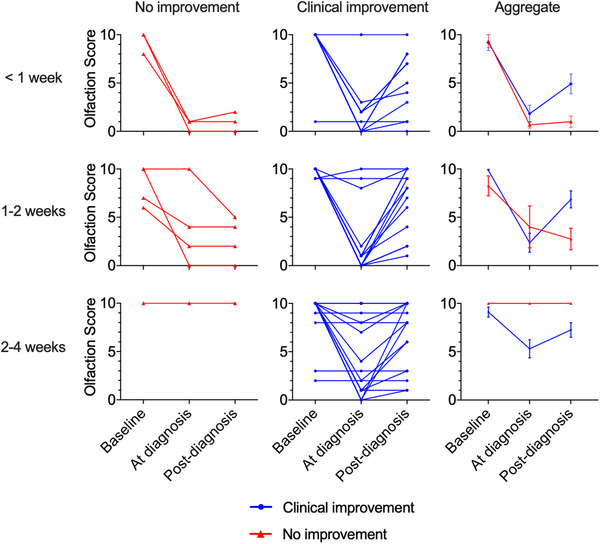
Temporal association of olfactory score and COVID‐19 testing. Spaghetti plot of all COVID‐19–positive individuals (n = 59) reporting olfactory scores (0: no sense of smell, 10: normal sense of smell) at baseline, at time of COVID‐19 diagnosis, and at time of survey completion (post–COVID‐19 diagnosis either <1 week, 1 to 2 weeks, or 2 to 4 weeks). Rows represent time elapsed between testing positive for COVID‐19 and completion of survey. The left and middle columns reflect patient stratification into groups who failed to improve (red lines, left column) and those who achieved improvement/resolution of clinical symptoms (blue lines, middle column) at the time of survey completion. The right column displays aggregated results (mean, SEM) stratified by clinical improvement. COVID‐19 = coronavirus 2019; SEM = standard error of the mean.
FIGURE 2.
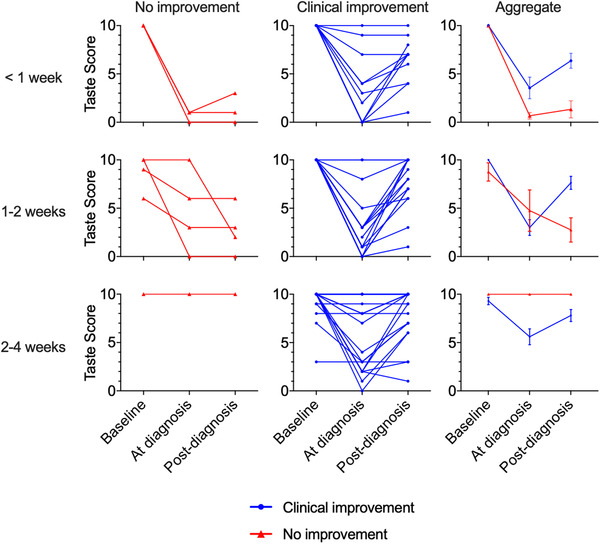
Temporal association of taste score and COVID‐19 testing. Spaghetti plot of all COVID‐19–positive individuals (n = 59) reporting taste scores (0: no sense of taste, 10: normal sense of taste) at baseline, at time of COVID‐19 diagnosis, and at time of survey completion (post–COVID‐19 diagnosis either <1 week, 1 to 2 weeks, or 2 to 4 weeks). Rows represent time elapsed between testing positive for COVID‐19 and completion of survey. The left and middle columns reflect patient stratification into groups who failed to improve (red lines, left column) and those who achieved improvement/resolution of clinical symptoms (blue lines, middle column) at the time of survey completion. The right column displays aggregated results (mean, SEM) stratified by clinical improvement. COVID‐19 = coronavirus 2019; SEM = standard error of the mean.
The majority of COVID‐19–positive patients had improvement of olfaction and taste that temporally correlated with clinical resolution of illness (Fig. 3A). Seventy‐four percent (28/38, 2 failed to respond) of affected patients reported both improvement of olfactory dysfunction and overall illness symptoms. Those who did not experience improvement in smell also had not felt improvement in other clinical symptoms. Four patients reported clinical improvement without olfactory improvement; however, these patients were less than 2 weeks from onset of symptoms. Similarly, of those who reported no improvement of smell loss, 82% were diagnosed <2 weeks prior (Fig. 3B).
