Abstract
Background
Severe acute respiratory syndrome‐coronavirus‐2 (SARS‐CoV‐2), the virus that causes coronavirus disease 2019 (COVID‐19), is responsible for the largest pandemic since the 1918 influenza A virus subtype H1N1 influenza outbreak. The symptoms presently recognized by the World Health Organization are cough, fever, tiredness, and difficulty breathing. Patient‐reported smell and taste loss has been associated with COVID‐19 infection, yet no empirical olfactory testing on a cohort of COVID‐19 patients has been performed.
Methods
The University of Pennsylvania Smell Identification Test (UPSIT), a well‐validated 40‐odorant test, was administered to 60 confirmed COVID‐19 inpatients and 60 age‐ and sex‐matched controls to assess the magnitude and frequency of their olfactory dysfunction. A mixed effects analysis of variance determined whether meaningful differences in test scores existed between the 2 groups and if the test scores were differentially influenced by sex.
Results
Fifty‐nine (98%) of the 60 patients exhibited some smell dysfunction (mean [95% CI] UPSIT score: 20.98 [19.47, 22.48]; controls: 34.10 [33.31, 34.88]; p < 0.0001). Thirty‐five of the 60 patients (58%) were either anosmic (15/60; 25%) or severely microsmic (20/60; 33%); 16 exhibited moderate microsmia (16/60; 27%), 8 mild microsmia (8/60; 13%), and 1 normosmia (1/60; 2%). Deficits were evident for all 40 UPSIT odorants. No meaningful relationships between the test scores and sex, disease severity, or comorbidities were found.
Conclusion
Quantitative smell testing demonstrates that decreased smell function, but not always anosmia, is a major marker for SARS‐CoV‐2 infection and suggests the possibility that smell testing may help, in some cases, to identify COVID‐19 patients in need of early treatment or quarantine.
Keywords: chronic rhinosinusitis, olfactory disorders, olfaction, olfactory test, UPSIT, COVID‐19, biomarker
Recently there have been numerous reports in the media that anosmia occurs in persons who have contracted coronavirus disease 2019 (COVID‐19) by exposure to the severe acute respiratory syndrome‐coronavirus‐2 (SARS‐CoV‐2) virus. These include 1 published single case report, 1 and self‐report surveys from Germany, 2 Great Britain, 3 Iran, 4 Italy, 5 and the United States, 6 with smell loss reports ranging from 34% to 68% of COVID‐19–positive patients. Otorhinolaryngology authorities have warned that loss of smell and taste, in combination with other symptoms, appears to be a strong predictor of COVID‐19 infection. 7 , 8
To date, validated quantitative olfactory testing has not been performed in a cohort of COVID‐19 patients to verify or determine the true magnitude of their deficits and whether less‐than‐total loss occurs in some patients. Moreover, the proportion of COVID‐19 patients exhibiting true olfactory disturbances is unknown. Most studies suggest that, in general, a significant number of persons with smell loss are unaware of their deficit until formal testing 9 and that self‐reports of both smell and taste abilities correlate poorly with the results of quantitative smell and taste tests. 10 , 11
In this case‐control study, we administered the Persian version of the 40‐item University of Pennsylvania Smell Identification Test (UPSIT) 12 to 60 confirmed COVID‐19 patients and 60 age‐ and sex‐matched controls to assess the presence, magnitude, and frequency of their olfactory dysfunction. We determined whether the smell loss was related to the sex of the subjects and inquired, for those patients who were aware of their dysfunction before testing, when they first noticed their chemosensory disorder.
Patients and methods
Subjects
The age, sex, comorbidities, smoking status, and complaints of chemosensory dysfunction of the 120 study participants are presented in Table 1. The 60 SARS‐COV‐2–positive subjects had been admitted with the symptoms of COVID‐19 to the Masih Daneshvari Hospital, Tehran, Iran, between March 21, 2020, and March 23, 2020, or March 31, 2020, and April 5, 2020. At the time of the olfactory testing, all were inpatients in the recovery period of the disease and were ready to be discharged within 4 days. The study was explained in detail to 68 such patents, of which 8 declined to participate (ie, the participation rate was 88%).
TABLE 1.
Patient and control subject demographics *
| Parameter | COVID‐19 patients | Controls |
p (Fisher exact probability test) |
|---|---|---|---|
| Sample size, n | 60 | 60 | |
| Age (years), mean ± SD | 46.55 ± 12.17 | 46.55 ± 12.07 | |
| Gender (male/female), n | 40/20 | 40/20 | |
| Smoker (current/never), n | 2/58 | 11/49 | 0.016 |
| Taste/smell complaints, n | 21 | 0 | 0.001 |
| Comorbidities, n | |||
| Asthma | 3 | 0 | 0.244 |
| Atherosclerosis | 0 | 2 | 0.496 |
| Autoimmune disease | 4 a | 0 | 0.119 |
| Carcinoma | 2 b | 0 | 0.496 |
| Congenital melanocytic nevi | 1 | 0 | 1.000 |
| Diabetes | 8 c | 0 | 0.007 |
| Hemophilia | 0 | 1 | 1.000 |
| Hepatic failure | 0 | 1 | 1.000 |
| Hyperlipidemia | 1 | 1 | 1.000 |
| Hypertension | 6 c | 5 | 1.000 |
| Hypothyroidism | 4 c | 2 | 0.679 |
| Migraine | 0 | 1 | 1.000 |
| Osteoporosis | 0 | 1 | 1.000 |
| Sinusitis | 2 | 0 | 0.496 |
*Significant p differences indicated in bold.
