Abstract
An ongoing outbreak of pneumonia associated with the severe acute respiratory coronavirus 2 (SARS-CoV-2) started in Wuhan, China, with cases now confirmed in multiple countries. The clinical course of patients remains to be fully characterized, clinical presentation ranges from asymptomatic infection to acute respiratory distress syndrome and acute renal failure, and no pharmacological therapies of proven efficacy yet exist. We report a case of SARS-CoV-2 infection in a renal transplant recipient with excellent outcome. This case states the importance of close monitoring of the concentration of cyclosporine in patients treated with lopinavir/ritonavir; the routine treatment of corticosteroid can be continued. This is a rare report of SARS-CoV-2 infection in a renal transplant recipient. Further data are needed to achieve better understanding of the impact of immunosuppressive therapy on the clinical presentation, severity, and outcome of SARS-CoV-2 infections in solid organ transplant recipients.
KEYWORDS: clinical decision-making, clinical research/practice, immunosuppressive regimens-maintenance, infection and infectious agents-viral, infectious disease, kidney transplantation/nephrology
Abbreviations: COVID-19, coronavirus disease 2019; CsA, cyclosporine; CT, computed tomography; MERS-CoV, Middle East respiratory syndrome coronavirus; RT-PCR, reverse transcription–polymerase chain reaction; SARS-CoV, severe acute respiratory coronavirus; SARS-CoV-2, severe acute respiratory coronavirus 2; USTC, University of Science and Technology of China
1. INTRODUCTION
Coronaviruses are frequent causes of self-limited community-acquired upper respiratory tract infections.1 A novel coronavirus strain named SARS-CoV-2 (formerly called 2019-nCoV) was isolated from human airway epithelial cells2 and reported in December 2019 in Wuhan, China. As it spread rapidly, outbreaks and case clusters were reported.3 , 4 As of April 24, 2020, the World Health Organization has reported 2 631 839 confirmed cases of coronavirus disease 2019 (COVID-19) globally.5
Because specific drugs to treat SARS-CoV-2 have not yet been revealed, the National Health Committee of China published a series of “Novel Coronavirus Pneumonia Diagnosis and Treatment Plan” to standardize the clinical diagnosis and treatment of COVID-19. According to this plan, interferon-α inhalation, lopinavir/ritonavir, and ribavirin are recommended as experimental antiviral treatment.6 , 7 Furthermore, the clinical picture of SARS-CoV-2 infection varies widely, ranging from asymptomatic carrier state to severe rapidly fatal pneumonia.8 , 9 SARS-CoV-2 has been associated with severe morbidity and mortality in elderly patients, complicated with cardiovascular or pulmonary comorbidities and in immunoincompetent populations.8 , 10 Clinical information of COVID-19 on solid organ transplant recipients and the effect of the immunosuppression regimens on the clinical course of COVID-19 are still lacking. We report a case of a renal transplant recipient with atypical pneumonia as a manifestation of COVID-19.
2. CASE REPORT
A 29-year-old male patient, with a clinical history of arterial hypertension and later chronic kidney disease, underwent hemodialysis for 9 months and then received a living kidney transplant donated by his mother on December 27, 2018. His routine immunosuppressive regimen consisted of mycophenolate mofetil 0.5 g q12h, cyclosporine (CsA) q12h (75 mg every morning and 75 mg/100 mg alternating every other night), and methylprednisolone 8 mg daily. Simultaneously, he was given a maintenance treatment with esomeprazole 20 mg daily, aspirin 0.1 g daily, metoprolol succinate 47.5 mg daily, felodipine 5 mg twice daily, valsartan 80 mg every night, and diammonium glycyrrhizinate 150 mg 3 times daily.
