Abstract
Following the demonstration of the efficacy of hydroxychloroquine against severe acute respiratory syndrome coronavirus 2 in vitro, many trials started to evaluate its efficacy in clinical settings. However, no systematic review and meta‐analysis have addressed the issue of the safety and efficacy of hydroxychloroquine (HCQ) in coronavirus disease 2019. We conducted a systematic review and meta‐analysis with the objectives of evaluation of safety and efficacy of HCQ alone or in combination in terms of “time to clinical cure,” “virological cure,” “death or clinical worsening of disease,” “radiological progression,” and safety. RevMan was used for meta‐analysis. We searched 16 literature databases out of which seven studies (n = 1358) were included in the systematic review. In terms of clinical cure, two studies reported possible benefit in “time to body temperature normalization” and one study reported less “cough days” in the HCQ arm. Treatment with HCQ resulted in less number of cases showing the radiological progression of lung disease (odds ratio [OR], 0.31, 95% confidence interval [CI], 0.11‐0.9). No difference was observed in virological cure (OR, 2.37, 95% CI, 0.13‐44.53), death or clinical worsening of disease (OR, 1.37, 95% CI, 1.37‐21.97), and safety (OR, 2.19, 95% CI, 0.59‐8.18), when compared with the control/conventional treatment. Five studies reported either the safety or efficacy of HCQ + azithromycin. Although seems safe and effective, more data are required for a definitive conclusion. HCQ seems to be promising in terms of less number of cases with radiological progression with a comparable safety profile to control/conventional treatment. We need more data to come to a definite conclusion.
Keywords: 2019‐nCoV, COVID‐19, hydroxychloroquine, meta‐analysis, SARS CoV‐2
Research Highlights
Following the demonstration of the efficacy of hydroxychloroquine (HCQ) against severe acute respiratory syndrome coronavirus 2 in vitro, many trials were started to evaluate its efficacy in clinical settings.
However, no systematic review and meta‐analysis have addressed the issue to the safety and efficacy of HCQ in coronavirus disease 2019 (COVID‐19).
We searched 16 literature databases finally seven articles were identified, which evaluated the therapeutic efficacy of HCQ either alone or in combination with azithromycin in COVID‐19.
RevMan was used for meta‐analysis. For continuous outcome, mean difference and for a dichotomous outcome, odds ratio with 95% confidence interval was calculated. Model selection (fixed effect or random effect) was done on the basis of heterogeneity among different studies.
Three studies reported the safety and efficacy of HCQ vs control/conventional treatment in terms of safety and efficacy in COVID‐19.
Treatment with HCQ resulted in benefit in terms of less number of cases with radiological progression with no increase in adverse events when compared with control/conventional/standard treatment.
We can also expect a benefit in terms of a number of days to body temperature normalization and number of cough days.
However, at this current point of time, no difference was seen in terms of virological cure on day 6 to 7 postinitiation of therapy and composite death or worsening of the disease.
Four studies reported efficacy and five studies reported safety of the HCQ + azithromycin combination (out of which three studies from the same group).
Although the combination of HCQ + azithromycin seems to be effective and safe, we need more controlled studies to come to an effective conclusion. However, electrocardiographic monitoring is essential while using this combination due to the risk of QT prolongation.
Abbreviations
- Azi
azithromycin
- COVID‐19
coronavirus disease 2019
- HCQ
hydroxychloroquine
- OR
odds ratio
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) has been declared a global pandemic by WHO. 1 , 2 However, we have limited therapeutic options. 2 , 3 With the rising toll of infected populations and rising number of deaths, the preferential global concern is the development of safe and effective therapeutics against COVID‐19. Although lopinavir/ritonavir was initially a first‐line agent in the management of the disease, a study conducted by Cao et al 4 compared lopinavir‐ritonavir with the standard of care and no benefit was observed in the primary endpoint.
However, at the same time chloroquine (CQ) emerged as a potent inhibitor of severe acute respiratory syndrome‐coronavirus‐2 (SARS‐CoV‐2) in vitro. 5 In SARS‐CoV‐2 infected Vero‐E6 cell lines, low‐micro molar concentration of CQ inhibited virus infection (EC50 = 1.13 μM) with high selectivity index (SI > 88.50). 5 CQ increases endosomal pH and also alters glycosylation of angiotensin‐converting enzyme 2 receptors, thus altering the pathogenesis in vitro. 6 , 7 , 8 Besides, CQ also has immunomodulatory activity 5 and enhances the activity of regulatory T cells. 9 Beneficial effect of CQ came to light in recent clinical studies also. In a randomized clinical trial, CQ (n = 10 patients) performed better than lopinavir‐ritonavir (n = 12) combination 10 any may represent an effective and in‐expensive option. However, CQ is one of the major treatment options against malaria and overuse may lead to resistance. Again the toxicity profile of CQ is also a major concern. 11
Hydroxychloroquine (HCQ), being a less toxic derivative, came to the limelight soon. 12 One group found CQ to be more potent than HCQ, 12 however, another research group found HCQ to be more potent. 13 In the study by Yao et al, 13 the EC50 to inhibit the virus in SARS‐CoV infected Vero cells was 0.72 µM 13 and using a physiologically based pharmacokinetic modelling model, they established that this concentration can be attained by a loading dose of HCQ 400 mg BD on the first day, followed by 200 mg BD for SARS‐CoV‐2. 13 Following these findings many clinical trial started worldwide, comparing HCQ to other treatment regimes, however, until now the results of only seven studies are reported. In this regard, we have conducted the first systematic review and meta‐analysis to evaluate the efficacy and safety of HCQ in clinical settings.