FIGURE 3.
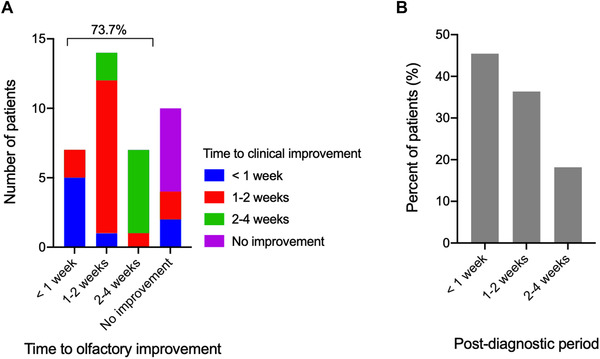
Temporal relationship between olfactory improvement and clinical improvement following COVID‐19 infection. (A) Histogram demonstrating the reported time to improvement in COVID‐19–positive subjects with olfactory loss and its relationship to patient‐reported time to overall clinical improvement (n = 38, 2 subjects did not answer time to clinical improvement). (B) Histogram demonstrating the time post–COVID‐19 diagnosis (approximate time elapsed since testing positive) in subjects who reported no improvement of smell loss (n = 11); 81.8% were diagnosed <2 weeks prior. COVID‐19 = coronavirus 2019.
Discussion
This study shows the prevalence and unique presentation of chemosensory impairment in COVID‐19–positive compared to COVID‐19–negative individuals, both presenting with similar influenza‐like symptoms. We found a significant association between smell/taste loss and COVID‐19 infection because these chemosensory impairments were at least 10‐fold more common in COVID‐19–positive cases. Of those who reported olfactory dysfunction, the loss was typically profound rather than mild. Despite the slightly higher reported incidence of ageusia compared to anosmia, we know that loss of taste is linked with one's loss of smell and the differences in reporting can be attributed to the few patients with baseline rhinosinusitis‐induced anosmia.
We have also shown that most patients reported improvement of smell and taste at the time of the survey, typically less than 2 weeks postdiagnosis. Similarly, the overall symptoms of disease improved or resolved during that time frame. In select cases (10%), patients reported early resolution of clinical symptoms without return of olfaction. It is possible that some of these individuals may regain sense of smell with more time because 82% of them had been tested for COVID‐19 less than 2 weeks prior. Overall, these findings may offer reassurance that patients with ambulatory COVID‐19 infection and associated anosmia/hyposmia may recover olfactory function within weeks paralleling resolution of other disease‐related symptoms.
Of the COVID‐19–positive respondents in this study, most did not require hospitalization, and none required intubation, suggesting that a relatively mild subset of COVID‐19 infection was captured. This is in contrast to the hospital‐based survey of COVID‐19 infections by Giacomelli et al. 3 that reported rates of chemosensory loss at one‐half the level of our subjects. This suggests that ambulatory and inpatient COVID‐19 cases may follow fundamentally different clinical courses. We hypothesize that perhaps ambulatory cases are in part the result of nasal‐centric viral spread, whereas patients requiring hospitalization may be experiencing a more pulmonary‐centric viral infection leading to increased rate of respiratory failure and need for hospitalization. Future studies are warranted to investigate this hypothesis because, if found to be true, beyond potential screening markers for COVID‐19 infection positivity, anosmia/ageusia may carry some prognostic potential on severity of disease.
Postviral anosmia is a common cause of smell loss in adults and is known to be associated with many human viral strains, including other coronaviruses. 4 Early studies evaluating mechanisms of SARS‐CoV2–mediated olfactory loss have suggested neurotrophic targeting of olfactory neurons vs infection of non‐neural olfactory epithelial cells. 5 , 6 The short‐lived COVID‐19–related olfactory loss found in our study favors a model in which SARS‐CoV2 targets the olfactory epithelium, which can rapidly regenerate and repair after viral clearance.