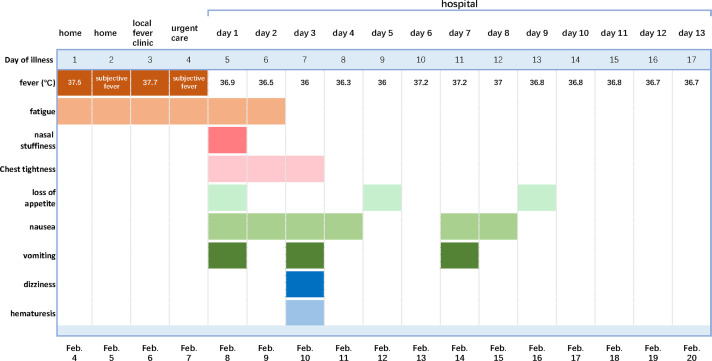
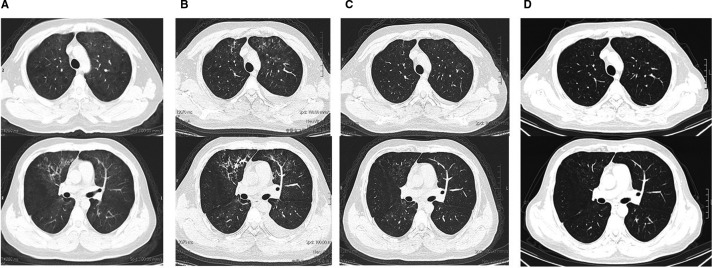
He presented to the fever clinic of the local hospital in Fuyang city, Anhui province, on February 6, 2020, complaining of generalized fatigue and chills for 2 days and a low-grade fever of 37.7℃ ( Figure 1). He had no rigors, cough, sputum, hemoptysis, shortness of breath, myalgia, malaise, diarrhea, abdominal pain, vomiting, or headache. There was no history of contact with similarly ill or febrile patients. An oropharyngeal swab obtained from the patient tested negative for SARS-CoV-2 on reverse transcription–polymerase chain reaction (RT-PCR) assay, and chest computed tomography (CT) images showed bilateral diffuse ground-glass opacity ( Figure 2A). Trimethoprim/sulfamethoxazole was initiated as empiric treatment for possible Pneumocystis jirovecii pneumonia.
FIGURE 1.
Symptoms and maximum body temperatures according to day of illness and day of hospitalization, February 4 to February 20, 2020 [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 2.
Shown are chest CT obtained on day 3 (February 6, A), day 9 (February 12, B), day 15 (February 18, C) of illness, and fifth day after discharge (February 24, D)
Due to persistent fatigue and chills and to monitor his renal transplant, the patient was transferred to the First Affiliated Hospital of University of Science and Technology of China on February 8, 2020. After admission, he was started on broad-spectrum antibiotic therapy with moxifloxacin in addition to trimethoprim/sulfamethoxazole; the immunosuppressive regimen and maintenance treatment were not changed. Mild chest tightness, nasal stuffiness, loss of appetite, nausea, and vomiting developed 12 hours later (Figure 1). His laboratory testing revealed an elevated white cell count, mild thrombocytopenia, hyponatremia, and hypoalbuminemia ( Table 1). Oropharyngeal swab and sputum were both positive for SARS-CoV-2 on RT-PCR results within 24 hours. According to the Novel Coronavirus Pneumonia Diagnosis and Treatment Plan (trial version 5) and in accordance with current best practice, the patient was put on antiviral therapy with oral lopinavir/ritonavir (1000 mg/d) and supportive therapy with probiotics on hospital day 2. Moxifloxacin was discontinued in consideration of the lack of evidence of bacterial pneumonia.
TABLE 1.