2. MATERIALS AND METHODS
This systematic review was done according to the fundamentals laid in the Cochrane Handbook for Systematic Reviews of Interventions 14 and described as stated by Preferred Reporting Items for Systematic reviews and Meta‐Analysis (PRISMA) statement. 15
Objective: To evaluate the safety and efficacy of hydroxychloroquine in the treatment of patients with COVID‐19.
2.1. Criteria for including studies in this review
Setting: In the systematic review part of the study, we included case series, single‐group prospective observational/interventional studies, other observational study designs and randomized controlled trials (RCTs). However, in the meta‐analysis part of the study, only comparative clinical studies (both prospective and retrospective observational studies) and RCTs were included.
Participants: Patients with lab‐confirmed COVOID‐19 of any age were included.
Intervention: Hydroxychloroquine.
Control: Standard/conventional therapy.
Objectives:
-
1.
Clinical cure (time to body temperature normalization and time to cough relief).
-
2.
Virological cure on day 6 to 7 postinitiation of therapy.
-
3.
Death or clinical worsening of disease condition during treatment.
-
4.
Radiological progression during drug treatment.
-
5.
Recurrence of infection during treatment.
-
6.
Safety and tolerability of HCQ.
Comparisons:
-
A.
HCQ vs conventional therapy/control.
-
B.
HCQ in combination of other agents vs conventional therapy/control.
Definitions:
-
1.
Clinical recovery:
Time to clinical recovery is calculated in terms of time to normalization of body temperature and total no of cough days. 16 The clinical cure parameters are important as one of the patient, who became polymerase chain reaction (PCR) negative following therapy, still died of the disease in the study by Gautret et al 17 and another in India. 18
-
2.
Virological cure: Nondetection of SARS‐CoV‐2 in nasopharyngeal swab. 17
-
3.
Recurrence of infection: Defined as swab becoming negative for SARS‐CoV‐2, which is again becoming positive after some days. 17
Search strategy:
Electronic searches: We searched a total of 16 literature databases (Pubmed, CINAHL, SCOPUS, OVID, Wiley online library, Web of Science, Cochrane CENTRAL, Embase, medRxiv and bioRxiv, Trip Database, Nature, Epistemonikos, Science Direct, Virtual Health Library; Pan American Health Organization, CNKI, and mediterranee‐infection.com/pre‐prints‐ihu) from the date of genesis to the 8th April 2020. Reference list of all identified articles was screened to find out more relevant articles. There were no language restrictions. The search keywords included 2019‐nCoV, 2019 novel coronavirus, COVID‐19, coronavirus disease 2019, chloroquine, hydroxychloroquine, Plaquenil, hydroxychloroquine sulfate, and hydroxychloroquine sulfate. Details of the search strategy of each of the databases are represented in Table S1.
2.2. Selection of studies
After a search of databases and removal of duplicates, two authors (HK and HRDK) independently screened the titles/abstracts using the selection criteria. For relevant articles, full texts were obtained for further evaluation. In case of any discrepancy PS and BM were consulted and the issue was resolved.
2.2.1. Data extraction
Data extraction was done separately by two authors (PS and HRDK) using pretested data extraction forms following the template provided by the Cochrane data extraction form. In articles published in a language other than English, Google translate was used to identify relevant data.
2.2.2. Risk of bias evaluation of included studies
For RCTs, we used the Cochrane risk of bias tool for randomized controlled studies. 14 In the case of nonrandomized interventional studies, we used ROBINS‐I tool. 19 In the case of observational studies, Newcastle Ottawa Scale was used. 20 Three investigators (DM, HRDK, and PS) separately evaluated the possibility of bias using these tools. Publication bias was not evaluated by funnel plot as there was only three studies which were included in the meta‐analysis part of the study. 21
2.3. Assessment of heterogeneity
Statistical heterogeneity was evaluated by χ 2 test and I 2statistics. 14 An I 2 value of 0 < 40% is not considered as significant, 30% to 60% is taken as moderate heterogeneity, 50% to 90% considered as substantial heterogeneity, and 75% to 100% is considered as significant heterogeneity.