aAutoimmune disease included Behcet's disease in combination with Crohn's disease (n = 1), multiple sclerosis (n = 2), and rheumatoid arthritis (n = 1).
bProstate and cervical cancers.
c Although, in rare cases, changes in dosage and medications may have occurred during the course of inpatient treatments, most patients remained on their preadmission medications.
COVID‐19 = coronavirus disease 2019; SD = standard deviation.
The control subjects were from a database of 141 subjects collected in Iran for an earlier study. They were tested in the olfactory laboratory of the Institute for Research in Fundamental Sciences, Tehran, Iran, and comprised a convenience sample obtained from e‐mail lists, flyers, and word of mouth. None had influenza or common cold symptoms at the time of testing. The recruitment period for this database (August 8, 2019, to February 13, 2020) preceded the first reported confirmed cases of COVID‐19 in Iran (February 19, 2020). A control subject was individually matched as closely as possible to each COVID‐19 patient. Exact age matches were possible for 34 subjects, 1‐year differences for 22 subjects, and 2‐year differences for 4 subjects. In cases where >1 match was possible, the first match in the database sequence was used.
Informed written consent was obtained from each patient and control, and the study protocol was approved by the local ethics committee and the Iranian Ministry of Health (license number IR.SBMU.NRITLD.REC.1399.013). All testing was performed with the highest regard for examiner safety with appropriate personal protective equipment.
Diagnosis and clinical severity classification of COVID‐19 patients
COVID‐19 diagnosis was based on the COVID‐19 detection protocol of Masih Daneshvari Hospital. All of the patients underwent 16‐slice chest computed tomography (CT) imaging (Scope Power Siemens CT Scan, Munich, Germany) and had positive chest CT findings. 13 Subsequently, the diagnosis of COVID‐19 disease was confirmed by quantitative detection of SARS‐CoV‐2 RNA using the real‐time reverse‐transcription polymerase chain reaction (rRT‐PCR) in respiratory specimens. 14 The RT‐PCR assays were performed using Sansure Biotech's 2019‐nCoV 30‐Minute Nucleic Acid Reagent Kits (Sansure Biotech, Inc., Development Zone, Changsha, China). The respiratory specimens were collected from the patients’ nasopharyngeal wash/aspirate or nasal aspirate.
COVID‐19 clinical severity was classified as mild, moderate, or severe according to the Massachusetts General Hospital COVID‐19 treatment guidance algorithm. 15 Mild clinical COVID‐19 presentation was defined as having oxygen saturation (SpO2) >90% along with or without risk factors. Moderate clinical COVID‐19 presentation was considered for patients who had at least 1 epidemiological risk factor along with a risk factor in vital signs or laboratory findings at the admission point of time. Patients in the intensive care unit (ICU) or with progressive disease were classified as having severe clinical presentation of COVID‐19. Epidemiological risk factors included age >55 years or preexisting pulmonary disease, chronic kidney disease, diabetes with glycated hemoglobin (A1c) >7.6%, history of hypertension or cardiovascular disease or transplant, or immunosuppression or human immunodeficiency virus (HIV). Risk factors of vital signs comprised respiratory rate >24 breaths/minute, heart rate >125 beats/minute, and SpO2 <90% on ambient air. In laboratory findings, fibrin degradation product D‐dimer >1000 ng/mL, creatine phosphokinase (CPK) more than twice the upper limit of normal, C‐reactive protein (CRP) >100 mg/L, lactate dehydrogenase (LDH) >245 U/L, elevated troponin, admission absolute lymphocyte count <0.8, and ferritin >300 μg/L. For COVID‐19 patients with mild disease with SpO2 >90%, supportive care was provided and hydroxychloroquine administration was started (200 mg twice per day [BID] × 2 doses, then 100 mg BID for 5 days). For the patients with moderate to severe COVID‐19 presentations, lopinavir/ritonavir 200/50 mg BID for up to 10 days) was prescribed. In patients with progressive COVID‐19 disease admitted to the ICU, intravenous immunoglobulin (IVIG) at standard dose of 0.5 g/kg/day daily for 5 days was administered. 16
Olfactory testing
A modified and validated Persian version of the UPSIT was administered in this study (Sensonics International, Haddon Heights, NJ). The UPSIT is a well‐validated and reliable (test‐retest r = 0.94) test that employs microencapsulated “scratch and sniff” odorants. 11 , 12 , 17 , 18 It provides an index of absolute dysfunction (ie, anosmia, severe microsmia, moderate microsmia, mild microsmia, normosmia, malingering), as well as relative dysfunction based upon age‐ and gender‐adjusted normative percentile ranks. The total number of odorant stimuli out of 40 that is correctly identified serves as the test measure. Scores on this test correlate well with other types of olfactory tests, including threshold tests. 19 Although the UPSIT is designed to be self‐administered, to be certain that the COVID‐19 patients correctly performed the test during the limited clinical time window, the testing was assisted by a trained examiner.