Clinical laboratory results
| Measure | Reference range | Illness day 5, hospital day 1 | Illness day 6, hospital day 2 | Illness day 7, hospital day 3 | Illness day 10, hospital day 6 | Illness day 12, hospital day 8 | Illness day 15, hospital day 11 | Discharge day 6 February 25 |
|---|---|---|---|---|---|---|---|---|
| White-cell count (109/L) | 3.5-9.5 | 11.14a | — | 6.73 | — | 8.78 | 13.21a | 6.19 |
| Lymphocyte count (109/L) | 1.1-3.2 | 1.5 | — | 1.01b | — | 1.82 | 1.62 | 1.31 |
| Platelet count (109/L) | 125-350 | 104b | — | 142 | — | 82b | 254 | 147 |
| Hemoglobin (g/L) | 130-175 | 157 | — | 136 | — | 144 | 132 | 114b |
| Sodium (mmol/L) | 135-145 | — | 126b | 122b | 128b | 133b | 136 | 133.2b |
| Creatinine (μmol/L) | 57-111 | — | 102 | 167a | 122a | 104 | 101 | 113.8 |
| Albumin (g/L) | 40-55 | — | 38.7b | 41 | 33.6b | 42.2 | 33.5b | 32.3b |
| Total bilirubin (μmol/L) | 3.4-21 | — | 7.8 | 13 | 24.1a | 16 | 9.2 | 11.3 |
| Alanine aminotransferase (U/L) | 9-50 | — | 20 | 20 | 19 | 72a | 49 | 119 |
| Aspartate aminotransferase (U/L) | 15-45 | — | 23 | 32 | 19 | 47a | 28 | 60 |
The value in the patient was above normal.
The value in the patient was below normal.
Dizziness and hematuria developed on day 3 of hospitalization, apart from the development of intermittent nausea and vomiting (Figure 1). Laboratory results showed an elevated creatinine level and hyponatremia on that evening (Table 1). The 24-hour urine volume on hospital day 3 was 500 mL. To improve the oliguria and hyponatremia, individually tailored fluid administration based on real-time urine volume was conducted, and 24-hour urine volume for hospital days 4 and 5 was 2000 and 2500 mL, respectively.
Chest CT was performed on hospital day 5 (Figure 2B) and revealed extensive but slightly improved ground-glass shadowing over both lungs and a strip-like sign over bilateral superior lobes and right lower lobe. Both the sputum and oropharyngeal swab specimens later tested negative on RT-PCR for SARS-CoV-2.
Given unavailable laboratory testing of CsA concentration in blood, our major concern was the metabolic interactions on cytochrome P450 (CYP) between CsA and lopinavir/ritonavir. This issue was addressed in a multidisciplinary team meeting, performed online on hospital day 6, in which it was decided to discontinue esomeprazole and reduce lopinavir/ritonavir by 25% to 750 mg daily. Dosage of baseline immunosuppression remained unchanged. Laboratory results on day 6 of hospitalization showed improved creatinine level and hyponatremia; again, the sputum and oropharyngeal swab specimens tested negative on RT-PCR for SARS-CoV-2.
Given the stable clinical presentation, chest CT revealed significant improvement on day 12 of hospitalization (Figure 2C) and an almost normal laboratory test, persistently negative for SARS-CoV-2; hence, he was discharged on hospital day 13 after recovering from COVID-19. Based on Novel Coronavirus Pneumonia Diagnosis and Treatment Plan (trial version 5), he was treated for 10 days (February 9 to February 18th) with lopinavir/ritonavir and for 3 days with γ-globulin.
Testing of the CsA concentration in patients with COVID-19 was not available before February 22, 2020 due to organizational issues, so when the patient was discharged from our hospital, we tested the concentration of CsA on stored blood specimens frozen in a −80℃ refrigerator collected on February 8 and February 19, 2020. The results revealed a C0 of 126.7 ng/mL on February 8 and a C2 of 1375.6 ng/mL on February 19, respectively ( Table 2).
TABLE 2.
Concentration of CSA in venous blood
| Time | November 26, 2019 | December 27, 2019 | January 21, 2020 | February 8, 2020, hospital day 1 | February 19, 2020, hospital day 12 | February 26, 2020, discharge day 7 |
|---|---|---|---|---|---|---|
| C0 (ng/mL)a | 101.2 | 121.4 | 114.4 | 126.7 | — | 260.58 |
| C2 (ng/ml)b | — | — | — | — | 1375.6 | 319.19 |
C0, concentration of cyclosporine on fasting specimens.