2.3.1. Statistical analysis
As all the data were dichotomous data, we used either odds ratio (OR) or risk ratio (RR) as appropriate for estimating the point estimate along with 95% confidence interval (CI). In the absence of significant clinical heterogeneity, we have performed the meta‐analysis using the Mantel Hazel method or inverse variance method for dichotomous data and continuous data respectively. When statistical heterogeneity was low to moderate, we used a fixed‐effect model for pooling of data otherwise, the random‐effects model was applied. 14
3. RESULT
We screened a total of 16 literature databases and identified 278 nonduplicate articles, which were evaluated for possible inclusion using title and abstract. Out of these, 25 articles were selected for full‐text screening and finally, seven articles (total participants = 1358) were included in the systematic review and three articles were included in the meta‐analysis. About 18 articles were excluded following full‐text screening (reasons: editorial = 3, review = 5, expert consensus/recommendation = 3, chloroquine = 3, in vitro = 2, in‐silico = 1, and kinetic study = 1). The PRISMA chart for the included studies is showed in Figure 1. The details of the included studies are shown in Table 1. Among these four articles 16 , 22 , 24 were in preprint versions.
Figure 1.
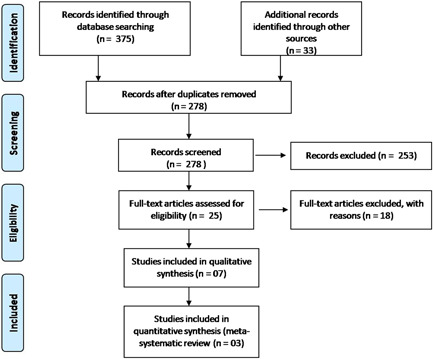
PRISMA flow chart of the included studies. PRISMA, Preferred Reporting Items for Systematic reviews and Meta‐Analysis
Table 1.
Details of included studies
| Study (author) | Population | Intervention | Control | Outcome | AE of HCQ | AE in control arm | Remark |
|---|---|---|---|---|---|---|---|
| Gautret et al 17 | Confirmed COVID‐19 cases age > 12 y | 36 Patients received HCQ. Among these, six cases lost to follow‐up | N = 16 | Virological cure on d 3: | One patient in HCQ arm died despite PCR negativity on d 3 | Not mentioned | In HCQ + azithromycin arm, one case negative on d 6, positive at low titer on d 8 |
| Both groups comparable in terms of gender, clinical status, and duration of symptoms | H alone (n = 14) | No details of the treatment of the control population | H alone: 35.7% | One patient stopped treatment due to nausea and vomiting | |||
| HCQ treated patients were older (51.2 y) compared with control (37.3 y) | H + A (n = 6). | H + A: 83.3% | |||||
| HCQ 200 mg three times a d during 10 d | C: 6.3% | ||||||
| Mean serum HCQ concentration = 0.46 µg/mL | Virological cure on d 6: | ||||||
| Dose of azithromycin: 500 mg day1, followed by 250 mg daily for next 4 d. | H: 57.1% | ||||||
| Daily ECG | H + A: 100% | ||||||
| C: 12.5% | |||||||
| Complete HCQ failure: two cases (mother and son) | |||||||
| Jun et al 25 | 30 Treatment‐naive patients with confirmed COVID‐19 | HCQ (n = 15) | Control (n = 15) | No difference in viral cure between the two groups on d 7 | Four cases (26.7%) of the HCQ group transient diarrhea and abnormal LFT | Three cases (20%) of the control group had transient diarrhea and abnormal LFT | Data extracted only from abstract |
| Patients in HCQ group were given HCQ 400 mg per d for 5 d plus conventional treatments | Conventional treatment alone. However, in the abstract, we could not find details of treatment of control population | ||||||
| Zhaowei et al 16 | 62 Patients with confirmed COVID‐19 | Patients in the HCQ treatment group received additional oral HCQ (HCQ sulfate tablets) 400 mg/d (200 mg/bid) between d 1 and 5 | Standard treatment (oxygen therapy, antiviral agents, antibacterial agents, and immunoglobulin, with or without corticosteroids) | HCQ treatment decreased time to body temperature normalization and number of cough days compared with control. Less number of patients in the HCQ arm showed evidence of radiological progression | Two patients with mild adverse reactions, one patient developed a rash, and one patient experienced headache | None | Detailed use of antiviral in the control group is not given |
| Molina et al 26 | 11 Consecutive patients, however, had significant comorbidity, higher age (58.7, 20‐77), comorbidities present in 8 cases (cancer: 5, HIV: 1, obesity = 2) | HCQ (600 mg/d for 10 d) and azithromycin (500 mg d 1 and 250 mg d 2‐5) | NA | Within 5 d, one died. Two patients required ICU | One patient had QT prolongation, leading to discontinuation of the combination | NA | Patient population were of severe disease and had significant comorbidities |