Statistical analyses
Statistical analyses were performed using either SYSTAT 13 (Systat Software, Inc., San Jose, CA) 20 or MATLAB 2019b (The MathWorks, Inc., Natick, MA). A subject group by gender mixed factor analysis of variance (ANOVA) was used to determine whether the UPSIT scores differed significantly between the patient and control groups and whether gender influenced the test scores. Standard ANOVAs were used to compare other means. Differences in frequencies were assessed using the Fisher's exact probability test.
Results
The COVID‐19 patients’ non‐mutually exclusive presenting symptoms were fever (n = 46, 77%), cough (n = 35, 58%), shortness of breath (n = 31, 52%), headache (n = 22, 37%), myalgia (n = 5, 8%), sweating (n = 2, 3%), shivering (n = 2, 3%), anorexia (n = 2, 3%), stomachache (n = 1, 2%), and tinnitus (n = 1, 2%). The mean (95% CI) time between the onset of symptoms and the olfactory testsing was 12.76 (11.47, 14.06) days.
The UPSIT testing revealed that, relative to controls and published normative data, the COVID‐19 patients exhibited marked olfactory dysfunction. Thus, as illustrated in Figure 1, the mean (95% confidence interval [CI]) UPSIT score for the COVID‐19 patients was 20.98 (19.47, 22.48), reflecting severe microsmia, 21 whereas the mean UPSIT score (95% CI) for the age‐ and sex‐matched controls fell within the normal range (34.10 [33.31, 34.88]; ANOVA group main effect F [1,58] = 232.99, p < 0.0001, η2 = 0.80). The COVID‐19 deficit was not specific to any 1 UPSIT odorant, being evident for all 40 stimuli (Fig. 2).
FIGURE 1.
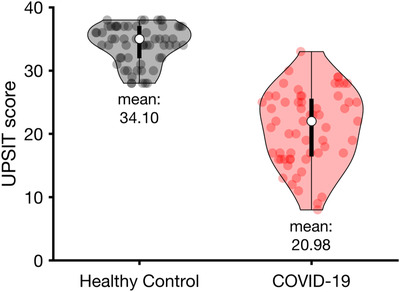
UPSIT scores of the COVID‐19 patients compared to those of healthy controls. The distribution of the participants’ scores in each group is depicted in violin plot. The white circles indicate the median of the score for each group. COVID‐19 = coronavirus disease 2019; UPSIT = University of Pennsylvania Smell Identification Test.
FIGURE 2.
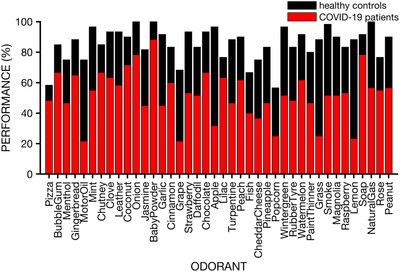
Performance on individual UPSIT odorants for the COVID‐19 patients and matched healthy controls. Note that dysfunction was evident for all 40 UPSIT odorants. Performance for each group is calculated as the percent of individuals having correctly identified the odorant. COVID‐19 = coronavirus disease 2019; UPSIT = University of Pennsylvania Smell Identification Test.
Importantly, all but 1 of the 60 patients with COVID‐19 had some degree of measured olfactory dysfunction (98%). Thirty‐five of the 60 patients (58%) were either anosmic (15/60; 25%) or severely microsmic (20/60; 33%); 16/60 (27%) exhibited moderate microsmia, 8/60 (13%) mild microsmia, and 1/60 (2%) normosmia according to Persian‐adjusted UPSIT norms (Table 2). 21 This contrasts markedly from the controls, of which 49 of 60 (82%) were normal with the remaining 11 of 60 (18%) having only mild borderline dysfunction. Relative to the normal controls, the 11 controls with mild borderline dysfunction tended to be disproportionately men (10/11 [91%] vs 30/49 [61%]; p = 0.08) of older age (respective mean ages [95% CIs] = 51.18 [42.63, 59.73] and 45.51 [42.11, 48.90]; p = 0.18). Even though there was a tendency for women, overall, to outperform men on the UPSIT (respective mean [95% CI] UPSIT scores: 22.55 [20.13, 24.97] and 20.20 [18.27, 22.13]; F [1,58] = 3.82, p = 0.055, η2 = 0.06), this was unrelated to COVID‐19 (sex by group interaction F [1,58] = 0.396, p = 0.53).