C2 blood specimen was collected about 2 h after the patient taking the CsA.
The patient was seen in the outpatient clinic on February 26 (7 days after discharge), and his appetite had improved, and he had no fever, cough, dyspnea, nausea, vomiting, headache, or diarrhea. His 24-hour urine volume fluctuated from 2000 mL to 3000 mL each day since February 20. The laboratory testing on February 25 revealed mild anemia and hypoalbuminemia, and creatinine and aminotransferase levels were slightly elevated (Table 1). Chest CT showed that the ground-glass lesions were significantly absorbed; fibrosis was absent (Figure 2D). Both oropharyngeal swab and sputum were negative for SARS-CoV-2 on RT-PCR twice, on February 25 and February 26. C0 and C2 of CsA were measured on February 26; C0 was 260.58 ng/mL and C2 was 319.19 ng/mL. No changes were made to the dosing at this time, and further evaluation will be performed.
3. DISCUSSION
We report a case of COVID-19 occurring in a kidney transplant recipient. Diagnosis was confirmed using RT-PCR assays. Although the source of his SARS-CoV-2 infection is unknown, evidence of person-to-person transmission has been confirmed.4 Long-term administration of immunosuppression in solid organ transplant recipients may increase the risk of infection, though there is no clinical evidence for an increased morbidity/mortality in respiratory tract infections caused by respiratory viruses such as the known group of coronaviruses so far (with exception of SARS and Middle East respiratory syndrome coronavirus [MERS]).1 , 11 Although the number of patients currently is too small to allow a prediction whether the natural history of SARS-CoV-2 infection is different or more severe in transplant recipients, this issue clearly needs further observation and careful monitoring in order to assess its impact on this specific patient group.
Specific drugs to treat SARS-CoV-2 will take several years to develop and evaluate. In an attempt to treat the COVID-19, interferon and ribavirin were deemed to be not suitable for a renal transplantation recipient due to their side effects, including the potential to trigger an acute rejection. Therefore, the protease inhibitors lopinavir/ritonavir were initiated based on Novel Coronavirus Pneumonia Diagnosis and Treatment Plan (trial version 5). Lopinavir and ritonavir are the inhibitors of CYP3A enzyme12 causing irreversible inhibition of CYP3A4 with increased blood levels of ciclosporin/tacrolimus.13 While we were unable to monitor C0 of CsA in blood because laboratory testing was restricted by biosafety level, we empirically adjusted the dosage of lopinavir/ritonavir to three quarters of the recommended dose. We retrospectively tested CsA levels on stored specimens which coincidentally had been collected from one day before and after the treatment with lopinavir/ritonavir. We found the C0 of CsA before treatment within the patients usual CsA exposure while the C2 level after treatment was higher than the C2 recommended level.14 To confirm the interaction between CsA and lopinavir/ritonavir, follow-up monitoring of C0 and C2 levels were implemented 1 week after discharge (8 days after lopinavir/ritonavir discontinued). The C0 was elevated, but C2 was decreased indicating that lopinavir/ritonavir may strongly interact via CYP450 with the concentration of CsA even after discontinuation of the protease inhibitors. Nevertheless, no serious hepatic and renal dysfunction was observed in our case and we assume further normalization of CsA levels.
Methylprednisolone was routinely used as baseline immunosuppression in this case, although its use for the treatment of SARS-CoV-2 infection remains controversial.15 No clinical data exist to indicate that net benefit is derived from corticosteroids in the treatment of respiratory infection due to coronavirus included SARS-CoV and MERS-CoV.15 Furthermore, corticosteroid treatment may be associated with delayed clearance of MERS-CoV viral RNA from respiratory tract secretions.16 Nevertheless, there are other studies supporting the use of corticosteroids at low-to-moderate dose in patients with coronavirus infection, proper use of corticosteroids was found to reduce mortality and shorten the length of stay in hospital for critically ill patients with SARS without causing secondary infection and other complications.17 While the average clearance time of SARS-CoV-2 from respiratory tract secretions was not reported, the majority of patients were tested persistently positive for SARS-CoV-2 for longer than 1 week.18 In this case, the SARS-CoV-2 from respiratory tract secretions was not detected after hospital day 5, no obvious delayed clearance was observed in this case under immunosuppression. Secondly, secondary infection and other complications were not presented in this case under immunosuppression as well. These findings indicate that routine low dose of corticosteroids probably may have no significant negative impact on patients infected with SARS-CoV-2; on the other hand, a positive impact cannot be concluded from one single case.