| Severe COVID‐19 disease population | Higher mean trough concentration of HCQ 0.678 µg/mL (381‐891) after 3‐7 d of treatment initiation | At 5‐6 d after treatment starting, 8 out of remaining 10 patients were still positive for the virus in nasopharyngeal swab | |||||
| Gautret et al 17 | COVID‐19 positive patients (n = 80) | HCQ 200 mg TDS for 10 d + azithromycin (first day 500 mg, 250 mg OD from d 2 to 5) | NA | Death=1 | Minor and infrequent adverse events, which included nausea, vomiting, diarrhea, and blurred vision | NA | Out of 80 patients, details of six patients were also reported in the first study by Gautret et al 17 |
| Discharge = 65/80 (81.25%) | |||||||
| Virological clearance on d 7: 83% | |||||||
| Mean length of hospital stay = 5 d | |||||||
| Chorin at al 22 | COVID‐19 patients (n = 84) | Patients were on HCQ + azithromycin combination | NA | Torsades de pointes = 0 | Significant QTc prolongation in around 11%. | NA | Development of ARF was a strong predictor of extreme QTc prolongation |
| QTc increase by more than 40 ms = 30%, | |||||||
| QTc more than 500 ms = 11% | |||||||
| Million et al 23 | 1061 COVID‐19 patients treated for minimum 3 d with HCQ + azithromycin combination | HCQ + azithromycin | NA | Virological cure on 10th d: 91.7% | No cases of cardiac toxicity were observed. However, details of the procedure for assessment of cardiac toxicity were not available | NA | Predictors of poor outcome were older age, initial higher severity of disease and low HCQ serum concentration |
| Mortality: 0.47% | |||||||
| Total cured till the publication of study report = 98% |
Abbreviations: AE, adverse event; ARF, acute renal failure; COVID‐2019, coronavirus disease 2019; ECG, electrocardiogram; HCQ, hydroxychloroquine; ICU, intensive care unit; LFT, liver function test; NA, not applicable.
3.1. Risk of bias of the included studies
We used Cochrane risk of bias tool for RCTs for evaluation of risk of bias in case of two RCTS. 16 , 25 Data showed in Figure S1. Risk of bias assessment of the study by Gautret et al 24 was done by using ROBINS‐I scale 19 and it was ranked as low, moderate, serious, or critical risk of bias 27 (Data shown in Table S2). The study by Molina et al, 26 Chorin at al, 22 the second study of Gautret et al, 24 and Million et al 23 were single‐group studies. Details of risk of bias analysis of these studies are showed in Table S3.
3.2. Comparison 1: HCQ vs control/conventional/standard therapy
A total of three studies 16 , 17 , 25 reported HCQ vs control/conventional therapy (total n = 128) in terms of efficacy and safety.
-
1.
Clinical cure:
-
a.
Time to body temperature normalization:
Two studies reported mean/media time to temperature normalization (normalization sustained minimum for 72 hours). In the study by Jun et al, 25 time to normalization (median and range) of body temperature was 1 (0‐3) days in the HCQ group, while in the control group it was 1 (0‐2) days. In the study by Zhaowei et al, 16 treatment with HCQ resulted in significantly less time for normalization of body temperature (2.2 ± 0.4 days) compared with the control group (3.2 ± 1.3 days). 16
-
b.
Duration of cough
A single study by Zhaowei et al, 16 the number of cough days was significantly lower in the HCQ group (2.0 ± 0.2 days) compared to the control group (3.1 ± 1.5 day).
-
2.
HCQ vs control: Virological cure at day 6 to 7 postinitiation of therapy:
Both the studies reported virological cure (n = 29 in HCQ alone arm vs n = 31 in control arm) on day 6 to 7. No difference was observed between the two arms in terms of virological cure (OR of virological cure 2.37; 95% CI, [0.13‐44.53]). As there was high heterogeneity (I 2 = 72%), we used the random‐effect model. Data showed in Figure 2.
-
3.
HCQ vs control: Death or clinical worsening of disease/progression to severe disease (LOCF model):
All the three studies (n = 66 in HCQ alone arm and n = 62 in the control arm) reported death or clinical worsening of disease despite treatment with the designated interventions. In the study by Gautret et al, 24 a total of 20 patients were given HCQ alone (out of which six lost to follow‐up). In the analysis, we used the last observation carried forward (LOCF) model for analysis. In case the patient required intensive care unit (ICU) during treatment, it was considered worsening of a clinical condition.