TABLE 2.
Classification of olfactory function of the UPSIT scores of COVID‐19 patients and matched controls
| UPSIT function category | COVID‐19 patients n (%) | Controls n (%) | UPSIT score range |
|---|---|---|---|
| Normosmia | 1 (2) | 49 (82) | 31–40 |
| Mild microsmia | 8 (13) | 11 (18) | 28–30 |
| Moderate microsmia | 16 (27) | 0 | 24–27 |
| Severe microsmia | 20 (33) | 0 | 17–23 |
| Anosmia | 15 (25) | 0 | 6–16 |
| Probable malingering | 0 | 0 | 0–5 |
COVID‐19 = coronavirus disease 2019; UPSIT = University of Pennsylvania Smell Identification Test.
Thirty‐five percent (21/60) of the COVID‐19 patients reported a loss in either smell or taste function, with 12% (7/60) reporting smell loss only, 7% (4/60) taste loss only, and 17% (10/60) both taste and smell loss. There was no significant difference between UPSIT scores of patients who were aware or unaware of their chemosensory loss (p = 0.28). All 21 reported that the onset of the olfactory dysfunction occurred at the same time or immediately after the onset of their other COVID‐19 symptoms. None reported recognizing any smell or taste deficits prior to their other COVID‐19 symptoms, namely fever, cough, or shortness of breath. In the healthy control group, none of the participants reported any smell or taste problems.
As shown in Table 1, significantly fewer smokers were present in the COVID‐19 group than in the control group (2/60 vs 11/60; p = 0.016). Eight patients with diabetes were present in the COVID‐19 group, unlike the control group (8/60 vs 0/60; p = 0.007). However, the respective mean (95% CI) UPSIT scores for COVID‐19 patients with and without diabetes did not differ (21.38 [18.18, 24.56] vs 20.92 [19.32, 22.62], respectively; F [2,57] = 1.43, p = 0.24, η2 = 0.05). No association of UPSIT scores with disease severity, as per the Massachusetts General Hospital COVID‐19 treatment guidance algorithm, was apparent (Table 3; F [2,57] = 1.45, p = 0.24, η2 = 0.05).
TABLE 3.
Relationship between COVID‐19 clinical disease severity and mean (95% CI) scores on the UPSIT
| COVID‐19 disease severity | n (%) |
UPSIT score mean (95%CI) |
|---|---|---|
| Mild | 25 (42) | 22.04 (20.11–24.72) |
| Moderate | 29 (48) | 19.69 (17.24–21.99) |
| Severe | 6 (10) | 22.83 (17.65–25.77) |
CI = confidence interval; COVID‐19 = coronavirus disease 2019; UPSIT = University of Pennsylvania Smell Identification Test.
Discussion
This study quantitatively evaluated olfaction in a sizable cohort of patients diagnosed with the SARS‐CoV‐2 virus infection. By employing a well‐validated 40‐item smell test, COVID‐19 patients were able to be classified into distinct categories of olfactory dysfunction, with 35 of 60 (58%) exhibiting either anosmia or severe microsmia. In the present study, only 35% of the patients were aware of their olfactory deficit before testing, a percentage near to that of 34% reported in an interview with COVID‐19 inpatients in Italy, 5 but lower than those reported in 2 online surveys (59% 3 and 68% 6 ). This difference between self‐report rate and quantified smell assessment conceivably reflects a disproportionate sampling of hospital admitted cases and/or the well‐documented underestimation of self‐reported smell and taste dysfunction present for the general population 9 , 10 and for such diseases as Alzheimer's disease (AD) 11 and Parkinson's disease (PD). 22 , 23 In general, smell loss is most noticeable when marked loss, such as anosmia, is present. 11 , 22 It should be pointed out that the present study's sample resembles the demographic and clinical characteristics of COVID‐19 patients reported in a compilation of 43 studies involving 3600 patients, 24 implying it is likely representative of COVID‐19 patients in general.
The basis for the smell loss due to SARS‐CoV‐2 is not entirely clear, although it is well established that viruses and other xenobiotics can damage the olfactory neuroepithelium. Indeed, acute viral upper respiratory viral infections that damage this epithelium are the major cause of chronic olfactory dysfunction and numerous viruses are known to enter the brain through cellular and pericellular transport via this epithelium. 25 In North America, the peak period of non‐influenza–related smell loss, including that possibly due to coronaviruses, occurs during the months of April, May, and June, whereas influenza‐related smell loss peaks in December, January, and February. 26 Currently, the prevalence of COVID‐19 in North America seems to follow a similar function to that observed for olfactory deficits due to other viruses, including other coronaviruses. What seems unique, however, is that nearly everyone who contacts COVID‐19 appears to exhibit measurable loss of smell seemingly independent of severe nasal congestion or inflammation.