In conclusion, we report COVID-19 in a renal transplant recipient successfully treated with lopinavir/ritonavir. The patient had a relatively mild illness, atypical pneumonia resolved rapidly under the antiviral therapy and an excellent clinical recovery was observed. No significant complications were found during the infection although baseline immunosuppression was continued. Maintenance treatment with corticosteroids was continued as well since no negative impact was found. Furthermore, lopinavir/ritonavir has a strong effect on CsA blood concentration, hence these patients need close monitoring and if necessary, adjustment such as dosage reduction or discontinuation. More data are needed to gain better understanding of SARS-CoV-2 infections in solid organ transplant recipients.
Acknowledgments
DISCLOSURE
The authors of this manuscript have no conflict of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1.Paulsen GC, Danziger-Isakov L. Respiratory viral infections in solid organ and hematopoietic stem cell transplantation. Clin Chest Med. 2017;38(4):707–726. doi: 10.1016/j.ccm.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan J-W, Yuan S, Kok K-H, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. The Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phan LT, Nguyen TV, Luong QC, et al. Importation and human-to-human transmission of a novel coronavirus in Vietnam. N Engl J Med. 2020;382(9):872–874. doi: 10.1056/NEJMc2001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO. Coronavirus disease 2019 (COVID-19) Situation Report –95. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/
- 6.office Nh. Novel Coronavirus Pneumonia Diagnosis and Treatment Plan (Provisional 5th Edition). http://www.gov.cn/zhengce/zhengceku/2020-02/05/content_5474791.html. 2020.
- 7.Dayer MR, Taleb-Gassabi S, Dayer MS. Lopinavir; A potent drug against coronavirus infection: insight from molecular docking study. Arch Clin Infect Dis. 2017;12(4):e13823. [Google Scholar]
- 8.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. New Engl J Med. 2020;382(10):970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020; 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed]
- 11.Haidar G, Singh N. Viral infections in solid organ transplant recipients: novel updates and a review of the classics. Curr Opin Infect Dis. 2017;30(6):579–588. doi: 10.1097/QCO.0000000000000409. [DOI] [PubMed] [Google Scholar]
- 12.Cvetkovic RS, Goa KL. Lopinavir/ritonavir: a review of its use in the management of HIV infection. Drugs. 2003;63(8):34. doi: 10.2165/00003495-200363080-00004. [DOI] [PubMed] [Google Scholar]
- 13.Monostory K. Chapter 20–Metabolic Drug Interactions with Immunosuppressants. Organ Donation and Transplantation-Current Status and Future Challenges. 2018:409–440. 10.5772/intechopen.74524 [DOI]
- 14.Nashan B, Bock A, Bosmans JL, et al. Use of neoral C monitoring: a European consensus. Transpl Int. 2005;18(7):768–778. doi: 10.1111/j.1432-2277.2005.00151.x. [DOI] [PubMed] [Google Scholar]
- 15.Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. The Lancet. 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arabi YM, Mandourah Y, Al-Hameed F, et al. Corticosteroid therapy for critically Ill patients with middle east respiratory syndrome. Am J Respir Crit Care Med. 2018;197(6):11. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 17.Chen R-C, Tang X-P, Tan S-Y, et al. Treatment of severe acute respiratory syndrome with glucosteroids: the Guangzhou experience. Chest. 2006;129(6):12. doi: 10.1378/chest.129.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zou L, Ruan F, Huang M, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. New Engl J Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.