In terms of death or clinical worsening of disease (composite), no difference was seen between the two arms with OR, 1.37 (95% CI, 0.09‐21.97). As there was moderate heterogeneity (I 2 = 59%), we used random‐effect model. Data showed in Figure 3.
-
4.
HCQ vs control: Radiological progression during treatment:
In terms of CT evidence of radiological progression of pneumonia/lung damage (n = 46 both the HCQ group and control/standard treatment group), treatment with HCQ resulted in a significant decrease in the radiological progression with OR, 0.31; 95% CI, (0.11‐0.9). As the heterogeneity among the studies was low (I 2 = 16%), we used the fixed‐effect model. Data showed in Figure 4.
-
5.
HCQ vs control: Recurrence (PCR negativity initially during treatment, which is followed by recurrence of PCR positivity) of infection during treatment:
In the study by Gautret et al, 24 one patient who was on both HCQ and azithromycin (Azi) tested negative on day 6 post inclusion, however, he became PCR positive gain on day 8 post inclusion. 17
-
6.
HCQ vs control: Safety:
Figure 2.
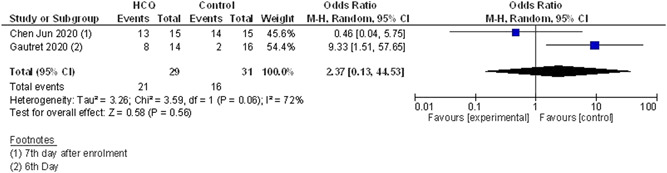
Virological cure (HCQ vs control/conventional treatment). CI, confidendence of interval; df, degrees of freedom; HCQ, hydroxychloroquine
Figure 3.
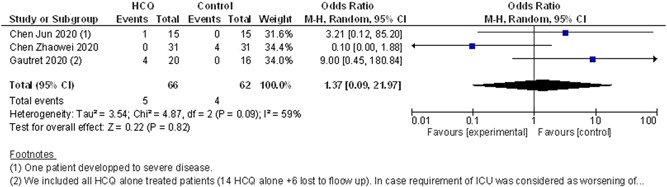
Composite endpoint of death or clinical worsening of disease/progression to severe disease (HCQ vs control/conventional treatment). CI, confidendence of interval; HCQ, hydroxychloroquine; ICU, intensive care unit
Figure 4.
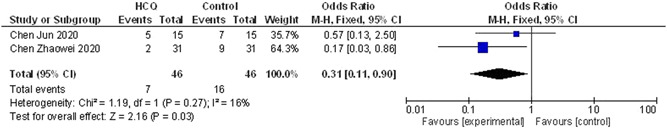
Number of cases showing evidence of radiological progression during therapy (HCQ vs control/conventional treatment). CI, confidendence of interval; HCQ, hydroxychloroquine
A total of seven adverse events is reported in the HCQ group (n = 66). On the other hand, in the standard treatment group, three adverse events were reported (n = 62). In the study by Jun et al, 25 four cases (26.7%) of the HCQ group and 3 cases (20%) of the control group had transient diarrhea and abnormal liver function. In the study by Zhaowei et al, 16 two patients in the HCQ arm showed mild adverse reactions, one patient developed a rash, and one patient experienced headache. In the study by Gautret et al, 17 one patient stopped treatment on day 3 due to nausea and one case of death in the HCQ arm (as there was no information regarding the details of the death case, the authors did not include it as adverse effect). However, when the results were combined, no significant difference was seen between the two arms with regard to the occurrence of adverse effects (OR, 2.19; 95% CI, [0.59‐8.18]). As there was very low heterogeneity (I 2 = 0%), we used the fixed‐effect model (data shown in Figure 5).
Figure 5.
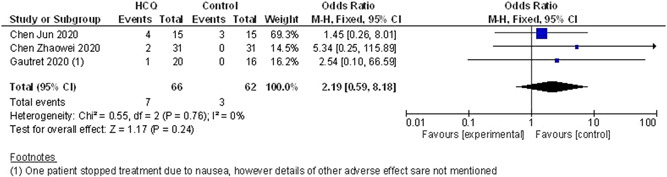
Safety issues (HCQ vs control/conventional treatment). CI, confidendence of interval; HCQ, hydroxychloroquine
3.2.1. Sensitivity analysis
As there is a huge criticism 28 about the first study on HCQ by Gautret et al, 17 and the high risk of bias of the study, we conducted a sensitivity analysis and conducted the same set of analysis as mentioned above by removing the study data of Gautret et al 17 from the analysis. However, irrespective of removal of the study, the study conclusions did not change in terms of virological cure on day 6 to 7 postinitiation of therapy, death or clinical worsening of disease/progression to severe disease, the radiological progression of lung disease during treatment and safety. The details of the sensitivity analysis are illustrated in Figure S2 to S4. After removing the first study by Gautret et al, 17 also, in meta‐analysis part of the study, a benefit was seen only in terms of radiological progression of lung disease during treatment with comparable safety to the control/conventional treatment.