Although SARS‐CoV‐2 has the ability to enter epithelial cells by directly binding to the angiotensin converting enzyme 2 (ACE2) protein on the cell surface, 27 olfactory receptor cells do not express ACE2, as well as another gene involved in SARS‐CoV‐2 entry (TMPRSS2), unlike epithelial sustentacular and stem cells. 28 Thus, damage to the olfactory receptors may be mediated indirectly through SARS‐CoV‐2 uptake into other cells critical for sustaining the olfactory receptor cell population. For example, olfactory ensheathing glial cells that surround the olfactory receptor cell axons and form the olfactory fila are 1 candidate by which ACE2‐independent virus transfer can occur into olfactory receptor neurons by way of exosomes. A possible scenario suggests that at this point olfactory receptor neurons may initiate a rapid immune response in the host with the manifestation of olfactory dysfunction. 29 That being said, the olfactory neuroepithelium has considerable propensity for regeneration if the stem cell layer is not markedly damaged 30 , 31 , 32 – regeneration that is likely related to spontaneous improvement in olfactory function over time. 33
It is of interest that significantly fewer smokers were found in our COVID‐19 cohort than in the control cohort. Our findings correspond with studies that report current smokers as rare as 1.4% and 1.3% in Chinese 34 and U.S. 35 COVID‐19 patient populations, respectively. A recent study reported that smoking upregulated the expression of ACE‐2 in the airways, potentially predisposing individuals to increased risk of coronavirus infection but, paradoxically, protecting the host against acute lung injury. 36 Interestingly, nonsmokers appear to be much more susceptible than smokers to olfactory dysfunction from industrial exposures to acrylate and methacrylate 37 and smoking appears to protect, to some degree, against the olfactory loss of PD. 38 Future research is needed to determine to what degree the reported low frequency of smokers in COVID‐19 populations is impacted by selection bias (eg, more smokers may have died before reaching the hospital) and reverse causation (ie, cessation of smoking in patients with severe symptoms prior to entering the hospital, thereby being counted as nonsmokers). The latter is unlikely in our study, however, because each patient was specifically asked whether they currently smoked or had smoked in the past.
The complaint of taste loss by a small number of our COVID‐19 patients most likely reflects, to a significant degree, damage to the olfactory system, rather than damage to the taste buds or taste afferents, per se. Thus, the vast majority of individuals who clinically present with complaints of taste loss actually exhibit smell dysfunction, including those with a viral etiology. 39 Taste bud–mediated sensations are largely limited to the basic taste qualities of sweet, sour, bitter, salty, and umami. With the exception of such sensations, all “tastes” are flavor sensations from olfactory receptor stimulation by volatiles entering from the nasopharynx during deglutition. 40 This tendency for many persons with smell loss to misconstrue their problem as taste loss 39 must be considered in studies relying only on self‐report. Future research employing quantitative taste tests is clearly needed to definitively establish whether SARS‐CoV‐2 also can damage taste afferents or, in rare cases, more central taste‐related brain regions.
More men than women were present in our sample, in accord with the reported demographic and clinical characteristics of COVID‐19 patients. 24 However, the magnitude of olfactory dysfunction, as measured by the UPSIT, was essentially the same in both sexes. This implies that there is little or no protection from being a female in terms of the degree to which SARS‐CoV‐2 damages the olfactory system, in accord with some other studies of postviral olfactory deficits. 41 If this observation is confirmed with larger samples, it would appear that the olfactory dysfunction of COVID‐19 differs from that of AD and PD, where women significantly outperform men. 11 , 22 , 38
It is important to note that the COVID‐19–positive patients evaluated in this study had severe enough symptoms to be admitted to the hospital. It is unknown whether less severe cases also exhibit the same degree of smell dysfunction as documented in this study, although within our hospitalized cohort no relationship was evident between the olfactory test scores and disease severity. This is similar to what is seen in the smell loss of PD, where no clear association is present between the magnitude of the classic motor signs and the amount of olfactory dysfunction. 22
Even though the COVID‐19 patients in this study were undergoing drug treatments for their disease, it is unlikely that the involved drugs were a meaningful cause of their olfactory dysfunction. Despite the fact that a significant number of medications are reported to have taste side effects, 42 alterations in smell function are relatively rare and have not been associated with hydroxychloroquine, lopinavir‐ritonavir, or IVIG. Because the same degree of smell function was evident among patients with COVID‐19 taking each of these medications, it is improbable that any one medication would have produced the smell deficits observed in this study.