Larger‐scale studies on epidemiologically balanced datasets are warranted to accurately determine the overall incidence and prevalence of COVID‐19–related anosmia/hyposmia, and to determine its predictive value for COVID‐19 infection. This study is limited by a short sampling period at a single institution, as well as the subjective assessment used to determine smell/taste impairment. Furthermore, by surveying respondents after COVID‐19 testing, we risk post hoc interpretations of smell and taste loss through their knowledge of their diagnosis, a potential recall bias especially in the context of pervasive anecdotal reports of COVID‐19–related anosmia. Specifically, it remains possible that patients with smell loss in the COVID‐19–positive group were more likely to respond based on media reporting of smell loss and/or desire to share their experience, as compared to the COVID‐19–negative group. However, the comparison of clinical characteristics and outcomes between the COVID‐19–positive and COVID‐19–negative cohorts was valuable in determining true associations because all individuals were deemed persons under investigation (PUIs) prior to study enrollment and demonstrated minor differences in baseline characteristics beyond COVID19 status. One must also consider the sensitivity and potential false‐negativity of the PCR‐based COVID‐19 assays. Two of our subjects had previously tested negative for COVID‐19 infection and subsequent testing resulted in a positive test.
In this study, olfaction was evaluated using a subjective olfaction score of 1 to 10 because a true visual analog scale (VAS) was unable to be performed through the online survey platform. It has been previously shown that compared to more objective batteries of olfactory testing, subjective reporting of sense of smell is specific but not sensitive. 7 Typically, people do not recognize their loss of smell and thus tend to underreport smell loss. However, we must weigh this possibility against the potential information bias of COVID‐19 test positivity. Future studies using well‐validated instruments of olfaction, will be important to corroborate these patient‐reported subjective assessments of olfactory loss.
Conclusion
There is a strong association of olfactory and gustatory impairment with COVID‐19 infection and a temporal relationship of improvement of these symptoms with resolution of overall clinical illness in this predominantly ambulatory population. This study offers support for using smell/taste loss as a symptom for heightened screening of COVID‐19 infections in an effort to decrease the risk of disease transmission from mildly symptomatic cases.
How to Cite this Article:Yan CH, Faraji F, Prajapati DP, Boone CE, DeConde AS. Association of chemosensory dysfunction and COVID‐19 in patients presenting with influenza‐like symptoms. Int Forum Allergy Rhinol. 2020;10:806–813.
Funding sources for the study: National Institutes of Health (Clinical and Translational Science Awards [CTSA], UL1TR001442).
Potential conflict of interest: A.S.D. is a consultant for Stryker endoscopy, Olympus, IntersectENT, Sanofi, and Optinose. The other authors have no financial disclosures.
References
- 1. Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID‐19. JAMA. 2020;323:1406‐1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bagheri SHR, Asghari AM, Farhadi M, et al. Coincidence of COVID‐19 epidemic and olfactory dysfunction outbreak. medRxiv. 2020.03.23.20041889. Epub 27 March 2020. 10.1101/2020.03.23.20041889. Accessed May 14, 2020. [DOI] [Google Scholar]
- 3. Giacomelli A, Pezzati L, Conti F, et al. Self‐reported olfactory and taste disorders in SARS‐CoV‐2 patients: a cross‐sectional study. Clin Infect Dis. (in press). Epub 26 March 2020. 10.1093/cid/ciaa330. Accessed May 14, 2020 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Suzuki M, Saito K, Min W‐P, et al. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope. 2007;117:272‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID‐19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11:995‐998. [DOI] [PubMed] [Google Scholar]
- 6. Brann D, Tsukahara T, Weinreb C, Logan DW, Datta SR. Non‐neuronal expression of SARS‐CoV‐2 entry genes in the olfactory epithelium suggests mechanisms underlying anosmia in COVID‐19 patients. bioRxiv. 2020.03.25.009084. Epub 09 April 2020. 10.1101/2020.03.25.009084. Accessed May 14, 2020. [DOI] [Google Scholar]
- 7. Boesveldt S, Postma EM, Boak D, et al. Anosmia—a clinical review. Chem Senses. 2017;42:513‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]