3.3. Comparision 2: HCQ along in combination with other agents vs control/standard therapy
A total of four studies evaluated efficacy of HCQ + Azi 17 , 23 , 24 , 26 and five studies 17 , 22 , 24 , 26 evaluated safety of the combination. Among these two studies were by Gautret et al 17 and the population of patients in the first study was also part of the second study.
Coming to the efficacy of the combination in patients with COVID‐19, in the first study by Gautret et al, 17 the use of HCQ + Azi (n = 6) combination resulted in 100% virological cure, compared with 57.1% virological cure in the HCQ alone arm (n = 14) and 12.5% virological cure in the control arm (n = 16). However detailed safety data is not available for the same. In the second single arm study by the same authors with a higher sample size (n = 80 COVID‐19 cases), a virological cure was seen in 83% of patients on day 7 and in 93% of patients on day 8. At the time of publishing the study report, 65 patients were discharged from the hospital with a mean length of hospital stay of 4.6 days. 24 In another report by the same group (n = 1061), the virological cure was seen in 91.7% of participants by day 10 and poor outcome was seen in only 4.3% patients with a mortality rate of 0.47%. 23 On the other hand, another published single‐group prospective study, 26 which evaluated the efficacy of the same combination at the same dose did not find significant efficacy of the combination in terms of virological cure (8 out of 10 positive for the virus at 5‐6 days after therapy). Among the 11 patients enrolled, one patient died, two needed ICU. However, the patient population was of severe COVID‐19 with significant comorbidities present in eight cases out of 11.
Coming to the safety profile of the combination, In the second study by Gautret et al, 24 the reported adverse effects of the combination treatment were nausea and vomiting (2.5%), diarrhea (5%), and blurring of vision (1.2%). In the study by Million et al, 23 none of the participants showed sign of cardiac toxicity. 23 In the study by Molina et al, 26 a single patient showed persistent QT prolongation and the medications had to be withdrawn. Chorin et al, 22 found that among 84 patients they recruited, in 30% of patients, QTc prolonged by more than 40 milliseconds and 11% of patients showed QTc of more than 500 milliseconds. In multivariate analysis, they found that development of acute renal failure was a more stringent predictor of extreme QTc prolongation. 22
4. DISCUSSION
While comparing HCQ vs control/conventional treatment, two studies reported clinical cure parameters. The importance of clinical cure parameters can be highlighted by the fact that in the study by Gautret et al, 17 one patient died despite having a virological cure. In our study, in terms of clinical cure parameters, two studies reported that time to body temperature normalization was less in the HCQ arm compared with the control/conventional treatment/standard treatment arm. On the other hand, total cough days were also lower in the HCQ treated arm compared with the control/conventional treatment/standard treatment arm. The benefit was also seen in terms of radiological progression (two studies) with less number of patients showing radiological progression in the HCQ arm compared with the control/conventional treatment/standard treatment arm (OR for radiological progression of disease during treatment with OR, 0.31; 95% CI, [0.11‐0.9]). On the other hand, no difference was found in terms of virological cure on day 6 to 7 (two studies), death or clinical worsening of disease condition (three studies). However, limited sample size and nondefining the treatment of control group/standard treatment/conventional treatment are major limitations. Coming to safety, there was no difference seen between the conventional/standard treatment/control arm and the HCQ arm.
Regarding recurrence of the disease during therapy, in the study by Gautret et al, 17 one patient had a recurrence of the disease (being PCR negative initially, followed by a return of PCR positivity, while still on treatment with HCQ and Azi). 17 So, the development of resistance to the drugs during therapy may be a possibility, which we need to be monitored closely.
As the study by Gautret et al, 17 showed a high risk of bias, we conducted a sensitivity analysis and reanalyzed the same endpoints while excluding the data of Gautret et al. 17 However, the final conclusions remained the same despite the exclusion of the study. Details of the sensitivity analysis can be found in Figure S2 to S4.
To address the issue of combining HCQ with other drugs, the two studies by Gautret et al, 17 , 24 and another study by the same group 23 showed benefit in terms of efficacy of a combination of HCQ + Azi in COVID‐19. In the first study, 17 treatment with the combination resulted in 100% virological cure on day 6 post inclusion in the combination arm (n = 6), compared with 57.1% virological cure in the HCQ alone arm (n = 14) and 12.5% virological cure in the control arm (n = 16). In the second study also, 24 they found virological clearance (total sample size = 80) in 83% of patients on day 7. Treatment with HCQ resulted in a shorter duration of hospital stay. 24 However the six patients in the first study were also included in the second study. In the third study by the same group, 23 virological cure was seen among 91.7% of the patients on day 10 with a very low mortality rate (0.47%). On the contrary, Molina et al, 26 failed to replicate the findings of the obtained by Gautret et al. 17 However, in the study by Molina et al, 26 the patient population also had significant comorbidities (cancer, HIV, and obesity) and the patient population included belonged to severe COVID‐19 category. We need further controlled studies for an effective conclusion.