Although RT‐PCR was by far the frontline response to the SARS‐CoV‐2 outbreak, the accuracy and conditions under which the results of RT‐PCR were achieved must be kept in context, because a false‐negative rate of at least 15% has been reported. 43 , 44 , 45 The present findings, along with the wealth of anecdotal data, suggest that quantitative testing of the sense of smell might serve as a rapid and inexpensive alternative diagnostic means to screen for COVID‐19 in large numbers of individuals. Indeed, the sensitivity and specificity of olfactory tests for COVID‐19–positive patients under the age of 65 years would seem to be quite strong, because age‐related changes in smell function occur mainly after the age of 65 years. 17
The present study has both strengths and weaknesses. Among its strengths are (1) the use of a sensitive test of olfactory function that allows for determining different degrees of olfactory function, (2) testing of well‐validated COVID‐19 patients whose clinical severity was well documented, and (3) the use of controls matched closely to those of the patients on the basis of age and sex who were sampled outside of the period in which COVID‐19 was first identified in Iran. Its major limitation is the sampling of the study population at only 1 point in time relative to the onset of COVID‐19 symptoms. Future studies are needed to establish (1) the exact time of onset of smell symptoms, (2) whether the olfactory dysfunction is transient, long‐lasting, or permanent, (3) whether such symptoms are evident in those who fail to develop other COVID‐19 symptoms, and (4) whether the deficits follow seasonal patterns such as those noted for other virus‐related cases of smell dysfunction.26 Information as to permanency is of considerable significance, because loss of the ability to smell significantly impacts quality of life, the flavor of foods, and beverages, and safety from spoiled food, fire, and leaking natural gas. Importantly, smell loss can be a harbinger of a number of neurological diseases, most notably AD and PD—diseases which, in some cases, have been associated with a number of viruses. 46 , 47 Although the reasons are poorly understood, older persons with smell loss are 3 times more likely to die over the course of an ensuring half‐decade than older persons with a normal sense of smell. 48 , 49
Conclusion
The present study provides a quantitative assessment of the olfactory function of a cohort of patients with COVID‐19. Its findings strongly suggest that some degree of loss of smell function is present in nearly all COVID‐19 patients near the end of their acute recovery period. However, anosmia, per se, was present in only about one‐quarter of COVID‐19 positive patients in our sample, with about one‐third evidencing severe microsmia. In light of the current findings and pandemic environment, and the widespread anecdotal evidence of smell dysfunction in COVID‐19, it does not seem unreasonable that testing the olfaction of persons who may be at risk or have subtle COVID‐19 signs, such as low‐grade fevers, may aid in identifying COVID‐19 patients who are in need of early treatment or quarantine.
Acknowledgments
We thank Mr. Iraj Fotouhi for his invaluable help in assisting patients during smell testing, Ms. Crystal Wylie for comments on a draft of the paper, and Dr. Kambiz Pourrezaei for insightful discussions of the research.
How to Cite this Article:Moein ST, Hashemian SM, Mansourafshar B, Khorram‐Tousi A, Tabarsi P, Doty RL. Smell dysfunction: a biomarker for COVID‐19. Int Forum Allergy Rhinol. 2020;10:944–950.
Potential conflicts of interest: R.L.D. is a consultant to Acorda Therapeutics, Eisai Co, Ltd, Merck Pharmaceuticals, the Michael J. Fox Foundation for Parkinson's Research, and Johnson & Johnson; receives royalties from Cambridge University Press, Johns Hopkins University Press, and John Wiley & Sons, Inc.; he is president of, and a major shareholder in, Sensonics International, a manufacturer and distributor of smell and taste tests, including the test used in this study. A. K‐T. is a medical advisor to Cobel Darou in Iran. The other authors have nothing to disclose.
References
- 1. Eliezer M, Hautefort C, Hamel AL, et al. Sudden and complete olfactory loss function as a possible symptom of COVID‐19. JAMA Otolaryngol Head Neck Surg. (in press). Epub 08 April 2020. 10.1001/jamaoto.2020.0832. [DOI] [PubMed] [Google Scholar]
- 2. Luers JC, Klussmann JP, Guntinas‐Lichius O. [The Covid‐19 pandemic and otolaryngology: what it comes down to?]. Laryngorhinootologie. 2020;99(5):287–291. 10.1055/a-1095-2344. German. [DOI] [PubMed] [Google Scholar]
- 3. Menni C, Valdes A, Freydin MB, et al. Loss of smell and taste in combination with other symptoms is a strong predictor of COVID‐19 infection. medRxiv. 2020.04.05.20048421. Epub 07 April 2020. 10.1101/2020.04.05.20048421. [DOI] [Google Scholar]