Coming to safety issues of the combination, mild adverse events were reported by Gautret et al, 2020 which included nausea, vomiting, diarrhea and blurring of vision. 24 In the study by Molina et al, 2020 a single patient showed electrocardiographic evidence of QT prolongation. 26 Chorin et al, 2020 found that around 11% of the population on the combination therapy showed evidence of significant QTc prolongation (> 500 ms) and development of acute renal failure was an important predictor of extreme QTc prolongation. 22
The key limitations of our study were a limited number of clinical studies with a limited number of participants and three studies being reported from the same group.
Another major limitation was the lack of control/conventional/standard group. In case of Jun et al, 25 the control group was treated with conventional treatment. However, it is unknown what was basically given to treat the control arm. Many of the blogs claim that the control arm was treated with Kelatra (lopinavir‐ritonavir combination) and arbidol. All the patients in both the arms were also treated with interferon‐alpha. 29 But the authenticity of this blog is unknown though. In the study by Gautret et al, 17 patients who met exclusion criteria or who are not given hydroxychloroquine were treated as controls. However, how these controls were treated is unknown. In the study by Chen Z et al, 2020 also, details of treatment of the control/conventional treatment group is not available. 16
Another concern is the return of PCR positivity in a patient who was turned PCR negative by treatment with HCQ + Azi. This highlights the possibility of occurrence of resistance to this regime. However this needs further validation.
5. CONCLUSION
Treatment with HCQ may result in a benefit in terms of less number of cases showing radiological progression, with comparable adverse events profile when compared to control/conventional/standard treatment. We can expect benefit in terms of time to body temperature normalization and a number of cough days. However, at this current point of time, no difference was seen in terms of virological cure on day six to seven postinitiation of therapy and composite death or worsening of disease. The benefit of the HCQ + Azi treatment is uncertain at this current point of time with most of the data being reported by the same research group. We need more clinical studies to come to a definite conclusion.
CONFLICT OF INTERESTS
All the authors declared that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
PS helped in developing the concept; PS, HRDK, MP, NS, SK, RS, AS, DPD, and BM assisted in protocol making; PS, HRDK, and DM conducted database search; HRDK and HK did the title abstract screen; PS, HRDK, HK, MP, NS, and SK helped in screening the full‐text; PS, PA, DPD, AP, and BM handled the discrepancy management; PS and HRDK conducted the data extraction; PS, HRDK, and BM did the data analysis; PS, HRDK, DM, and MP helped in ROB; PA, AB, MP, NS, SK, RS, AS, DPD, and AP wrote the manuscript; and all the authors gave the final approval for the article.
Supporting information
Supporting information
ACKNOWLEDGMENTS
The authors acknowledge PGIMER, Chandigarh Library for their kind support during this period of lockdown.
Sarma P, Kaur H, Kumar H, et al. Virological and clinical cure in COVID‐19 patients treated with hydroxychloroquine: A systematic review and meta‐analysis. J Med Virol. 2020;92:776–785. 10.1002/jmv.25898
Phulen Sarma and Hardeep Kaur contributed equally to this study.
REFERENCES
- 1. Coronavirus Disease (COVID‐19) ‐ events as they happen. https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/events‐as‐they‐happen. Accessed March 31, 2020.
- 2. Sarma P, Sekhar N, Prajapat M, et al. In‐silico homology assisted identification of inhibitor of RNA binding against 2019‐nCoV N‐protein (N terminal domain). J Biomol Struct Dyn. 2020;0(ja):1‐11. 10.1080/07391102.2020.1753580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sarma P, Prajapat M, Avti P, Kaur H, Kumar S, Medhi B. Therapeutic options for the treatment of 2019‐novel coronavirus: an evidence‐based approach. Indian J Pharmacol. 2020;52(1):1. 10.4103/ijp.IJP_119_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid‐19. N Engl J Med. 2020;0(0), 10.1056/NEJMoa2001282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐nCoV) in vitro. Cell Res. 2020;30:269‐271. 10.1038/s41422-020-0282-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prajapat M, Sarma P, Shekhar N, et al. Drug targets for corona virus: a systematic review. Indian J Pharmacol. 2020;52(1):56. 10.4103/ijp.IJP_115_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2(1):69. 10.1186/1743-422X-2-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Touret F, de Lamballerie X. Of chloroquine and COVID‐19. Antiviral Res. 2020;177:104762. 10.1016/j.antiviral.2020.104762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thomé R, Moraes AS, Bombeiro AL, et al. Chloroquine treatment enhances regulatory t cells and reduces the severity of experimental autoimmune encephalomyelitis. PLoS One. 2013;8(6):e65913. 10.1371/journal.pone.0065913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang M, Tang T, Pang P, et al. Treating COVID‐19 with chloroquine. J Mol Cell Biol. 2020. 10.1093/jmcb/mjaa014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weniger H, Organization WH Review of side effects and toxicity of chloroquine. 1979. https://apps.who.int/iris/handle/10665/65773. Accessed March 31, 2020.
- 12. Liu K, Tang M, Liu Q, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS‐CoV‐2 infection in vitro. Cell Discov. 2020;6(1):1‐4. 10.1038/s41421-020-0156-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Clin Infect Dis. 2020;ciaa237. 10.1093/cid/ciaa237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cochrane Handbook for Systematic Reviews of Interventions. /handbook. Accessed December 7, 2018. [Google Scholar]
- 15. PRISMA. http://prisma‐statement.org/. Accessed December 7, 2018.
- 16. Zhaowei C, Hu J, Zhang Z, et al. Efficacy of hydroxychloroquine in patients with COVID‐19: results of a randomized clinical trial. MedRxiv. 2020. 10.1101/2020.03.22.20040758 [DOI] [Google Scholar]
- 17. Gautret P, Lagier J‐C, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial. Int J Antimicrob Agents. 2020:105949. 10.1016/j.ijantimicag.2020.105949 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18. Italian tourist, who had recovered from COVID‐19, dies in Jaipur hospital from cardiac arrest. Deccan Herald. https://www.deccanherald.com/national/italian‐tourist‐who‐had‐recovered‐from‐covid‐19‐dies‐in‐jaipur‐hospital‐from‐cardiac‐arrest‐815720.html. Accessed April 2, 2020.
- 19. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomised studies of interventions. BMJ. 2016;355:355. 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ottawa Hospital Research Institute . http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed April 1, 2020.
- 21. Maneeton N, Maneeton B, Putthisri S, Woottiluk P, Narkpongphun A, Srisurapanont M. Risperidone for children and adolescents with autism spectrum disorder: a systematic review. Neuropsychiatr Dis Treat. 2018;14:1811‐1820. 10.2147/NDT.S151802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chorin E, Matthew D, Shulman E, et al. The QT interval in patients with SARS‐CoV‐2 Infection treated with hydroxychloroquine/azithromycin. medRxiv. 2020:20047050. 10.1101/2020.04.02.20047050. www.medrxiv.org [DOI] [Google Scholar]
- 23. Million M, Lagier J‐C, Gautret P, Colson P, Fournier P‐E, Amrane S. Early treatment of 1061 COVID‐19 patients with hydroxychloroquine and azithromycin, Marseille, France. Mediterr‐Infect. 2020. https://www.mediterranee‐infection.com/early‐treatment‐of‐1061‐covid‐19‐patients‐with‐hydroxychloroquine‐and‐azithromycin‐marseille‐france/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gautret P, Lagier J‐C, Parola P, Hoang VT, Meddeb L, Sevestre J. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID‐19 patients with at least a six‐day follow up: an observational study. Mediterr‐Infect. 2020:101663. https://www.mediterranee‐infection.com/wp‐content/uploads/2020/03/COVID‐IHU‐2‐1.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jun C, Danping L, Li L, et al. A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease‐19 (COVID‐19). J Zhejiang Univ Med Sci. 2020;49(1):0‐0. 10.3785/j.issn.1008-9292.2020.03.03 [DOI] [Google Scholar]
- 26. Molina JM, Delaugerre C, Le Goff J, et al. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID‐19 infection. Médecine Mal Infect. 2020. 10.1016/j.medmal.2020.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dhiman P, Lee H, Kirtley S, Collins GS. A systematic review showed more consideration is needed when conducting nonrandomized studies of interventions. J Clin Epidemiol. 2020;117:99‐108. 10.1016/j.jclinepi.2019.09.027 [DOI] [PubMed] [Google Scholar]
- 28. Marcus AA. Hydroxychloroquine‐COVID‐19 study did not meet publishing society's “expected standard.” Retraction Watch . April 2020. https://retractionwatch.com/2020/04/06/hydroxychlorine‐covid‐19‐study‐did‐not‐meet‐publishing‐societys‐expected‐standard/. Accessed April 10, 2020.
- 29. Did chloroquine really fail a COVID‐19 study—or was the trial design to blame? FiercePharma. https://www.fiercepharma.com/pharma‐asia/did‐chloroquine‐really‐fail‐a‐covid‐19‐study‐or‐was‐it‐just‐trial‐design‐s‐fault. Accessed March 30, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