- 4. Bagheri SHR, Asghari AM, Farhadi M, et al. Coincidence of COVID‐19 epidemic and olfactory dysfunction outbreak. medRxiv. 2020.03.23.20041889. Epub 27 March 2020. 10.1101/2020.03.23.20041889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Giacomelli A, Pezzati L, Conti F, et al. Self‐reported olfactory and taste disorders in SARS‐CoV‐2 patients: a cross‐sectional study. Clin Infect Dis. (in press). Epub 26 March 2020. 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yan CH, Faraji M, Prajapati DP, Boone CE. Association of chemosensory dysfunction and Covid‐19 in patients presenting with influenza‐like symptoms. Int Forum Allergy Rhinol. (in press). Epub 12 April 2020. 10.1002/alr.22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. American Academy of Otolaryngology–Head and Neck Surgery Foundation . ENT Health. Hyposmia and anosmia. Alexandria, VA: American Academy of Otolaryngology–Head and Neck Surgery Foundation; 2020. https://www.enthealth.org/conditions/hyposmia-and-anosmia/. Accessed May 14, 2020. [Google Scholar]
- 8. Hopkins C, Kumar N. Loss of sense of smell as marker of COVID‐19 infection 2020. London, UK: ENT UK, The Royal College of Surgeons of England; 2020. https://www.entuk.org/loss-sense-smell-marker-covid-19-infection-0. Accessed May 14, 2020. [Google Scholar]
- 9. Wehling E, Nordin S, Espeseth T, Reinvang I, Lundervold AJ. Unawareness of olfactory dysfunction and its association with cognitive functioning in middle aged and old adults. Arch Clin Neuropsychol. 2011;26:260‐269. [DOI] [PubMed] [Google Scholar]
- 10. Soter A, Kim J, Jackman A, Tourbier I, Kaul A, Doty RL. Accuracy of self‐report in detecting taste dysfunction. Laryngoscope. 2008;118:611‐617. [DOI] [PubMed] [Google Scholar]
- 11. Doty RL, Reyes PF, Gregor T. Presence of both odor identification and detection deficits in Alzheimer's disease. Brain Resh Bull 1987;18:597‐600. [DOI] [PubMed] [Google Scholar]
- 12. Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav. 1984;32:489‐502. [DOI] [PubMed] [Google Scholar]
- 13. Chung M, Bernheim A, Mei X, et al. CT imaging features of 2019 novel coronavirus (2019‐nCoV). Radiology. 2020;295:202‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zou L, Ruan F, Huang M, et al. SARS‐CoV‐2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177‐1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Massachusetts General Hospital . Massachusetts General Hospital COVID‐19 Treatment Guidance. Boston, MA: Massachusetts General Hospital; 2020. https://www.massgeneral.org/assets/MGH/pdf/news/coronavirus/covid-19_domID_treatmentGuide.pdf. Accessed May 14, 2020. [Google Scholar]
- 16. Jamaati H DF, Tabarsi P, Marjani M, Saffaei A, Hashemian SM. A fourteen‐day experience with coronavirus disease 2019 (COVID‐19) induced acute respiratory distress syndrome (ARDS): an Iranian treatment protocol. Iran J Pharm Res 2020;19(1):31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Doty RL, Shaman P, Applebaum SL, Giberson R, Siksorski L, Rosenberg L. Smell identification ability: changes with age. Science 1984; 226: 1441‐1443. [DOI] [PubMed] [Google Scholar]
- 18. Doty RL, Frye RE, Agrawal U. Internal consistency reliability of the fractionated and whole university of Pennsylvania smell identification test. Percept Psychophys 1989;45(5): 381‐384. [DOI] [PubMed] [Google Scholar]
- 19. Doty RL, Wylie C, Potter M, Beston R, Cope B, Majam K. Clinical validation of the olfactory detection threshold module of the Snap & Sniff(R) olfactory test system. Int Forum Allergy Rhinol 2019;9(9): 986‐992. [DOI] [PubMed] [Google Scholar]
- 20. Wilkinson L. SYSTAT: The System for Statistics. Evanston, IL: SYSTAT, Inc.; 1990. [Google Scholar]
- 21. Doty RL. The Smell Identification TestTM Administration Manual. 3rd ed. Haddon Heights, NJ: Sensonics, Inc.; 1995. [Google Scholar]
- 22. Doty RL, Deems DA, Stellar S. Olfactory dysfunction in parkinsonism: a general deficit unrelated to neurologic signs, disease stage, or disease duration. Neurology. 1988;38:1237‐1244. [DOI] [PubMed] [Google Scholar]
- 23. Doty RL, Perl DP, Steele JC, et al. Odor identification deficit of the parkinsonism‐dementia complex of Guam: equivalence to that of Alzheimer's and idiopathic Parkinson's disease. Neurology. 1991;41(5 Suppl 2):77‐80. [DOI] [PubMed] [Google Scholar]
- 24. Fu L, Wang B, Yuan T, et al. Clinical characteristics of coronavirus disease 2019 (COVID‐19) in China: a systematic review and meta‐analysis. J Infect. 2020;80:656‐665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Doty RL. The olfactory vector hypothesis of neurodegenerative disease: is it viable? Ann Neurol. 2008;63:7‐15. [DOI] [PubMed] [Google Scholar]
- 26. Potter MR, Chen JH, Lobban N‐S, Doty RL. Olfactory dysfunction from acute upper respiratory infections: relationship to season of onset. Int Forum Allergy Rhinol. 2020;10(6):706–712. 10.1002/alr.22551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Qi F, Qian S, Zhang S, Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun. 2020;526:135‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brann DH, Tsukahara T, Weinreb C, et al. Non‐neural expression of SARS‐ClV‐2 entry genes in the olfactory epithelium suggests mechanisms underlying anosmia in COVD‐19 patients. bioRxiv 2020.03.25.009084. Epub 09 April 2020. 10.1101/2020.03.25.009084. [DOI] [Google Scholar]
- 29. Butowt R, Bilinska K. SARS‐CoV‐2: olfaction, brain infection, and the urgent need for clinical samples allowing earlier virus detection. ACS Chem Neurosci. 2020;11:1200‐1203. [DOI] [PubMed] [Google Scholar]
- 30. Chang SY, Glezer I. The balance between efficient anti‐inflammatory treatment and neuronal regeneration in the olfactory epithelium. Neural Regen Res. 2018;13:1711‐1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Choi R, Goldstein BJ. Olfactory epithelium: cells, clinical disorders, and insights from an adult stem cell niche. Laryngoscope Investig Otolaryngol. 2018;3:35‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Joiner AM, Green WW, McIntyre JC, Allen BL, Schwob JE, Martens JR. Primary cilia on horizontal basal cells regulate regeneration of the olfactory epithelium. J Neurosci. 2015;35:13761‐13672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. London B, Nabet B, Fisher AR, White B, Sammel MD, Doty RL. Predictors of prognosis in patients with olfactory disturbance. Ann Neurol. 2008;63:159‐166. [DOI] [PubMed] [Google Scholar]
- 34. Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. (in press). Epub 19 February 2020. 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 35. CDC COVID‐19 Response Team . Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 ‐ United States, February 12‐March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:382‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Leung JM, Yang CX, Tam A, et al. ACE‐2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID‐19. Eur Respir J. 2020;55:2000688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schwartz BS, Doty RL, Monroe C, Frye R, Barker S. Olfactory function in chemical workers exposed to acrylate and methacrylate vapors. Am J Public Health. 1989;79:613‐618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sharer JD, Leon‐Sarmiento FE, Morley JF, Weintraub D, Doty RL. Olfactory dysfunction in Parkinson's disease: positive effect of cigarette smoking. Mov Disord. 2015;30:859‐862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Deems DA, Doty RL, Settle RG, et al. Smell and taste disorders, a study of 750 patients from the University of Pennsylvania Smell and Taste Center. Arch Otolaryngol Head Neck Surg. 1991;117:519‐528. [DOI] [PubMed] [Google Scholar]
- 40. Burdach KJ, Doty RL. The effects of mouth movements, swallowing, and spitting on retronasal odor perception. Physiol Behav. 1987;41:353‐356. [DOI] [PubMed] [Google Scholar]
- 41. Tian J, Wei YX, Li L, Sun ZF, Wang BQ. [Analysis of clinical characteristics of 141 patients with postviral olfactory dysfunction]. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2017;31:749‐752. Chinese. [DOI] [PubMed] [Google Scholar]
- 42. Schiffman SS. Influence of medications on taste and smell. World J Otorhinolaryngol Head Neck Surg 2018;4:84‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li D, Wang D, Dong J, et al. False‐negative results of real‐time reverse‐transcriptase polymerase chain reaction for severe acute respiratory syndrome coronavirus 2: role of deep‐learning‐based CT diagnosis and insights from two cases. Korean J Radiol. 2020;21:505‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ai J‐W, Zhang H‐C, Xu T, et al. Optimizing diagnostic strategy for novel coronavirus pneumonia, a multi‐center study in Eastern China. medRxiv. 2020.02.13.20022673. Epub 17 February 2020. 10.1101/2020.02.13.20022673. [DOI] [Google Scholar]
- 45. Liang Y, Liang J, Zhou Q, et al. Prevalence and clinical features of 2019 novel coronavirus disease (COVID‐19) in the Fever Clinic of a teaching hospital in Beijing: a single‐center, retrospective study. medRxiv. 2020.02.25.20027763. Epub 28 February 2020. 10.1101/2020.02.25.20027763. [DOI] [Google Scholar]
- 46. Balin BJ, Hudson AP. Herpes viruses and Alzheimer's disease: new evidence in the debate. Lancet Neurol. 2018;17:839‐841. [DOI] [PubMed] [Google Scholar]
- 47. Olsen LK, Cairns AG, Aden J, et al. Viral mimetic priming enhances alpha‐synuclein‐induced degeneration: implications for Parkinson's disease. Brain Behav Immun. 2019;80:525‐535. [DOI] [PubMed] [Google Scholar]
- 48. Pinto JM, Wroblewski KE, Kern DW, Schumm LP, McClintock MK. Olfactory dysfunction predicts 5‐year mortality in older adults. PLoS One. 2014;9:e107541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Devanand DP, Lee S, Manly J, et al. Olfactory deficits predict cognitive decline and Alzheimer dementia in an urban community. Ann Neurol. 2015;84:182‐189. [DOI] [PMC free article] [PubMed] [Google Scholar]


